Abstract
Objective
Our objective was to describe the characteristics of patients admitted, discharged and readmitted, due to COVID-19, to a central London acute-care hospital during the second peak, in particular in relation to corticosteroids use.
Methods
We reviewed patients admitted from the community to University College Hospital (UCH) with COVID-19 as their primary diagnosis between 1st-31st December 2020. Re-attendance and readmission data were collected for patients who re-presented within 10 days following discharge. Data were retrospectively collected.
Results
196 patients were admitted from the community with a diagnosis of COVID-19 and discharged alive in December 2020. Corticosteroids were prescribed in hospital for a median of 5 days (IQR 3–8). 20 patients (10.2%) were readmitted within 10 days. 11/20 received corticosteroids in the first admission of which 10 had received 1–3 days of corticosteroids. Readmission rate in those receiving 1–3 days of corticosteroids was 25%.
Conclusions
Most international guidelines have recommended providing up to 10 days of corticosteroids for severe COVID-19 but stopping on discharge. Our findings show shorter courses of corticosteroids during admission are associated with an increased risk of being readmitted and support continuing the course of corticosteroids after hospital discharge monitored in the virtual ward setting.
Keywords: COVID-19, Hospital, Readmissions, Corticosteroids, Dexamethasone
Dear Editor,
Introduction
Outcomes for patients with COVID-19 infection have been widely reported for the initial peak of the pandemic.1 However, there is a lack of data describing outcomes and characteristics of readmitted patients in the resurgent peak, after corticosteroids became standard of care.
Based on data and protocols from randomised controlled trials, most international treatment guidelines recommend 6 mg dexamethasone daily (or equivalent) for up to 10 days in those hospitalised with severe COVID-19 but stopping on discharge.2, 3, 4, 5, 6
The UK's second COVID-19 wave peaked on 9/1/2021.7 Here we describe the characteristics of patients admitted, discharged and readmitted, due to COVID-19, to our hospital, during this second wave. We explored the relationship between clinical and biochemical variables, treatment received during a patient's first admission, and readmission risk, in relation to corticosteroid use.
Methods
We reviewed patients admitted from the community to University College Hospital (UCH) with COVID-19 as their primary diagnosis between 1st-31st December 2020. Re-attendance and readmission data were collected for patients who re-presented within 10 days following discharge from their first admission.
Data were retrospectively collected, including patient demographics, clinical data on first admission and readmission, steroid treatment and any treatment received on discharge from the first admission.
In the primary analysis, appropriate corticosteroid dosage was defined as receiving 6 mg dexamethasone daily. Statistical analysis was conducted in Stata ver. 12.1 (StataCorp). Independent data were compared using Mann-Whitney U test or t-test. Paired data were compared by Wilcoxon signed-rank and proportions by χ2 test.
We fitted a logistic regression model to assess relationships between demographic and clinical factors and readmission risk. We conducted a sensitivity analysis considering anyone receiving a dose equivalent to 75% of 6 mg dexamethasone daily as having received steroids, using the outcome of readmission or re-attendance.
The study met the NHS definition of a quality improvement project with the departmental governance lead and did not require ethical approval.
Results
271 patients were admitted to UCH with COVID-19 in December. 25 patients were transferred from external hospitals or had nosocomially-acquired COVID-19 and 50 patients died during their first admission or remained an inpatient throughout the data collection period and were excluded from subsequent analysis. 196 patients were included in the analysis.
Median age was 58 years (IQR 47–71); 48% female; 133/196 (67.9%) had ≥1 comorbidity (as defined by the ISARIC 4C score),8 32 (16.3%) had diabetes mellitus. Median length of stay was 4 days (IQR 2–8). 125/196 (63.8%) required oxygen of whom 30 (15.3%) required respiratory support. 124/196 (63.3%) received corticosteroids on their first admission for a median of 5 days (IQR 3–8). All patients had acceptable peripheral oxygen saturations (SpO2) at discharge(≥92% on air or within their target range). 10/196 (5.1%) were discharged with corticosteroids. 53/196 (27.0%) were followed up in a virtual clinic post-discharge.
26/196 (13.3%) patients re-attended UCH due to COVID-19, a median of 3 days (IQR 2–5) following discharge. Of these, 20 (10.2%) were readmitted. Median CRP (mg/L) rose significantly in those readmitted from 43.2 (IQR 29.4–71.6) on discharge to 91.8 (IQR 37.3–139.6) on readmission (p = 0.021). 17/20 (85%) required oxygen and corticosteroids on readmission of whom 6 (30%) required respiratory support.
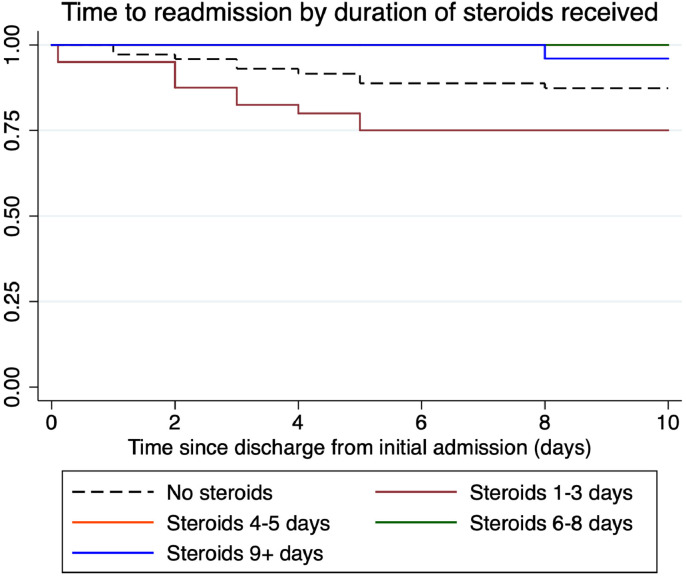
The 11/20 patients receiving steroids during their first admission, subsequently readmitted, had a shorter initial admission (median 2 days [IQR 1–3] vs 5 days [3–9] p = 0.005), received shorter courses of steroids (median 2 days [IQR 1–3] vs 5 days [3–8] p<0.001) and were discharged earlier in their illness course (median day 8 [IQR 6–11] vs day 13 [IQR 9–18], p = 0.005) than those that were not readmitted. There was no difference in SpO2 on air at discharge (95% IQR [94%–96%] for both) or in remdesivir use (27.2% vs 33.6%, p = 0.669). Data for patients receiving inpatient corticosteroids on their first admission were quartiled based on their duration of steroids. In the first quartile, (1–3 days) readmission rates were highest at 25% (Fig. 1 and Table 1 ). In an exploratory logistic regression analysis, only treatment with dexamethasone significantly reduced odds of readmission (OR 0.77 per day of dexamethasone 95% CI 0.61–0.92, p = 0.012). Results were similar in the sensitivity analysis considering both equivalent doses of other steroids and both re-attendance and readmission to hospital (supplementary data).
Fig. 1.
Survival plot showing the probability of being out of hospital in the 10 days following discharge by duration of corticosteroids received during initial admission. The line showing readmission in the group receiving steroids for 4–5 days is not visible as masked by the line for steroids for 6–8 days – i.e. there were no readmissions for this group.
Table 1.
Patients admitted from the community who were discharged alive from their first admission. Excluding ITU transfers and nosocomial transmissions. Comparing characteristics of those receiving different steroid course durations by quartile (n = 196).
| Characteristics |
Number of days of dexamethasone received as inpatient on 1st admission |
||||
|---|---|---|---|---|---|
| Did not receive (n = 72) | 1st Quartile1–3 (n = 40) | 2nd Quartile4–5 (n = 26) | 3rd Quartile6–8 (n = 32) | 4th Quartile≥9 (n = 26) | |
| Number readmitted (%) | 9 (12.5) | 10 (25.0) | 0 (0.0) | 0 (0.0) | 1 (3.8) |
| Median age (IQR) - years | 61 (49–78) | 51 (41–65) | 56 (40–66) | 64 (48–70) | 55 (49–67) |
| Sex | |||||
| Males (%) | 37 (51.4) | 23 (57.5) | 12 (46.2) | 15 (46.9) | 15 (57.7) |
| Females (%) | 35 (48.6) | 17 (42.5) | 14 (53.8) | 17 (53.1) | 11 (42.3) |
| Ethnicity (%) | |||||
| White | 27 (37.5) | 19 (47.5) | 10 (38.5) | 10 (31.3) | 9 (34.6) |
| Black | 11 (15.3) | 8 (20.0) | 1 (3.8) | 5 (15.6) | 5 (19.2) |
| South Asian | 7 (9.7) | 1 (2.5) | 3 (11.5) | 3 (9.4) | 3 (11.5) |
| Other Asian | 7 (9.7) | 0 (0.0) | 3 (11.5) | 1 (3.1) | 2 (7.7) |
| Unknown | 20 (27.8) | 12 (30.0) | 9 (34.6) | 13 (40.6) | 7 (26.9) |
| Median day of illness on admission (IQR) | 4 (2–8) | 7 (4–9) | 8 (5–10) | 8 (5–10) | 8 (6–9) |
| Median length of admission (IQR) - days | 2 (1–5) | 2 (2–3) | 4 (4–5) | 7 (6–8) | 10 (9–13) |
| Number of comorbidities (%) | |||||
| 0 | 22 (30.6) | 15 (37.5) | 11 (42.3) | 8 (25.0) | 7 (26.9) |
| 1 | 19 (26.4) | 8 (20.0) | 7 (26.9) | 11 (34.4) | 11 (42.3) |
| >2 | 31 (43.1) | 17 (42.5) | 8 (30.8) | 13 (40.6) | 8 (30.8) |
| Number with infiltrates of Chest X-ray (%) | 34 (47.2) | 34 (85.0) | 23 (88.5) | 31 (96.9) | 26 (100.0) |
| Number of asymptomatic COVID (%) | 18 (25.0) | 0 (0.0) | 0 (0.0) | 1 (3.1) | 0 (0.0) |
| Median admission sats (IQR) -% | 97 (95–98) | 93 (91–97) | 92 (90–95) | 92 (88–95) | 92 (88–94) |
| Median CRP on admission (IQR) – mg/L | 24.0 (8.0–63.4) | 41.2 (24.8–77.5) | 68.0 (51.8–102.8) | 102.1 (59.8–168.9) | 98 (73–164) |
| Median Urea on 1st admission (IQR) – mmol/L | 4.5 (3.6–6.9) | 4.2 (3.5–5.9) | 4.5 (3.5–5.6) | 4.4 (3.3–6.3) | 6.0 (4.8–7.3) |
| Median lymphocytes on 1st admission (IQR) – x109/L | 1.01 (0.80–1.47) | 1.06 (0.79–1.32) | 0.87 (0.63–1.23) | 0.87 (0.69–1.31) | 1.00 (0.73–1.10) |
| Median peak oxygen requirement (IQR) -% | 21 (21–21) | 28 (24–33) | 32 (32–36) | 40 (35–53) | 60 (40–64) |
| Number of patients by peak respiratory support (%) | |||||
| Hospitalised, no oxygen | 61 (84.7) | 8 (20.0) | 1 (3.8) | 1 (3.1) | 0 (0.0) |
| Oxygen by mask or nasal prongs | 10 (13.9) | 31 (77.5) | 24 (92.3) | 20 (62.5) | 9 (34.6) |
| NIV or high-flow oxygen | 1 (1.4) | 0 (0.0) | 1 (3.8) | 11 (34.4) | 16 (61.5) |
| Intubation and Mechanical Ventilation | 0 (0.0) | 1 (2.5) | 0 (0.0) | 0 (0.0) | 1 (3.8) |
| Ventilation and additional organ support (Pressors, RRT, ECMO) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Number receiving remdesivir (%) | 0 (0.0) | 6 (15.0) | 11 (42.3) | 11 (34.4) | 13 (50.0) |
| Median CRP on discharge from 1st admission (IQR) – mg/L | 22.5 (4.6–53.9) | 28.0 (16.3–62.2) | 19.9 (13.2–41.4) | 16.8 (10.2–31.1) | 12 (5.3–40.6) |
| % Sats drop on exercise at discharge from 1st admission (%) | |||||
| 0–1 | 21 (29.2) | 14 (35.0) | 10 (38.5) | 10 (31.3) | 6 (23.1) |
| 2–3 | 2 (2.8) | 8 (20.0) | 5 (19.2) | 5 (15.6) | 3 (11.5) |
| ≥4 | 5 (6.9) | 3 (7.5) | 7 (26.9) | 4 (12.5) | 11 (42.3) |
| Unknown | 44 (61.1) | 15 (37.5) | 4 (15.4) | 13 (40.6) | 6 (23.1) |
| Number discharged with steroids on 1st admission (%) | 2 (2.8) | 6 (15.0) | 1 (3.8) | 0 (0.0) | 1 (3.8) |
| Number discharged with thromboprophylaxis on 1st admission (%) | 6 (8.3) | 3 (7.5) | 3 (11.5) | 7 (21.9) | 7 (26.9) |
| Number discharged with saturations probe on 1st admission (%) | 8 (11.1) | 10 (25.0) | 6 (23.1) | 3 (9.4) | 6 (23.1) |
| Median ISARIC 4C mortality% (IQR) | 11.7 (4.8–19.2) | 7.8 (2.3–19.2) | 11.7 (4.8–19.2) | 19.2 (11.7–26.9) | 14.4 (7.8–26.9) |
| Median ISARIC 4C deterioration%(IQR) | 18.4 (12.3–24.4) | 29.4 (20.1–44.9) | 46.7 (26.6–59.7) | 56.4 (35.9–65.7) | 57.4 (40.3–65.4) |
| Number receiving telephone clinic follow up on 1st admission (%) | 19 (26.4) | 16 (40.0) | 12 (46.2) | 3 (9.4) | 3 (11.5) |
Discussion
To our knowledge this is the first study to evaluate readmission rate in the recent COVID-19 wave, in the context of corticosteroid use.
Despite the majority meeting safe discharge criteria, the readmission rate is significant, but concordant with rates from the first wave in similar hospitals9. Readmitted patients presented to and were discharged from hospital earlier in their COVID-19 illness than patients who were not readmitted. They returned to hospital after a short time after reaching their illness peak, displaying a proinflammatory phenotype as evidenced by their rising CRP. Significant oxygen requirements were observed and an appreciable proportion of patients required respiratory support.
Shorter courses of steroids on first admission increased risk of being readmitted to hospital with COVID-19. Those who received 1–3 days of steroids experienced quick clinical improvement and were discharged from hospital and corticosteroids were stopped at discharge. 25% of this subgroup were readmitted.
Our data suggest that short courses of corticosteroids may not be sufficient for patients requiring hospital admission with severe COVID-19. As patients are readmitted with evidence of ongoing inflammation, it is biologically plausible that increasing corticosteroid duration would reduce the chance of deterioration post-discharge. Many hospitals have now instigated virtual follow up with daily calls. It is therefore reasonable to consider continuing a course of corticosteroids after hospital discharge as treatment can be given within these frameworks to monitor side-effects of steroids. UK national guidelines now recognise that patients may be discharged to a virtual ward where continuation of steroids may be appropriate. Our data support this.
Despite the limitations of small sample size and retrospective data collection, our data demonstrate a high readmission rate amongst patients with COVID-19 who received shorter courses of steroids. Further research is required to establish the optimal duration of steroids and how to identify patients who require ongoing steroids at discharge.
Declaration of Competing Interest
No conflicts of interests declared by an author.
Funding
Bryan Williams is supported by Health Data Research UK Better Care Catalyst Award (CFC0125) and Health Data Research UK (LOND1).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jinf.2021.03.002.
Appendix. Supplementary materials
References
- 1.Lavery Amy M., Ellyn P.L., Ko Jean Y., Chevinsky Jennifer R., DeSisto Carla L., Pennington Audrey F., et al. Characteristics of hospitalized COVID-19 patients discharged and experiencing same-hospital readmission - United States, March-August 2020. MMWR Morb Mortal Wkly Rep. 2020;69(45):1695–1699. doi: 10.15585/mmwr.mm6945e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Group The Recovery Collaborative. The RECOVERY Collaborative Group Dexamethasone in hospitalized patients with covid-19 — preliminary report. N Engl J Med. 2020 doi: 10.1056/nejmoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Angus D.C., Derde L., Al-Beidh F., Annane D., Arabi Y., Beane A., et al. Effect of hydrocortisone on mortality and organ support in patients with severe COVID-19: the REMAP-CAP COVID-19 corticosteroid domain randomized clinical trial. JAMA. 2020;324(13) doi: 10.1001/jama.2020.17022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Institutes of Health. Corticosteroids. Available at https://www.covid19treatmentguidelines.nih.gov/immunomodulators/corticosteroids/. Accessed February 12, 2021, n.d.
- 5.National Institute for Health and Care Excellence (NICE) National Institute for Health and Care Excellence (NICE); 2021. COVID-19 prescribing briefing: corticosteroids. [Google Scholar]
- 6.World Health Organization. Corticosteroids for COVID-19. Available at https://www.who.int/publications/i/item/WHO-2019-nCoV-Corticosteroids-2020.1. Accessed February 12, 2021, n.d.
- 7.The United Kingdom: WHO coronavirus disease (COVID-19) dashboard. Available at https://covid19.who.int. Accessed February 11, 2021, n.d.
- 8.Knight Stephen R., Antonia H., Riinu P., Iain B., Gail C., Drake Thomas M., et al. Risk stratification of patients admitted to hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: development and validation of the 4C Mortality Score. BMJ. 2020;370 doi: 10.1136/bmj.m3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rokadiya S., Gil E., Stubbs C., Bell D., Herbert R. COVID-19: outcomes of patients with confirmed COVID-19 re-admitted to hospital. J Infect. 2020;81(3) doi: 10.1016/j.jinf.2020.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.