Abstract
The use of negative‐pressure wound therapy (NPWT) has displayed significant clinical benefits in the healing of infected wounds. However, the effects of NPWT on bacterial colonisation and infection of traumatic wounds has been controversial. The aim of this study is to evaluate the impact of NPWT treatment in rabbits with a contaminated full‐thickness wound on bacterial behaviour, including colony morphology, spatial distribution, fissional proliferation, and bacterial bioburden. Full‐thickness wounds were created on the back of rabbits, and were inoculated with bioluminescent Staphylococcus aureus. The wounds were treated with sterile gauze dressings and NPWT with continuous negative pressure (−125 mm Hg). Wound samples were harvested on days 0 (6 hours after bacterial inoculation), 2, 4, 6, and 8 at the centre of wound beds before irrigation. Scanning electron microscopy and transmission electron microscopy (TEM) analyses were performed to determine the characteristic bacteriology. Laser scanning confocal microscopy was performed to obtain bioluminescent images, which were used to observe spatial distribution of the GFP‐labelled S. aureus within the tissue and quantify the bacterial bioburden. NPWT resulted in sparse amounts of scattered bacteria on the wound surface or as sparsely spaced single colonies within the tissue. Wound bioburden on day 8 in the NPWT and gauze groups was 34.6 ± 5.5% and 141.9 ± 15.4% of the baseline values (N = 6), respectively (P < .0001). TEM showed a lack of S. aureus active fission within NPWT‐treated tissue. NPWT can impact S. aureus colony morphology and spatial distribution both on the surface and within wound tissue, and reduce S. aureus as early as 48 hours after therapy initiation. Additionally, NPWT inhibits bacterial fissional proliferation in microcolonies.
Keywords: bioluminescent imaging, contamination, infection, negative‐pressure wound therapy, wound management
1. INTRODUCTION
Wound infection occurs when contamination and colonisation lead to bacterial invasion of the wound and surrounding tissue and can lead to severe complications that delay wound healing and may even prove fatal. Gardner et al1 reported that the mortality rate associated with bacteraemia because of pressure‐ulcer infection was 15.4%.To prevent infection, open‐wound management and promotion of wound healing have been the focus to stimulate development of new techniques and wound‐care products. In the last 20 years, negative‐pressure wound therapy (NPWT) has been widely used in clinics for the treatment of various wounds.2, 3, 4, 5, 6, 7, 8 It is a sealing‐dressing system that applies negative pressure to a wound bed through an open‐cell sponge covered with an occlusive dressing. Based on good clinical results, NPWT has become an important therapeutic method to prevent wound infection. The current literature suggests that primary mechanisms of action of the NPWT device may include drawing the wound edges together, stabilisation of the wound environment, decrease in wound oedema, removal of wound exudate, and micro‐deformation of the wound surface.9
Several studies found that NPWT also displayed significant clinical benefits in the healing of infected wounds.3, 10, 11, 12, 13 However, the effects of NPWT on bacterial colonisation and infection of traumatic wounds have been controversial. The initial studies using a swine model showed exciting results that NPWT could significantly decrease the amount of bacteria in the wound after 5 days of treatment.14 These results have not been duplicated in clinical studies, in particular, Staphylococcus aureus increased in these wounds.15, 16 Boone et al17 found the bacterial burden continued to increase and broaden in a porcine wounds model. In addition, Lalliss et al18 indicated that in the previous studies looking at bacterial clearance from wounds, bacterial contamination was determined using quantitative tissue‐culture biopsies, swabs from various sections of the wound, or by clinical evidence of infection; therefore, these data offered little information regarding the total surface area of wound contamination. Because of these limitations, the previous results cannot comprehensively elucidate the impact of NPWT on the bacteria. Meanwhile, the cooperative social trait between bacterial cells in a S. aureus bacterial species can provide a fitness benefit at the group level.19 We hypothesise that the change in bacterial behaviour treated by NPWT is one possible contributor. Therefore, the bacterial colony morphology, spatial distribution, proliferation activity, and bacterial burden within tissue need to be investigated simultaneously.
The aim of this study was to investigate the hypothesis that the application of NPWT could alter bacterial behavioural characteristics and the degree of bacterial bioburden within tissue in a bioluminescent S. aureus‐contaminated full‐thickness soft tissue‐trauma wound. Bioluminescent imaging not only provides more comprehensive data during an infection study, but also generates greater statistical meaning to the data. Transgenic bioluminescent bacterial pathogens were used to evaluate the depth and amount of bacterial penetration and retention in soft tissue.20 This approach readily allows observation of either the bacterial colony morphology or spatial distribution as well as quantification of the bacteria within wound tissue. Moreover, scanning electron microscopy (SEM) and transmission electron microscopy (TEM) are also used to determine the characteristics of bacteriology on NPWT treatment.
2. MATERIALS AND METHODS
2.1. Animals
All study protocols were performed in accordance with the protocols approved by the Medical Ethics Committee of the General Hospital of People's Liberation Army. Thirty young, adult, female Japanese white rabbits (pathogen free, aged 3‐5 months, approximately 3 kg) were acclimated to standard housing and fed ad libitum. All animals were housed in individual cages at constant temperature (22°C) and humidity (45%), with a 12‐hour light‐dark cycle.
2.2. Wound creation
The backs of the animals were shaved prior to the experimental procedure. Smooth and hairless skin was obtained using a commercial depilatory cream (Veet, Reckitt Benckiser, Inc., India). Two paraspinal surgical sites were marked using a circular standard template 2.5 cm in diameter. All animals were anaesthetised intramuscularly by injection with ketamine (50 mg/kg) and xylazine (5 mg/kg) before the surgical procedure. The surgical area was disinfected by sequential scrubbing for at least 4 minutes with povidone‐iodine solution and then removed with 70% isopropyl alcohol. Two bilateral symmetrical standardised 2.5 cm diameter full‐thickness wound was created between the crest of the shoulders. The incision was made down to the dorsal muscle, followed by fasciotomies to expose the underlying muscle. The wounds were bandaged with sterile gauze dressings. The wound area approximately 5 cm2 was created, and the total wound area was less than 5% of the animal's total body surface area. All procedures were performed by the same surgeon.
2.3. Bacterial preparation and inoculation
S. aureus (RN6390‐GFP) with constitutive expression of the green fluorescent protein (GFP) was obtained from the Chinese PLA Institute for Disease Control and Prevention (Beijing, China). Once the wound model was created, the rabbits were inoculated with 0.5 mL of 108 CFU/mL. Before treatment, bacterial data of wound were collected as described by Morykwas et al14 in day 0 (6 hours after bacterial inoculation). The data were recorded as the number of colony‐forming units per gram tissue (CFU/g tissue). The mean ± standard was calculated and set as the starting point for the wound colonisation.
2.4. Treatment and wound harvesting
The animals were randomised to each of the treatment options. The NPWT group was covered with a polyvinyl alcohol open‐cell foam dressing, and the control group was covered with a sterile gauze dressing. The NPWT devices were all set to continuous suction at a negative pressure of −125 mm Hg. Gauze dressings and the NPWT dressings were changed every 48 hours in both groups.
Three animals in both groups were, respectively, sacrificed on days 0 (6 hours after bacterial inoculation), 2, 4, 6, and 8, and wound samples were harvested from the erector spinae muscle at the centre of the wound beds. Six biopsy samples in each study group were excised at each time point. The wound specimens were excised as 1 × 1 × 0.2 cm3 and 1 × 1 × 0.5 mm3 cubes from the surface of the wound, quickly fixed in 2.5% glutaraldehyde in 0.1 M phosphate‐buffered saline (PBS) (pH 7.2) and stored at 4°C overnight for preparation of SEM and TEM samples. For fluorescence imaging using laser scanning confocal microscopy, the specimens were longitudinally excised as 1 × 1 × 0.5 cm3 cubes perpendicular to the surface of the wound and embedded in O.C.T. Compound (Sakura Finetek, Torrance, California), snap frozen in liquid nitrogen, and stored at −80°C.
2.5. Scanning electron microscopy
Wound samples were washed three times in PBS, dehydrated through an ethanol series and hexamethyldisilazane and mounted using double‐sided tape to specimen stubs, followed by gold‐platinum (50:50) ion coating (108 Auto Sputter Coater; TedPella, Inc., Redding, California). Imaging of the sample wound surface was accomplished using SEM (S‐3400 N, Hitachi, Tokyo, Japan).
2.6. Laser scanning confocal microscopy and fluorescence quantitation
The muscle specimens were cut into 6‐μm thick sections using a cryostat and mounted on glass slides. Section slides were observed using an argon confocal laser scanning microscope (Olympus FV1000, Tokyo, Japan) to capture the spatial distribution and quantitation of the GFP‐labelled S. aureus. To observe the general spatial distribution of the GFP‐labelled bacteria, laser scanning confocal microscopy images of one view were captured at ×40 magnification within the superficial layer of tissue.
The images to quantify the fluorescence intensity of GFP‐labelled S. aureus were captured at ×20 magnification. Three views were created in the centre of wound continuously perpendicular to the surface along the Y‐axis. A stack of images was created from a series of five consecutive images taken along the Z‐axis at 1‐μm intervals for each view. The three sequential images of the same Z‐axis were conjoined to a seamless image using the FV10‐ASW 4.1 software embedded on an Olympus FV‐1000. Such five images were created to analyse the mean fluorescence intensity for one sample (Figure 1). The tissue boundary was identified by referring to differential interference contrast images. The scan speed was set at 4 ms/pixel with a scan area of 512 × 512 pixels, and the power of the 488‐nm laser was set at 4.5% according to the power slider.
Figure 1.
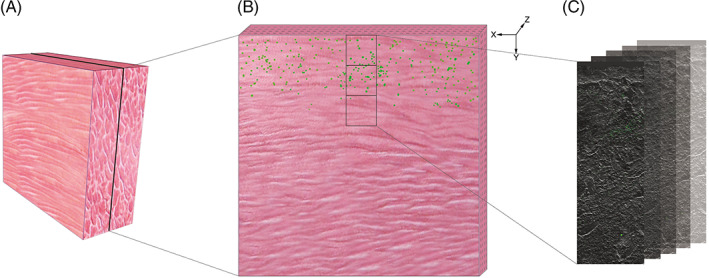
The samples and observation setup are diagrammed. (A) The muscle specimens were longitudinally excised as 1 × 0.5 × 1 cm3 cubes perpendicular to the surface of the wound. (B) The specimen was sectioned with 6‐μm thickness, and three views were created continuously along the Y‐axis perpendicular to the surface of the wound. (C) Five consecutive images were created taken along the Z‐axis at 1‐μm intervals at each view
Digital images were captured using the same parameters and were analysed by two blinded independent observers. The fluorescence intensity was observed in the region of interest, and the fluorescence signal from samples was quantified using the FV10‐ASW 4.1 software. The mean percentage of relative fluorescence intensity was obtained at each time point compared with the mean baseline fluorescence intensity (day 0) for each animal.
2.7. Transmission electron microscopy
Samples were washed with PBS buffer then post‐fixed with 1% OsO4 for 1 hour. The specimens were dehydrated in a graded series of ethanol and absolute acetone, followed by embedding in capsules. The specimens were stained using uranyl acetate and alkaline lead citrate, and observed in a transmission electron microscope (HT7700, Hitachi, Tokyo, Japan).
2.8. Statistical analysis
All data are presented as the mean ± SD, and statistical comparisons of the means were performed on measured samples using the paired Students t‐test. All statistical analyses were performed with SPSS software (SPSS 19.0, SPSS Inc., Chicago, Illinois). The significance level was set at P < .05.
3. RESULTS
Thirty animals were survived according to the scheduled experimental plan. The successful creation of contaminated wounds (bacterial counts = 6.5 ± 0.32 × 107 CFUs/g tissue) was confirmed at 6 hours after bacterial inoculation (day 0). Under SEM, the wound‐bed surface was abundantly colonised with planktonic bacteria 6 hours after inoculation. In the gauze group, S. aureus congregated to multiply and populated the surface with grapelike clusters gradually. In the NPWT group, bacteria were sparsely visible and scattered on the surface at the end of the study (Figure 2).
Figure 2.
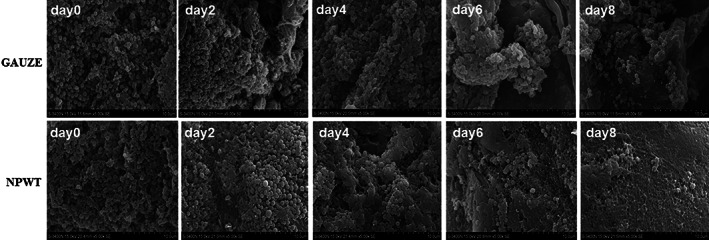
Scanning electron microscopy of wound bed surfaces infected with Staphylococcus aureus. (GAUZE group) S. aureus congregated to multiply and populated the surface with grapelike clusters gradually; (NPWT group) bacteria were sparsevisible and scattered on the surface at the end of the study
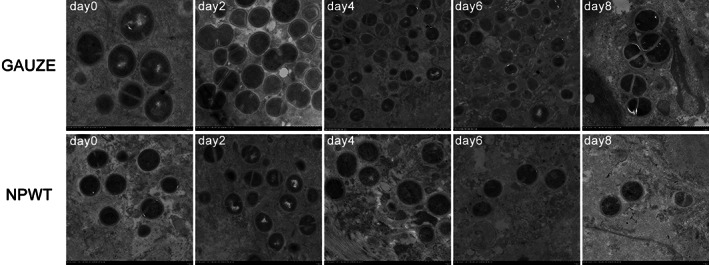
In general terms, there was a clustering of bacteria (green) within the shallow tissue of the wound bed 6 hours after inoculation in confocal imaging. In the control group, the bacteria invaded deep into the wound, with dense and numerous colonies within the infected tissue by day 8. NPWT resulted in single colonies of S. aureus scattered sparsely (Figure 3).
Figure 3.
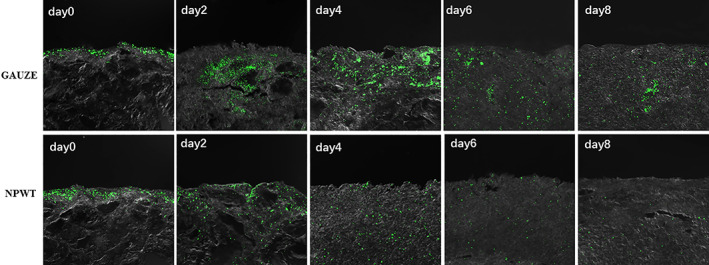
Laser scanning confocal microscopy of the GFP‐labelled S. aureus (green) within infected tissue (magnification ×40). (GAUZE group) The bacteria invaded deep into the wound, with dense and numerous colonies at the indicated time points; (NPWT group) Single colonies of S.aureus scattered sparsely
Quantitative analysis of the relative fluorescence intensity of GFP‐labelled S. aureus in tissue was also performed to quantify the bacteria amount within the wound at each time point. The bacterial fluorescence intensity before treatment was similar between the two groups (N = 6) (P = .72). NPWT effectively reduced the amount of S. aureus within the contaminated wounds compared with gauze dressings at day 2, 4, 6, and day 8 (P < .001). At the final time point, the wound tissue treated with NPWT or gauze contained 34.6 ± 5.5% and 141.9 ± 15.4% of the baseline values (N = 6), respectively (Figure 4). There was a significant difference of bioburden in wounds between two groups.
Figure 4.
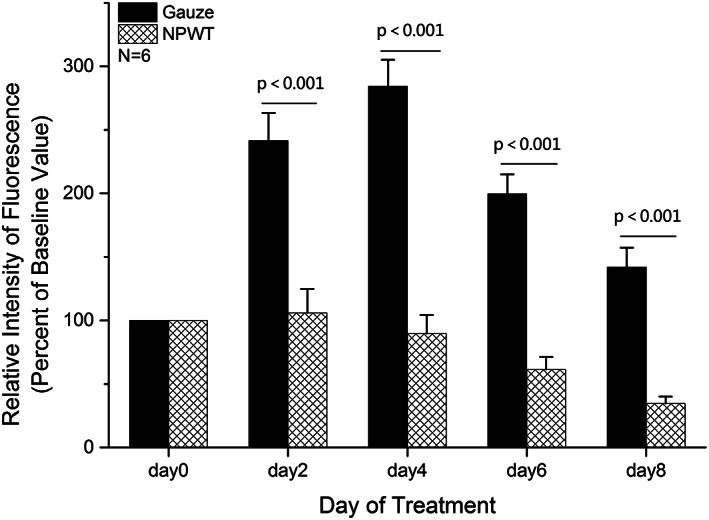
Bacterial fluorescence quantitation in the wound compared with baseline levels. Comparison of the percentage of Staphylococcus aureus remaining in the wound at various time points
Before treatment, the bacteria that had successfully colonised the wounds were fissiparous. At day 8 of NPWT treatment, fissiparous S. aureus cells were rare in bacterial microcolony. In contrast, actively dividing bacteria and every fissional phase of S. aureus could be detected within tissue with gauze dressings (Figure 5).
Figure 5.
Transmission electron microscopy of the bacterial fissiparous state. (GAUZE group) Actively dividing phase of S. aureus could be detected within tissue; (GAUZE group) A few number of fissiparous S. aureus cells were detected
4. DISCUSSION
In the present study, we found a significant effect of NPWT on both distributive morphology, proliferation state, and amount of bacteria in the wound compared with conventional gauze treatment. Using SEM, we demonstrated sparse amounts of scattered bacteria on the planar wound surface treated with NPWT when compared with gauze treatment. Within wound tissue, NPWT resulted in single S. aureus colonies scattered sparsely under laser scanning confocal microscopy, and using fluorescence quantitation, the bacterial‐load reduction was even more pronounced within wound tissue, where it was approximately four times lower in NPWT‐treated wounds than in gauze‐treated wounds (34.6 ± 5.5% and 141.9 ± 15.4% vs baseline, respectively). Under TEM, fissiparous bacteria were rare within NPWT‐treated tissue.
The microbial bioburden in a wound can range from contamination, colonisation, localised infection, spreading infection, and ultimately to systemic infection if not controlled appropriately.21 The organisms were readily able to infect the wounds, with levels of approximately 108 organisms per gram of tissue.14 In our model, the microbial bioburden (bacterial counts = 6.5 ± 0.32 × 107 CFUs/g tissue) was able to reproduce infection model reliably.
The bacteria colonial morphology and distribution characteristics observed on the surface of the wound using SEM were not reported previously. To observe the original bacterial situation of the wound, only aseptic saline was used to douche the wound without formal debridement. Images of colonies were successfully captured in our model using SEM. The difference of colonial morphology and distribution characteristics between the two groups was clear. We hypothesise that the major mechanism was because of the shear forces at the wound interface between the tissue and the foam on continuous negative pressure suction.22 The compressive forces prevent bacteria from congregating as grapelike clusters of cocci.
With confocal imaging, the use of bioluminescent engineered bacterial strains enables a visual assessment of the bacterial colonisation and infection process. There was a clustering of bacteria (green) within the shallow tissue after contamination, and the bacteria gradually colonised and invaded deep into the wound. It is important to note that the bacteria deep within the wound only formed discrete single colonies on NPWT treatment, and differ from the numerously gathered bacteria that formed large localised colonies in the gauze group. Our results indicate that distinct bacterial behaviour occurs within tissue depending on the form of treatment.
The effect of NPWT on bacterial load has been controversial.15, 16, 17, 23 There are several possible reasons for studies presenting inconsistent bacterial wound count results in controlled experiments. First, the variety of wound models and the level of initial contamination in these studies potentially play a role in bacterial clearance. Extensive surgical debridement prior to NPWT dressing was not applied in all studies. The technique applied to bacterial quantification can lead to spurious results. Quantitative cultures in in vitro culture used in all of the previous studies could not assess the real‐time bacterial phenotypes in wound. The types of bacteria contaminating the wounds can lead to disparities in the degree of infection.
Using this model, we have shown that NPWT decreases S. aureus bacterial counts in a contaminated full‐thickness soft‐tissue wound as early as 48 hours after initiating therapy. The wounds had more bacteria in the gauze group compared with the NPWT group throughout the treatment process; this difference was significant. The results of the present study do not reflect the trend reported by Lalliss, who also used bioluminescent bacteria for quantitative analysis, and found that NPWT treatment did not significantly reduce the counts.18 We suggest the following potential reasons: first, the soft tissue wound we used was different from the complex open fracture wound they created. NPWT can create a better wound‐healing environment by increasing blood flow and granulation tissue within the wound bed, which can augment the host's response to injury and bacterial contamination.24 NPWT can clear more bacteria in a soft‐tissue wound than in an open‐fracture wound. In addition, there was a significant disparity between the bacteria quantification on the surface of wounds analysed by Lalliss using a photon‐counting camera and within tissue using fluorescence quantitation in our study. The greater blood perfusion within the soft tissue than on the surface of an open‐fracture wound is more conductive to bacterial removal.
Bacterial colony of S. aureus in the wounds that received NPWT displayed an inactive proliferative state compared with the bacteria in the gauze group. The fissional proliferation of bacteria requires a suitable environment and the necessary nutrients. The continuous suction during NPWT may physically force out the oedema and nutrients required for bacterial multiplication. The decrease in proliferation further reduces the bacterial load in the wound.
There are limitations to this study. An animal‐model study using a single type of wound and only one species of bacteria was performed. Standard debridement was not performed on the animals to eliminate this as a confounder for bacterial behaviour, and the treatment period was relatively short. In addition, although systemic antibiotics are normally administered in the treatment of infected wounds, they were not used in the present study in an effort to minimise confounding variables and to allow independent assessment of the tested interventions. Despite these limitations, the results clearly demonstrate that wounds treated with NPWT had a significant effect on bacterial behaviour and reduced the S. aureus present. It is possible that this bacteriologically relevant data may only be demonstrated when using an animal wound model similar to that in the present study.
5. CONCLUSIONS
In summary, this study demonstrates that in a contaminated full‐thickness wound model, NPWT can impact the S. aureus colony morphology and spatial distribution either on the surface or within tissue of the wound, and reduce S. aureus as early as 48 hours after initiation of therapy. In addition, NPWT inhibits bacterial fissional proliferation in microcolonies.
CONFLICT OF INTEREST
All authors declare that they have no conflict of interests.
AUTHORS CONTRIBUTIONS
Z.L., Q.Y., and S.W. conceived and designed the study and performed research and they contributed equally to this work. G.W. wrote the article. T.L. analysed the data. P.T. and D.L. conceived the study and contributed new methods. All authors read and approved the manuscript.
ACKNOWLEDGEMENTS
This study was supported by the National Natural Science Foundation of China (No. 7172211); Project of Capital Clinical Characteristic Application of Beijing Science and Technology Commission (No. Z161100000516179).
Li Z, Yu Q, Wang S, et al. Impact of negative‐pressure wound therapy on bacterial behaviour and bioburden in a contaminated full‐thickness wound. Int Wound J. 2019;16:1214–1221. 10.1111/iwj.13197
Contributor Information
Pei‐fu Tang, Email: pftang301@163.com.
Daohong Liu, Email: domb@vip.sina.com.
REFERENCES
- 1. Gardner SE, Frantz RA, Doebbeling BN. The validity of the clinical signs and symptoms used to identify localized chronic wound infection. Wound Repair and Regen. 2001;9:178‐186. [DOI] [PubMed] [Google Scholar]
- 2. Fleischmann W, Strecker W, Bombelli M, Kinzl L. Vacuum sealing as treatment of soft tissue damage in open fractures. Unfallchirurg. 1993;96:488‐492. [PubMed] [Google Scholar]
- 3. Morykwas MJ, Argenta LC. Nonsurgical modalities to enhance healing and care of soft tissue wounds. J South Orthop Assoc. 1997;6:279‐288. [PubMed] [Google Scholar]
- 4. Mooney JF 3rd, Argenta LC, Marks MW, Morykwas MJ, DeFranzo AJ. Treatment of soft tissue defects in pediatric patients using the V.a.C. system. Clin Orthopaed Relat Res. 2000;376:26‐31. [DOI] [PubMed] [Google Scholar]
- 5. Song DH, Wu LC, Lohman RF, Gottlieb LJ, Franczyk M. Vacuum assisted closure for the treatment of sternal wounds: the bridge between debridement and definitive closure. Plast Reconstr Surg. 2003;111:92‐97. [DOI] [PubMed] [Google Scholar]
- 6. Clare MP, Fitzgibbons TC, McMullen ST, Stice RC, Hayes DF, Henkel L. Experience with the vacuum assisted closure negative pressure technique in the treatment of non‐healing diabetic and dysvascular wounds. Foot Ankle Int. 2002;23:896‐901. [DOI] [PubMed] [Google Scholar]
- 7. DeFranzo AJ, Argenta LC, Marks MW, et al. The use of vacuum‐assisted closure therapy for the treatment of lower‐extremity wounds with exposed bone. Plast Reconstr Surg. 2001;108:1184‐1191. [DOI] [PubMed] [Google Scholar]
- 8. Liu DS, Sofiadellis F, Ashton M, MacGill K, Webb A. Early soft tissue coverage and negative pressure wound therapy optimises patient outcomes in lower limb trauma. Injury. 2012;43:772‐778. [DOI] [PubMed] [Google Scholar]
- 9. Orgill DP, Manders EK, Sumpio BE, et al. The mechanisms of action of vacuum assisted closure: more to learn. Surgery. 2009;146:40‐51. [DOI] [PubMed] [Google Scholar]
- 10. Fleischmann W, Lang E, Russ M. Treatment of infection by vacuum sealing. Unfallchirurg. 1997;100:301‐304. [DOI] [PubMed] [Google Scholar]
- 11. Pinocy J, Albes JM, Wicke C, Ruck P, Ziemer G. Treatment of periprosthetic soft tissue infection of the groin following vascular surgical procedures by means of a polyvinyl alcohol‐vacuum sponge system. Wound Repair Regen. 2003;11:104‐109. [DOI] [PubMed] [Google Scholar]
- 12. Fleck TM et al. The vacuum‐assisted closure system for the treatment of deep sternal wound infections after cardiac surgery. Ann Thoracic Surg. 2002;74:1596‐1600; discussion 1600. [DOI] [PubMed] [Google Scholar]
- 13. Blum ML, Esser M, Richardson M, Paul E, Rosenfeldt FL. Negative pressure wound therapy reduces deep infection rate in open tibial fractures. J Orthop Trauma. 2012;26:499‐505. [DOI] [PubMed] [Google Scholar]
- 14. Morykwas MJ, Argenta LC, Shelton‐Brown EI, McGuirt W. Vacuum‐assisted closure: a new method for wound control and treatment: animal studies and basic foundation. Ann Plast Surg. 1997;38:553‐562. [DOI] [PubMed] [Google Scholar]
- 15. Weed T, Ratliff C, Drake DB. Quantifying bacterial bioburden during negative pressure wound therapy: does the wound VAC enhance bacterial clearance? Ann Plastic Surg. 2004;52:276‐279; discussion 279–280. [DOI] [PubMed] [Google Scholar]
- 16. Moues CM, Vos MC, van den Bemd GJ, Stijnen T, Hovius SE. Bacterial load in relation to vacuum‐assisted closure wound therapy: a prospective randomized trial. Wound Repair Regen. 2004;12:11‐17. [DOI] [PubMed] [Google Scholar]
- 17. Boone D, Braitman E, Gentics C, et al. Bacterial burden and wound outcomes as influenced by negative pressure wound therapy. Wounds. 2010;22:32‐37. [PubMed] [Google Scholar]
- 18. Lalliss SJ, Stinner DJ, Waterman SM, Branstetter JG, Masini BD, Wenke JC. Negative pressure wound therapy reduces pseudomonas wound contamination more than Staphylococcus aureus . J Orthop Trauma. 2010;24:598‐602. [DOI] [PubMed] [Google Scholar]
- 19. Rumbaugh KP, Diggle SP, Watters CM, Ross‐Gillespie A, Griffin AS, West SA. Quorum sensing and the social evolution of bacterial virulence. Curr Biol. 2009;19:341‐345. [DOI] [PubMed] [Google Scholar]
- 20. Hassinger SM, Harding G, Wongworawat MD. High‐pressure pulsatile lavage propagates bacteria into soft tissue. Clin Orthop Relat Res. 2005;439:27‐31. [DOI] [PubMed] [Google Scholar]
- 21. Kairinos N, Solomons M, Hudson DA. Negative‐pressure wound therapy I: the paradox of negative‐pressure wound therapy. Plast Reconstr Surg. 2009;123:589‐598; discussion 599–600. [DOI] [PubMed] [Google Scholar]
- 22. Leaper DJ, Schultz G, Carville K, Fletcher J, Swanson T, Drake R. Extending the TIME concept: what have we learned in the past 10 years?(*). Int Wound J. 2012;9(Suppl 2):1‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Assadian O, Assadian A, Stadler M, Diab‐Elschahawi M, Kramer A. Bacterial growth kinetic without the influence of the immune system using vacuum‐assisted closure dressing with and without negative pressure in an in vitro wound model. Int Wound J. 2010;7:283‐289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Morykwas MJ, Faler BJ, Pearce DJ, Argenta LC. Effects of varying levels of subatmospheric pressure on the rate of granulation tissue formation in experimental wounds in swine. Ann Plast Surg. 2001;47:547‐551. [DOI] [PubMed] [Google Scholar]


