Abstract
To assess the efficacy and safety of dexmedetomidine (DEX) as an adjuvant to local wound infiltration anaesthesia in abdominal surgery, we conducted this meta‐analysis. First, the systematic search strategy was performed on PubMed, Embase, and Cochrane Library and five randomised controlled trials (RCTs) involving 294 patients were included. Then, the outcome data were extracted from the studies and their effect sizes were calculated using Review Manager 5. As a result, the addition of DEX significantly reduced visual analogy scores at 6 hours after surgery (mean difference = −0.53[−0.82, −0.25], P < .001), 12 hours after surgery (mean difference = −0.39 [−0.73, −0.05]; P = .03), and 24 hours after surgery (mean difference = −0.20 [−0.29, −0.11], P < .001) and reduced total analgesic consumption within 24 hours after surgery (mean difference = −4.92 [−9.00, −0.84]; P = .02) compared with placebo groups. However, there was no difference in the incidence of postoperative nausea and vomiting (risk ratio = 0.68 [0.41, 1.14]; P = .14). In summary, DEX as a local anaesthetic adjuvant added for local wound infiltration anaesthesia in abdominal surgery could reduce visual analogy scores and postoperative analgesic consumption without changing incidence of postoperative nausea and vomiting.
Keywords: abdominal surgery, adjuvant, dexmedetomidine, local wound infiltration
1. INTRODUCTION
Presently, minimally invasive surgery and open surgery are two methods in abdominal operations.1 Both methods cannot avoid a postoperative acute wound pain. Acute wound pain is a nociceptive pain in the wound region of surgery, including peripheral sensitisation, secondary hyperalgesia, and spontaneous pain. It not only affects rapid rehabilitation but also reduces the perioperative quality of life and, therefore, has a negative effect on the patient's prognosis.2 At present, the treatments for postoperative wound pain are mainly based on intravenous or oral opioids and non‐steroidal anti‐inflammatory drugs (NSAIDs), such as fentanyl, morphine, and flurbiprofen. However, the use of opioids may cause a series of adverse reactions: postoperative nausea and vomiting (PONV), itching, respiratory depression, urinary retention, etc.3, 4
To reduce the side effects of opioids, multimodal analgesia has been used in perioperative pain management. As a new method of multimodal analgesia, local wound infiltration anaesthesia plays an important role in alleviating postoperative acute wound pain, reducing opioid use, and facilitating fast recovery.5, 6 This method uses only local anaesthetic drugs, such as ropivacaine and bupivacaine,7, 8, 9, 10, 11, 12 and provides adequate analgesic effects. In recent years, it has been found that adding adjuvants to local anaesthetics can improve the quality and duration of analgesia. Common adjuvants include adrenaline, clonidine, opioids, etc.13 Although these adjuvants can help reduce postoperative acute pain, they are not devoid of side effects.14, 15, 16
Dexmedetomidine (DEX) is a highly selective alpha 2‐adrenergic receptor agonist, which has been used as an adjuvant in local anaesthetics. A few studies have shown that DEX could be used as an adjuvant for peripheral nerve block and spinal anaesthesia.17, 18, 19, 20, 21 Does DEX provide a similar effect on local wound infiltration in abdominal operation? At present, some clinical randomised controlled trials (RCTs) have studied this problem.22, 23, 24, 25, 26 Therefore, we wanted to see whether DEX could improve analgesia when used in combination with local anaesthetics for wound infiltration after abdominal surgery.
2. METHODS
2.1. Search strategy
Based on the quality of reporting of meta‐analysis guidelines and the recommendations from the Cochrane Collaboration, a systematic search was performed on MEDLINE, Embase, and the Cochrane Central Register of Controlled Trials. The search strategy contained the following keywords: (DEX) and (local anaesthetics, ropivacaine, and bupivacaine) and (local wound infiltration, abdominal operation). The retrieval time was from the time of database establishment to November 2018. A manual search was also performed for selected articles and published reviews. Because this study is a meta‐analysis, ethical recognition was unnecessary, and informed consent was not given.
2.2. Study selection
Studies were included if they met the following criteria: (a) RCTs, (b) local wound infiltration was performed before or after operation, (c) adult patients (≥18 years old), (d) the experimental group included the comparison of DEX with local anaesthetics and local anaesthetics alone, at least,(e) abdominal operation, including minimally invasive surgery and open surgery, and (f) availability of full‐text publication in English. Studies were excluded if they: (a) were abstracts only, (b) were duplications, (c) had data loss, and (d) inaccurate statistical analysis was performed in the study. The operation technique, the dosage of DEX, and the dosage and type of local anaesthetics were not considerations for inclusion.
2.3. Data retrieval
The extracted information included the name of the main author, the country, the year of publication, the method of surgery, the size of the sample, the dosage in the DEX‐with‐anaesthetic group and in the anaesthetic group, and the outcomes: visual analogue scores (VAS, ranging from 0 to 10; 0 corresponding to no pain and 10 representing worst imaginable pain) at 6, 12, and 24 hours postoperatively, the total analgesic consumption in the 24‐hour postoperative period, the incidence of PONV. The consumption of analgesic drugs was converted to a morphine equivalent and the unit was milligrams; the original data were represented by a median and interquartile range, data conversions were made to a mean and standard deviation (SD) through the methods described by Wan et al.27
2.4. Qualitative assessment
All the selected documents were reviewed by two reviewers (SW and RYF) to evaluate the methodological quality of the included RCTs independently, using the Cochrane Collaboration's risk of bias assessment tool. They evaluated the quality of each article from the random methods, the allocation of the hidden methods, the blind law of the research objects and the implementers, the blind method of the results measurement, the integrity of the result data, the selective report bias, and the other bias sources. Finally, the low‐bias, high‐bias, and unclear judgments were obtained. When they disagreed with each other, they discussed the disagreements to reach consensus or the issue was decided by two other reviewers (NCG and ZXM).
2.5. Statistical analysis
The software Review Manager 5.3 was used for statistical analysis. The effect size of the total analgesic consumption within the first 24 hours and the VAS scores at 6, 12, and 24 hours after surgery were expressed by mean difference (MD) and its 95% confidence interval (CI). The incidence of PONV was expressed by relative risk (RR) and its 95% CI. The Q (χ2) test and I 2 statistics were used for assessing the studies' heterogeneity. If the P value for the Q test <.1 and I 2 < 50%, heterogeneity was considered not significant and the fixed‐effects model was used; otherwise, we assumed that there was significant heterogeneity and used the random‐effects model to calculate effect size and, furthermore, performed the sensitivity analysis to analyse the sources of heterogeneity. P value for effect size <.05 was considered statistically significant.
3. RESULTS
3.1. Search process
The search identified 251 studies, of which 198 were eliminated from further review because of not controlled trials or were duplications; after reviewing the abstracts, an additional 48 trials were excluded because they were not relevant to our study. Finally, the articles considered to be suitable for the meta‐analysis consisted of five RCTs,22, 23, 24, 25, 26 enrolling a total of 294 adult patients. The search process is shown in Figure 1.
Figure 1.
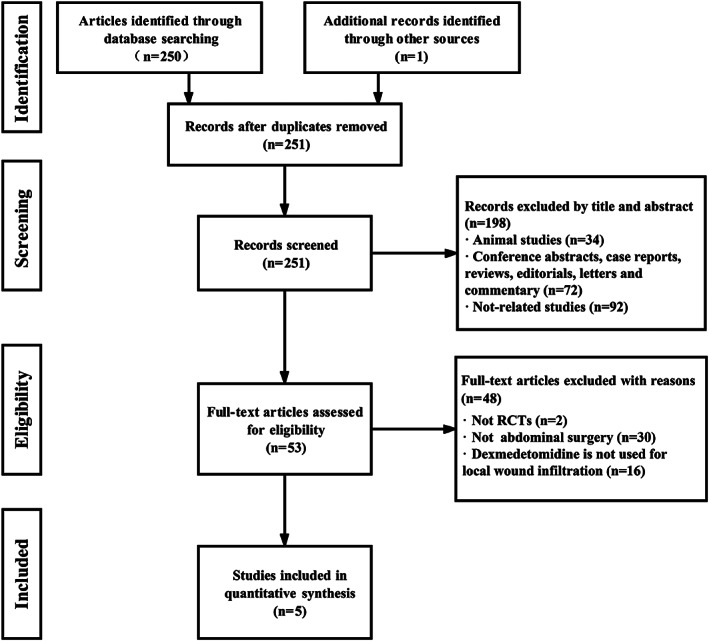
Study flow diagram for inclusion
3.2. Characteristics of included studies
Of the five studies, two studies22, 25 were from India, two studies23, 26 were from China, and one study24 was from Egypt. Four studies22, 23, 24, 25 involved local wound infiltration at the end of the operation, and one study26 concerned local wound infiltration before the operation. Among them, local anaesthetics were ropivacaine in three studies,22, 23, 26 and bupivacaine in the other two studies.24, 25 The concentration range of ropivacaine was 0.3%‐0.75%; concentration of bupivacaine was 0.25%. The dosage of DEX fluctuated between 0.5 μg/kg and about 1.5 μg/kg in all studies. Three studies23, 24, 26 evaluated the VAS scores at 6, 12, and 24 hours in the postoperative period, four studies22, 23, 24, 25 described the total analgesic consumption within 24 hours after surgery and analysed the incidence of PONV. The detailed characteristics of all the included studies are shown in Table 1.
Table 1.
Study characteristics of all randomised trials included in the meta‐analysis
| Studies | Surgery | Measures | Groups (n): Treatment |
|---|---|---|---|
| Bhardwaj et al (2017, India)22 | Lower segment caesarean section | Total analgesic consumption, PONV | DEX (30): 0.75%ropivacaine 3 mg/kg with 1.5 μg/kg DEX |
| PLA (30): 0.75% ropivacaine 3 mg/kg without DEX | |||
| Luan et al (2017, China)23 | Open gastrectomy | VAS scores at 6, 12 and 24 h; Total analgesic consumption; PONV | DEX (23): 0.3% ropivacaine with 1.0 μg/kg DEX |
| PLA (23): 0.3% ropivacaine without DEX | |||
| Mohamed et al (2018, Egypt)24 | Abdominal hysterectomy | VAS scores at 6, 12 and 24 h; Total analgesic consumption; PONV | DEX (30): 0.25% bupivacaine with 2 μg/kg DEX |
| PLA (30): 0.25% bupivacaine without DEX | |||
| Singh and Prasad (2017, India)25 | Abdominal hysterectomy | Total analgesic consumption; PONV | DEX (30): 0.25% bupivacaine with 1.0 μg/kg DEX |
| PLA (28): 0.25% bupivacaine without 1.0 μg/kg DEX | |||
| Yu et al (2016, China)26 | Laparoscopic cholecystectomy | VAS scores at 6, 12 and 24 h | DEX (35): 0.5% ropivacaine with 1.0 μg/kg DEX |
| PLA (35): 0.5% ropivacaine with without DEX |
Abbreviations: DEX, dexmedetomidine; PLA, placebo; PONV, postoperative nausea and vomiting; VAS, visual analogue score.
3.3. Risk of bias of the included studies
The risk assessment was performed to judge the study quality and potential bias. Four studies22, 23, 24, 25 reported the randomisation procedure in detail. All studies mentioned allocation concealment and reported their double‐blinded administration. However, one study26 had high bias risk because of partial data loss in outcomes. The risk‐of‐bias analysis for these studies is detailed in Figure 2.
Figure 2.

Methodological quality and bias risk. Green circle = low bias risk, red circle = high bias risk, yellow circle = unclear bias risk
3.4. The VAS scores at 6, 12, and 24 hours postoperatively
The VAS is often used for assessing pain degree. Three studies23, 24, 26 reported VAS scores at 6 hours after surgery. There was significant heterogeneity among the studies (P = .09, I 2 = 59%), and there was a statistical difference between the two groups (MD = −0.53[−0.82, −0.25], P < .001) (shown in Figure 3). To explore the source of heterogeneity, we did a sensitivity analysis. After the study26 reported by Yu et al was deleted, the statistical heterogeneity was no longer significant, and the absolute value of mean difference was smaller (data not shown).
Figure 3.
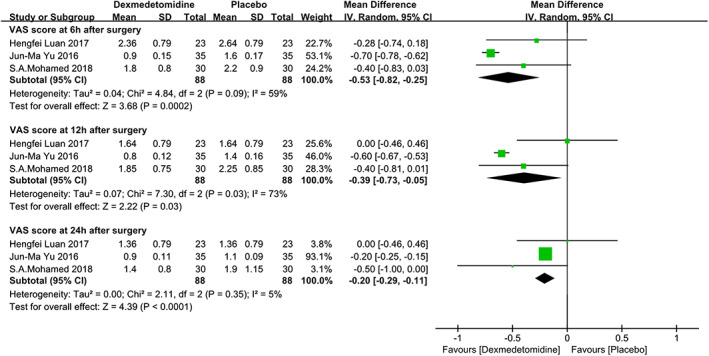
The forest plots of VAS pain score at 6, 12, and 24 hours after surgery. VAS, visual analogue scale
The same studies reported results of the VAS scores at 12 hours after surgery. We found there was still a significant heterogeneity among the studies (P = .03, I 2 = 73%) and there was a statistical difference between the two groups (MD = −0.39 [−0.73, −0.05], P = .03) (see Figure 3). In sensitivity analysis, when we deleted the same study,26 the significant heterogeneity turned over, and the mean difference between the two groups became smaller, similar to the result at 6 hours (data not shown).
For the VAS scores at 24 hours after surgery, the same studies still reported it. There was no significant heterogeneity among the studies (P = .35, I 2 = 5%), and there was a statistical difference between the two groups (MD = −0.20 [−0.29, −0.11], P < .001) (shown in Figure 3).
3.5. Total analgesic consumption at 24 hours postoperatively
Four studies22, 23, 24, 25 reported analgesic requirements within 24 hours after surgery. There was significant heterogeneity among the studies (P < .00001, I 2 = 98%). A random‐effects model was used, and there was significant heterogeneity in analgesic requirements at 24 hours among the studies. A significant difference was detected between the groups (MD = −4.92 [−9.00, −0.84], P = .02; see Figure 4).
Figure 4.
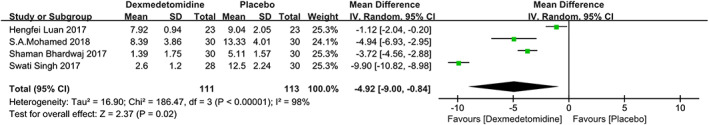
The forest plots of the total analgesic consumption within 24 hours after surgery
3.6. The incidence of PONV
Four studies22, 23, 24, 25 reported the incidence of PONV at 24 hours after surgery. A fixed‐effects model was used, and there was no significant heterogeneity among the studies (P = .96, I 2 = 0%). There was no statistical difference (RR = 0.68 [0.41, 1.14], P = .14; see Figure 5).
Figure 5.
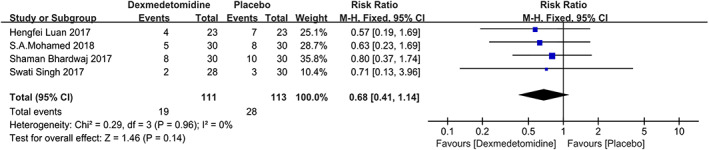
The forest plots of the incidence of PONV within 24 hours after surgery. PONV, postoperative nausea and vomiting
4. DISCUSSION
This meta‐analysis shows that DEX as a local anaesthetic adjuvant used in local wound infiltration of abdominal surgery could reduce the VAS score at 6, 12, and 24 hours postoperatively, decrease the patient's needs for postoperative analgesia, and prolong the duration of analgesia.
The primary outcome was postoperative pain in this meta‐analysis. The VAS pain scores were considered the gold standard for judgement of the degree of pain.28 Because postoperative wound pain is an unpleasant feeling in many patients who undergo abdominal surgery, it has attracted great attention from surgeons.29 Our research found that pain scores were significantly lower in the anaesthetic‐with‐DEX group at 6, 12, and 24 hours after surgery. So what is the mechanism of analgesic effect of DEX used as a local anaesthetic adjuvant for local wound analgesia? We speculate that it might be: First, anti‐inflammatory and analgesic effects by reducing the production of inflammatory cytokines (IL‐6, TNF‐α)30; Second, vasoconstriction mediated by the action of a vascular α‐2 adrenoceptor around the site of the injection, which delays the absorption of the local anaesthetic and prolongs its efficacy31; Third, its action on the peripheral nerve and blockage of an activity‐dependent cation current, blocking the transmission of pain signals.32
In the analysis of the VAS scores at 6 and 12 hours after surgery, however, we found that the results had high heterogeneity among three studies.23, 24, 26 When we performed the sensitivity analysis, the heterogeneity of the results was significantly reduced when the study by Yu et al26 was deleted. We found that the MD of this study is larger than in the other two studies. We consider that two factors may have led to the larger MD. First, although the surgical site was abdomen in all included studies, the surgical methods were different. Especially in this study, the surgical method was laparoscopic cholecystectomy (LC). LC is minimally invasive surgery, which led to less trauma after the operation and less usage of postoperative analgesics.12 Because of this, the pain scores in this study may be more sensitive to local wound infiltration with DEX than the other studies. Second, the local incision infiltration analgesia of this study was performed before the laparoscopy. This method is similar to pre‐emptive analgesia, which can reduce the production of inflammatory factors in advance. The addition of DEX in the experimental group not only produced an early analgesic effect, it also prolonged the duration of local anaesthetic action,33 therefore, lowered the degree of pain much more than in the control group.
PONV are common complications for patients who received opioids. Many researches have agreed that DEX could reduce the incidence and severity of PONV in different surgery.34, 35, 36, 37, 38 Although our meta‐analysis found that the incidence of PONV was decreased in group DEX in our study, there was no significant difference between the anaesthetic‐with‐DEX and the without‐DEX groups. Comparing with the systemic using of DEX, there may be insufficient drug concentration and different mechanisms in local using of DEX. The small sample size and different mode of administration should also be considered.
Furthermore, several limitations should also be considered when explaining our results. First, only five studies were included in this meta‐analysis and all of these studies had a sample size of less than 100 patients; thus, our results may be subject to small study‐effect bias. Second, several continuous outcomes in the included studies were expressed by medians and interquartile ranges instead of mean values and 95% Cl. After we transformed the values into means and standard deviations, there was a risk of bias. Third, there are some clinical heterogeneities between the included studies: the types and dosages of local anaesthetics and the dosages of DEX in the included studies were varied, which may more or less affect the credibility of pooling effects. Fourth, VAS scores at 6, 12, and 24 hours postoperatively were decreased and this is statistically correct (P < .05), but the magnitude of decrease was very small: less than 1. Typically, this is not considered clinically significance.39 Fifth, adding adjuvants to local anaesthetics is an important topic at present, but because of the limited number of studies included, the optimal dosages of DEX are the remaining question, which needed to be addressed in future research.
In conclusion, DEX as a local anaesthetic adjuvant added for local wound infiltration anaesthesia in abdominal surgery could reduce VAS scores and postoperative opioid consumption without changing the incidence of PONV. Meanwhile, more large‐sample and high‐quality RCTs are needed to increase the credibility identified in the current meta‐analysis.
CONFLICT OF INTEREST
No conflicts of interest declared.
ACKNOWLEDGEMENTS
This study was supported by the National Natural Science Foundation of China (81800395) and Science and Technology Department of Henan Province (182102310159).
Ren Y, Shi W, Chen C, et al. Efficacy of dexmedetomidine as an adjuvant to local wound infiltration anaesthesia in abdominal surgery: A meta‐analysis of randomised controlled trials. Int Wound J. 2019;16:1206–1213. 10.1111/iwj.13195
Yifeng Ren and Wei Shi contributed equally as first authors.
Funding information National Natural Science Foundation of China, Grant/Award Number: 81800395; Science and Technology Department of Henan Province, Grant/Award Number: 182102310159
Contributor Information
Xuemei Zheng, Email: zxmmails@163.com.
Chenguang Niu, Email: asdncg@henu.edu.cn.
REFERENCES
- 1. Cash JC, Zehetner J, Hedayati B, et al. Outcomes following laparoscopic transhiatal esophagectomy for esophageal cancer. Surg Endosc. 2014;28(2):492‐499. [DOI] [PubMed] [Google Scholar]
- 2. Cepeda MS, Africano JM, Polo R, Alcala R, Carr DB. What decline in pain intensity is meaningful to patients with acute pain? Pain. 2003;105(1–2):151‐157. [DOI] [PubMed] [Google Scholar]
- 3. Gehling M, Tryba M. Risks and side‐effects of intrathecal morphine combined with spinal anaesthesia: a meta‐analysis. Anaesthesia. 2009;64(6):643‐651. [DOI] [PubMed] [Google Scholar]
- 4. Lee M, Silverman SM, Hansen H, Patel VB, Manchikanti L. A comprehensive review of opioid‐induced hyperalgesia. Pain Physician. 2011;14(2):145‐161. [PubMed] [Google Scholar]
- 5. Rao Z, Zhou H, Pan X, et al. Ropivacaine wound infiltration: a fast‐track approach in patients undergoing thoracotomy surgery. J Surg Res. 2017;220:379‐384. [DOI] [PubMed] [Google Scholar]
- 6. Sun JX, Bai KY, Liu YF, et al. Effect of local wound infiltration with ropivacaine on postoperative pain relief and stress response reduction after open hepatectomy. World J Gastroenterol. 2017;23(36):6733‐6740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hinarejos P, Capurro B, Santiveri X, et al. Local infiltration analgesia adds no clinical benefit in pain control to peripheral nerve blocks after total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2016;24(10):3299‐3305. [DOI] [PubMed] [Google Scholar]
- 8. Kong TW, Park H, Cheong JY, Min SK, Ryu HS. Efficacy of continuous wound infiltration of local anesthetic for pain relief after gynecologic laparoscopy. Int J Gynaecol Obstet. 2014;124(3):212‐215. [DOI] [PubMed] [Google Scholar]
- 9. Kuthiala G, Chaudhary G. Ropivacaine: a review of its pharmacology and clinical use. Indian J Anaesth. 2011;55(2):104‐110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lee KC, Lu CC, Lin SE, Chang CL, Chen HH. Infiltration of local Anesthesia at wound site after single‐incision laparoscopic colectomy reduces postoperative pain and analgesic usage. Hepatogastroenterology. 2015;62(140):811‐816. [PubMed] [Google Scholar]
- 11. Sellami M, Feki S, Triki Z, et al. Bupivacaine wound infiltration reduces postoperative pain and analgesic requirement after thyroid surgery. Eur Arch Otorhinolaryngol. 2018;275(5):1265‐1270. [DOI] [PubMed] [Google Scholar]
- 12. Loizides S, Gurusamy KS, Nagendran M, Rossi M, Guerrini GP, Davidson BR. Wound infiltration with local anaesthetic agents for laparoscopic cholecystectomy. Cochrane Database Syst Rev. 2014;3:CD007049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Demiraran Y, Ilce Z, Kocaman B, Bozkurt P. Does tramadol wound infiltration offer an advantage over bupivacaine for postoperative analgesia in children following herniotomy? Paediatr Anaesth. 2006;16(10):1047‐1050. [DOI] [PubMed] [Google Scholar]
- 14. Choi S, Rodseth R, McCartney CJ. Effects of dexamethasone as a local anaesthetic adjuvant for brachial plexus block: a systematic review and meta‐analysis of randomized trials. Br J Anaesth. 2014;112(3):427‐439. [DOI] [PubMed] [Google Scholar]
- 15. Milic MS, Brkovic B, Krsljak E, Stojic D. Comparison of pulpal anesthesia and cardiovascular parameters with lidocaine with epinephrine and lidocaine with clonidine after maxillary infiltration in type 2 diabetic volunteers. Clin Oral Investig. 2016;20(6):1283‐1293. [DOI] [PubMed] [Google Scholar]
- 16. Nazir N, Jain S. Randomized controlled trial for evaluating the analgesic effect of Nalbuphine as an adjuvant to bupivacaine in supraclavicular block under ultrasound guidance. Anesth Essays Res. 2017;11(2):326‐329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bajwa SJ, Bajwa SK, Kaur J, et al. Dexmedetomidine and clonidine in epidural anaesthesia: a comparative evaluation. Indian J Anaesth. 2011;55(2):116‐121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bisui B, Samanta S, Ghoshmaulik S, Banerjee A, Ghosh TR, Sarkar S. Effect of locally administered Dexmedetomidine as adjuvant to Levobupivacaine in supraclavicular brachial plexus block: double‐blind controlled study. Anesth Essays Res. 2017;11(4):981‐986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Elyazed MMA, Mogahed MM. Comparison of magnesium Sulfate and Dexmedetomidine as an adjuvant to 0.5% Ropivacaine in Infraclavicular brachial plexus block. Anesth Essays Res. 2018;12(1):109‐115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gupta R, Verma R, Bogra J, Kohli M, Raman R, Kushwaha JK. A comparative study of intrathecal dexmedetomidine and fentanyl as adjuvants to bupivacaine. J Anaesthesiol Clin Pharmacol. 2011;27(3):339‐343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hussain N, Grzywacz VP, Ferreri CA, et al. Investigating the efficacy of Dexmedetomidine as an adjuvant to local Anesthesia in brachial plexus block: a systematic review and meta‐analysis of 18 randomized controlled trials. Reg Anesth Pain Med. 2017;42(2):184‐196. [DOI] [PubMed] [Google Scholar]
- 22. Bhardwaj S, Devgan S, Sood D, Katyal S. Comparison of local wound infiltration with Ropivacaine alone or Ropivacaine plus Dexmedetomidine for postoperative pain relief after lower segment Cesarean section. Anesth Essays Res. 2017;11(4):940‐945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Luan H, Zhu P, Zhang X, et al. Effect of dexmedetomidine as an adjuvant to ropivacaine for wound infiltration in patients undergoing open gastrectomy: a prospective randomized controlled trial. Medicine (Baltimore). 2017;96(38):e7950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mohamed SA, Sayed DM, El Sherif FA, Abd El‐Rahman AM. Effect of local wound infiltration with ketamine versus dexmedetomidine on postoperative pain and stress after abdominal hysterectomy, a randomized trial. Eur J Pain. 2018;22(5):951‐960. [DOI] [PubMed] [Google Scholar]
- 25. Singh S, Prasad C. Post‐operative analgesic effect of dexmedetomidine administration in wound infiltration for abdominal hysterectomy: a randomised control study. Indian J Anaesth. 2017;61(6):494‐498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yu JM, Sun H, Wu C, Dong CS, Lu Y, Zhang Y. The analgesic effect of Ropivacaine combined with Dexmedetomidine for incision infiltration after laparoscopic cholecystectomy. Surg Laparosc Endosc Percutan Tech. 2016;26(6):449‐454. [DOI] [PubMed] [Google Scholar]
- 27. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Myles PS, Urquhart N. The linearity of the visual analogue scale in patients with severe acute pain. Anaesth Intensive Care. 2005;33(1):54‐58. [DOI] [PubMed] [Google Scholar]
- 29. Hung CH, Wang JC, Strichartz GR. Spontaneous chronic pain after experimental thoracotomy revealed by conditioned place preference: morphine differentiates tactile evoked pain from spontaneous pain. J Pain. 2015;16(9):903‐912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kim MH, Hahn TH. The effect of clonidine pretreatment on the perioperative proinflammatory cytokines, cortisol, and ACTH responses in patients undergoing total abdominal hysterectomy. Anesth Analg. 2000;90(6):1441‐1444. [DOI] [PubMed] [Google Scholar]
- 31. Yoshitomi T, Kohjitani A, Maeda S, Higuchi H, Shimada M, Miyawaki T. Dexmedetomidine enhances the local anesthetic action of lidocaine via an alpha‐2A adrenoceptor. Anesth Analg. 2008;107(1):96‐101. [DOI] [PubMed] [Google Scholar]
- 32. Brummett CM, Hong EK, Janda AM, Amodeo FS, Lydic R. Perineural dexmedetomidine added to ropivacaine for sciatic nerve block in rats prolongs the duration of analgesia by blocking the hyperpolarization‐activated cation current. Anesthesiology. 2011;115(4):836‐843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jianda X, Yuxing Q, Yi G, Hong Z, Libo P, Jianning Z. Impact of Preemptive analgesia on inflammatory responses and rehabilitation after primary Total knee Arthroplasty: a controlled clinical study. Sci Rep. 2016;6:30354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Song Y, Shim JK, Song JW, Kim EK, Kwak YL. Dexmedetomidine added to an opioid‐based analgesic regimen for the prevention of postoperative nausea and vomiting in highly susceptible patients: a randomised controlled trial. Eur J Anaesthesiol. 2016;33(2):75‐83. [DOI] [PubMed] [Google Scholar]
- 35. Nie Y, Tu W, Shen X, et al. Dexmedetomidine added to Sufentanil patient‐controlled intravenous analgesia relieves the postoperative pain after Cesarean delivery: a prospective randomized controlled Multicenter study. Sci Rep. 2018;8(1):9952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mohta M, Kalra B, Sethi AK, Kaur N. Efficacy of dexmedetomidine as an adjuvant in paravertebral block in breast cancer surgery. J Anesth. 2016;30(2):252‐260. [DOI] [PubMed] [Google Scholar]
- 37. Gao Y, Deng X, Yuan H, et al. Patient‐controlled intravenous analgesia with combination of Dexmedetomidine and Sufentanil on patients after abdominal operation: a prospective, randomized, controlled, blinded Multicenter Clinical Study. Clin J Pain. 2018;34(2):155‐161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dong CS, Zhang J, Lu Q, et al. Effect of Dexmedetomidine combined with sufentanil for post‐ thoracotomy intravenous analgesia:a randomized, controlled clinical study. BMC Anesthesiol. 2017;17(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Myles PS, Myles DB, Galagher W, et al. Measuring acute postoperative pain using the visual analog scale: the minimal clinically important difference and patient acceptable symptom state. Br J Anaesth. 2017;118(3):424‐429. [DOI] [PubMed] [Google Scholar]


