Abstract
In this study, we evaluated a new aspect of negative pressure wound therapy (NPWT) as an analytical tool for pharmacokinetic studies. Twenty‐one patients with soft tissue defects scheduled to receive NPWT were included in this study. Concomitant to NPWT, all patients received intravenous moxifloxacin (MX). At different time intervals, blood plasma levels of MX were sampled and compared with synchronous concentrations of MX in the exudate obtained from the NPWT drainage system. Serial measurements were performed upon initiation of the therapy as well as in the steady state (after 5 days). At steady state, wound tissue was obtained intraoperatively. High‐performance liquid‐chromatography (HPLC) was used for analysis. At 1 hour post‐administration, the exudate/plasma levels (mg/L) were 1.92/3.07; at 12 hours, 0.80/1.14; at 24 hours, 0.26/0.43; and at 120 hours (steady state), 0.42/0.47. There was a correlation between exudate and plasma levels reaching approximately 0.75. Until now, methods for pharmacokinetic studies concerning interstitial fluid are difficult to apply in the clinical context. The presented method showed limitations, but we believe that, after methodological improvements, measurements of substances in the interstitial fluid by means of NPWT are feasible.
Keywords: interstitial fluid, moxifloxacin, negative pressure wound therapy (NPWT), pharmacokinetics, wound infection
1. BACKGROUND
The question of whether antibiotics that are systemically administered do reach the wound tissue and surface is an essential clinical issue and remains not completely answered up to now. For most antibiotics, interstitial fluid is the biospace where an interaction between the substance and the pathogen takes place.1, 2 Therefore, measurements of concentration in this target interstitial space are of cardinal importance. However, the task at hand is not an easy one. In the past, several approaches were used to accomplish this, for example, microdilution,3 equilibrium chamber,4 and cantharis‐induced or perioperative blisters after excisional surgery.5, 6, 7 All these methods are either too expensive or too complex for use in the clinical routine.
More than 10 million wounds have been treated by negative pressure wound therapy (NPWT) worldwide since its introduction in 1995.8, 9 Indications for NPWT therapy include management of acute and chronic open wounds, immobilisation of skin grafts, antimicrobial conditioning, and drainage of cavitary defects.10 The potential use of an NPWT device as a collector of interstitial fluid for further pharmacokinetic studies has not been reported before.
Moxifloxacin (MX) is a fourth‐generation fluoroquinolone with excellent pharmacokinetic and pharmacodynamic properties.11 Its pharmacokinetic properties have been extensively evaluated in the past, and it was therefore used for this study.
2. METHODS
2.1. Patients
Twenty‐one patients with an open wound met all inclusion criteria and were included in the study. None of the wounds was secondary to an open abdomen (Table 3). The study was performed with individual patient's consent and with institutional board review of the Ethic Committee of the University Erlangen‐Nurnberg (nr.:3698). For a patient to be included in the study, there had to be an indication for NPWT with concomitant antibiotic chemotherapy as well as informed consent of the patient. Patients under 21 years of age, patients with another contraindication for MX administration, or patients with a contraindication for NPWT therapy were not included in the study. Inclusion and exclusion criteria are summarised in Table 1.
Table 3.
Demographic data of patients
| Patient‐Nr. | Gender (m/w) | Age | Weight (kg) | Height (cm) | BMI (kg/m2) | Wound localisation | Diagnosis |
|---|---|---|---|---|---|---|---|
| 2 | w | 70 | 98 | 165 | 36 | Lower extremity | Venous ulcer |
| 3 | m | 54 | 60 | 174 | 198 | Lower extremity | Sarcoma |
| 5 | w | 70 | 75 | 162 | 28.6 | Lower extremity | Diabetic ulcer |
| 6 | w | 53 | 75 | 174 | 24.8 | Lower extremity | Necrotising fasciitis |
| 7 | m | 38 | 85 | 170 | 29.4 | Upper extremity | Wrist infection |
| 8 | w | 52 | 75 | 160 | 29.3 | Lower extremity | Compartment syndrome |
| 10 | m | 40 | 70 | 178 | 22.1 | Lower extremity | Melanoma |
| 11 | w | 48 | 75 | 165 | 27.5 | Lower extremity | De‐gloving trauma |
| 12 | m | 49 | 93 | 178 | 29.4 | Upper extremity | Tumour |
| 13 | m | 21 | 70 | 180 | 21.6 | Upper extremity | Amputation trauma |
| 14 | w | 48 | 81 | 175 | 26.4 | Lower extremity | Chronic infection |
| 15 | w | 51 | 70 | 174 | 23.1 | Lower extremity | postOP infection |
| 16 | w | 69 | 75 | 167 | 26.9 | Thorax | Costal osteomyelitis |
| 17 | w | 65 | 69 | 170 | 23.9 | Lower extremity | Venous ulcer |
| 18 | m | 33 | 89 | 176 | 28.7 | Back | Tumour |
| 19 | w | 67 | 65 | 166 | 23.6 | Lower extremity | Ulcer |
| 20 | m | 31 | 70 | 169 | 24.5 | Lower extremity | Open fracture |
| 21 | w | 50 | 74 | 170 | 25.6 | Lower extremity | Open fracture |
| n | 18 | 18 | 18 | 18 | |||
| MW | 50.5 | 76.1 | 170.7 | 26.2 | |||
| SD | 14.2 | 9.8 | 5.8 | 3.8 | |||
| RSD | 36 | 24 | 11 | 69 | |||
| Median | 51 | 75 | 170 | 26 | |||
| Min | 21 | 60 | 160 | 19.8 | |||
| Max | 70 | 98 | 180 | 36 |
Table 1.
Study exclusion criteria
| Known adverse reaction to fluoroquinolones |
| Age under 21 years |
| Bacteriological proof or clinical suspicion of Pseudomonas aeruginosa infection or bacteriological proof of a pathogen with resistance against moxifloxacin |
| Pregnancy or lactation |
| Negative pressure wound therapy (NPWT) with an indication for therapeutic instillation with antiseptic solution |
| Liver or kidney disease. |
| Serious heart disease (QTc prolongation) |
| Contraindication to NPWT |
| History of tendinopathy associated with fluoroquinolone therapy |
| History of convulsions |
| Concomitant therapy with antacids and sucralfate |
2.2. Study design and sampling schedule
Upon application of the NPWT system, an initial dose of 400 mg MX was administered intravenously. In accordance with the manufacturers' instructions, the antibiotic solution was administered over a 1‐hour time period. This is depicted as time point 0. At 1, 12, and 24 hours after administration of MX, venous blood samples and exudate from the draining lines of the NPWT system were obtained synchronously. This first 24 hours will be referred to as the loading phase. After 5 days, before administration of MX, other pairs of venous blood samples and exudate samples were obtained (120 hours to 5 days). These samples were meant to assess the steady‐state phase. MX was then administered prior to the operative change of the NPTW system. During operation, samples from the wound tissue were obtained, along with blood and exudate samples. Time interval between last administration of MX and sampling was documented. Sample quantities were defined as in Table 2.
Table 2.
Definition of sample quantities
| Ca. 0.1 g wound tissue |
| Ca. 100 μL exudate |
| Ca. 2.6 mL blood |
After acquisition, all samples were stored immediately at −20 ° C.
2.3. Technique of sample acquisition
Processing of samples was staged. In the first stage, aspiration, acquisition, centrifugation, and freezing was performed. In the second stage, the sample was processed in a standardised protocol for liquid chromatogtraphy. Blood samples were obtained from the median cubital vein when possible. Approximately 2.6 mL of blood was suctioned into a lithium‐heparin Sarstedt Monovette® (Sarstedt AG & Co, Nuembrecht). The obtained plasma was centrifuged for 20 minutes in 4000 rotations per minute in room temperature (Megafuge 10 R, Heraeus Sepatech Gmbh, Osterode) and was then stored at −20 °C. Exudate (approximately 100 μL) was obtained from the drainage line of the NPWT and was transferred into a standard probe (Eppendorf‐Cup) and then stored at −20 °C.
At day five, in the operating theatre, at least 0.1 g of wound tissue was surgically excised from the tissue after removal of the NPWT system. At the same time, a venous blood sample was obtained. All samples were transferred into an Eppendorf Cup and then stored at −20 °C. The time interval between last MX administration and excision was documented. When more than one tissue sample was obtained, the values were pooled and averaged. Maintaining a continuous cooling chain, all probes were transferred to the department for pharmacology and toxicology of the University of Regensburg, where the analysis was conducted place.
2.4. Analysis with high‐performance liquid chromatography (HPLC)
The method for the measurement of MX through HPLC with a fluorescent assessment has been described extensively and validated in previous publications.12, 13, 14 After MX (Bayer AG, Leverkusen), gatifloxacin (Grünenthal, Aachen) was also used as an internal standard. Gatifloxacin was not in any way added to the solutions but was only used in the past for validation reasons, as described in previous publications.12, 13, 14
A total of 100 μL of plasma or exudate is diluted in 100 μL 0.2% EDTA (pH 6‐7) and treated with 400 μL methanol to separate them from protein. After centrifugation, 400 μL of the supernatant is transferred into a HPLC vial and diluted with 400 μL 0.01 M HCl.
The tissue biopsies from the wound tissue (ca. 0.1 g) were shock‐frozen in liquid nitrogen and were pulverised with a custom‐made stainless steel mortar (Bessman Tissue Pulverizer, Spectum Europe, Breda, Netherlands). They were then homogenised in a methanol‐water‐perchloric acid solution. After centrifugation, the supernatant was analysed. Gatifloxacin was used as an internal standard. The following HPLC system was used: Prominence series (pump LC 20AT, AS SIL‐20AC HAT, control box CBM‐20A, Integration software LCSolution), column oven CTO‐AS VP, and Fluorescence detector RF10AXL (Shimadzu, Duisburg).
The tolerance level of the method for exudate or plasma was 10 ng/mL, <50 ng/g in tissue. For every analysis, secondary probes of plasma or tissue were acquired as controls for double assay. Inter‐assay and intra‐assay variation (CVinter, CVintra) as well as deviations from nominal values (Bias) were better than 5%. Values are generally expressed as average with standard deviation (±).
2.5. Pharmacokinetic calculations and statistical evaluation
The results were documented with Excel 2010 (Microsoft Corporation One, Microsoft Way, Redmond, WA). The pharmacokinetic calculation was performed with Phoenix WinNonlin 6.1 (Pharsight—A Certara Com‐pany, 1699 South Hanley Road, St. Louis, MO). The pharmacokinetic parameters in plasma were calculated with a non‐compartmental as well as a compartmental model and were found to coincide. The results are presented in a one‐compartment model (Table 4). For pharmacokinetic analysis of the concentration in the wound exudate, a non‐compartmental model was used because of high dispersion of concentration values and because of peak concentrations being reached later than 12 hours (Table 5). The statistical evaluation of correlation analysis was performed with SPSS Version 18 (SPSS Inc, IBM Corporation, Somers, NY). Significance level was set to P < 0.05. Simple linear regression analysis was used as a statistical method. Coefficient calculation was performed by means of the Spearman test.
Table 4.
Pharmacokinetic parameters of moxifloxacin (MX) in plasma after a 1‐hour infusion of 400 mg MX
| Pat. | C max (Mg/L) | t 1/2 (h) | AUC oo (Mg*h/L) | V d (L) | CL (l/h) |
|---|---|---|---|---|---|
| 2 | 2.68 | 19.5 | 76.7 | 146.4 | 5.2 |
| 3 | 2.47 | 4.4 | 16.8 | 149.9 | 23.7 |
| 5 | 3.90 | 9.9 | 57.4 | 99.0 | 7.0 |
| 6 | 5.17 | 6.7 | 52.7 | 73.6 | 7.6 |
| 7 | 2.15 | 4.1 | 13.9 | 171.4 | 28.8 |
| 8 | 3.81 | 5.7 | 33.2 | 98.7 | 12.0 |
| 10 | 2.95 | 9.1 | 40.4 | 130.4 | 9.9 |
| 11 | 3.65 | 11.2 | 61.0 | 106.1 | 6.6 |
| 12 | 2.28 | 5.1 | 17.8 | 164.3 | 22.5 |
| 13 | 3.38 | 7.4 | 37.7 | 113.0 | 10.6 |
| 14 | 3.65 | 9.2 | 50.3 | 105.5 | 7.9 |
| 15 | 3.24 | 3.9 | 20.0 | 113.0 | 20.0 |
| 16 | 2.04 | 14.2 | 42.9 | 191.4 | 9.3 |
| 17 | 3.46 | 5.7 | 30.3 | 108.9 | 13.2 |
| 18 | 2.27 | 8.9 | 30.2 | 169.8 | 13.2 |
| 19 | 4.96 | 5.7 | 43.1 | 75.8 | 9.3 |
| 20 | 3.00 | 8.6 | 38.9 | 128.2 | 10.3 |
| 21 | 3.83 | 6.7 | 38.9 | 99.3 | 10.3 |
| n | 18 | 18 | 18 | 18 | 18 |
| Mean | 3.27 | 8.1 | 39.0 | 124.7 | 12.6 |
| SD | 0.90 | 3.9 | 16.7 | 34.0 | 6.7 |
| RSD | 27 | 48 | 43 | 27 | 53 |
| Median | 3.31 | 7.1 | 38.9 | 113.0 | 10.3 |
| Q25 | 2.52 | 5.7 | 30.2 | 100.9 | 8.3 |
| Q75 | 3.77 | 9.2 | 48.5 | 149.0 | 13.2 |
| Min | 2.04 | 3.9 | 13.9 | 73.6 | 5.2 |
| Max | 5.17 | 19.5 | 76.7 | 191.4 | 28.8 |
Abbreviations: AUC oo, area under curve; CL, clearance; C max, peak concentration; t 1/2, concentration half‐life; V d, volume of distribution.
The calculations were performed by means of a one‐compartment model.
Table 5.
Pharmacokinetic parameters of moxifloxacin (MX) in wound exudate after a 1‐hour infusion of 400 mg MX
| Pat. | C max (mg/L) | Q E/P of C max | T max (h) | T last (h) | C last (mg/L) | AUC t (mg*h/L) | AUC t Plasma (mg*h/L) | Q E/P of AUC |
|---|---|---|---|---|---|---|---|---|
| 2 | 4.55 | 1.69 | 1.0 | 12.0 | 1.28 | 34.36 | 26.1 | 1.32 |
| 3 | 0.41 | 0.17 | 12.0 | 12.0 | 0.41 | 3.54 | 17.2 | 0.21 |
| 5 | 1.23 | 0.31 | 12.0 | 24.0 | 0.54 | 17.99 | 48.6 | 0.37 |
| 6 | 4.51 | 0.87 | 1.0 | 12.0 | 1.31 | 34.25 | 40.1 | 0.85 |
| 7 | 0.82 | 0.38 | 1.0 | 24.0 | 0.11 | 12.06 | 17.2 | 0.70 |
| 8 | 0.19 | 0.05 | 12.0 | 12.0 | 0.19 | 1.14 | 28.4 | 0.04 |
| 10 | 3.18 | 1.18 | 2.2 | 24.0 | 0.48 | 35.67 | 33.4 | 1.07 |
| 11 | 1.51 | 0.44 | 12.0 | 24.0 | 0.15 | 23.31 | 46.1 | 0.51 |
| 12 | 1.89 | 0.83 | 1.0 | 24.0 | 0.09 | 14.55 | 20.1 | 0.72 |
| 13 | 0.77 | 0.26 | 13.8 | 24.0 | 0.43 | 12.04 | 32.8 | 0.37 |
| 14 | 0.94 | 0.29 | 12.0 | 24.0 | 0.40 | 13.67 | 39.7 | 0.34 |
| 15 | 1.87 | 0.80 | 2.9 | 24.0 | 0.14 | 18.47 | 19.7 | 0.94 |
| 16 | 3.34 | 1.76 | 2.2 | 30.2 | 0.13 | 36.08 | 33.0 | 1.09 |
| 17 | 0.71 | 0.22 | 12.0 | 24.0 | 0.14 | 9.11 | 31.1 | 0.29 |
| 18 | 1.92 | 0.99 | 3.0 | 24.0 | 0.18 | 17.30 | 24.2 | 0.71 |
| 19 | 4.38 | 0.97 | 1.8 | 25.5 | 0.33 | 34.47 | 44.5 | 0.77 |
| 20 | 1.91 | 0.65 | 1.3 | 25.0 | 0.49 | 25.30 | 35.0 | 0.72 |
| 21 | 0.39 | 0.10 | 12.0 | 24.0 | 0.15 | 5.54 | 38.3 | 0.14 |
| n | 18 | 18 | 18 | 18 | 18 | 18 | 18 | 18 |
| Mean | 1.92 | 0.67 | 6.4 | 21.8 | 0.39 | 19.4 | 32.0 | 0.62 |
| SD | 1.47 | 0.52 | 5.4 | 5.6 | 0.36 | 11.7 | 9.9 | 0.36 |
| RSD | 76 | 77 | 84 | 26 | 95 | 60 | 31 | 58 |
| Median | 1.69 | 0.54 | 3.0 | 24.0 | 0.26 | 17.6 | 32.9 | 0.71 |
| Q25 | 0.78 | 0.27 | 1.4 | 24.0 | 0.14 | 12.0 | 24.7 | 0.35 |
| Q75 | 2.86 | 0.95 | 12.0 | 24.0 | 0.47 | 32.0 | 39.3 | 0.83 |
| Min | 0.19 | 0.05 | 1.0 | 12.0 | 0.09 | 1.1 | 17.2 | 0.04 |
| Max | 4.55 | 1.76 | 13.8 | 30.2 | 1.31 | 36.1 | 48.6 | 1.32 |
Abbreviations: AUC t, area under curve for exudate concentration; AUC t‐plasma, area under curve for corresponding plasma concentration; C last, concentration at last time point; C max, peak concentration; Q E/P, quotient between exudate and plasma; T last, time point of last probe; T max; time point of probe with peak concentration.
The calculations were performed by means of a non‐compartmental model because of wide value dispersion.
3. RESULTS
3.1. Demographic data of patients, study inclusion
Fifty‐three patients were considered for the study; however, only 21 met all inclusion criteria. The study was approved by the ethical committee of the University of Erlangen. Informed consent was obtained from all patients prior to recruitment. In five cases, patients had to be taken off MX prior to completing the protocol. Their data are used in phase 1 calculations but not in the measurements in the steady state (phase 2). Patient No 1 was considered a pilot patient. In another five patients, the nurse accidentally administered MX prior to the 24‐hour sampling period because of standard dosage schedules. Altogether, data obtained from 18 Patients were utilised in this study. Demographic data are shown in Table 3.
3.2. MX concentration in plasma and exudate
Altogether, 222 probes were sampled (107 plasma, 91 exudate, 24 wound tissue).
MX concentration in plasma after 1 hour was n = 18, 3.07 mg/L ± 0.90. After 12 hours, MX concentration in plasma was n = 18, 1.14 mg/L ± 0.45. After 24 hours, MX concentration in plasma was n = 14, 0.43 mg/L ± 0.26. After 120 hours, concentration of MX in plasma was n = 12, 0.47 mg/L ± 0.31.
MX concentration in exudate after 1 hour was n = 16, 1.92 mg/L ± 1.62. After 12 hours, MX concentration in exudate was n = 17, 0.80 mg/L ± 0.41. After 24 hours, MX concentration in exudate was n = 13, 0.26 mg/L ± 0.17. After 120 hours, concentration of MX in exudate was n = 9, 0.42 mg/L ± 0.29.
For the time point 1 hour, there was no significant correlation between plasma and exudate concentrations (n = 16, r 2 = 0.07; r 2 corr 0.004; r = 0.27; P = 0.32).
For the time point 12 hours, there was a highly significant correlation between plasma and exudate concentrations (n = 17, r 2 = 0.50; r 2 corr 0.47; r = 0.71; P = 0.0015).
For the time point 24 hours, there was correlation between plasma and exudate. However, we could not establish a statistical significance (n = 13, r 2 = 0.24; r 2 corr 0.17; r = 0.49; P = 0.09).
For the time point 120 hours (steady state), there was a highly significant correlation between plasma and exudate concentrations (n = 9, r 2 = 0.64; r 2 corr 0.59; r = 0.80; P = 0.001).
A correlation coefficient of around 0.75 was established between plasma and exudate concentrations (Figure 1).
Figure 1.
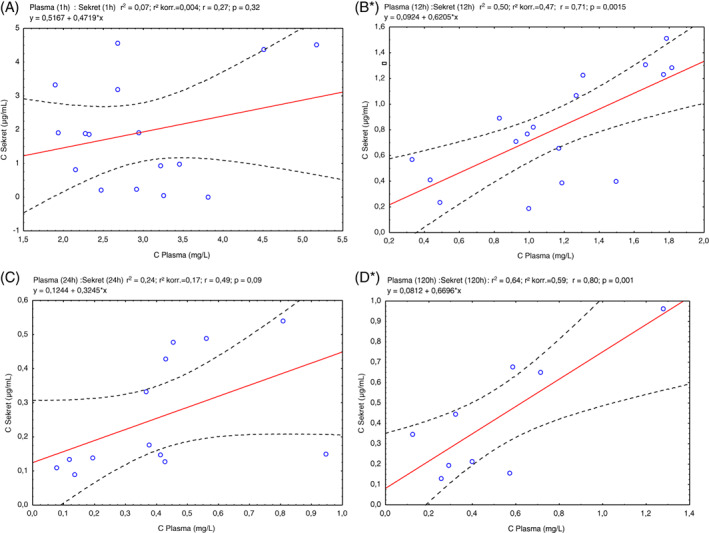
Scatter plots of wound exudate (Y axis) to blood plasma (X axis) concentrations after administration of moxifloxacin. A, 1 hour after administration. B, 12 hours. C, 24 hours. D, 5 days (steady state). Correlation was highly significant in (B) and (D) time points
3.3. MX concentration in wound tissue
We could obtain wound tissue from 11 patients on day five of therapy. The time interval from last MX administration was variable, depending on the time point or operation. The correlation coefficient of tissue/plasma concentration was 1.315 (n = 11, r2 = 0.133; r = 0.36; P = 0.270) (Spearman test, R = 0.76364, P = 0.00623). In other words, concentration of MX in interstitial tissue achieved at steady state was 135% of the corresponding concentration in plasma (Figure 2).
Figure 2.
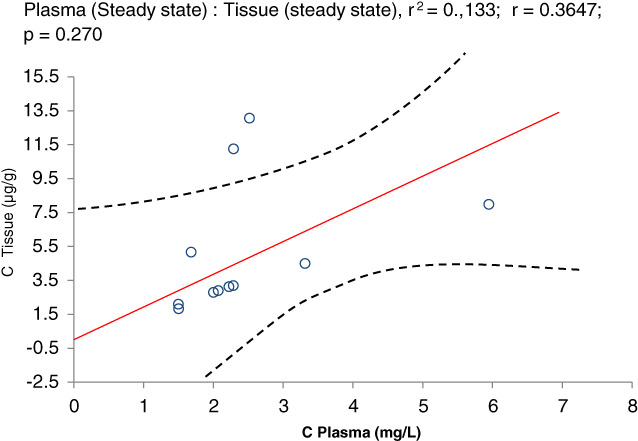
Scatter plots of wound tissue (Y axis) to blood plasma (X axis) concentrations after administration of moxifloxacin. Correlation was not significant
3.4. Pharmacological considerations
For concentration in plasma (one‐compartment model), a peak concentration of 3.27 mg/L after infusion of MX was calculated. Half‐life period was calculated to be 8.1 hours. Calculated AUC was 39 mg*h/L, and volume of distribution was 125 L, with a clearance of 12.6 L/h (Table 4). The quotient of abbreviated area under curve AUC t and area under curve AUC oo was 17.5 ± 14.4%. Measurements were therefore valid, with an acceptable error margin. For concentration in exudate (non‐compartment model), a peak concentration of 1.92 mg/L was calculated. Maximum concentration was reached at 6.4 hours. Calculated AUC t was 19.4 mg*h/L. The quotient between AUC t in exudate and AUC t in plasma was found to be 0.62 (Table 5). The calculated quotient of the AUC (0.62) using the non‐compartmental model was lower than the quotient of concentrations (0.75) in the analysis of the individual values seen above.
Pharmacokinetic parameters of MX in plasma were measured after a 1‐hour infusion of 400 mg MX. The calculations were performed by means of a one‐compartment model. C max: peak concentration, t 1/2: concentration half‐life, AUC oo: area under curve, V d: volume of distribution, CL: clearance.
4. DISCUSSION
While guidelines on the perioperative administration of antibiotics have been established for decades, it remains a matter of discussion whether antibiotic drugs do reach significant and sufficient concentrations in wounds. The wound tissue, interstitial wound fluid, and exudate have been subject to various studies. Antibiotic concentrations have been measured in wound fluid from Redon drains with regard to short time effectiveness in orthopaedic surgery, indicating that antibiotic concentrations at therapeutic levels can be reached for a short period perioperatively.15, 16 Bone samples in joint replacement have also shown that systemically administered drugs can be found within the bone.17 In the context of colorectal surgery, attempts have been made to clarify the pharmacokinetics of prophylactically administered antibiotic in serum, intestinal tissue, and subcutaneous adipose tissue in patients who underwent surgery for colorectal cancer, but in subcutaneous tissue, the concentrations were found to be low at the time of measurement.18 This has repeatedly led surgeons to administer antibiotics locally into wounds with the aim of reaching effective dosages.19 In obese patients, problems of achieving sufficient drug levels in the fatty tissue have been described,20 and it was found that, in obese women undergoing caesarean delivery, prophylaxis with 3 g of cefazolin did not significantly increase adipose tissue concentration.21
NPWT has been proposed to increase the antibiotic concentration in damaged and infected tissues because of the mechanisms of topical negative pressure.22 In tissue biopsies, a significant increase in the concentration of antibiotics under NPWT was described, while in a control group, the improvement was sensibly lower. This would foster the assumption that maybe even a significant increase of antibiotic concentration in the wound tissue could be expected.22
In this study, we tried to evaluate the idea of NPWT as a sampling method of interstitial fluid for pharmacokinetic studies, although this study is not a pharmacological study of MX. During NPWT, the system collects wound fluid in a continuous or intermittent manner. Speaking in general terms, we managed to demonstrate the following facts. For one, there is a significant correlation between blood plasma and exudate levels of MX. Second, levels of MX in plasma and tissue were similar to those reported in previous studies.13, 23, 24 However, results were far from consistent, and there were several difficulties.
For example, flow of exudate in NPWT is highly variable. Directly after surgical debridement, the wound produces ample blood and fluids, and collection of exudate from the draining lines is easy and reliable. At later stages, the system runs “dry”, and exudate, if any, stagnates. In everyday clinical life, patients often ask their surgeons if it is alright that the pump makes “funny noises” and “no more fluid” is seen “circulating in their drains”. Technically, abduction of fluid from the wound is only a by‐product of NWPT. Ideally, the exudate should be collected directly from the wound bed rather than from the drains.
These limitations can be easily overcome. First, the wound could be directly rinsed for adequate amount of exudate to be collected. The rinsing solution could carry a marker substance. Rate of dilution could then be calculated by the concentration of the marker, and exact calculations of concentrations of the target substance could be obtained directly from the wound.
Another source of inconsistency was surgical acquisition of tissue from the wound during surgical debridement. Tissue samples were sometimes too small for evaluation. Tissue fluids and blood adherent to the tissue produced false low measurements. On the other hand, drying out of the sample during the freezing procedure produced false high measurements. To overcome this, we had to switch to highly standardised pre‐weighted Eppendorf cups.
Interaction between daily clinical routine and study protocol posed difficulties. In the 1‐hour and 24‐hours time points of plasma and exudate sampling, high variance of measurements was partially because of erratic administration of MX. According to the manufacturer's instructions, the substance has to be slowly administered throughout a 60‐minute interval. Variations in the rate of administration might have had an impact on differences in plasma and exudate concentrations. On the other hand, there might indeed be a time lag between administration of MX and the time to achieving maximum concentration in the interstitial fluid. That would be in concordance with findings in the literature. Other investigators showed that concentration of MX in tissue reaches a C maximum in approximately 3 hours post‐administration.24
Concentration of MX in ejaculate and prostatic fluid was the same or higher than the plasma concentration.12 In our study, levels of MX in interstitial fluid reached approximately 0.75 of levels in plasma. This is in concordance with findings in the literature25 where MX concentrations in interstitial fluid and plasma reached a ratio of 0.81 to 0.86 in 24 hours. Concerning wound tissue, we found a ratio of tissue/plasma of 1.4. In the literature, levels of MX were found to be even higher in the tissue in comparison with plasma levels.24
Non‐compartmental analysis is suitable for demonstrating how imprecise data are. Abbreviated area under curve (AUC) is calculated until the last measurement point (AUC t). This residual extrapolated area (from the last time point until infinite) is a good indicator of erratic data. In a Note for Guidance on the investigation of Bioavailability and Bioequivelence, Committee for Proprietary Medicinal Products, London, July 26th, 2001, it was postulated that this AUC t should by no means exceed 20% of AUC oo. In the present study, on calculating pharmacokinetic values for plasma concentration of MX, the AUC t was within these limits. The parameters in the one‐compartment pharmacokinetic analysis correlated well to data of healthy individuals found in the literature,26 maybe with the exception of a shorter half‐life.
A central point of this study was the calculation of a quotient between plasma concentrations and concentrations in wound exudate. Values were extracted from the individual measurements by means of regression analysis as well as by means of parallel pharmacokinetic calculations. At the end, we found a slight discrepancy between these two quotients (regression analysis: 0.75 versus pharmacokinetic calculations: 0.62). Furthermore, we observed an overall lower quotient than in the literature. We know from bibliographic data27 that this quotient is higher in inflammatory tissue than in healthy tissue. This phenomenon can be partially explained by the fact that NPWT can reduce regional oedema and inflammatory response.
Overall, we were able to demonstrate that NPWT can be used for the measurement of concentrations of different substances in the interstitial fluid. Although further investigations appear necessary to standardise the procedure, this method appears able to gain clinical importance in principle. A direct comparison between the microdilution method and our proposed method would be of great interest. If the technique became more easily accessible, it could be helpful in a variety of hard‐to‐heal wounds to determine whether antibiotic levels within the wound are sufficient or not.
ACKNOWLEDGEMENTS
This study was supported by a grant provided by Bayer Healthcare AG for a project named “Measurement of concentration of Avalox in wounds treated by vacuum assisted closure.”
Polykandriotis E, Horch RE, Jost M, Arkudas A, Kees F, Schmitz M. Can systemically administered antibiotics be detected in wound tissues and surfaces under negative pressure wound therapy? Int Wound J. 2019;16:503–510. 10.1111/iwj.13063
Funding information Bayer Healthcare AG
REFERENCES
- 1. Eichler HG, Muller M. Drug distribution. The forgotten relative in clinical pharmacokinetics. Clin Pharmacokinet. 1998;34(2):95‐99. [DOI] [PubMed] [Google Scholar]
- 2. Brunner M, Stabeta H, Moller JG, et al. Target site concentrations of ciprofloxacin after single intravenous and oral doses. Antimicrob Agents Chemother. 2002;46(12):3724‐3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Elmquist WF, Sawchuk RJ. Application of microdialysis in pharmacokinetic studies. Pharm Res. 1997;14(3):267‐288. [DOI] [PubMed] [Google Scholar]
- 4. Tan JS, Trott A, Phair JP, Watanakunakorn C. A method for measurement of antibiotics in human interstitial fluid. J Infect Dis. 1972;126(5):492‐497. [DOI] [PubMed] [Google Scholar]
- 5. Bagley DH, Mac Lowry J, Beazley RM, Gorschboth C, Ketcham AS. Antibiotic concentration in human wound fluid after intravenous administration. Ann Surg. 1978;188(2):202‐208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kanellakopoulou K, Pagoulatou A, Stroumpoulis K, et al. Pharmacokinetics of moxifloxacin in non‐inflamed cerebrospinal fluid of humans: implication for a bactericidal effect. J Antimicrob Chemother. 2008;61(6):1328‐1331. [DOI] [PubMed] [Google Scholar]
- 7. Hussmann B, Johann I, Kauther MD, Landgraeber S, Jager M, Lendemans S. Measurement of the silver ion concentration in wound fluids after implantation of silver‐coated megaprostheses: correlation with the clinical outcome. Biomed Res Int. 2013;2013:763096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Morykwas MJ, Argenta LC. Nonsurgical modalities to enhance healing and care of soft tissue wounds. J South Orthop Assoc. 1997;6(4):279‐288. [PubMed] [Google Scholar]
- 9. Morykwas MJ, Simpson J, Punger K, Argenta A, Kremers L, Argenta J. Vacuum‐assisted closure: state of basic research and physiologic foundation. Plast Reconstr Surg. 2006;117(7 suppl):121s‐126s. [DOI] [PubMed] [Google Scholar]
- 10. Huang C, Leavitt T, Bayer LR, Orgill DP. Effect of negative pressure wound therapy on wound healing. Curr Probl Surg. 2014;51(7):301‐331. [DOI] [PubMed] [Google Scholar]
- 11. Stefanopoulos PK, Tarantzopoulou AD. Facial bite wounds: management update. Int J Oral Maxillofac Surg. 2005;34(5):464‐472. [DOI] [PubMed] [Google Scholar]
- 12. Wagenlehner FM, Kees F, Weidner W, Wagenlehner C, Naber KG. Concentrations of moxifloxacin in plasma and urine, and penetration into prostatic fluid and ejaculate, following single oral administration of 400 mg to healthy volunteers. Int J Antimicrob Agents. 2008;31(1):21‐26. [DOI] [PubMed] [Google Scholar]
- 13. Wagenlehner FM, Lunz JC, Kees F, Wieland W, Naber KG. Serum and prostatic tissue concentrations of moxifloxacin in patients undergoing transurethral resection of the prostate. Journal of Chemotherapy (Florence, Italy). 2006;18(5):485‐489. [DOI] [PubMed] [Google Scholar]
- 14. Stass H, Dalhoff A. Determination of BAY 12‐8039, a new 8‐methoxyquinolone, in human body fluids by high‐performance liquid chromatography with fluorescence detection using on‐column focusing. J Chromatogr B Biomed Sci Appl. 1997;702(1–2):163‐174. [DOI] [PubMed] [Google Scholar]
- 15. Wewalka G, Endler M. Antibiotic concentration in postoperative wound fluid during short‐term prevention with cephalosporins. Arzneimittelforschung. 1978;28(1):72‐75. [PubMed] [Google Scholar]
- 16. Rosenfeld MB, Campos J, Ratzan KR, Uredo I. Chemoprophylaxis with cefoxitin and cephalothin in orthopedic surgery: a comparison. Antimicrob Agents Chemother. 1981;19(5):826‐830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bue M, Tottrup M, Hanberg P, et al. Bone and subcutaneous adipose tissue pharmacokinetics of vancomycin in total knee replacement patients. Acta Orthop. 2018;89(1):95‐100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nakamura T, Tomizawa A, Inano H, Sato T, Yago K, Watanabe M. Tissue concentrations of antibiotics given prophylactically during colorectal cancer surgery. Hepatogastroenterology. 2013;60(126):1371‐1375. [DOI] [PubMed] [Google Scholar]
- 19. Eder C, Schenk S, Trifinopoulos J, et al. Does intrawound application of vancomycin influence bone healing in spinal surgery? Eur Spine J. 2016;25(4):1021‐1028. [DOI] [PubMed] [Google Scholar]
- 20. Kram JJF, Greer DM, Cabrera O, Burlage R, Forgie MM, Siddiqui DS. Does current cefazolin dosing achieve adequate tissue and blood concentrations in obese women undergoing cesarean section? Eur J Obstet Gynecol Reprod Biol. 2017;210:334‐341. [DOI] [PubMed] [Google Scholar]
- 21. Maggio L, Nicolau DP, Dacosta M, Rouse DJ, Hughes BL. Cefazolin prophylaxis in obese women undergoing cesarean delivery: a randomized controlled trial. Obstet Gynecol. 2015;125(5):1205‐1210. [DOI] [PubMed] [Google Scholar]
- 22. Lo Torto F, Ruggiero M, Parisi P, Borab Z, Sergi M, Carlesimo B. The effectiveness of negative pressure therapy on infected wounds: preliminary results. Int Wound J. 2017;14(6):909‐914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Breilh D, Jougon J, Djabarouti S, et al. Diffusion of oral and intravenous 400 mg once‐daily moxifloxacin into lung tissue at pharmacokinetic steady‐state. Journal of Chemotherapy (Florence, Italy). 2003;15(6):558‐562. [DOI] [PubMed] [Google Scholar]
- 24. Esposito S, Noviello S, D'Errico G, et al. Concentration of moxifloxacin in plasma and tonsillar tissue after multiple administration in adult patients. J Antimicrob Chemother. 2006;57(4):789‐792. [DOI] [PubMed] [Google Scholar]
- 25. Muller M, Stass H, Brunner M, Moller JG, Lackner E, Eichler HG. Penetration of moxifloxacin into peripheral compartments in humans. Antimicrob Agents Chemother. 1999;43(10):2345‐2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Keating GM, Scott LJ. Moxifloxacin: a review of its use in the management of bacterial infections. Drugs. 2004;64(20):2347‐2377. [DOI] [PubMed] [Google Scholar]
- 27. Joukhadar C, Stass H, Muller‐Zellenberg U, et al. Penetration of moxifloxacin into healthy and inflamed subcutaneous adipose tissues in humans. Antimicrob Agents Chemother. 2003;47(10):3099‐3103. [DOI] [PMC free article] [PubMed] [Google Scholar]


