ABSTRACT
For optimal wound bed preparation, wound debridement is essential to eliminate bacterial biofilms. However, it is challenging for clinicians to determine whether the biofilm is completely removed. A newly developed biofilm detection method based on wound blotting technology may be useful. Thus, we aimed to investigate the effect of biofilm elimination on wound area decrease in pressure ulcers, as confirmed using the wound blotting method. In this retrospective observational study, we enrolled patients with pressure ulcers who underwent sharp debridement with pre‐ and post‐debridement wound blotting. Biofilm was detected on the nitrocellulose membrane using ruthenium red or alcian blue staining. Patients were included if the test was positive for biofilm before wound debridement. Percent decrease in wound area after 1 week was calculated as an outcome measure. We classified the wounds into a biofilm‐eliminated group and a biofilm‐remaining group based on the post‐debridement wound blotting result. Sixteen wound blotting samples from nine pressure ulcers were collected. The percent decrease in wound area was significantly higher in the biofilm‐eliminated group (median: 14.4%, interquartile range: 4.6%‐20.1%) than in the biofilm‐remaining group (median: −14.5%, interquartile range: −25.3%‐9.6%; P = .040). The presence of remaining biofilms was an independent predictor for reduced percent decrease in wound area (coefficient = −22.84, P = .040). Biofilm‐based wound care guided by wound blotting is a promising measure to help clinicians eliminate bacterial bioburden more effectively for wound area reduction.
Keywords: debridement, point‐of‐care, wound blotting
1. INTRODUCTION
Promoting wound healing in chronic wounds requires adequate management of the bacterial bioburden.1 A recent systematic review revealed that 78% of non‐healing chronic wounds harbours biofilms.2 Once biofilms are well established, they prevent leucocytes and other immune cells to physically reach and kill bacteria, causing prolonged inflammation, antibiotic tolerance, and wound chronicity. This eventually causes a higher morbidity and medical cost.3 Biofilms persist and invade host tissue, which causes prolonged and excessive inflammation. Therefore, the physical elimination of bacterial biofilms is indispensable.4 The international guideline for pressure ulcers describes the importance of both identifying and eliminating biofilms.5 For optimal wound bed preparation, wound debridement is crucial. A previous report indicated that frequent sharp debridement improves the healing process in chronic wounds.6 This might attribute to bacterial biofilm removal, which however, has not been proven.
Determining whether a biofilm exists on the wound surface requires wound biopsy and electron microscopic analysis. However, being invasive, and time‐ and cost‐consuming, it is not always clinically applicable.7, 8, 9 Our recent effort revealed a wound blotting method using nitrocellulose membrane and polysaccharide‐specific staining dye that could identify the biofilm component on wound surface.10 This system visualises the biofilm component by blotting the small molecules of biofilm components within the wound to the nitrocellulose membrane, followed by a simple staining procedure. The concurrent validity of this technology has been confirmed in animal experiments involving infected, full‐thickness wounds. A high correlation was observed between the biofilm mass quantified using native polyacrylamide gel electrophoresis and the wound blotting intensity (unpublished data). This advanced technology for biofilm visualisation does not involve any invasive or time‐ and cost‐consuming procedures. Furthermore, it showed a high odds ratio of 9.37 for slough formation within 1 week if the blotting result was positive. Although the predictive validity of this method has been confirmed in pressure ulcers in a clinical setting, there is no data supporting that wound healing can be promoted through biofilm elimination based on biofilm detection.
The purpose of this preliminary study was to investigate the effect of biofilm elimination on wound area decrease in pressure ulcers, as confirmed using the wound blotting method.
2. MATERIALS AND METHODS
The study protocol was approved by the ethics committee of the Graduate School of Medicine of The University of Tokyo and was conducted in accordance with the Helsinki Declaration of 1975. Since this study is a retrospective observational design, obtaining informed consent from each patient was waived.
2.1. Study design
This retrospective cohort study was conducted at a university hospital in an urban centre in Tokyo, Japan. This hospital was selected because wound blotting has been routinely performed here for all patients with pressure ulcers since March 2012. Furthermore, wound ostomy and continence nurses provide standardised treatment based on the Japanese Society of Pressure Ulcers guidelines.11 At each visit, if a slough or eschar was judged to be hindering wound healing, a dermatologist or a plastic surgeon performed conservative sharp wound debridement with a minimally invasive technique using scissors and/or scalpel, while the result of biofilm visualisation was blinded to the operator. The wounds were cleansed with a pH‐balanced cleanser and rinsed with normal saline prior to wound blotting. Following wound blotting, the wounds were dressed using clinical judgement based on exudate levels and clinical signs of inflammation.
2.2. Participants
We recruited inpatients who were diagnosed with pressure ulcers and had wounds during their weekly observation by the interdisciplinary pressure ulcer team between 15 July 2014 and 13 June 2017. The inclusion criteria were as follows: wounds which had undergone debridement and wound blotting for biofilm detection. The exclusion criteria were as follows: (a) not being followed‐up for 1 week and (b) testing negative for biofilm at pre‐debridement. During this time window, we observed 76 records from 32 inpatients. Among these, the final data set included 16 records from 9 patients (Figure 1). Some individual pressure ulcers were observed once, whereas others were observed up to five times.
Figure 1.

Biofilm detection after sharp debridement
2.3. Data collection
Wound blotting and photography of the pressure ulcers were performed at the time of the interdisciplinary pressure ulcer team's rounds. Patients' demographic characteristics (age and sex) and pressure ulcer characteristics [location, DESIGN‐R score, wound area (cm2), and treatment options] were collected from the patients' medical records. DESIGN‐R was used to characterise the ulcers. DESIGN‐R is a wound assessment tool that evaluates the severity of pressure ulcers and monitors the wound healing process on the basis of seven parameters: depth (pressure ulcer severity category), amount of exudate, size (width × length), inflammation/infection, granulation tissue (percentage of healthy granulation tissue relative to the whole wound area), necrotic tissue (presence of soft or hard necrotic tissue), and undermining.12, 13, 14 Higher DESIGN‐R scores represent severer pressure ulcer status.
2.4. Outcome measure
The areas of the wound on digitised photographs were measured using the ImageJ software (National Institutes of Health, Bethesda, Maryland). Digital photographs of the pressure ulcers were taken after debridement and 1 week after collection of the wound blotting samples. The measurements were performed with blinding to the blotting results to avoid potential biases. Wound area was measured at the baseline after the wound debridement and at 1 week after the blotting. The percent wound area decrease was calculated according to the following formula: (wound area at the baseline − wound area after 1 week after blotting)/wound area at the baseline × 100.
2.5. Wound blotting procedure
The pressure ulcer interdisciplinary team performed wound blotting on the wound surfaces before and after debridement.15 The wound was washed, and the wound and the surrounding skin were then wiped dry. A pre‐wet nitrocellulose membrane (supported nitrocellulose membrane, 0.2 μm pore size, Bio‐Rad Laboratories, Inc., California) was firmly pressed to the wound bed for 10 seconds. The blotted membranes were stored at 4°C until they were stained using ruthenium red or alcian blue dyes. Ruthenium red staining was performed as follows: The membranes were hydrated with phosphate‐buffered saline. Ruthenium red (5 mg/mL; Wako Pure Chemical Industries, Tokyo, Japan) was used to detect mucopolysaccharides in the bacterial biofilm.10 After 1 minute of staining, the membrane was washed by soaking in a 40% methanol/10% acetic acid solution three times for 30 minutes to decrease the amount of non‐specifically bound staining solution and thus facilitate clearer visualisation. Alcian blue staining was performed as follows: Each nitrocellulose membrane was soaked in the first cation detergent solution (Saraya Co., Ltd., Tokyo, Japan) for 30 seconds with shaking, then stained with alcian blue solution for 30 seconds with shaking, and distained with the second cation detergent solution for 60 seconds with shaking to clearly visualise the biofilm signals. The stained membranes were then scanned and stored as digital images. Then they were evaluated by researchers, who were blinded to the wound outcome, to determine whether the biofilm staining was positive or negative on the pressure ulcer surface.
We classified the wounds into the biofilm‐remaining group and the biofilm‐eliminated group based on the post‐debridement wound blotting result. If the biofilm signal was eliminated (negative result) after the debridement, the wound was classified into the biofilm‐eliminated group. Conversely, if the biofilm signal remained (positive result) on the wound surface even after wound debridement, the wound was classified into the biofilm‐remaining group (Figure 2).
Figure 2.
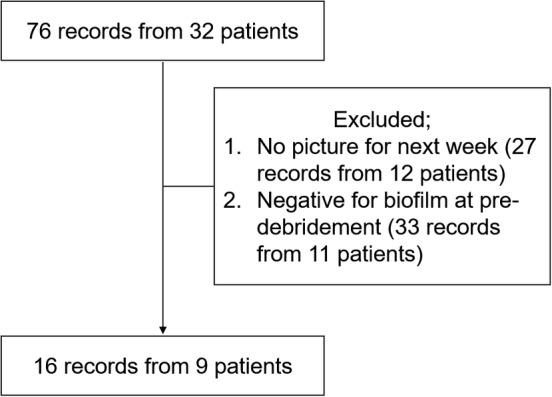
Flow chart of patient enrolment
2.6. Statistical analysis
Descriptive statistics are presented as n (%) or median (interquartile range). Fisher's exact probability test or Wilcoxon's rank sum test was performed to compare the variables between the biofilm‐eliminated and remaining groups. A mixed linear regression analysis was used to assess the effect of the presence or absence of biofilm after debridement on the percent wound area decrease by accounting for within‐patient correlations because we repeatedly included the same patients. The possible confounders for percent wound area decrease were included in the multivariate analysis. All statistical analyses were performed using Stata/SE 15.1 (StataCorp LP, Collage Station, Texas).
3. RESULTS
The characteristics of the nine included patients are shown in Table 1. Four patients had pressure ulcers on their sacral area. The number of times the same pressure ulcer was assessed ranged from 1 to 5. Of the 16 samples obtained from these patients, 7 were classified into the biofilm‐eliminated group and 9 into the biofilm‐remaining group. Baseline wound characteristics did not differ between the two groups (Table 2). The percent area decrease was significantly higher in the eliminated group than in the remaining group (14.4 (range: 4.6‐20.1) versus −14.5 (range: −25.3 to −9.6), P = .050) (Figure 3). Table 3 indicates that the presence of biofilm after sharp debridement can predict percent area decrease in 1 week. Negative results in biofilm detection after the debridement significantly related to increased levels of percent area decrease (coefficient = −22.84, P = .040).
Table 1.
Patient characteristics, pressure ulcer locations, the number of ulcers per patient, and number of times the same ulcer was assessed
| Variables | |
|---|---|
| Age (years, N = 9) | 75 (63‐82) |
| Sex (female, N = 9) | 3 (33.3) |
| Location (N = 9) | |
| Sacrum | 4 (44.4) |
| Others | 5 (55.6) |
| Times assessed for the same pressure ulcer (n = 16) | |
| Once | 9 (56.3) |
| Twice | 4 (25.0) |
| Three times | 1 (6.3) |
| Four times | 1 (6.3) |
| Five times | 1 (6.3) |
Note: Median (interquartile range) or number of participants or pressure ulcers (%).
Table 2.
The characteristics of pressure ulcers whose biofilm was eliminated or remaining
| Eliminated (N = 7) | Remaining (N = 9) | P‐value | |
|---|---|---|---|
| Area, cm2 | 5.9 (1.3‐22.1) | 8.9 (7.1‐23.8) | .315 |
| DESIGN‐R® | |||
| Depth (unstageable) | 6 (85.7) | 8 (88.9) | .356 |
| Exudate | 3.0 (1.0‐6.0) | 3.0 (3.0‐3.0) | .637 |
| Size | 6.0 (3.0‐8.0) | 8.0 (6.0‐8.0) | .565 |
| Inflammation | 0.0 (0.0‐0.0) | 0.0 (0.0‐0.0) | .378 |
| Granulation tissue | 6.0 (5.0‐6.0) | 5.0 (5.0‐6.0) | .593 |
| Necrotic tissue | 3.0 (3.0‐3.0) | 3.0 (3.0‐3.0) | .378 |
| 0.0 (0.0‐24.0) | 0.0 (0.0‐24.0) | .852 | |
| Total score | 18.0 (13.0‐46.0) | 19.0 (17.0‐43.0) | .710 |
| Albumin, g/dL | 2.6 (2.4‐2.9) | 2.3 (2.0‐2.6) | .090 |
| C‐reactive protein, mg/dL | 2.5 (0.9‐4.3) | 2.9 (1.0‐4.1) | .729 |
| Topical treatment | |||
| Antiseptics | 4 (57.1) | 6 (66.7) | 1.000 |
| Trafermin | 1 (14.3) | 3 (33.3) | .585 |
| Foam/silicone dressing | 1 (14.3) | 2 (22.2) | 1.000 |
Note: Median (interquartile range) number of participants or pressure ulcers (%). Nine pressure ulcers were included. Some of the pressure ulcers were assessed several times, providing 16 observations in total. Fisher's exact probability test or the Wilcoxon's rank‐sum test was used for group comparison.
Figure 3.
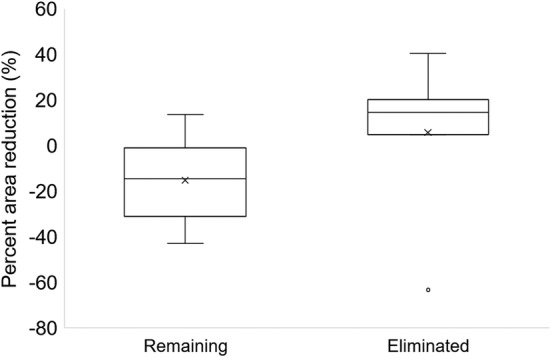
Comparison of percent reduction in wound area. Boxplot for percent reduction in wound area 1 week after the debridement. A higher value indicates a higher reduction in wound area. Negative values indicate wound area increase in 1 week
Table 3.
Mixed linear regression for percent area reduction 1 week after wound debridement
| Variables | Coefficient | Standard error | P value |
|---|---|---|---|
| Biofilm remaining (ref: eliminated) | −22.84 | 11.11 | .040 |
| Area | 0.79 | 0.46 | .088 |
4. DISCUSSION
Determining the presence of biofilm on the wound surface has been considered crucial for realising biofilm‐based wound care.16 This study used the novel biofilm detection system based on wound blotting technology to visualise the biofilm. We observed that when sharp debridement eliminated the biofilm, wound healing was significantly improved in pressure ulcers compared with that when the biofilm remained. This implies that optimal debridement or wound cleansing guided by biofilm detection offers better wound healing outcome in pressure ulcers.
A bacterial biofilm retards wound healing by inducing excessive inflammation. Therefore, its removal is essential.17 For biofilm‐based wound care, clinicians need to identify the biofilm's presence. The efficient removal of biofilm using sharp debridement further needs locating of the biofilm on the wound surface because it is impossible to presume the biofilm's location through the naked eyes. Scanning electron microscopy or other microscopic observation has been considered as a gold standard to identify the biofilm on the wound surface. However, this procedure requires invasive wound biopsy, which potentially increases the risk of bacteremia.18 Furthermore, biopsy samples are only available from limited locations on the wound, which do not represent the wound characteristics. Therefore, the biofilm is sometimes overlooked.9 This technique thus does not help wound clinicians to determine the accurate location of biofilms that need to be eliminated. On the contrary, wound blotting technology enables clinicians to map the biofilm's distribution on the wound surface in a non‐invasive manner because this technology can acquire two‐dimensional location information of the small molecules on the wound surface.19
Moreover, some pressure ulcers had biofilms on their wound surface even after sharp debridement, which indicates the effectiveness of biofilm removal by debridement varies among procedures. Visualisation of the biofilm is therefore needed to determine if the debridement was effectively performed. Biofilm‐based wound care using wound blotting is a promising measure to guide clinicians to perform elimination of bacterial bioburden more precisely. Wound blotting technology can visualise the biofilm within 2 minutes using alcian blue stain and a destaining solution. Thus, point‐of‐care biofilm detection can further facilitate biofilm‐based wound care as a clinically applicable measure for optimal wound management. This concept can be applied to TIME concept in which clinicians can determine the appropriate intervention based on pathophysiology of the chronic wound.1 Information of the biofilm distribution by this point‐of‐care biofilm detection system guides the clinician to determine where to debride or intensively cleanse at the bed side, and this will enhance the effectiveness of the efforts against the bacterial bioburden.
This study has several limitations. As this study is a preliminary retrospective observational study, further interventional study is required to prove the effectiveness of biofilm removal based on wound blotting for improving wound healing outcome. The wound healing outcome used in the present study was percent decrease in wound area 1 week after debridement. This outcome does not fully represent the wound healing process. Detailed analysis with a larger sample size will contribute to determining the optimal timing of biofilm removal guided by the wound blotting technology.
In conclusion, this retrospective observational study revealed the effectiveness of biofilm removal from the wound surface using sharp debridement, guided by wound blotting technology, on wound healing in pressure ulcers. Further investigation will better validate the effectiveness of biofilm‐based wound care guided by wound blotting technology, which facilitates the wound healing in pressure ulcers by controlling bacterial bioburden.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
ACKNOWLEDGEMENTS
The authors would like to thank all patients and clinical staff for their cooperation in this study. This study was supported by Japan Society for the Promotion of Science KAKENHI grant number, 17H04455. The author, G.S., holds the US patent of biofilm detection method. The author, T.M., belongs to the department sponsored by Saraya Cooperation, which had no role in the study concept, design, data collection and analysis, and manuscript drafting.
Nakagami G, Schultz G, Kitamura A, et al. Rapid detection of biofilm by wound blotting following sharp debridement of chronic pressure ulcers predicts wound healing: A preliminary study. Int Wound J. 2020;17:191–196. 10.1111/iwj.13256
This study was performed in the Department of Gerontological Nursing/Wound Care Management, Dermatology, and Nursing of The University of Tokyo Hospital, Tokyo, Japan.
Funding information Japan Society for the Promotion of Science, Grant/Award Number: 17H04455
REFERENCES
- 1. Leaper DJ, Schultz G, Carville K, Fletcher J, Swanson T, Drake R. Extending the TIME concept: what have we learned in the past 10 years?(*). Int Wound J. 2012;9 (Suppl 2):1–19. doi:10.1111/j.1742‐481X.2012.01097.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Malone M, Bjarnsholt T, McBain AJ, et al. The prevalence of biofilms in chronic wounds: a systematic review and meta‐analysis of published data. J Wound Care. 2017;26(1):20‐25. 10.12968/jowc.2017.26.1.20. [DOI] [PubMed] [Google Scholar]
- 3. Wolcott R, Dowd S. The role of biofilms: are we hitting the right target? Plast Reconstr Surg. 2011;127(suppl 1 S):28S‐35S. 10.1097/PRS.0b013e3181fca244. [DOI] [PubMed] [Google Scholar]
- 4. Wolcott RD, Rhoads DD. A study of biofilm‐based wound management in subjects with critical limb ischaemia. J Wound Care. 2008;17:145‐148, 150‐152, 154‐155. 10.12968/jowc.2008.17.4.28835. [DOI] [PubMed] [Google Scholar]
- 5. National Pressure Ulcer Advisory Panel, European Pressure Ulcer Advisory Panel and Pan Pacific Pressure Injury Alliance . In: Emily H, ed. Prevention and Treatment of Pressure Ulcers: Clinical Practice Guideline. Perth, Australia: Cambridge Media; 2014.
- 6. Wilcox JR, Carter MJ, Covington S. Frequency of debridements and time to heal: a retrospective cohort study of 312 744 wounds. JAMA Dermatol. 2013;149(9):1050‐1058. 10.1001/jamadermatol.2013.4960. [DOI] [PubMed] [Google Scholar]
- 7. Oates A, Bowling FL, Boulton AJM, Bowler PG, Metcalf DG, McBain AJ. The visualization of biofilms in chronic diabetic foot wounds using routine diagnostic microscopy methods. J Diabetes Res. 2014;2014:153586‐153588. 10.1155/2014/153586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fritz B, Stavnsbjerg C, Markvart M, et al. Shotgun sequencing of clinical biofilm following scanning electron microscopy identifies bacterial community composition. Pathog Dis. 2019;77(1):1‐7. 10.1093/femspd/ftz013. [DOI] [PubMed] [Google Scholar]
- 9. Wu Y‐K, Cheng N‐C, Cheng C‐M. Biofilms in chronic wounds: pathogenesis and diagnosis. Trends Biotechnol. 2019;37(5):505‐517. 10.1016/j.tibtech.2018.10.011. [DOI] [PubMed] [Google Scholar]
- 10. Nakagami G, Schultz G, Gibson DJ, et al. Biofilm detection by wound blotting can predict slough development in pressure ulcers: a prospective observational study. Wound Repair Regen. 2017;25(1):131‐138. 10.1111/wrr.12505. [DOI] [PubMed] [Google Scholar]
- 11. Committee TJS of PUGR . JSPU guidelines for the prevention and management of pressure ulcers. (4th ed.). Jpn J PU 2016;18:455–544.
- 12. Matsui Y, Furue M, Sanada H, et al. Development of the DESIGN‐R with an observational study: an absolute evaluation tool for monitoring pressure ulcer wound healing. Wound Repair Regen. 2011;19(3):309‐315. 10.1111/j.1524-475X.2011.00674.x. [DOI] [PubMed] [Google Scholar]
- 13. Sanada H, Iizaka S, Matsui Y, et al. Clinical wound assessment using DESIGN‐R total score can predict pressure ulcer healing: pooled analysis from two multicenter cohort studies. Wound Repair Regen. 2011;19(5):559‐567. 10.1111/j.1524-475X.2011.00719.x. [DOI] [PubMed] [Google Scholar]
- 14. Iizaka S, Sanada H, Matsui Y, et al. Predictive validity of weekly monitoring of wound status using DESIGN‐R score change for pressure ulcer healing: a multicenter prospective cohort study. Wound Repair Regen. 2012;20(4):473‐481. 10.1111/j.1524-475X.2012.00778.x. [DOI] [PubMed] [Google Scholar]
- 15. Koyano Y, Nakagami G, Iizaka S, et al. Exploring the prevalence of skin tears and skin properties related to skin tears in elderly patients at a long‐term medical facility in Japan. Int Wound J. 2016;13(2):189‐197. 10.1111/iwj.12251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Malone M, Swanson T. Biofilm‐based wound care: the importance of debridement in biofilm treatment strategies. Br J Community Nurs. 2017;22(suppl 6):S20‐S25. 10.12968/bjcn.2017.22.Sup6.S20. [DOI] [PubMed] [Google Scholar]
- 17. Nakagami G, Morohoshi T, Ikeda T, et al. Contribution of quorum sensing to the virulence of Pseudomonas aeruginosa in pressure ulcer infection in rats. Wound Repair Regen. 2011;19(2):214‐222. 10.1111/j.1524-475X.2010.00653.x. [DOI] [PubMed] [Google Scholar]
- 18. Healy B, Freedman A. Infections. BMJ. 2006;332(7545):838‐841. 10.1136/bmj.332.7545.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kitamura A, Yoshida M, Minematsu T, et al. Prediction of healing progress of pressure ulcers by distribution analysis of protein markers on necrotic tissue: a retrospective cohort study. Wound Repair Regen. 2015;23(5):772‐777. 10.1111/wrr.12316. [DOI] [PubMed] [Google Scholar]


