Dear Editor,
Pyoderma gangrenosum (PG) is an inflammatory disease that has previously been associated with many systemic disorders. 1 Sometimes, it may be idiopathic; otherwise, it may occur together with systemic conditions or could arise in the context of auto‐inflammatory syndromes such as pyogenic arthritis, PG, and acne (PAPA), PG, acne, and suppurative hidradenitis (PASH), or pyogenic arthritis, PG, acne, and suppurative hidradenitis (PAPASH). 2 As PG and the above syndromes are recently recognised disorders with very few reported cases available in the literature, validated treatment recommendations still do not exist. Tumour necrosis factor (TNF)‐alpha has been found to play an important role in the pathogenesis of these syndromes: indeed, anti‐TNF‐α treatments have shown a great positive impact on skin manifestations. 3 Here, we report the case of a patient presenting with PASH syndrome successfully treated with a combination of local wound care and adalimumab. A 39‐year‐old Caucasian male patient presented to our outpatient dermatology clinic showing a cutaneous ulcer on the anterior part of the left leg. The patient had been suffering from this lesion for 6 months. His medical history was positive for hypertension, obesity, dyslipidaemia, diabetes (type II), acne, and hidradenitis suppurativa (HS). Dermatological examination revealed papulo‐pustules and nodules and sinus tracts with malodorous discharge in the axillary region consistent with a worsening of the HS lesions (Hurley staging system: stage II) (Figure 1A); on the face, there were evident acne scars (Figure 1B), while a severe cutaneous ulcer with a slightly undermined margin was found on the left leg, along with inflamed perilesional skin, with the bottom of the ulcer covered by a greenish fibrinous exudate that had a strong smell (Figure 2). In addition, it was associated with a spontaneous tenderness. In order to assess the presence of comorbidities, a multidisciplinary medical approach was required. Vascular consultation with an eco‐colour Doppler excluded the presence of venous insufficiency or arterial disease. A nutritional and endocrinological consultation was required because of the patient's obesity (125 kg; body mass index [BMI]: 38.5) and high serum glycaemic levels (glycaemia: 185 mg/dL, n.v.: 60‐110 mg/dL; glycated haemoglobin: 8.5%, n.v. 4%‐6%). Thus, diet and physical exercise were prescribed. Because of the severity of the lesion and the clinical suspicion of PG, a biopsy and a swab of the lesion were performed. The cutaneous swab revealed the presence of Pseudomonas aeruginosa, while histological examination revealed an ulcerated area with exudation of fibrin and leucocytes associated with a dermal infiltration prevalent in the neutrophilic component, which was consistent with the suspicion diagnosis of PG. The concomitant presence of acne, HS, and PG led to the diagnosis of PASH syndrome. Routine laboratory examinations were within normal reference values, aside from an increase in C‐reactive protein up to 59.6 mg/L, glycaemia: 185 mg/dL, (n.v.: 60‐110 mg/dL), and glycated haemoglobin: 8.5%, (n.v. 4%‐6%). Chest x‐ray and abdominal ultrasound ruled out the concomitant presence of other major diseases. Oral antibiotic treatment with rifampicin and clindamycin was administered for 8 weeks (instead of starting the treatment by combining the two antibiotics, the patient started the therapy taking only 300 mg rifampicin twice a day for 7 days and, after the first week, added clindamycin at a dose of 300 mg twice a day), 4 associated with adalimumab. Adalimumab therapy was started with a loading dose of 160 mg, subcutaneously injected at week 0, 80 mg at week 2, and 40 mg every week from week 4 onwards. Systemic treatment was associated with an intensive local treatment based an accurate local cleaning and disinfection combined with the use of compressive dressing, hydrogel dressing, and hydrofibre dressing with silver. The patient was followed up for a dressing changed twice a week. After 8 weeks of combined treatment, the patient's lesions showed great improvement. At week 16, the patient showed remission of the leg lesions, with a great improvement in HS manifestations (Figure 2). These results were still maintained at the last follow‐up visit (week 28). Moreover, his weight reduced from 125 to 107 kg (BMI from 38.5 to 33), also achieving great control of glycaemia. Several authors have recently described auto‐inflammatory syndromes such as PAPA, PASH, etc. 5 Even if the exact pathogenesis of these syndromes is still unproven, there is emerging evidence that PG and HS share dysfunctional signalling within inflammatory signalling cascades that facilitate the development of sterile neutrophilic auto‐inflammation. TNF‐α could particularly play a central role in the pathogenesis of the skin manifestations. 4 Indeed, different cases reported the efficacy of TNF inhibitors in these auto‐inflammatory conditions. 2 , 3 , 4 , 5 , 6 , 7 Treatment of PG remains largely anecdotal, with only two randomised controlled trials; therefore, its management is based largely on case series and poorly evidenced publications. 8 , 9 Different treatments have been proposed for PG patients, including topical agents (potent topical corticosteroids and tacrolimus ointment) and systemic conventional treatments (corticosteroids, cyclosporine, colchicine, sulphasalazine, dapsone, minocycline, apremilast, and thalidomide). 10 There is now a growing body of evidence to support biologic therapy as a treatment of PG, with anti‐TNF drugs as a well‐known efficient treatment option, while promising data are being obtained from treatment with etanercept, ustekinumab, anakinra, and canakinumab. 10 Moreover, several cases showed the increased efficacy of the combination of different treatments. 11 Here, we reported the case of a PASH syndrome patient successfully treated with a combination of anti‐TNF‐α (adalimumab) and a local intensive treatment combined with a multidisciplinary approach. Indeed, we highlighted how this combination treatment could help patients achieve a faster and long‐term resolution of the skin lesions. We particularly found that, with combined treatment and a multidisciplinary approach, the patient reached faster clinical improvement. 3 Furthermore, recent findings showed that PASH syndrome could represent a biofilm disease, increasing the importance of an associated local treatment. 12 In this context, evidence shows that the application of a supportive wound dressing plays a key role in achieving a successful result. Indeed, wound dressing can improve the three clinically identified key local factors that largely do not allow wound healing, that is, exudate, infection, and biofilm. 13 Moreover, according to clinical experience, the healing of inflammatory skin diseases (eg, erythema nodosum, PG) is supported by compression therapy. 14 On the other hand, we note the importance of a multidisciplinary approach to these particular patients. Therefore, the patient reduced his weight from 125 to 107 kg (BMI from 38.5 to 33), also achieving great control of glycaemia. All these treatments lead to important improvement in the quality of life of the patient above the clinical manifestations. Patients suffering from PASH syndrome and from all other auto‐inflammatory diseases are often fragile patients because of various possible comorbidities such as auto‐inflammatory bowel diseases or cardiologic comorbidities, 7 which can lead to a worsening of the skin manifestations and to a reduction in the treatments' effectiveness. Indeed, several studies have demonstrated a relationship between increased weight, obesity, diabetes, hypertension, and inflammatory skin diseases. Furthermore, nutritional assessment has been found to be a predictive tool in HS patients, 15 while weight loss interventions have been shown to improve psoriasis, as well as HS, and increase responsiveness to treatment. 16 , 17 According to our experience, this group of patients should be treated with a personalised approach based on their skin manifestations and on their comorbidities. To date, official guidelines for PASH syndrome patients are still lacking. Here, we propose a more intensive local treatment associated with adalimumab or other systemic drugs. However, more studies are needed to confirm the real efficacy of local treatments in this group of patients. Furthermore, we believe that a multidisciplinary approach with personalised treatment, in order to guarantee the most appropriate treatment for each patient, could represent the best option for patients' management.
FIGURE 1.
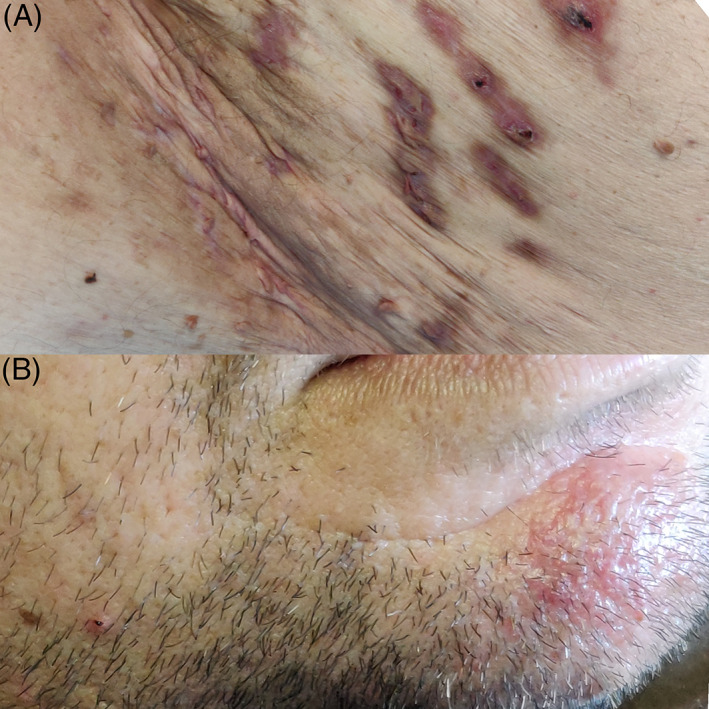
Hidradenitis suppurativa lesions (A) and acne scars (B) at the baseline
FIGURE 2.
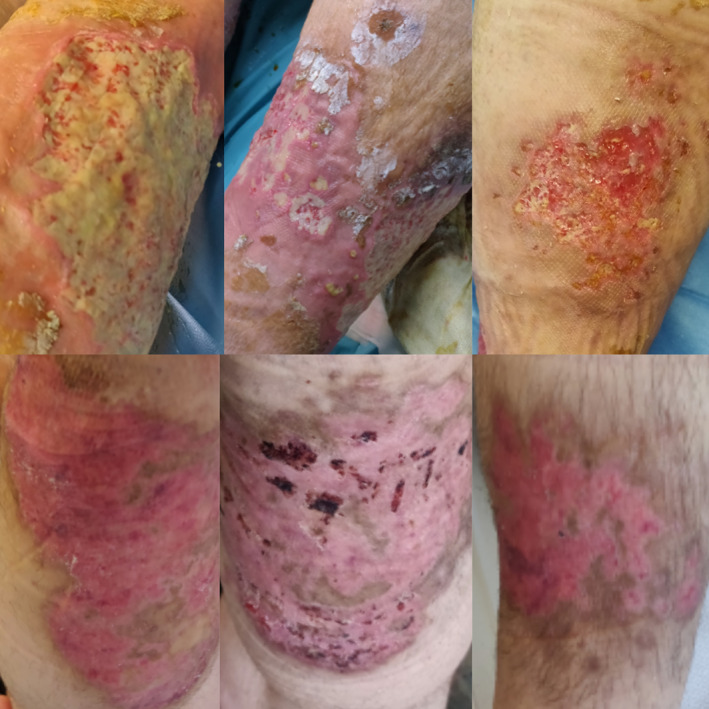
Pyoderma gangrenosum manifestations before, during, and after the treatment (adalimumab in combination with clindamycin and intensive local treatment)
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
ETHICS STATEMENT
Informed consent for the study and for the publication of the photos was obtained from the patient. The study complied with the Declaration of Helsinki.
REFERENCES
- 1. Hsiao JL, Antaya RJ, Berger T, Maurer T, Shinkai K, Leslie KS. Hidradenitis suppurativa and concomitant pyoderma gangrenosum: a case series and literature review. Arch Dermatol. 2010;146:1265‐1270. [DOI] [PubMed] [Google Scholar]
- 2. Cugno M, Borghi A, Marzano AV. PAPA, PASH and PAPASH syndromes: pathophysiology, presentation and treatment. Am J Clin Dermatol. 2017;18(4):555‐562. [DOI] [PubMed] [Google Scholar]
- 3. Saint‐Georges V, Peternel S, Kaštelan M, Brajac I. Tumor necrosis factor antagonists in the treatment of pyoderma gangrenosum, acne, and suppurative hidradenitis (PASH) syndrome. Acta Dermatovenerol Croat. 2018;26(2):173‐178. [PubMed] [Google Scholar]
- 4. Balato A, Caiazzo G, Annunziata MC, et al. Anti‐TNF‐α therapy modulates mTORC1 signalling in hidradenitis suppurativa. J Eur Acad Dermatol Venereol. 2019;33(1):e43‐e45. 10.1111/jdv.15160 Epub 2018 Jul 30. [DOI] [PubMed] [Google Scholar]
- 5. Marasca C, Masarà A, Annunziata MC, Bettoli V, Luciano MA, Fabbrocini G. Long‐term clinical safety of clindamycin and rifampicin combination for the treatment of hidradenitis suppurativa: a strategy to reduce side‐effects, improving patients' compliance. Br J Dermatol. 2019;180(4):949. [DOI] [PubMed] [Google Scholar]
- 6. Staub J, Pfannschmidt N, Strohal R, et al. Successful treatment of PASH syndrome with infliximab, cyclosporine and dapsone. J Eur Acad Dermatol Venereol. 2015;29(11):2243‐2247. [DOI] [PubMed] [Google Scholar]
- 7. Ahn C, Negus D, Huang W. Pyoderma gangrenosum: a review of pathogenesis and treatment. Expert Rev Clin Immunol. 2018;14(3):225‐233. [DOI] [PubMed] [Google Scholar]
- 8. Brooklyn TN, Dunnill MG, Shetty A, et al. Infliximab for the treatment of pyoderma gangrenosum: a randomised, double blind, placebo controlled trial. Gut. 2006;55:505‐509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ormerod AD, Thomas KS, Craig FE, et al. Comparison of the two most commonly used treatments for pyoderma gangrenosum: results of the STOP GAP randomised controlled trial. BMJ. 2015;350:h2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. George C, Deroide F, Rustin M. Pyoderma gangrenosum—a guide to diagnosis and management. Clin Med (Lond). 2019;19(3):224‐228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Marasca C, Fontanella G, Annunziata MC, Marasca D, Fabbrocini G. Adalimumab plus topical tacrolimus for the treatment of pyoderma gangrenosum: report of a case. Int Wound J. 2019;16(4):1047‐1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ead JK, Snyder RJ, Wise J, Cuffy C, Jafary H, Fischborn K. Is PASH syndrome a biofilm disease? A case series and review of the literature. Wounds. 2018;30(8):216‐223. [PubMed] [Google Scholar]
- 13. Tunuković S. ULOGA OBLOGA ZA UNISTENJE i REFORMACIJU BIOFILMA u VRIJEDU [The role of wound dressing in biofilm destruction and reformation]. Acta Med Croatica. 2016;70(1):43‐47. [PubMed] [Google Scholar]
- 14. Konschake W, Valesky E, Stege H, Jünger M. Evidenz der Kompressionstherapie [evidence of compression therapy]. Hautarzt. 2017;68(8):625‐631. 10.1007/s00105-017-3999-z. [DOI] [PubMed] [Google Scholar]
- 15. Barrea L, Fabbrocini G, Annunziata G, et al. Role of nutrition and adherence to the mediterranean diet in the multidisciplinary approach of hidradenitis suppurativa: evaluation of nutritional status and its association with severity of disease. Nutrients. 2018;11(1):57 Published 2018 Dec 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bettoli V, Naldi L, Cazzaniga S, et al. Overweight, diabetes and disease duration influence clinical severity in hidradenitis suppurativa‐acne inversa: evidence from the national Italian registry. Br J Dermatol. 2016. Jan;174(1):195‐197. 10.1111/bjd.13864 Epub 2015 Nov 8. [DOI] [PubMed] [Google Scholar]
- 17. Budu‐Aggrey A, Brumpton B, Tyrrell J, et al. Evidence of a causal relationship between body mass index and psoriasis: a mendelian randomization study. PLoS Med. 2019;16(1):e1002739. Published online 2019 Jan 31. 10.1371/journal.pmed.1002739. [DOI] [PMC free article] [PubMed] [Google Scholar]


