Abstract
The effective approach on pressure ulcer (PU) prevention regarding patient safety in the hospital context was evaluated. Studies were identified from searches in EBSCO host, PubMed, and WebofScience databases from 2009 up to December 2018. Studies were selected if they were published in English, French, Portuguese, or Spanish; incidence of PUs was the primary outcome; participants were adults (≥18 years) admitted in hospital wards and/or units. The review included 26 studies. Studies related to prophylactic dressings applied in the sacrum, trochanters, and/or heels, education for health care professionals, and preventive skin care and system reminders on‐screen inpatient care plan were effective in decreasing PUs. Most of the studies related to multiple intervention programmes were effective in decreasing PU occurrence. Single interventions, namely support surfaces and repositioning, were not always effective in preventing PUs. Repositioning only was effective when supported by technological pressure‐mapping feedback or by a patient positioning system. Risk‐assessment tools are not effective in preventing PUs. PUs in the hospital context are still a worldwide issue related to patient safety. Multiple intervention programmes were more effective in decreasing PU occurrence than single interventions in isolation. Single interventions (prophylactic dressings, support surfaces, repositioning, preventive skin care, system reminders, and education for health care professionals) were effective in decreasing PUs, which was always in compliance with other preventive measures. These results provide an overview of effective approaches that should be considered when establishing evidence‐based guidelines to hospital health care professionals and administrators for clinical practice effective in preventing PUs.
Keywords: effectiveness, hospital‐acquired pressure ulcers, patient safety, pressure injury, prevention
1. INTRODUCTION
Despite all advances in health care, pressure ulcers (PUs) remain an old worldwide public health problem related to patient safety.1, 2, 3 Hospital‐acquired PUs are one of the most harmful events in the clinical context.1, 2
PUs, recently known as pressure injuries,4, 5, 6 are defined as skin injuries and/or underlying tissue damage localised over a bony prominence, resulting from pressure force and/or pressure combined with shear.7 PUs result in significant physical, psychological, and social problems related to lower quality of life, increasing dependence, and frailty of patients.8 They increase health care costs2, 8, 9 and are recognised as an indicator of the quality of care provided in health care institutions.4 In most of the clinical contexts, PUs are predictable and preventable with interventions and evidence‐based practice guidelines.4, 7
PUs are a complex phenomenon and, although there are many risk factors identified,10 the most common are mobility, activity, skin moisture, nutritional status, and sensorial perception.11 Risk‐assessment tools are part of a structured process used to identify risks of individuals or patients to develop a PU.7, 12 However, there is no evidence that risk‐assessment scales were effective in reducing PUs.13 Multiple intervention programmes like “care bundles” in a clinical practice context seem to improve patient outcomes in terms of PU incidence with a small set of interventions performed collectively and reliably.4 Inconsistent adherence of health care professionals to evidence‐based guidelines remains a major issue in nursing practice.14, 15 Considering the negative outcomes emerging from PU occurrence, prevention is highlighted as the priority measure to be enhanced at both the national level7 and the international level.16 Furthermore, prevention of PUs is substantially cheaper than the treatment of these wounds in a long‐term scenario.2 Indeed, many interventions are recommended by the international PU prevention guidelines7 but it is not clearly known whether which interventions make difference in decreasing PU incidence in hospitalised patients. Therefore, this study aimed to evaluate the evidence available regarding effective approaches to PU prevention in hospitalised adults, using the range of decreasing incidence to measure effectiveness. In order to establish a solid search strategy and reporting, the Preferred Reporting Items for Systematic Reviews and Meta‐Analysis (PRISMA) guidelines were followed. The participants, interventions, comparisons, outcomes, and study design (PICOS) to be included in this review were as follows: participants, hospitalised adults; interventions, PU prevention strategies; comparisons, control group, usual care, or competing technologies/products; outcomes, PU incidence; study design, cross‐sectional, prospective and retrospective cohort, comparative, pre‐test and post‐test, quasi‐experimental, experimental, randomised control trial (RCT), and mixed‐method studies.
2. METHODS
This systematic review was performed and recorded in accordance with the PRISMA guidelines.17 The primary outcome measured was the incidence of PUs among adult patients cared for in acute settings.
2.1. Inclusion criteria
Primary source articles published from 2009 (regarding 1st edition of international guidelines—7 to December 2018 were eligible for inclusion if data were related to the effectiveness of PU prevention. All quantitative, original research studies including human studies were included considering the specific eligibility criteria as follows: (a) cross‐sectional, prospective and retrospective cohort, comparative, pre‐test and post‐test, quasi‐experimental, experimental, RCT, and mixed‐method study design (study design criterion); (b) studies on incidence of PUs (outcome measure criterion); (c) adults admitted in hospital wards or any type of acute unit (participants criterion); and (d) articles published in English, French, Portuguese, or Spanish were included (language criterion).
2.2. Search strategy and study selection
Studies were comprehensively identified by searching the following electronic databases: PubMed, Web of science, and EBSCO (CINAHL; MEDLINE; Nursing & Allied Health; Cochrane Central Register of Controlled Trials; Library, Information Science & Technology Abstracts; MedicLatina). The search aimed to identify peer‐reviewed articles published from January 2009 (regarding 1st edition of international guidelines—7 up to December 2018. The search terms were as follows: “pressure ulcer*” OR “pressure injur*” AND “prevent*” OR “incidence” AND “effectiv*” (Figure 1). Retrieved titles and abstracts were independently assessed for eligibility for inclusion by two authors (S.G., M.P.). Duplicate entries were removed. Relevant articles were then retrieved for a full reading. The references to those articles were searched to find any other relevant studies. The same two authors reviewed the text of potential studies, and decisions to include or exclude studies in the review were made by consensus.
Figure 1.
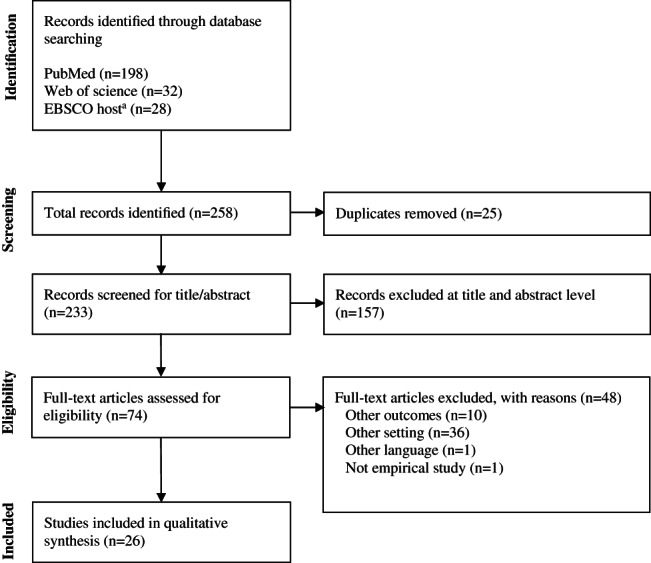
Flow diagram of studies. aEBSCO host database searches included CINAHL; MEDLINE; Nursing & Allied Health; Cochrane Central Register of Controlled Trials; Library, Information Science & Technology Abstracts; and MedicLatina
2.3. Data extraction and harmonisation
A data extraction form was developed based on the PRISMA statement.18 Relevant data were extracted from manuscripts by one author (S.G.); coding was verified by two authors (S.G., M.P.) according to the subjects of the international guidelines to PU prevention.7 Disagreements were resolved by discussion among the authors. Data extracted included guideline elements, author/year, study design, sample size/age, setting/country, study quality, and outcomes presented in a consistent manner.
2.4. Study analysis, quality, and risk of bias
Study quality was assessed using the evidence‐based librarianship (EBL) Critical Appraisal checklist.19 This tool assessing validity, applicability, and relevance of included studies was based on four domains of research: population, data collection, study design, and results. The overall validity (global rating) of the studies was determined based on the “Yes” scores ≥75% or “No/Unclear” scores ≤25%. Two researchers (S.G., M.P.) rated the articles, and discrepancies were resolved by agreement.
3. RESULTS
3.1. Literature search
The flow diagram of studies included in this systematic review is presented in Figure 1. The systematic literature searched yielded a total of 258 relevant records, of those, 233 abstracts were assessed for eligibility after excluding duplicates (n = 26). A total of 157 articles were rejected after title and abstract being screened. Subsequently, 74 full‐text potentially relevant articles assessed with a total of 26 articles were included.
3.2. Included study characteristics
The characteristics of the 26 studies included are described in Table 1. The most common study design was RCTs (n = 6). Regarding the PU preventive interventions, most of the featured studies focused on support surfaces,20, 21, 22, 23 health professional's education,24, 25 multiple intervention programmes,4, 26, 27, 28, 29, 30, 31 repositioning and early mobilisation,32, 33, 34 preventive skincare,35 prophylactic dressings,36, 37, 38, 39, 40 remind systems on patient care plan,41 and risk‐assessment tools.42 According to the care setting where the studies were carried out, most of them (n = 17) were developed in intensive care units (ICU) followed by medicine (n = 6), geriatrics (n = 2), surgical (n = 2), orthopaedics (n = 2), oncology (n = 1), rehabilitative (n = 1), community hospital (n = 1), and other specific units (n = 4). Regarding quality of studies (Table 2), most of the studies (n = 16) were a high quality ≥75% according to the EBL appraisal check list.19 Studies were not excluded based on quality. On average, the overall quality of the included studies was 74.73%.
Table 1.
Characteristics of the included studies
| Characteristics | Number of studies |
|---|---|
| Study design | |
| Randomised control trial | 10 |
| Quasi‐experimental | 5 |
| Pre‐test and post‐test study | 3 |
| Prospective controlled study | 2 |
| Mixed methods | 1 |
| Cohort study | 3 |
| Non‐randomized comparison design | 1 |
| Epidemiological, exploratory, comparative, and cross‐sectorial analytical | 1 |
| Prevention strategies | |
| NPUAP/EPUAP/PPPIA guidelines | |
| Prophylactic dressings | 6 |
| Support Surfaces | 4 |
| Repositioning | 4 |
| Health professional's education | 2 |
| Risk‐assessment tools | 1 |
| Preventive skin care | 1 |
| Other preventive strategies | |
| Multiple interventions | 7 |
| Reminder system | 1 |
| Sample characteristics | |
| Country | |
| United States of America | 7 |
| Australia | 4 |
| Spain | 4 |
| Canada | 4 |
| Turkey | 2 |
| Argentina | 1 |
| Brazil | 1 |
| Belgium | 1 |
| Italy | 1 |
| Saudi Arabia | 1 |
| Setting | |
| ICU (medical, surgical, cardiac, trauma, and neurointensive) | 17 |
| Medicine | 6 |
| Geriatrics | 2 |
| Surgical | 2 |
| Orthopaedics | 2 |
| Oncology | 1 |
| Rehabilitative | 1 |
| Community hospital | 1 |
| Other units (acute spine, coronary care, medical) | 4 |
Table 2.
Analysis of EBL appraisal checklist domains for the included study
| Studies | Validity (%) | Overall validity of study (%) | |||
|---|---|---|---|---|---|
| Population domain | Data collection domain | Study design domain | Results domain | ||
| Anderson et al26 | 66.66 | 66.66 | 80 | 66.66 | 69.57 |
| Behrendt et al32 | 83.33 | 50 | 100 | 50 | 71.43 |
| Chaboyer et al4 | 87.5 | 75 | 100 | 66.67 | 82.6 |
| Cobb et al27 | 62.5 | 57.14 | 80 | 83.33 | 69.23 |
| Demarre et al20 | 87.5 | 60 | 80 | 66.66 | 75 |
| Dutra et al36 | 87.5 | 80 | 80 | 50 | 75 |
| Forni et al37 | 100 | 100 | 100 | 66.67 | 91.67 |
| Kalowes et al38 | 85.71 | 100 | 100 | 83.33 | 91.3 |
| Loudet et al28 | 66.66 | 60 | 80 | 33.33 | 59.09 |
| Manzano et al21 | 55.55 | 40 | 80 | 50 | 52 |
| Manzano et al33 | 77.77 | 60 | 100 | 100 | 83.33 |
| Martin et al29 | 66.66 | 60 | 100 | 83.33 | 77.27 |
| Ozyurek & Yavuz22 | 75 | 20 | 80 | 83.33 | 66.66 |
| Picatoste et al24 | 60 | 60 | 80 | 66.67 | 66.67 |
| Powers et al44 | 62.5 | 83.33 | 80 | 83.33 | 76 |
| Rich et al34 | 85.7 | 66.66 | 100 | 100 | 87.5 |
| Richard‐Denis et al43 | 75 | 80 | 80 | 100 | 83.33 |
| Santamaria et al40 | 75 | 60 | 100 | 83.33 | 79.16 |
| Santamaria et al39 | 87.5 | 80 | 100 | 83.33 | 87.5 |
| Sebastian‐Viana et al41 | 71.43 | 100 | 80 | 83.33 | 82.61 |
| Shannon et al35 | 28.57 | 50 | 60 | 66.67 | 50 |
| Swafford et al30 | 50 | 28.57 | 40 | 33.33 | 37.5 |
| Tayyib et al,31 | 100 | 40 | 100 | 83.33 | 83.33 |
| Uzun et al25 | 22.22 | 50 | 100 | 66.67 | 56 |
| Vermette et al23 | 100 | 40 | 80 | 100 | 83.33 |
| Webster et al42 | 87.5 | 100 | 100 | 100 | 95.83 |
3.3. Main findings
Findings of the 26 studies included in this review are presented in Tables 3, 4, 5, 6. The present systematic review identified eight domains in terms of PU prevention among the included studies, namely: support surfaces, multiple intervention programmes, repositioning and early mobilisation, risk‐assessment tools, prophylactic dressings, education, skincare, and reminder system to prevent PUs.
Table 3.
Main findings and characteristics of studies focused on support surfaces and health education strategies
| Guidelines elements | Author, year | Study design | Sample size (mean age) | Setting; country | Study quality | Main finding(s) |
|---|---|---|---|---|---|---|
| Support surfacesa | Vermette et al23 | Prospective RCT | Sample n = 110 | Medical, surgical, active geriatric, ICU; Canada | 83.33 | No significant difference (P = 0.2706) in PU incidence between the MSO and/or LALDM (11%, n = 6) and the ISO (4%, n = 2). |
| Control n = 55 MSO and LALM group (77.7 ± 10.6 y) | ||||||
| Intervention n = 55 ISO group (77.9 ± 14.6 y) | ||||||
| Demarre et al20 | RCT | Sample n = 1057 patients (80 y) | Geriatrics, internal medicine wards; Belgium | 75.0 | The cumulative HAPU incidence was 4.9%. | |
| Multistage ALPAM reduce the incidence of HAPU (3.6%) when compared with the APAM overlay group (8.9%) (P = 0.047). | ||||||
| APAM overlay n = 447 (81 y) | No significant differences were found between APAM overlay and those on a one‐stage ALPAM. | |||||
| One‐stage ALPAM n = 312 (79 y) | ||||||
| Multistage ALPAM n = 298 (80 y) | ||||||
| Manzano et al21 | Prospective quasi‐experimental study | Sample n = 232 | ICU; Spain | 52.0 | Incidence of HAPU (grade ≥ II) was lower when using the APAM mattress compared with the APAM overlay (18.67 cases/1000 days vs 12.41 cases/1000 d, P = 0.003) | |
| APAM overlay n = 122 (63 y) | ||||||
| APAM mattress n = 110 (64 y) | ||||||
| Ozyurek & Yavuz22 | RCT | Sample n = 105 (64.99 ± 15.1 y) | ICU; Turkey | 66.66 | No significant difference in HAPU incidence was found between the viscoelastic foam 1 (22/53) and foam 2 (23/52). | |
| Viscoelastic foam 1 n = 53 (64.77 ± 15.09 y) | ||||||
| Viscoelastic foam 2 n = 52 (65.21 ± 15.26 y) | ||||||
| Health professional education | Uzun et al25 | Prospective study | Sample n = 186 | ICU; Turkey | 56.0 | The incidence of PU stage II was significantly (P < 0.01) lower on the intervention group (17%, n = 16) when compared with the control group (37%, n = 34). |
| Education regarding preventive care can be effective in reducing the incidence of PU. | ||||||
| Intervention group n = 93 (58.56 ± 18.02 y) | ||||||
| Control group n = 93 (61.36 ± 16.42) | ||||||
| Picatoste et al24 | Quasi‐experimental study | Sample n = 447 | Surgical ICU; Spain | 66.67 | The overall incidence of PU decreases from 19.4% to 16.0% with the education programme, however it was not statistically significant. The incidence of PU stage I significantly (0.008) decrease from 68.7% (n = 57) to 25% (n = 44.6). | |
| Pre‐intervention n = 247 (65.2 ± 16.2 y) | ||||||
| Post‐intervention n = 200 (65.7 ± 15.5 y) |
Abbreviations: ALPAM, alternating low‐pressure air mattress; APAM, alternating pressure air mattress; HAPU, hospital‐acquired pressure ulcers; ICU, intensive care unit; ISO, inflated static overlay; LALM, low‐air‐loss dynamic mattress; MSO, Microfluid static overlay; RCT, randomised controlled trial.
European Pressure Ulcer Advisory Panel, National Pressure Ulcer Advisory Panel, Pan Pacific Pressure Injury Alliance, 2009.
Table 4.
Main findings and characteristics of studies focused on multiple intervention strategies
| Guidelines elements | Author, year | Study design | Sample size (mean age) | Setting; country | Study quality | Main finding(s) |
|---|---|---|---|---|---|---|
| Multiple interventions | Cobb et al27 | Pre‐test and post‐test study | Sample n = 143 | Acute spine unit; Canada | 69.23 | The PU incidence based on nursing assessment (26%) and occupational therapist (36%) did not decrease significantly (P = 0.2). |
| Cohort 1 n = 70 (47.29 ± 20.54) | ||||||
| Cohort 2 n = 73 (46.90 ± 20.91 y) | ||||||
| PUPI protocol was successful in changing clinical practice in PUs prevention, but was not statistically significant seen on immediate or long‐term patient outcomes during the study period. | ||||||
| Anderson et al,26 | Quasi‐experimental | Sample n = 327 (62.71 ± 17.12 y) | ICU; USA | 69.57 | UPUPB with semi‐weekly WOC Nurse rounds is significantly (P < 0.001) effective in decreasing HAPU incidence from 15.5% (pre‐intervention) to 2.1% (post‐intervention). | |
| Pre‐intervention n = 181 | ||||||
| Post‐intervention n = 146 | ||||||
| Tayyib et al,31 | RCT | Sample n = 140 | ICU; Saudi Arabia | 83.33 | PUPB was effective in reducing HAPU incidence from 32.9% to 7.1%. | |
| Control n = 70 (52 ± 19.5 y) | ||||||
| Intervention n = 70 prevention bundle group (47.5 ± 22.5 y) | ||||||
| The cumulative incidence of HAPU was significantly (P < 0.001) different between the intervention group (7.1%, 5/70 patients) and the control group (32.9%, 23/70 patients). | ||||||
| Chaboyer et al4 | Pragmatic cluster randomised trial | Sample n = 1598 | Medical, surgical, and rehabilitative; Australia | 82.6 | There was no significant difference (P = 0.644) between intervention (6.1%) and control (10.5%) groups in terms of the effect of PUPCB on PU incidence. | |
| Control n = 799 (74 y) | ||||||
| Intervention (PUPCB care bundle) n = 799 (70 y) | ||||||
| Swafford et al30 | Pre‐test and post‐test study | Sample 2011 n = 461 (51.9 y) | Medical/surgical ICU; USA | 37.5 | The incidence of HAPUs decreases from 69% between 2011 (10%, n = 45) and 2013 (3%, n = 17), with a comprehensive, proactive, and collaborative PU prevention program (staff education, adherence to protocols for patient care) | |
| Sample 2012 n = 434 (50.5 y) | ||||||
| Sample 2013 n = 563 (52.2 y) | ||||||
| Loudet et al28 | Quasi‐experimental | Sample n = 124 | ICU; Argentina | 59.09 | A multifaceted interventional process (educational session, PU checklist, smartphone application for monitoring and decision‐making, and “family prevention bundle”) was effective in decreasing the incidence of HAPU in patients with MV ≥ 96 h, from 75% to 54% (P = 0.016). | |
| Pre‐Intervention n = 55 (47 ± 18 y) | ||||||
| Post‐Intervention n = 69 (39 ± 17 y) | ||||||
| Martin et al29 | Mixed methods study | Sample n = 239 (age not available) | Community hospital; Canada | 77.27 | The implementation of PUPP decreases the incidence from 15.5% in 2013 to 5.1% in 2014. | |
| On‐line tutorial (n = 80 health care professionals and assistants) | ||||||
| The on‐line tutorial improved staff knowledge level. |
Abbreviations: HAPU, hospital‐acquired pressure ulcers; ICU, intensive care unit; MV, mechanical ventilation; PUPB, pressure ulcer prevention bundle; PUPCB, pressure ulcer prevention care bundle; PUs, pressure ulcers; PUPP, pressure ulcer prevention program; RCT, Randomised Controlled Trial; UPUPB, universal pressure ulcer prevention bundle; WOC, wound ostomy continence.
Table 5.
Main findings and characteristics of studies focused on risk‐assessment tools, repositioning and early mobilisation, repositioning with technology feedback, and a reminder system
| Guidelines elements | Author, year | Study design | Sample size (mean age) | Setting; country | Study quality | Main finding(s) |
|---|---|---|---|---|---|---|
| Risk‐assessment toolsa | Webster et al42 | RCT | Sample n = 1231 | Internal Medicine or oncology wards; Australia | 95.83 | HAPU was similar between groups (clinical judgement, 6.8%; Waterlow 7.5%; Ramstadius, 5.4%; P = 0.44). |
| Waterlow scale n = 411 (62.6 ± 19.6 y) | ||||||
| Ramstadius scale n = 410 (63.2 ± 19.2 y) | ||||||
| Clinical judgement n = 410 (61.9 ± 19.0 y) | ||||||
| Repositioning and early mobilisationa (repositioning frequency) | Rich et al34 | Cohort study | Sample n = 269 (84.0 ± 6.5 y) | Orthopaedics ward; USA | 87.5 | HAPU incidence in patients repositioned at least every 2 h was 12%. |
| HAPU incidence in patients repositioned less frequently than every 2 h was 10%. | ||||||
| Repositioning patients at least every 2 h is not associated with a decreased incidence of HAPU. | ||||||
| Repositioned less frequently than every 2 h n = 130 (84.0 ± 6.5 y) | ||||||
| Repositioned at least every 2 h n = 139 (83.9 ± 6.4 y) | ||||||
| Manzano et al33 | RCT | Sample n = 329 | ICU; Spain | 83.33 | Increasing repositioning frequency (2 h vs 4 h) did not reduce the incidence of HAPU in MV patients (9.68 cases for the 2 h group vs 12.12 cases for the 4 h group, P = 0.48). | |
| Control n = 165 every 2 h turning group (62.1 ± 14.5 y) | ||||||
| Intervention n = 164 every 4 h turning group (61.1 ± 14.5 y) | ||||||
| Powers44 | Non‐randomized comparison design | Sample n = 59 | ICU (trauma/neurointensive); USA | 76 | There was a statistically significant difference in the number of HAPU turning methods (6 in the SOC group vs 1 in the PPS group; P = .042). | |
| SOC n = 29 (57.72 ± 18.45 y) | ||||||
| PPS = 30 (57.73 ± 17.67 y) | ||||||
| Repositioning with technology feedback | Behrendt et al32 | Prospective controlled study | Sample n = 422 | ICU; USA | 71.43 | HAPU incidence in the CPBM group was lower (0.99%) than in control group (4.78%) (P = 0.02). |
| Control n = 209 (57.2 ± 18.3 y) | ||||||
| Intervention n = 213 CBPM group (58.7 ± 14.9 y) | ||||||
| Reminder system in patient care plan | Sebastian‐Viana, et al,41 | Pre‐ and post‐test study | Sample n = 18 483 | Medical/surgical ICU; Spain | 82.61 | The implementation of a reminder system on care plan for health professionals to alert patients who are at risk for PU was effective in decreasing the incidence of PU from 0.9% to 06% (P = 0.03. |
| A list of on‐screen reminders at the beginning of a health care professional's shift to inform them of patients at risk for developing a PU was effective at reducing the incidence of PU. | ||||||
| 2009 (pre‐intervention) n = 9263 (60.1 y) | ||||||
| 2010 (post‐intervention) n = 9220 (60.4 y) |
Abbreviations: CBPM, continuous bedside pressure mapping; HAPUs, hospital‐acquired pressure ulcers; ICU, intensive care unit; MV, mechanical ventilation; PPS, patient positioning system; PU, pressure ulcers; RCT, randomised controlled trial; SOC, standard of care.
European Pressure Ulcer Advisory Panel, National Pressure Ulcer Advisory Panel, Pan Pacific Pressure Injury Alliance, 2009.
Table 6.
Main findings and characteristics of studies focused on preventive skin care and prophylactic dressings
| Guidelines elements | Author, year | Study design | Sample size (mean age) | Setting; country | Study quality | Main finding(s) |
|---|---|---|---|---|---|---|
| Preventive skincarea | Shannon et al35 | Retrospective, quasi‐experimental study | Sample n = 110 | Medical unit; USA | 50 | The replacement of a mixture of ad hoc skin care products (without silicone‐based emollients) for an implementation of a silicone‐based dermal nourishing emollient associated with a skincare regimen decreases the incidence of HAPU from 20% to 0% in 8 mo. |
| Pre‐intervention n = 46 (68.65 ± 18.109 y) | ||||||
| Post‐intervention (SBDNE) n = 64 (63.2 ± 19.2 y) | ||||||
| Prophylactic dressingsa | Santamaria et al40 | RCT | Sample n = 440 | ICU; Australia | 73.16 | Multilayered soft silicone foam dressings are effective in preventing HAPU on heel and sacrum. The incidence in the intervention group was significantly (P = 0.001) lower (3.1%, n = 5/161) when compared with the control group (13.1%, n = 20/152). |
| Intervention n = 219 multilayered soft silicone foam dressing applied on sacrum and both heels. (54 ± 20.8 y) | ||||||
| Control n = 221 (56 ± 20.5 y) | ||||||
| Dutra et al36 | Epidemiological, exploratory, comparative, and cross‐sectorial analytical study | Sample n = 160 | ICU; Coronary Care Unit; Medical Clinic, Brazil | 75.0 | The incidence of HAPU was significantly lower (P = 0.038) in the polyurethane film group (8.7%) compared with the hydrocolloid group (15.0%). | |
| Polyurethane film group n = 80 (65.15 y) | ||||||
| Hydrocolloid group n = 80 (64.13 y) | ||||||
| Santamaria et al39 | Border II trial: Prospective Cohort study | Sample n = 412 | ICU; Australia | 87.5 | The incidence of HAPU in the intervention group was null (0%, n = 0/150), and in the control group was 9.2% (n = 14/152). | |
| Reduced incidence of heels HAPU from 13% to 3%. | ||||||
| Intervention n = 191 (55 ± 19.7 y) | ||||||
| Hydrocolloid n = 221 (56 ± 20.5 y) | ||||||
| Kalowes et al38 | Prospective RCT | Sample n = 366 | Cardiac, medical, surgical, and trauma ICU; USA | 91.3 | The incidence rate of HAPUs was significantly lower in the intervention group (0.7%) than in the control group (5.9%, P = 0.01). | |
| Intervention (5‐layered soft silicone foam n = 184) (64.6 ± 17.7 y) | ||||||
| Control n = 182 (67.3 ± 16.2 y) | ||||||
| Forni et al37 | Pragmatic RCT | Sample n = 359 | Orthopaedics; Italy | 91.67 | The overall incidence of HAPU was 10% (n = 36). The incidence was lower on the intervention groups (4.5%, n = 8) when compared with the control group (15.4%, n = 28, P = 0.001) | |
| Intervention (sacral polyurethane foam) n = 177 (84.3 ± 7.7) | ||||||
| Control n = 182 (83.2 ± 7.7) | ||||||
| Richard‐Denis43 | Retrospective and prospective cohort study | Sample n = 315 | Trauma center (level I); Canada | 83.33 | Patients with complete paraplegia developed sacral PUs in similar proportions (20.8% vs 27.3%) for gel mattress and multilayered foam dressing, respectively (P = 0.63). | |
| Group 1 (gel mattress) n = 226 | ||||||
| Group 2 (multilayered foam on sacrum) n = 89 |
Abbreviations: HAPI, hospital‐acquired pressure injuries; HAPU, hospital‐acquired pressure ulcers; ICU, intensive care unit; RCT, Randomised Controlled Trial; SBDNE, silicone‐based dermal nourishing emollient.
European Pressure Ulcer Advisory Panel, National Pressure Ulcer Advisory Panel, Pan Pacific Pressure Injury Alliance, 2009.
3.3.1. Support surfaces
Support surfaces are interface devices for pressure redistribution in some patient body areas, specially designed for management of tissue loads, microclimate, and/or other therapeutic functions, for example, any mattress, integrated bed system, mattress replacement, overlay, or seat cushion, or seat cushion overlay.7 Four studies were found that meet the inclusion criteria regarding support surfaces (Table 3). Three of them were performed in the ICU setting and one study in geriatrics and internal medicine wards. These studies only evaluated mattresses, no studies related to cushion performance were found.
No statistically significant difference was found in terms of PU incidence related to the use of microfluid static overlays (MSO) and/or low‐air‐loss dynamic mattress (LALDM). The study was carried out among acute, surgical, geriatric, and ICU patients.23 Another study assessing the effectiveness of two types of viscoelastic mattresses (viscoelastic foam 1 was composed of two layers and viscoelastic foam 2 composed of three layers) in ICU patients22 also did not find statistically significant difference between the patients with viscoelastic foam 1 and patients with viscoelastic foam 2. A similar finding showed no statistically significant difference between alternating pressure air mattress (APAM) overlays and one‐stage alternating low‐pressure air mattress (ALPAM) in reducing incidence in patients admitted to geriatrics and internal medicine wards.20
In contrast, multistage ALPAM showed a reduction in the incidence of PUs when compared with APAM overlays,20 and APAM decreased the incidence of PU grade ≥ II when compared with APAM overlays in ICU patients.21
3.3.2. Education of health care professionals
Education of health care staff is an important component of PU prevention, education programmes should include a large variety of factors that reflect the multifactorial nature of PUs.7 Table 3 indicate the results regarding education of the health professionals (n = 2). Education with focus on preventive care can be effective in reducing the incidence of PUs in ICU setting.24, 25
3.3.3. Multiple intervention programmes
Multiple intervention programmes and care bundles are a set of evidence‐based interventions that when performed together had a better and positive impact on patient outcomes, when compared with individual interventions.4 Regarding Table 4, most of the multiple intervention programmes (n = 7) were effective in decreasing the incidence of PUs.4, 26, 28, 29, 30, 31 One study did not decrease the incidence of PUs with statistical significance, but was successful in changing clinical practice in PU prevention.27
3.3.4. Risk‐assessment tools
Risk‐assessment tools are a part of a structured risk assessment in PU prevention; risk‐assessment tools (scale) are used for identifying if a patient is at risk of developing PUs and for identifying which risk factor could lead to a pressure ulceration. Table 5 presents the included studies reporting incidence rates associated with the implementation of a risk‐assessment tool. One study met the inclusion criteria within the domain risk‐assessment tools. The study showed that the incidence of PUs was similar between patients admitted in internal medicine and oncology wards assessed by Waterlow, Ramstadius, or clinical judgement.42 The authors concluded that there was no evidence that the risk‐assessment tools used effectively to decrease the incidence of PUs compared with clinical judgement.
3.3.5. Repositioning and early mobilisation
Repositioning and early mobilisation of patients are an intervention to reduce the duration and magnitude of pressure over vulnerable areas of the body, such as bony prominences, to contribute to comfort, hygiene, dignity, and functional ability.7 Studies related to repositioning and early mobilisation are presented in Table 5. Only studies related to manual repositioning frequency and repositioning supported by technological feedback were found. When 2 hours vs 4 hours repositioning frequency was studied in patient with mechanical ventilation support managed on an APAM,33 or 2 hours at least vs more than 2 hours repositioning frequency in elderly orthopaedics patients with bed‐bound hip fracture,34 the results showed no decrease in the incidence rates of PUs among the participants. Interestingly, when using a continuous bedside pressure‐mapping system to assist a 2‐hourly repositioning regimen in ICU patients, Behrendt et al32 found a statistically significant decrease in the incidence of PUs. The authors32 pointed out that the bedside pressure‐mapping system was able to support clinical staff optimising positioning and permitting feedback, which allowed intervention towards early pressure relief. When two methods were compared for patient repositioning, the prevalon turn and position system was effective in decreasing the incidence of PUs when compared with the standard of care using pillows.44 All the included studies examined the effectiveness of repositioning of patients in bed. No studies were found related to seating repositioning or repositioning of heels and offloading as a strategy to prevent PUs.
3.3.6. Reminder system in patient care plan
Gaps between recommend practice and daily routine care are known and given the challenge of changing the behaviour of health care staff, reminders in patient plan care on a screen computer are a promising strategy for better patient outcomes.45 A list of screen reminder at the beginning of a health shift and a reminder to alert which patients are at risk of PU development was effective in decreasing the incidence of PUs41
3.3.7. Preventive skin care
Maintaining skin integrity is an important factor to reduce PU occurrence.35 Table 6 shows the results of preventive skin care, implementation of a silicone‐based dermal creams on the skin care regimen decreases PU incidence in patients admitted to medical wards.35
3.3.8. Prophylactic dressings
It is evident that some dressings provide added benefits in preventing PUs, like helping to redistribute pressure and protect skin from shear and friction forces, and also contributing to microclimate balance.46 Table 6 presents the incidence of the included studies assessing the effectiveness of dressings for PU prevention. Among all the studies that assessed dressings as a PU preventive measure (n = 6), there was at least one type of dressing that showed a statistically significant decrease in incidence rates of PU occurrence. The study conducted by Dutra et al36 showed that polyurethane film dressings were more effective than hydrocolloid dressings in decreasing PU incidence in the trochanteric and sacral areas for patients cared for in the ICU, coronary unit, and in one general medicine unit.36 Other studies found that a multilayered silicone foam dressing was effective in reducing PUs on heels39, 40 and sacrum37, 38, 40 in orthopaedics patients37 and patients in ICU38, 40 when previously applied in emergency department39 or within 24 hours of admission to the ICU.38 Multilayered silicone foam dressing applied on sacrum in a special population like patients with spinal cord injury did not show a higher effectiveness in terms of PU prevention when compared with a gel mattress.43 Forni et al37 found that a polyurethane foam dressing applied on the sacral area is effective in decreasing the incidence of PUs in orthopaedics patients.37
4. DISCUSSION
The present review summarises studies that analysed effective approaches to PU prevention in hospitalised adults, published from 2009 up to December 2018. Twenty‐six studies were retrieved following the inclusion criteria and were systematically reviewed to address the effectiveness of hospital‐acquired PU prevention.
4.1. Support surfaces
Generally, and despite setting and country, evidence from these studies suggests that support surfaces of multistage ALPAM and APAM were effective in preventing PUs when compared with APAM overlay.20, 21 When the patient is immobile,47 the pressure force over the bone prominence is the main factor that exposes patients to the risk of tissue damage, being also influenced by the length of time and intensity of pressure force applied to the patient's bone‐tissue interface. The main function of the supporting surfaces is the redistribution and, therefore, partial relief of pressure through the surface's immersion and envelopment capacity. Dynamic support surfaces such as ALPAM and APAM are characterised by cycles of insufflation and mechanical deflation of air that is alternately transmitted to different segments of cells of the mattress.20 Thus, the surface's performance is influenced by the material constitution, depth, and number of air cells of the mattress, as well as by the time of the insufflation‐deflation cycles programmed. Thus, the pressure intensity throughout the cycle and the duration of the same influence its effectiveness in the prevention of PUs. The use of APAM, when compared with APAM overlay, seems to perform better in reducing PUs.21 This study was performed in high‐risk patients and measured effectiveness regarding the incidence of PU grade II.21 A recent meta‐analysis showed a moderate‐certainty evidence that dynamic surfaces, such as powered active and hybrid air surfaces, may reduce PU incidence when compared with standard hospital surfaces.48 As the development of PUs is the result of a complex interplay of pathological pathways and risk factors,10, 11 it is not clear which mattresses' specifications are effective in decreasing the incidence of PUs.20, 21 There is some evidence that support surfaces prevent PU development,20, 21 however, the evidence level of the studies retrieved was low or very low. Thus, more research is needed to understand in a greater depth the relationship between supporting surfaces and PU prevention.48 Support surfaces should be chosen based on setting/wards characteristics and mainly on patients' individual needs. Multistage ALPAM seems to have a good performance on patients admitted into geriatric and acute medical wards.20 Additionally, APAMs may be a better choice to acute patients admitted to ICUs.20, 21
4.2. Health professional's education
Two studies show that education of health care staff on PU prevention can be effective in reducing the incidence of PUs in ICU setting.24, 25 Education of health care professionals is a recognised element of PU prevention guidelines5 and also influences behaviour change to encourage preventative practices with the aim of reducing the incidence of PU development.49 However, a recent systematic review shows that there are some issues related to education of health care professionals influencing PU incidence or knowledge of nurses.49 Those studies provided very low‐certainty evidence.26, 49 Therefore, the impact of education of health care professionals on PU prevention still needs clarification.
4.3. Multiple intervention programmes
Despite the setting, country, or specific approach, most of the multiple intervention programmes was effective in preventing PUs,4, 26, 27, 28, 29, 30, 31 but those effectiveness was not always with statistical significance.4, 27 Multiple intervention programmes and care bundles had a positive impact on patient outcomes in terms of PUs, like incidence and severity.31
Multifactorial and comprehensive programmes help to reduce PUs in hospitalised patients mainly those that include: teamwork approaches,29 education of health care staff,28, 29, 31 nutritional assessment,29, 31 risk‐assessment tools,27, 28, 30, 31 visual skin assessment,26, 27, 29, 31 support surfaces,26, 27, 29, 30, 31 offloading heels,26, 29 repositioning mainly with use of sliders,29 disposable soaker pads to manage moisture and incontinence,29 skin care,26, 27, 30, 31 medical devices related to PU assessment,31 prophylactic dressings,30 smartphone applications,28 patient and family involvement,4, 28 and semi‐weekly Wound Ostomy Continence (WOC) nurse rounds.26
Multiple interventions also increase staff knowledge, patient and family involvement, supporting clinical decision‐making, and improving health outcomes.31 Teamwork is an important part to successfully prevent PUs.29 Nurses are the link professionals who ensure that all members of the health care team are involved in PU strategies, whilst mitigating the tendency of ritualistic practices.29 Multiple intervention programmes associated with wound WOC nurse rounds and audits are effective in decreasing the probability of PU occurrence.26 WOC nurses round is the complement element of a bundle and their presence allows health care coaching in a consistent way.26 General education of health staff on PU prevention might not be enough to effectively prevent PUs, rounds, and presence of expert professionals like tissue viability nurses or WOC nurses.26 They served to maintain staff focus on PU prevention, stimulating questions and helping to find alternative and effective solutions to patients' problems.26 This suggests that a bundle performed individually by health care staff, probably not decrease the PUs without ongoing WOC nurses rounds.26 Patients and families should be seen by healthcare professionals as a partner and an important resource to avoid PUs.4, 6, 28 Limitations in terms of health staff ratios, miscommunication between health administrators and staff in direct clinical practice, and unavailability of materials and equipment are some of the identified barriers to PU prevention.29 Need of new and innovate approaches seemed to be an extra key to prevent PUs, a smartphone application was used as a telemedicine tool to simultaneously provide information to all the members of the health team.28 The compliance of single interventions to other strategies to avoid PUs is described in most of the studies included in this review. Therefore, multiple intervention programmes are more effective, even not always with statistical significance, than single interventions for PU prevention in hospitalised patients.
4.4. Risk‐assessment tools
The use of a risk‐assessment tool is recommended by national12 and international guidelines.7 Clinical judgement should be used to recognise other risk factors that were not screened by a risk‐assessment tool.7 Risk‐assessment tools (Waterlow, Ramstadius) and clinical judgement were not effective in decreasing PU occurrence.42 Therefore, it is suggested that the time spent to screening patients with risk‐assessment tools should be replaced by careful and daily skin inspection and in specific interventions tailored to patient's individual risk factors.42 No studies, with the inclusion criteria, were found with the focus on skin assessment as part of structured risk assessment. In some cases, skin changes were not visually detected properly,23 indeed subepidermal moisture (SEM) measurement and ultrasound are promising technologies in the early detection and prediction of early deep tissue damage and PU presence.50
4.5. Repositioning and early mobilisation
Repositioning of patients is recommended to relieve pressure and improve comfort in bedfast patients.7 The frequency of repositioning may differ according to the patient medical condition and the type of support surface in use.33, 34 Frequent repositioning for every 2 hours is considered to be the standard time interval to prevent PUs,32 and when this frequency is addressed with technology feedback, such as continuous bedside pressure mapping, these may decrease PU incidence and improve immediate pressure relief and consequently patient comfort.32 A continuous bedside pressure mapping is a sensing interface, which measures whole body pressure and alerts staff to execute the prescription of 2‐hours repositioning frequency.32 Bedside visual reminders, or in patients care plan, might be useful to remind health professional the need of repositioning and mobilisation.23 In contrast, findings of other two studies suggest that more frequent manual repositioning of patients did not decrease the incidence of PUs,33, 34 but increase the adverse events related to medical devices and nursing workload.33, 34 On the other hand, the evaluation of the degree of turn in patients with the use of a patient positioning system is effective in decreasing PU incidence.44 These positioning devices can perform sacral offloading, skin microclimate control, anti‐shear strap, and two body wedges to facilitate turning and positioning and maintaining the recommended 30° angle.44 Also, these devices require significantly less nurses for positioning and maintaining the patient in 30° when compared with standard of care.44 These contrast findings suggest a possible compliance related to support surfaces, manual repositioning frequency, and other addressed strategies like visual or sound reminders,51 which support the need of healthcare professionals for repositioning in bed and chair.
4.6. Reminder system in patient care plan
As stated in many recommendations of care for the prevention of PUs, identifying the patients developing PUs can facilitate health professionals to adopt adequate preventive measures and monitoring protocols to decrease PU incidence.5, 41 A list of on‐screen reminders (date of admission, the last assessment of PU risk, the status of current PUs, and the last recorded location, and extent of PUs) effectively decreases the cumulative incidence of PUs.41 New strategies need to be researched to effectively decrease the occurrence of complex worldwide problems such as PUs.
4.7. Preventive skin care
Maintaining skin integrity is the focus of health care professionals in daily practice, particularly in bedfast patients. Applying topical agents, like a cream or an ointment on skin, is one of the strategies to prevent PUs (52). The replacement of a mixture of ad hoc skin care products (without silicone‐based emollients) for the implementation of a silicone‐based dermal nourishing emollient associated with a skincare regimen effectively decreases the incidence of PUs.35 It is not clearly known which mechanisms of silicone‐based dermal‐nourishing emollient are truly effective, but it is believed that the major contributory effect is keeping the skin moist and hydrated, and also prevent skin damage based on the antioxidant component protection.35 The impact of topical agents on PU incidence is not a clear benefit or harm (53). More research is need to show which of these therapies provide potential benefit to patients (52).
4.8. Prophylactic dressings
The role of dressings in PU prevention, regarding the capacity of reducing pressure, friction, and shear, as well as effectively managing skin moisture, has been explored by many investigators.36, 37, 38, 39, 40 Multilayered foam dressings are effective in preventing PUs on heels39, 40 and sacrum37, 38, 40 in patients admitted to ICUs when they were in the emergency department40 or in the first 24 hours of ICU admission.38 Many PUs may have their beginning in the period of pre‐hospital wards admission.54 The hospital patients admission way (e.g. through emergency department) and lenght of stay in the emergency department, should be considered as an additional and individual risk factor to develop a PU.40 Multilayered foam dressing applied on sacrum in spinal cord injured patients did not develop fewer PUs when compared with patients in gel mattress.43 Indeed, prophylactic dressings should be used with precautions in this special population, mainly in complete tetraplegic patients.43
Dressings may also contribute to the reduction of PUs associated with medical devices and mainly in immobile ICU patients.46 Polyurethane films (PF) had a better performance and were more effective in preventing PUs when compared with hydrocolloids.36 The advantage of PF is its own system of gas exchange like the skin performance, which allows the diffusion of gases. Its elastic and adhesive characteristics permit it to be applied to different anatomical areas and allow resistance to friction and shear forces.36 Hospital policies should consider prophylactic dressings for high risk admitted patients in the emergency department and ICUs in new or revised clinical guidelines for PU prevention.39 However, prophylactic dressings to prevent PUs should be performed in combination with other preventive measures to minimise friction and shear.38, 39 Immobility is the main factor indicating that maybe a dressing could be considered as a prevention strategy.46 Other indications could be taken into account for the use of dressings in PU prevention, like planned immobility, alterations in sensorial perception, reduced or restricted mobility, atypical movements, and presence of medical devices.46
These findings show that preventing PUs is still a heterogeneous and complex process within diverse samples and settings, which remains a clinical challenge. In future, other strategies need to be considered to effectively prevent PUs. Innovations in daily clinical practice need to be considered like music cueing intervention that can improve staff adherence of PU guidelines and increase patient movement, which can result in a reduction of PUs.55 More high‐quality studies should be performed in different settings besides ICU. Research in other hospital settings is needed to examine the effectiveness of the same strategies in compliance with hospital ward specifications. PUs are adverse events that affect patient safety in hospitals. Although they are related to quality of care provided, a positive approach regarding health care professionals work should be integrated into health care institutional approaches. Health care professionals should be motivated in a positive way, for being involved in patient safety improvement, namely increasing the adherence to evidence‐based guidelines.
4.9. Strengths and limitations
This review was conducted following the PRISMA recommended checklist.18 All steps implemented on search strategy for each database were thoroughly reported, therefore this review can be replicated. In the studies included, heterogeneity was present, mainly arising as a result of issues surrounding the study populations, settings, and interventions under investigation. Therefore, it was not possible to perform a meta‐analysis.
5. CONCLUSION
Multiple intervention programmes in compliance with advanced practice wound nurse's regulation are more effective in decreasing PU incidence in hospitalised patients than single interventions by itself. Indeed, studies of different single interventions emphasise that the single intervention was effective when it was combined with other preventive measures. Prophylactic dressing applied early in sacrum or heels addressing other preventive measures is a recent promising strategy to effectively prevent PUs. Continuous bedside pressure‐mapping technology is a resource that improves repositioning of patients and helps health care professionals to prevent PUs in bedfast patients. Reminder systems in patient care plan help health care staff to identify patients at high risk of developing PUs and provide early tailored preventive measures.
CONFLICT OF INTERESTS
All authors declare that they have no conflict of interests.
ACKNOWLEDGEMENTS
S.G. received a scholarship from the University of Lisbon (BD2016/609). M.P. received a scholarship from Foundation for Science and Technology (SFRH/BD/122219/2016).
Gaspar S, Peralta M, Marques A, Budri A, Gaspar de Matos M. Effectiveness on hospital‐acquired pressure ulcers prevention: a systematic review. Int Wound J. 2019;16:1087–1102. 10.1111/iwj.13147
Funding information Fundação para a Ciência e a Tecnologia, Grant/Award Number: SFRH/BD/122219/2016; Universidade de Lisboa, Grant/Award Number: BD609/2016
REFERENCES
- 1. Lyder CH, Wang Y, Metersky M, et al. Hospital‐acquired pressure ulcers: results from the national Medicare patient safety monitoring system study. J am Geriatr Soc. 2012;60(9):1603‐1608. 10.1111/j.1532-5415.2012.04106.x. [DOI] [PubMed] [Google Scholar]
- 2. Slawomirski, L. , Auraaen A., Klazinga N. The Economics of Patient Safety: Stengthening a Value‐Based Approach to Reducing Patient Harm at National Level. OECD Health Working Papers, No. 96, Paris, France: OECD Publishing; 2017.
- 3. Vanderwee K, Clark M, Dealey C, Gunningberg L, Defloor T. Pressure ulcer prevalence in Europe: a pilot study. J Eval Clin Pract. 2007;13(2):227‐235. 10.1111/j.1365-2753.2006.00684.x. [DOI] [PubMed] [Google Scholar]
- 4. Chaboyer W, Bucknall T, Webster J, et al. The effect of a patient centred care bundle intervention on pressure ulcer incidence (INTACT): a cluster randomised trial. Int J Nurs Stud. 2016;64:63‐71. 10.1016/j.ijnurstu.2016.09.015. [DOI] [PubMed] [Google Scholar]
- 5. Edsberg LE, Black JM, Goldberg M, McNichol L, Moore L, & Sieggreen M. Revised National Pressure Ulcer Advisory Panel Pressure Injury Staging System: Revised Pressure Injury Staging System. Journal of Wound, Ostomy, and Continence Nursing. 2016;43(6):585‐597. 10.1097/WON.0000000000000281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schank JE. The NPUAP Meeting ‐ This was No Consensus Conference. J Am Coll Clin Wound Spec. 2015;7(1‐3):19‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Emily H, ed. National Pressure Ulcer Advisory Panel , European Pressure Ulcer Advisory Panel and Pan Pacific Pressure Injury Alliance . (2014). Prevention and Treatment of Pressure Ulcers: Quick Reference Guide. Osborne Park, Australia: Cambridge Media; 2014. [Google Scholar]
- 8. Latimer S, Chaboyer W, Gillespie B. Patient participation in pressure injury prevention: giving patient's a voice. Scand J Caring Sci. 2014;28(4):648‐656. 10.1111/scs.12088. [DOI] [PubMed] [Google Scholar]
- 9. Direção Geral de Saúde . (2015). Plano Nacional para a Segurança dos Doentes 2015–2020. Diário da República. https://dre.pt/application/file/66457154. Accessed August 10, 2018.
- 10. Coleman S, Gorecki C, Nelson EA, et al. Patient risk factors for pressure ulcer development: systematic review. Int J Nurs Stud. 2013;50(7):974‐1003. 10.1016/j.ijnurstu.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 11. Garcia‐Fernandez FP, Agreda JJ, Verdu J, Pancorbo‐Hidalgo PL. A new theoretical model for the development of pressure ulcers and other dependence‐related lesions. J Nurs Scholarsh. 2014;46(1):28‐38. 10.1111/jnu.12051. [DOI] [PubMed] [Google Scholar]
- 12. Direção Geral de Saúde . (2011). Escala de Braden: Versão Adulto e Pediátrica. https://www.dgs.pt/departamento-da-qualidade-na-saude/ficheiros-anexos/orientacao_ulceraspdf-pdf.aspx. Accessed August 10, 2018.
- 13. Moore ZE, Cowman S. Risk assessment tools for the prevention of pressure ulcers. Cochrane Database Syst Rev. 2014;2:Cd006471. 10.1002/14651858.CD006471.pub3. [DOI] [PubMed] [Google Scholar]
- 14. Barakat‐Johnson M, Barnett C, Wand T, White K. Knowledge and attitudes of nurses toward pressure injury prevention: a cross‐sectional multisite study. J Wound Ostomy Continence Nurs. 2018;45(3):233‐237. 10.1097/won.0000000000000430. [DOI] [PubMed] [Google Scholar]
- 15. Meesterberends E, Halfens R, Lohrmann C, de Wit R. Pressure ulcer guideline development and dissemination in Europe. J Clin Nurs. 2010;19(11–12):1495‐1503. 10.1111/j.1365-2702.2010.03229.x. [DOI] [PubMed] [Google Scholar]
- 16. Moore Z, Soriano JV, Pokorná A, Schoonhoven L, Markova A, Kristensen J. The joint EPUAP & EWMA pressure ulcer prevention & patient safety advocacy project. Wounds UK. 2017;13(3):4. [Google Scholar]
- 17. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264‐269. W264. [DOI] [PubMed] [Google Scholar]
- 18. Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta‐analysis protocols (PRISMA‐P) 2015 statement. Syst Rev. 2015b;4:1. 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Glynn L. A critical appraisal tool for library and information research. Library Hi Tech. 2006;24(3):387‐399. 10.1108/07378830610692154. [DOI] [Google Scholar]
- 20. Demarre L, Verhaeghe S, Van Hecke A, et al. The effectiveness of three types of alternating pressure air mattresses in the prevention of pressure ulcers in Belgian hospitals. Res Nurs Health. 2013;36(5):439‐452. 10.1002/nur.21557. [DOI] [PubMed] [Google Scholar]
- 21. Manzano F, Perez AM, Colmenero M, et al. Comparison of alternating pressure mattresses and overlays for prevention of pressure ulcers in ventilated intensive care patients: a quasi‐experimental study. J Adv Nurs. 2013;69(9):2099‐2106. 10.1111/jan.12077. [DOI] [PubMed] [Google Scholar]
- 22. Ozyurek P, Yavuz M. Prevention of pressure ulcers in the intensive care unit: a randomized trial of 2 viscoelastic foam support surfaces. Clin Nurse Spec. 2015;29(4):210‐217. 10.1097/nur.0000000000000136. [DOI] [PubMed] [Google Scholar]
- 23. Vermette S, Reeves I, Lemaire J. Cost effectiveness of an air‐inflated static overlay for pressure ulcer prevention: a randomized controlled trial. Wounds‐a Compendium of Clinical Research and Practice. 2012;24(8):207‐214. [PubMed] [Google Scholar]
- 24. Picatoste W, Salgado Barreira MJ, Pestonit M, et al. Effectiveness of an educational intervention in pressure ulcer prevention in a surgical intensive care unit: a quasi experimental study. GEROKOMOS. 2012;23(3):128‐131. [Google Scholar]
- 25. Uzun O, Aylaz R, Karadag E. Prospective study: reducing pressure ulcers in intensive care units at a Turkish medical center. J Wound Ostomy Continence Nurs. 2009;36(4):404‐411. 10.1097/WON.0b013e3181aaf524. [DOI] [PubMed] [Google Scholar]
- 26. Anderson M, Finch Guthrie P, Kraft W, Reicks P, Skay C, Beal AL. Universal pressure ulcer prevention bundle with WOC nurse support. J Wound Ostomy Continence Nurs. 2015;42(3):217‐225. 10.1097/won.0000000000000109. [DOI] [PubMed] [Google Scholar]
- 27. Cobb JE, Belanger LM, Park SE, et al. Evaluation of a pilot Pressure Ulcer Prevention Initiative (PUPI) for patients with traumatic spinal cord injury. J Wound Care. 2014;23(5):211‐212, 214, 216–218 passim. 10.12968/jowc.2014.23.5.211. [DOI] [PubMed] [Google Scholar]
- 28. Loudet CI, Marchena MC, Maradeo MR, et al. Reducing pressure ulcers in patients with prolonged acute mechanical ventilation: a quasi‐experimental study. Rev Bras Ter Intensiva. 2017;29(1):39‐46. 10.5935/0103-507x.20170007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Martin D, Albensi L, Van Haute S, et al. Healthy skin wins: a glowing pressure ulcer prevention program that can guide evidence‐based practice. Worldviews Evid Based Nurs. 2017;14(6):473‐483. 10.1111/wvn.12242. [DOI] [PubMed] [Google Scholar]
- 30. Swafford K, Culpepper R, Dunn C. Use of a comprehensive program to reduce the incidence of hospital‐acquired pressure ulcers in an intensive care unit. Am J Crit Care. 2016;25(2):152‐155. 10.4037/ajcc2016963. [DOI] [PubMed] [Google Scholar]
- 31. Tayyib N, Coyer F, Lewis PA. A two‐arm cluster randomized control trial to determine the effectiveness of a pressure ulcer prevention bundle for critically ill patients. J Nurs Scholarsh. 2015a;47(3):237‐247. 10.1111/jnu.12136. [DOI] [PubMed] [Google Scholar]
- 32. Behrendt R, Ghaznavi AM, Mahan M, Craft S, Siddiqui A. Continuous bedside pressure mapping and rates of hospital‐associated pressure ulcers in a medical intensive care unit. Am J Crit Care. 2014;23(2):127‐133. 10.4037/ajcc2014192. [DOI] [PubMed] [Google Scholar]
- 33. Manzano F, Colmenero M, Perez‐Perez AM, et al. Comparison of two repositioning schedules for the prevention of pressure ulcers in patients on mechanical ventilation with alternating pressure air mattresses. Intensive Care Med. 2014;40(11):1679‐1687. 10.1007/s00134-014-3424-3. [DOI] [PubMed] [Google Scholar]
- 34. Rich SE, Margolis D, Shardell M, et al. Frequent manual repositioning and incidence of pressure ulcers among bed‐bound elderly hip fracture patients. Wound Repair Regen. 2011;19(1):10‐18. 10.1111/j.1524-475X.2010.00644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shannon RJ, Coombs M, Chakravarthy D. Reducing hospital‐acquired pressure ulcers with a silicone‐based dermal nourishing emollient‐associated skincare regimen. Adv Skin Wound Care. 2009;22(10):461‐467. 10.1097/01.Asw.0000361384.89866.85. [DOI] [PubMed] [Google Scholar]
- 36. Dutra RA, Salome GM, Alves JR, et al. Using transparent polyurethane film and hydrocolloid dressings to prevent pressure ulcers. J Wound Care. 2015;24(6):268, 270–261, 273–265. 10.12968/jowc.2015.24.6.268. [DOI] [PubMed] [Google Scholar]
- 37. Forni C, D'Alessandro F, Gallerani P, et al. Effectiveness of using a new polyurethane foam multi‐layer dressing in the sacral area to prevent the onset of pressure ulcer in the elderly with hip fractures: a pragmatic randomised controlled trial. Int Wound J. 2018;15(3):383‐390. 10.1111/iwj.12875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kalowes P, Messina V, Li M. Five‐layered soft silicone foam dressing to prevent pressure ulcers in the intensive care unit. Am J Crit Care. 2016;25(6):e108‐e119. 10.4037/ajcc2016875. [DOI] [PubMed] [Google Scholar]
- 39. Santamaria N, Gerdtz M, Liu W, et al. Clinical effectiveness of a silicone foam dressing for the prevention of heel pressure ulcers in critically ill patients: border II trial. J Wound Care. 2015;24(8):340‐345. [DOI] [PubMed] [Google Scholar]
- 40. Santamaria N, Gerdtz M, Sage S, et al. A randomised controlled trial of the effectiveness of soft silicone multi‐layered foam dressings in the prevention of sacral and heel pressure ulcers in trauma and critically ill patients: the border trial. Int Wound J. 2013;12(3):302‐308. 10.1111/iwj.12101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sebastian‐Viana T, Losa‐Iglesias M, Gonzalez‐Ruiz JM, Lema‐Lorenzo I, Nunez‐Crespo FJ, Salvadores Fuentes P. Reduction in the incidence of pressure ulcers upon implementation of a reminder system for health‐care providers. Appl Nurs Res. 2016;29:107‐112. 10.1016/j.apnr.2015.05.018. [DOI] [PubMed] [Google Scholar]
- 42. Webster J, Coleman K, Mudge A, et al. Pressure ulcers: effectiveness of risk‐assessment tools. A randomised controlled trial (the ULCER trial). BMJ Qual Saf. 2011;20(4):297‐306. 10.1136/bmjqs.2010.043109. [DOI] [PubMed] [Google Scholar]
- 43. Richard‐Denis A, Thompson C, Mac‐Thiong JM. Effectiveness of a multi‐layer foam dressing in preventing sacral pressure ulcers for the early acute care of patients with a traumatic spinal cord injury: comparison with the use of a gel mattress. Int Wound J. 2017;14(5):874‐881. 10.1111/iwj.12710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Powers J. Two methods for turning and positioning and the effect on pressure ulcer development: a comparison cohort study. J Wound Ostomy Continence Nurs. 2016;43(1):46‐50. 10.1097/won.0000000000000198. [DOI] [PubMed] [Google Scholar]
- 45. Shojania KG, Jennings A, Mayhew A, Ramsay CR, Eccles MP, Grimshaw J. The effects of on‐screen, point of care computer reminders on processes and outcomes of care. Cochrane Database Syst Rev. 2009;3:Cd001096. 10.1002/14651858.CD001096.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Clark M, Black J, Alves P, et al. Systematic review of the use of prophylactic dressings in the prevention of pressure ulcers. Int Wound J. 2014;11(5):460‐471. 10.1111/iwj.12212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Vitoriano A, Moore Z. The relationship between risk factors, risk assessment, and the pathology of pressure ulcer development. Česká a slovenská neurologie a neurochirurgie. 2017;80/113(suppl 1):S25‐S28. [Google Scholar]
- 48. Shi C, Dumville JC, Cullum N. Support surfaces for pressure ulcer prevention: a network meta‐analysis. PLoS One. 2018;13(2):e0192707. 10.1371/journal.pone.0192707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Porter‐Armstrong AP, Moore ZE, Bradbury I, McDonough S. Education of healthcare professionals for preventing pressure ulcers. Cochrane Database Syst Rev. 2018;5:Cd011620. 10.1002/14651858.CD011620.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Oliveira AL, Moore Z, O'Connor T, Patton D. Accuracy of ultrasound, thermography and subepidermal moisture in predicting pressure ulcers: a systematic review. J Wound Care. 2017;26(5):199‐215. 10.12968/jowc.2017.26.5.199. [DOI] [PubMed] [Google Scholar]
- 51. Yap TL, Kennerly SM, Simmons MR, et al. Multidimensional team‐based intervention using musical cues to reduce odds of facility‐acquired pressure ulcers in long‐term care: a paired randomized intervention study. J am Geriatr Soc. 2013;61(9):1552‐1559. 10.1111/jgs.12422. [DOI] [PubMed] [Google Scholar]
- 52. Moore Z, Webster J. Dressings and topical agents for preventing pressure ulcers. Cochrane Database Syst Rev. 2013;8:Cd009362. 10.1002/14651858.CD009362.pub2. [DOI] [PubMed] [Google Scholar]
- 53. Moore Z, Webster J. Dressings and topical agents for preventing pressure ulcers. Cochrane Database Syst Rev. 2018;12:1‐55. 10.1002/14651858.CD009362.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Cubit K, McNally B, Lopez V. Taking the pressure off in the emergency department: evaluation of the prophylactic application of a low shear, soft silicon sacral dressing on high risk medical patients. Int Wound J. 2013;10(5):579‐584. 10.1111/j.1742-481X.2012.01025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yap TL, Kennerly S, Corazzini K, Porter K, Toles M, Anderson RA. Evaluation of cueing innovation for pressure ulcer prevention using staff focus groups. Healthcare (Basel). 2014;2(3):299‐314. 10.3390/healthcare2030299. [DOI] [PMC free article] [PubMed] [Google Scholar]


