Abstract
The coronavirus disease 2019 (COVID-19) and Severe Acute Respiratory Syndrome (SARS) are associated with various psychiatric comorbidities. This is a systematic review and meta-analysis comparing the prevalence of psychiatric comorbidities in all subpopulations during the SARS and COVID-19 epidemics. A systematic literature search was conducted in major international (PubMed, EMBASE, Web of Science, PsycINFO) and Chinese (China National Knowledge Internet [CNKI] and Wanfang) databases to identify studies reporting prevalence of psychiatric comorbidities in all subpopulations during the SARS and COVID-19 epidemics. Data analyses were conducted using the Comprehensive Meta-Analysis Version 2.0 (CMA V2.0). Eighty-two studies involving 96,100 participants were included. The overall prevalence of depressive symptoms (depression hereinafter), anxiety symptoms (anxiety hereinafter), stress, distress, insomnia symptoms, post-traumatic stress symptoms (PTSS) and poor mental health during the COVID-19 epidemic were 23.9% (95% CI: 18.4%-30.3%), 23.4% (95% CI: 19.9%-27.3%), 14.2% (95% CI: 8.4%-22.9%), 16.0% (95% CI: 8.4%-28.5%), 26.5% (95% CI: 19.1%-35.5%), 24.9% (95% CI: 11.0%-46.8%), and 19.9% (95% CI: 11.7%-31.9%), respectively. Prevalence of poor mental health was higher in general populations than in health professionals (29.0% vs. 11.6%; Q=10.99, p=0.001). The prevalence of depression, anxiety, PTSS and poor mental health were similar between SARS and COVID-19 epidemics (all p values>0.05). Psychiatric comorbidities were common in different subpopulations during both the SARS and COVID-19 epidemics. Considering the negative impact of psychiatric comorbidities on health and wellbeing, timely screening and appropriate interventions for psychiatric comorbidities should be conducted for subpopulations affected by such serious epidemics.
Keywords: Psychiatric comorbidities, COVID-19, SARS, depression, anxiety, stress
1. Introduction
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was first reported in Wuhan, Hubei province, China in December 2019 (World Health Organization, 2020, World Health Organization, 2020). Subsequently, the WHO declared COVID-19 as a Public Health Emergency of International Concern (PHEIC) on 30 January 2020 (World Health Organization, 2020, World Health Organization, 2020). As of the end of February 2021, approximately 113 million cases had been confirmed and over 2.5 million deaths were reported worldwide (Johns Hopkins University, 2021). Severe acute respiratory syndrome (SARS) is an infectious disease caused by another coronavirus, severe acute respiratory syndrome coronavirus (SARS-CoV-1) (World Health Organization, 2004). SARS was first reported in southern China in November 2002, and later found in Hong Kong (World Health Organization, 2004) and many other Asian countries and territories. By 31 December 2003, a total of 8,096 SARS cases were confirmed worldwide (World Health Organization, 2003).
Clinical features of SARS and COVID-19 are similar in some aspects, but also different in others. For example, most patients with SARS suffered from a fever above 38.0°C, chills, headache, lethargy, and muscle pain. After 2 to 7 days, they may develop a dry, nonproductive cough with low blood oxygen levels. Most SARS patients developed shortness of breath and pneumonia subsequently, either primary viral pneumonia or secondary bacterial pneumonia (Centers for Diseases Control and Prevention, 2017). In contrast, COVID-19 patients usually experienced flu-like symptoms, including fever and/or dry cough. Severe cases may present difficult breathing, chest pain, sudden confusion, and bluish face or lips (Grant et al., 2020, Centers for Diseases Control and Prevention, 2020). Some COVID-19 patients eventually developed pneumonia, acute respiratory distress syndrome, sepsis, and kidney failure (World Health Organization, 2020). Further, SARS-CoV-1 and SARS-CoV-2 are different in both transmission characteristics and virulence. Compared to SARS-CoV-1, SARS-CoV-2 is more infectious with the reproduction number (R0) of around 3.3 (Liu et al., 2020, Xie et al., 2020), while the R0 of SARS-CoV-1 is around 2.7 (Riley et al., 2003, Lipsitch et al., 2003). The SARS-CoV-1 is more virulent than SARS-CoV-2. As of the end of 2003, SARS caused 774 deaths, resulting in a mortality rate of 9.2% (World Health Organization, 2003). In contrast, as of 18 October 2020, the mortality rate of COVID-19 was 2.8% (Johns Hopkins, 2020).
In any major catastrophes including bio-disasters, psychiatric comorbidities and related problems, such as depression, anxiety, sleep disturbances, fear, and stigmatization, are common and may act as barriers to accessing appropriate medical and mental health care. In order to prevent or minimise the negative outcomes caused by psychiatric comorbidities, understanding their patterns and associated factors is important. Previous studies on prevalence of psychiatric comorbidities found that confusion symptoms (27.9%), depression (32.6%), memory impairment (34.1%) insomnia (41.9%) and steroid-induced mania and psychosis (0.7%) were common in patients with SARS or Middle East respiratory syndrome (MERS) (Rogers et al., 2020). In addition, psychiatric comorbidities also persisted after the SARS epidemic, such as post-traumatic stress disorder (PTSD) (Hawryluck et al., 2004) and major depressive disorder (MDD) (Ma, 2009) in SARS survivors. Other subpopulations including family members and close contacts of SARS patients, health professionals, and the public also suffered from psychiatric problems during the epidemic (Cong et al., 2003), which could be associated with a range of negative consequences, such as decreased quality of life, increased treatment burden, and increased suicidality (Chinese Ministry of Health 2003). Similarly, psychiatric comorbidities, such as depression, anxiety, and sleep disturbance were common in COVID-19 patients (Deng et al., 2020), health professionals, and other subpopulations (Salazar de et al., 2020, Li et al., 2020).
To date, very few studies have compared the psychiatric comorbidities of SARS and COVID-19 epidemics. Understanding their differences would be important to identify high-risk subpopulations, allocate health resources and provide appropriate treatments. A number of meta-analyses focused on psychiatric comorbidities of coronavirus diseases (Rogers et al., 2020, Kisely et al., 2020), but only one compared the epidemiological data of psychiatric comorbidities between multiple coronavirus diseases among health professionals (Salazar de et al., 2020). Several meta-analyses on prevalence of psychiatric comorbidities during the COVID-19 pandemic have been conducted, but most only focused on specific subpopulations, such as infected or suspected patients (Deng et al., 2020), health professionals (Pappa et al., 2020), or the public (Salari et al., 2020).
In order to better understand the psychiatric comorbidities of SARS and COVID-19, it is necessary to compare the prevalence of psychiatric comorbidities in all subpopulations during the SARS and COVID-19 epidemics. Therefore, we conducted this systematic review and meta-analysis of observational studies to compare the overall prevalence of psychiatric comorbidities (e.g., depressive symptoms [depression hereinafter], anxiety symptoms [anxiety hereinafter], stress, distress, insomnia symptoms [insomnia hereinafter], post-traumatic stress symptoms [PTSS], post-traumatic stress disorder [PTSD], and poor mental health) during the SARS and COVID-19 epidemics across all subpopulations studied. We also explored the moderating effects of sociodemographic characteristics (e.g., sex, education level and marital status) on the results. We hypothesized that the overall prevalence of psychiatric comorbidities during the COVID-19 epidemic would be similar to that during the SARS epidemic; 2) the overall prevalence of psychiatric comorbidities in healthcare professionals would be higher than that in the general population during the COVID-19 epidemic.
2. Methods
2.1. Literature search and selection
This systematic review and meta-analysis were conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (Moher et al., 2009), with the PROSPERO registration number of CRD42020211604. Literature search was systematically and independently conducted by three researchers (WWR, YJ, WL) in PubMed, EMBASE, Web of Science, PsycINFO, China National Knowledge Internet (CNKI) and WanFang databases from their inception to May 25, 2020, using the following search terms: “novel coronavir*”, “alphacoronavirus”, “betacoronavirus”, “COVID”, “COVID-19”, “severe acute respiratory syndrome” and “SARS”. For the psychiatric outcome category, the following search terms were used: “psychiatr*”, “mental”, “psycholog*”, “depress*”, “anxiety”, “posttraumatic stress disorder”, “PTSD”, “insomnia”, “sleep”, “epidemiology” and “prevalence”. The references of retrieved articles were also searched by hand for additional studies.
The same three researchers independently screened titles and abstracts, and then two of the researchers (YJZ and YJ) read the full texts of relevant articles for eligibility. Inclusion criteria were: 1) studies that examined psychiatric comorbidities during the SARS or COVID-19 epidemics in any subpopulations; 2) studies with available data on the prevalence of psychiatric comorbidities or relevant data that could generate the prevalence of psychiatric comorbidities during the SARS or COVID-19 epidemics in any subpopulations, as measured by standardized scales or diagnostic instruments; 3) case-control studies, cross-sectional or cohort studies. Case studies, reviews, systematic reviews, meta-analyses or commentaries were excluded. If more than one article were published using the same dataset, only the one with the most complete information or highest quality assessment score was included. Disagreement was resolved by consensus.
2.2. Data extraction
Relevant data were independently extracted by two researchers (YJZ and YJ) using a pre-designed data extraction sheet, including sex, education level, marital status, the first author, publication year, study design, study location, study period, study population, sample size, sampling method, prevalence of specific psychiatric co-morbidities. Disagreement was resolved by consensus, or a discussion with a senior researcher (YTX).
2.3. Quality assessment
The quality of included studies was evaluated using the Loney's 8-item scale (Loney et al., 1998) which has been widely used previously (Boyle, 1998, Yang et al., 2016). This scale assesses the quality of observational studies in eight domains: target population, probability sampling, response rate, non-responders, sample representative of the target population, standardized data collection method, validated criteria for outcomes, and confidence intervals (CI) of the prevalence of target outcomes. The total quality score ranges from 0 to 8, with ‘7-8’ as “high quality”, ‘4-6’ as “moderate quality” and ‘0-3’ as “low quality”. Two researchers (YJZ and YJ) independently evaluated the study quality, and disagreement was resolved by consensus or a discussion with the senior researcher (YTX).
2.4. Data analysis
Data analyses were performed using Comprehensive Meta-Analysis Version 2.0 (CMA V2.0, Biostat Inc., Englewood, New Jersey, USA). I2 test was used to evaluate heterogeneity between studies, with I2 > 50% indicating significant heterogeneity. The random-effects model was used in data syntheses due to different demographic characteristics between studies. In SARS related studies, December 31, 2003 was used as the cutoff date to classify acute SARS phase and SARS recovery phase. At least three articles were needed for data synthesis in each phrase. If the number of articles in either SARS phase was less than three, the relevant data in the two phrases were pooled.
Subgroup and meta-regression analyses were conducted to explore moderating effects of categorical (e.g. study population, sex, education level and marital status) and continuous variables (e.g., female percentage and quality assessment score) respectively, on the prevalence of psychiatric comorbidities in COVID-19 patients. Publication bias was examined by funnel plots, Egger's test and Duval and Tweedie trim-and-fill method. Two-tailed tests were conducted with the significance level of 0.05.
3. Results
3.1. Study characteristics
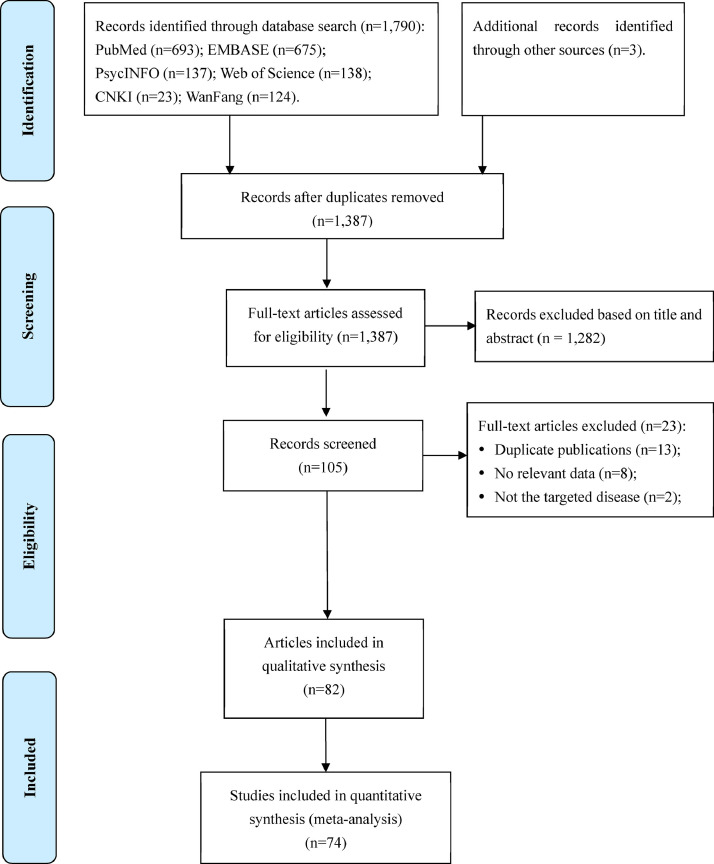
A total of 1,793 studies were identified in the literature search, and 82 met the eligibility criteria; of them, 74 studies with available data were included in the meta-analysis. Details of literature search, screening and selection are shown in Figure 1 . Study characteristics are presented in Table 1 . The included studies were conducted across 10 countries or areas including Asia, Europe, North America and South America.
Figure 1.
Flow diagram
Table 1.
Characteristics of studies included in this systematic review and meta-analysis.
| Study | Language | Disease | Study design | Survey period | Country/territory | Population | Sampling method | Sample size | Female percentage (%) | Age | Response rate (%) | Quality score | Reference | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Min | Max | |||||||||||||
| Ahmed, M. Z. et al. 2020 | English | COVID-19 | cross-sectional | 2020/NR | Mainland China | general population | NR | 1074 | 46.83 | 33.54 | 11.13 | 14 | 68 | NR | 4 | (Ahmed et al., 2020) |
| Bo, H. X. et al. 2020 | English | COVID-19 | cross-sectional | 2020.3 | Mainland China | infected people | NR | 714 | 50.90 | 50.2 | 12.9 | - | - | 97.80 | 6 | (Bo et al., 2020) |
| Cai, W. et al. 2020 | English | COVID-19 | cross-sectional | 2020/NR | Mainland China | health professionals | NR | 1521 | 75.54 | - | - | 18 | - | NR | 4 | (Cai et al., 2020) |
| Cao, W et al. 2020 | English | COVID-19 | cross-sectional | 2020/NR | Mainland China | university students | C | 7143 | 69.65 | - | - | - | - | 100.00 | 7 | (Cao et al., 2020) |
| Chan, A. O. M. et al. 2004 | English | acute SARS | cross-sectional | 2 months after first case in Singapore | Singapore | health professionals | NR | 661 | NR | - | - | - | - | 67.00 | 4 | (Chan and Huak, 2004) |
| Chang, J. et al. 2020 | Chinese | COVID-19 | cross-sectional | 2020.1-2020.2 | Mainland China | university students | convenient | 3881 | 63.05 | 20 | - | 18 | - | 91.38 | 5 | (Chang et al., 2020) |
| Chen, C. S. et al. 2005 | English | acute SARS | cross-sectional | 2003.5 | Taiwan | health professionals | NR | 128 | 100.00 | 26.5 | 3.1 | - | - | 69.57 | 4 | (Chen et al., 2005) |
| Chen, Y. et al. 2020 | English | COVID-19 | cross-sectional | 2020/NR | Mainland China | health professionals | NR | 105 | 90.5 | 32.6 | 6.5 | - | - | 84.70 | 5 | (Chen et al., 2020) |
| Cheng, S. K. et al. 2004 | English | acute SARS | cross-sectional | 2003.6 | Hong Kong | total sample | NR | 284 | 62.32 | - | - | - | - | 60.17 | 5 | (Cheng et al., 2004) |
| Chew, N. W. S. et al. 2020 | English | COVID-19 | cross-sectional | 2020.2-2020.4 | Singapore, India | health professionals | NR | 906 | 64.35 | 29 (median) | - | - | - | 90.60 | 5 | (Chew et al., 2020) |
| Chong, M. Y. et al. 2004 | English | acute SARS | cross-sectional | 2003.5-2003.6 | Taiwan | health professionals | NR | 1257 | 81.07 | 31.8 | 6.4 | 21 | 59 | 50.28 | 5 | (Chong et al., 2004) |
| Consolo, U. et al. 2020 | English | COVID-19 | cross-sectional | 2020.4 | Italy | health professionals | C | 356 | 39.61 | - | - | - | - | 40.73 | 5 | (Consolo et al., 2020) |
| Fang, Y. et al. 2004 | Chinese | acute SARS | cross-sectional | 2003.7-2003.10 | Mainland China | infected people | NR | 286 | 52.80 | 33.43 | 11.85 | 15 | 64 | 100.00 | 6 | (Fang et al., 2004) |
| Gao, J. et al. 2020 | English | COVID-19 | cross-sectional | 2020.1-2020.2 | Mainland China | general population | NR | 4827 | 67.68 | 32.3 | 10.0 | 10 | 85 | 82.50 | 6 | (Gao et al., 2020) |
| Hawryluck, L. et al. 2004 | English | acute SARS | cross-sectional | 2003.2-2003.6 | Canada | general population | convenient | 129 | NR | - | - | 18 | 66+ | 0.86 | 4 | (Hawryluck et al., 2004) |
| Hong, X. et al. 2009 | English | acute SARS | cohort | 2003.6-2007.9 | Mainland China | infected people | NR | 68 | 66.18 | 38.5 | 12.3 | - | - | 97.14 | 6 | (Hong et al., 2009) |
| Huang, J. Z. et al. 2020 | Chinese | COVID-19 | cross-sectional | 2020.2 | Mainland China | health professionals | NR | 230 | 81.30 | 32.6 | 6.2 | 22 | 59 | 93.50 | 5 | (Huang et al., 2020) |
| Huang, Y. et al. 2020 | English | COVID-19 | cross-sectional | 2020.2 | Mainland China | total sample | NR | 7236 | 54.62 | 35.3 | 5.6 | - | - | 85.30 | 6 | (Huang and Zhao, 2020) |
| Ko, C. H. et al. 2006 | English | SARS | cross-sectional | when the epidemic had just been controlled | Taiwan | general population | R | 1472 | 51.97 | - | - | 15 | 51+ | 94.85 | 6 | (Ko et al., 2006) |
| Kwek, S. K. et al. 2006 | English | SARS | cross-sectional | 3 month post-discharge | Singapore | infected people | NR | 63 | 79.37 | 34.83 | 10.49 | - | - | 43.45 | 5 | (Kwek et al., 2006) |
| Lai, J. et al. 2020 | English | COVID-19 | cross-sectional | 2020.1-2020.2 | Mainland China | health professionals | CMRS | 1257 | 76.69 | - | - | 18 | 40+ | 68.69 | 5 | (Lai et al., 2020) |
| Lam, M. H. B. et al.2009 | English | recovery SARS | cross-sectional | 2005.12-2007.7 | Hong Kong | infected people | NR | 181 | 68.51 | 43.3 | 13.7 | - | - | 49.05 | 5 | (Lam et al., 2009) |
| Lancee, W. J. et al. 2008 | English | recovery SARS | cohort | 2004.10-2005.9 | Canada | health professionals | NR | 139 | 87.05 | 45.0 | 9.6 | - | - | 23.68 | 6 | (Lancee et al., 2008) |
| Lau, J. T. F. et al. 2006 | English | acute SARS | cross-sectional | 2003.5-2003.6 | Hong Kong | general population | R | 818 | 50.24 | - | - | 18 | 50+ | 64.70 | 6 | (Lau et al., 2006) |
| Lee, A. M. et al. 2007 | English | recovery SARS | cohort | 2004.4-2004.5 | Hong Kong | infected people | NR | 96 | 63.54 | - | - | 18 | 61+ | 80.00 | 5 | (Lee et al., 2007) |
| Lei, L. et al. 2020 | English | COVID-19 | cross-sectional | 2020.2 | Mainland China | general population | convenient | 1593 | 61.27 | 32.3 | 9.8 | - | - | 80.17 | 5 | (Lei et al., 2020) |
| Li, X. et al. 2020 | English | COVID-19 | cross-sectional | 2020.2 | Mainland China | health professionals | NR | 948 | 76.79 | - | - | 20 | 60+ | NR | 4 | (Li et al., 2020) |
| Li, Y. et al. 2020 | English | COVID-19 | prospective cohort | 2020.2 | Mainland China | university students | NR | 1442 | 61.79 | - | - | - | - | 71.20 | 4 | (Li et al., 2020) |
| Liang, L. L. et al. 2020 | English | COVID-19 | cross-sectional | 2020.1 | Mainland China | general population | convenient | 584 | 61.82 | - | - | 14 | 35 | 95.70 | 5 | (Liang et al., 2020) |
| Liu, C. Y. et al. 2020 | English | COVID-19 | cross-sectional | 2020.2 | Mainland China | health professionals | NR | 512 | 84.57 | - | - | 18 | 60+ | 85.33 | 5 | (Liu et al., 2020) |
| Liu, N. et al. 2020 | English | COVID-19 | cross-sectional | 2020.1-2020.2 | Mainland China | general population | NR | 285 | 54.39 | - | - | - | - | 95.00 | 5 | (Liu et al., 2020) |
| Liu, X. et al. 2012 | English | recovery SARS | cross-sectional | 2006 | Mainland China | health professionals | SR | 549 | 76.50 | - | - | - | - | 83.00 | 6 | (Liu et al., 2012) |
| Liu, Z. R. et al. 2004 | Chinese | acute SARS | cross-sectional | 2003.5 | Mainland China | university students | CS | 6280 | 38.74 | 20.3 | 2.0 | - | - | 92.35 | 6 | (Liu et al., 2004) |
| Lü, S. H. et al. 2010 | Chinese | acute SARS | retrospective | 2003.3-2003.6 | Mainland China | general population | MS | 2379 | 45.61 | 39.12 | 13.67 | 18 | 69 | 93.96 | 6 | (Lü et al., 2010) |
| Lu, W. et al. 2020 | English | COVID-19 | cross-sectional | 2020.2 | Mainland China | health professionals | NR | 2299 | 77.64 | - | - | - | - | 94.88 | 5 | (Lu et al., 2020) |
| Lu, Y. C. et al. 2006 | English | acute SARS | cross-sectional | 2003.7-2004.3 | Taiwan | health professionals | NR | 127 | 58.27 | - | - | - | - | 94.07 | 5 | (Lu et al., 2006) |
| Lung, F. W. et al. 2009 | English | recovery SARS | longitudinal | 2004.7-2005.3 | Taiwan | health professionals | NR | 123 | NR | - | - | - | - | 96.85 | 5 | (Lung et al., 2009) |
| Mak, I. W. C. et al. 2009 | English | recovery SARS | cohort | 2005.9-2006.3 | Hong Kong | infected people | NR | 90 | 62.22 | 41.1 | 12.1 | - | - | 96.77 | 6 | (Mak et al., 2009) |
| Maunder, R. G. et al. 2006 | English | recovery SARS | cohort | 2004.10-2005.9 | Canada | health professionals | NR | 769 | 86.87 | - | - | - | - | 38.76 | 4 | (Maunder et al., 2006) |
| Mazza, C. et al. 2020 | English | COVID-19 | cross-sectional | 2020.3 | Italy | general population | NR | 2766 | 71.66 | 32.94 | 13.2 | 18 | 90 | 98.36 | 5 | (Mazza et al., 2020) |
| Mihashi, M. et al. 2009 | English | recovery SARS | cross-sectional | 2004.2-2004.3 | Mainland China | general population | NR | 187 | 36.90 | 26.3 | 8.0 | - | - | 62.33 | 3 | (Mihashi et al., 2009) |
| Ni, M. Y. et al. 2020 | English | COVID-19 | cross-sectional | 2020/NR | Mainland China | total sample | NR | 1791 | 61.75 | - | - | - | - | NR | 5 | (Ni et al., 2020) |
| Nickell, L. A. et al. 2004 | English | acute SARS | cross-sectional | 2003.4 | Canada | health professionals | NR | 510 | 80.59 | - | - | - | - | 11.91 | 4 | (Nickell et al., 2004) |
| Ozamiz-Etxebarria, N. et al. 2020 | English | COVID-19 | cross-sectional | 2020.3 | Spain | general population | NR | 976 | 81.15 | - | - | 18 | 78 | 40.67 | 4 | (Ozamiz-Etxebarria et al., 2020) |
| Peng, E. Y. C. et al. 2010 | English | acute SARS | cross-sectional | 2003.11 | Taiwan | general population | SR | 1278 | 49.69 | 41.6 | 16.6 | 18 | 89 | 68.31 | 5 | (Peng et al., 2010) |
| Reynolds, D. L. et al. 2008 | English | acute SARS | cross-sectional | 2003.3-2003.6 | Canada | total sample | NR | 1057 | 61.12 | - | - | - | - | 55.28 | 6 | (Reynolds et al., 2008) |
| Shacham, M. et al. 2020 | English | COVID-19 | cross-sectional | 2020.3-2020.4 | Israel | health professionals | NR | 338 | 58.58 | 46.39 | 11.18 | 24 | 74 | NR | 4 | (Shacham et al., 2020) |
| Sim, K. et al. 2004 | English | acute SARS | cross-sectional | 2003.7 | Singapore | health professionals | NR | 277 | 85.20 | 38.0 | 12.7 | - | - | 92.03 | 5 | (Sim et al., 2004) |
| Sim, K. et al. 2010 | English | acute SARS | cross-sectional | 2003.7 | Singapore | general population | consecutive | 415 | 40.72 | 36.6 | 13.9 | - | - | 78.01 | 4 | (Sim et al., 2010) |
| Su, T. P. et al. 2007 | English | acute SARS | prospective | 2003.4-2003.6 | Taiwan | health professionals | NR | 102 | 100.00 | 25.4 | 3.7 | - | - | 95.33 | 5 | (Su et al., 2007) |
| Tan, W. et al. 2020 | English | COVID-19 | cross-sectional | 2020.2 | Mainland China | general population | NR | 673 | 25.56 | 30.8 | 7.4 | - | - | 50.87 | 4 | (Tan et al., 2020) |
| Tang, W. et al. 2020 | English | COVID-19 | cross-sectional | 2020.2 | Mainland China | university students | convenient | 2485 | 61.37 | 19.81 | 1.55 | 16 | 27 | 68.84 | 4 | (Tang et al., 2020) |
| Tham, K. Y. et al. 2004 | English | acute SARS | cross-sectional | 2003.11 | Singapore | health professionals | NR | 96 | 68.75 | - | - | - | - | 77.42 | 4 | (Tham et al., 2004) |
| Tian, B. C. et al. 2007 | Chinese | recovery SARS | cross-sectional | 2006.3-2006.4 | Mainland China | general population | convenient | 2424 | 45.46 | 39.12 | 13.67 | - | - | 101.00 | 5 | (Tian et al., 2007) |
| Tian, F. et al. 2020 | English | COVID-19 | cross-sectional | 2020.1-2020.2 | Mainland China | general population | convenient | 1060 | 48.21 | 35.01 | 12.8 | 13 | 76 | 93.64 | 5 | (Tian et al., 2020) |
| Wang, C. et al. 2020 | English | COVID-19 | cross-sectional | 2020.1-2020.2 | Mainland China | general population | convenient | 1210 | 67.27 | - | - | 12 | 59 | 92.79 | 5 | (Wang et al., 2020) |
| Wang, S. et al. 2020 | English | COVID-19 | cross-sectional | 2020.1-2020.2 | Mainland China | health professionals | NR | 123 | 90.24 | 33.75 | 8.41 | 20 | 50+ | 50.00 | 4 | (Wang et al., 2020) |
| Wu, K. et al. 2020 | English | COVID-19 | cross-sectional | 2020/NR | Mainland China | health professionals | NR | 60 | 26.67 | 33.5 | 12.4 | 25 | 59 | NR | 4 | (Wu and Wei, 2020) |
| Yin, Q. et al. 2020 | English | COVID-19 | cross-sectional | 2020.2 | Mainland China | health professionals | convenient | 371 | 61.46 | 35.30 | 9.48 | 20 | 40+ | 98.41 | 5 | (Yin et al., 2020) |
| Zhang, C. et al. 2020 | English | COVID-19 | cross-sectional | 2020.1-2020.2 | Mainland China | health professionals | convenient | 1563 | 82.73 | - | - | 18 | 60+ | 80.32 | 6 | (Zhang et al., 2020) |
| Zhang, K. R. et al. 2005 | Chinese | acute SARS | cross-sectional | 2003.9-2003.10 | Mainland China | total sample | NR | 296 | 67.57 | 34 | 12 | 8 | 81 | NR | 4 | (Zhang et al., 2005) |
| Zhang, W. R. et al. 2020 | English | COVID-19 | cross-sectional | 2020.2-2020.3 | Mainland China | health professionals | NR | 2182 | 64.21 | - | - | 16 | 60+ | NR | 4 | (Zhang et al., 2020) |
| Zhang, Y. et al. 2020 | English | COVID-19 | cross-sectional | 2020.1-2020.2 | Mainland China | general population | convenient | 263 | 59.70 | 37.7 | 14.0 | 18 | 50+ | 65.75 | 5 | (Zhang and Ma, 2020) |
| Zhu, J. et al. 2020 | English | COVID-19 | cross-sectional | 2020.2 | Mainland China | health professionals | NR | 165 | 83.03 | 34.16 | 8.06 | - | - | 100.00 | 6 | (Zhu et al., 2020) |
| Zhu, S. et al. 2020 | English | COVID-19 | cross-sectional | 2020.2-2020.3 | Mainland China | total sample | NR | 2279 | 59.72 | - | - | - | - | NR | 4 | (Zhu et al., 2020) |
| Shi, T. Y. et al. 2005 | Chinese | acute SARS | cross-sectional | 2003.12-2004.1 | Mainland China | total sample | C | 162 | 79.63 | - | - | - | - | 93.1 | 6 | (Shi et al., 2005) |
| Zhang, X. J. et al. 2003 | Chinese | acute SARS | cross-sectional | 2003.4-2003.5 | Mainland China | general population | C | 1031 | 35.89 | 33.17 | - | 16 | 86 | 91.73 | 6 | (Zhang et al., 2003) |
| He, L. P. et al. 2004 | Chinese | acute SARS | cross-sectional | 2003.5 | Mainland China | general population | CR | 1016 | NR | 27.30 | 9.62 | - | - | 94.69 | 6 | (He et al., 2004) |
| Zhao, Q. et al. 2020 | Chinese | COVID-19 | cross-sectional | 2020.2 | Mainland China | infected people | NR | 106 | 56.60 | 35.90 | 11.92 | 21 | 65 | 100.00 | 6 | (Zhao et al., 2020) |
| Gao, H. S. et al. 2006 | Chinese | acute SARS | longitudinal | 2003.9-2004.6 | Mainland China | infected people | NR | 67 | 68.66 | 25.32 | 8.54 | 15 | 67 | 88.16 | 5 | (Gao et al., 2006) |
| Gao, H. S. et al. 2006 | Chinese | SARS | longitudinal | 2003.6-2004.6 | Mainland China | infected people | NR | 67 | 68.66 | - | - | - | - | NR | 4 | (Gao et al., 2006) |
| Wei, L. P. et al. 2005 | Chinese | SARS | longitudinal | within 2 weeks and after 3 months of post-charge | Mainland China | infected people | NR | 22 | 86.36 | - | - | - | - | NR | 4 | (Wei et al., 2005) |
| Cheng, S. K. et al. 2004 | English | acute SARS | cross-sectional | 2003.5-2003.7 | Hong Kong | infected people | NR | 180 | 66.67 | 36.9 | 11.1 | 18 | 70 | 42.35 | 5 | (Cheng et al., 2004) |
| Wu, K. K. et al. 2005 | English | SARS | longitudinal | at 1 month and 3 months after discharge from hospital | Hong Kong | infected people | NR | 131 | 56.49 | 41.82 | 14.01 | 18 | 84 | 27.52 | 4 | (Wu et al., 2005) |
| Lee, D. T. S. et al. 2006 | English | acute SARS | case-control | 2003.4-2003.6 | Hong Kong | pregnant women | consecutive | 235 | 100.00 | 29.6 | 5.4 | - | - | 57.6 | 4 | (Lee et al., 2006) |
| Wu, Y. et al. 2020 | English | COVID-19 | cross-sectional | 2020.1-2020.2 | Mainland China | pregnant women | NR | 1285 | 100.00 | - | - | 27 | 32 | NR | 4 | (Wu et al., 2020) |
| Xie, X. et al. 2020 | English | COVID-19 | cross-sectional | 2020.2-2020.3 | Mainland China | children | NR | 1784 | 43.27 | - | - | - | - | 76.57 | 4 | (Xie et al., 2020) |
| Zhou, S. J. et al. 2020 | English | COVID-19 | cross-sectional | 2020.3 | Mainland China | adolescents | NR | 8079 | 53.55 | 16 | - | 12 | 18 | 99.25 | 5 | (Zhou et al., 2020) |
| Yuan, R. et al. 2020 | English | COVID-19 | cross-sectional | 2020/NR | Mainland China | the parents of children hospitalized or not hospitalized | NR | 100 | 57.00 | - | - | - | - | NR | 4 | (Yuan et al., 2020) |
| Nguyen, H. C. et al. 2020 | English | COVID-19 | cross-sectional | 2020.2-2020.3 | Vietnam | outpatients | NR | 3947 | 55.66 | 44.4 | 17.0 | 18 | 60+ | 97.96 | 6 | (Nguyen et al., 2020) |
| Han, Z. H. et al. 2020 | Chinese | COVID-19 | longitudinal | 2020.1-2020.3 | Mainland China | suspected infected people | NR | 72 | 41.67 | - | - | 11 | 73 | 100 | 5 | (Han et al., 2020) |
| Wan, I. Y. P. et al. 2004 | English | acute SARS | cross-sectional | 2003.4 | Hong Kong | patients on a waiting list for thoracic surgery | NR | 57 | 31.58 | 59.77 | 14.5 | 17 | 83 | 31.67 | 4 | (Wan et al., 2004) |
Abbreviations: COVID-19: Coronavirus disease 2019; SARS: Severe acute respiratory syndrome; M: multistage; SD: standard deviation; S: stratified; C: cluster; R: random; NR: not reported.
3.2. Prevalence of psychiatric comorbidities during the COVID-19 epidemic
Of the 36 studies on COVID-19, 21 studies reported prevalence of depression during the COVID-19 epidemic and the pooled prevalence of depression was 23.9% (95% CI: 18.4% - 30.3%; I2=99.43%, p<0.001; Supplementary Figure 1). Twenty-four studies reported prevalence of anxiety during the COVID-19 epidemic and the pooled prevalence of anxiety was 23.4% (95% CI: 19.9% - 27.3%; I2=98.78%, p<0.001; Supplementary Figure 2). Five studies reported the prevalence of stress during the COVID-19 epidemic and the pooled prevalence was 14.2% (95% CI: 8.4% - 22.9%; I2=98.65%, p<0.001; Supplementary Figure 3). Three studies reported prevalence of distress the COVID-19 epidemic and the pooled prevalence of distress was 16.0% (95% CI: 8.4% - 28.5%; I2=97.77%, p<0.001; Supplementary Figure 4). Eight studies reported the prevalence of insomnia during the COVID-19 epidemic and the pooled prevalence of insomnia was 26.5% (95% CI: 19.1% - 35.5%; I2=98.79%, p<0.001; Supplementary Figure 5). Thirteen studies reported prevalence of PTSS during the COVID-19 epidemic and the pooled prevalence of PTSS was 24.9% (95% CI: 11.0% - 46.8%; I2=99.68%, p<0.001; Supplementary Figure 6). Five studies reported the prevalence of poor mental health during the COVID-19 epidemic and the pooled prevalence of poor mental health was 19.9% (95% CI: 11.7% - 31.9%; I2=98.92%, p<0.001; Supplementary Figure 7). Details of pooled prevalence of psychiatric comorbidities are presented in Table 2 .
Table 2.
Prevalence of psychiatric comorbidities during the COVID-19 epidemic in all subpopulations
| Psychiatric outcomes | Number of studies | Events | Sample size | Prevalence (%) | 95% CI (%) | I2 (%) | p | Publication bias (Egger's test) | |
|---|---|---|---|---|---|---|---|---|---|
| Depression | 21 | 10025 | 39542 | 23.9 | 18.4 - 30.3 | 99.43 | < 0.001 | t = 1.28, p = 0.22 | |
| Anxiety | 24 | 11690 | 45253 | 23.4 | 19.9 - 27.3 | 98.78 | < 0.001 | t = 1.28, p = 0.21 | |
| Stress | 5 | 1440 | 6531 | 14.2 | 8.4 - 22.9 | 98.65 | < 0.001 | t = 3.37, p = 0.04 | |
| Distress | 3 | 555 | 2840 | 16.0 | 8.4 - 28.5 | 97.77 | < 0.001 | t = 1.40, p = 0.39 | |
| Insomnia | 8 | 3481 | 14042 | 26.5 | 19.1 - 35.5 | 98.79 | < 0.001 | t = 0.61, p = 0.57 | |
| PTSS | 13 | 4268 | 11983 | 24.9 | 11.0 - 46.8 | 99.68 | < 0.001 | t = 2.26, p = 0.04 | |
| Poor mental health | 5 | 1216 | 6406 | 19.9 | 11.7 - 31.9 | 98.92 | < 0.001 | t = 0.14, p = 0.90 | |
Notes: I2 statistic was used to assess the heterogeneity of the studies.
The minimum number of studies required to synthesize data is 3.
3.3. Comparisons of prevalence of psychiatric comorbidities between COVID-19 and SARS epidemics
Of the 38 studies on SARS, 6 studies reported prevalence of depression during the acute SARS phase, while 3 studies reported that during the SARS recovery phase, with the pooled prevalence of 27.5% (95% CI: 17.3% - 40.6%; I2=94.95%, p<0.001) and 26.0% (95% CI: 15.6% - 40.0%; I2=87.59%, p<0.001), respectively. No significant difference in prevalence of depression between SARS and COVID-19 epidemics was found (Q=0.34, p=0.85). Nine studies reported prevalence of anxiety during the SARS epidemic and the pooled prevalence of anxiety was 17.7% (95% CI: 8.2% - 34.1%; I2=97.37%, p<0.001), with no significant difference compared to that during the COVID-19 epidemic (Q=0.59, p=0.44). Fifteen studies reported the prevalence of PTSS during the SARS epidemic and the pooled prevalence of PTSS was 16.8% (95% CI: 12.9% - 21.5%; I2=93.94%, p<0.001), with no significant difference compared to that during the COVID-19 epidemic (Q=0.89, p=0.35).
Nine studies reported prevalence of poor mental health in acute SARS phase while 3 studies reported that in SARS recovery phase, with the pooled prevalence of 26.6% (95% CI: 11.7% - 49.8%; I2=99.61%, p<0.001) and 32.8% (95% CI: 12.4% - 62.8%; I2=96.44%, p<0.001), respectively. The pooled prevalence of poor mental health in SARS was similar with that during the COVID-19 epidemic (Q=1.06, p=0.59). Three studies reported prevalence of PTSD in acute SARS phase while 3 studies reported that in SARS recovery phase, with the pooled prevalence of 29.4% (95% CI: 9.3% - 63.0%; I2=96.62%, p<0.001) and 15.3% (95% CI: 6.7% - 31.3%; I2=89.83%, p<0.001), respectively. No study on prevalence of PTSD during the COVID-19 epidemic was published by the date of literature search; therefore, comparison between SARS and COVID-19 could not be made. Detailed comparisons of psychiatric comorbidities between COVID-19 and SARS epidemics are shown in Table 3 .
Table 3.
Comparison of prevalence of psychiatric comorbidities during the COVID-19 and SARS epidemics
| Psychiatric outcomes | Condition | Number of studies | Events | Sample size | Prevalence (%) | 95% CI (%) | I2 (%) | p (within subgroup) | Q (p across subgroups) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Depression | COVID-19 | 21 | 10025 | 39542 | 23.9 | 18.4 - 30.3 | 99.43 | < 0.001 | Q = 0.34, p = 0.85 | |
| Acute SARS | 6 | 348 | 1780 | 27.5 | 17.3 - 40.6 | 94.95 | < 0.001 | |||
| SARS Recovery | 3 | 175 | 712 | 26.0 | 15.6 - 40.0 | 87.59 | < 0.001 | |||
| Anxiety | COVID-19 | 24 | 11690 | 45253 | 23.4 | 19.9 - 27.3 | 98.78 | < 0.001 | Q = 0.59, p = 0.44 | |
| SARS | 9 | 275 | 2892 | 17.7 | 8.2 - 34.1 | 97.37 | < 0.001 | |||
| PTSS | COVID-19 | 13 | 4268 | 11983 | 24.9 | 11.0 - 46.8 | 99.68 | < 0.001 | Q = 0.89, p = 0.35 | |
| SARS | 15 | 938 | 5653 | 16.8 | 12.9 - 21.5 | 93.94 | < 0.001 | |||
| Poor mental health | COVID-19 | 5 | 1216 | 6406 | 19.9 | 11.7 - 31.9 | 98.92 | < 0.001 | Q = 1.06, p = 0.59 | |
| Acute SARS | 9 | 2034 | 9907 | 26.6 | 11.7 - 49.8 | 99.61 | < 0.001 | |||
| SARS Recovery | 3 | 129 | 406 | 32.8 | 12.4 - 62.8 | 96.44 | < 0.001 | |||
| PTSD | Acute SARS | 3 | 89 | 421 | 29.4 | 9.3 - 63.0 | 96.62 | < 0.001 | Q = 0.95, p = 0.33 | |
| SARS Recovery | 3 | 71 | 410 | 15.3 | 6.7 - 31.3 | 89.83 | < 0.001 | |||
Note: Acute SARS refers to study period before January 1, 2004; Recovery SARS refers to study period after January 1, 2004.
Studies involving anxiety during SARS were not divided into “acute SARS/recovery SARS” because only 2 studies were conducted during recovery phase of SARS and they did not reach the minimum number of studies to synthesize data. Studies involving stress, distress, insomnia were not compared between COVID-19 and SARS due to the similar reason.
3.4. Subgroup analyses in prevalence of psychiatric comorbidities during the COVID-19 epidemic
The pooled prevalence of poor mental health in the general population and health professionals during the COVID-19 epidemic was 29.0% (95% CI: 18.1% - 43.1%) and 11.6% (95% CI: 9.2% - 14.6%), respectively. Subgroup analyses revealed that compared with health professionals, general populations were more likely to have poorer general mental health (Q=10.99, p=0.001). No significant difference was found between health professionals (28.0%, 95% CI: 9.5% - 59.0%) and general populations (19.2%, 95% CI: 4.6% - 54.2%) in prevalence of PTSS (Q=0.21, p=0.63). The prevalence estimates of depression and anxiety during the COVID-19 were similar between the general population and health professionals (Q=0.01, p=0.91 for depression; Q=0.23, p=0.64 for anxiety). Details of the comparisons are presented in Table 4 . No significant differences were found in prevalence of depression, anxiety, insomnia and PTSS during the COVID-19 epidemic between different sex, between different education levels and between different marital status (all p values > 0.05; Table 5 ).
Table 4.
Prevalence of psychiatric comorbidities during the COVID-19 epidemic in all subpopulations
| Psychiatric outcomes | Population | Number of studies | Events | Sample size | Prevalence (%) | 95% CI (%) | I2 (%) | p (within subgroup) | Q (p across subgroups) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Depression | General population | 10 | 6016 | 20644 | 23.2 | 16.6 - 31.4 | 99.38 | < 0.001 | Q = 0.01, p = 0.91 | |
| Health professionals | 11 | 2809 | 11922 | 23.9 | 15.0 - 35.9 | 99.32 | < 0.001 | |||
| Anxiety | General population | 10 | 5118 | 20599 | 21.2 | 16.6 - 26.7 | 98.74 | < 0.001 | Q = 0.23, p = 0.64 | |
| Health professionals | 14 | 3584 | 13020 | 23.2 | 17.1 - 30.8 | 98.77 | < 0.001 | |||
| PTSS | General population | 5 | 1164 | 3015 | 19.2 | 4.6 - 54.2 | 99.57 | < 0.001 | Q = 0.21, p = 0.63 | |
| Health professionals | 5 | 2190 | 4327 | 28.0 | 9.5 - 59.0 | 99.59 | < 0.001 | |||
| Poor mental health | General population | 3 | 742 | 2575 | 29.0 | 18.1 - 43.1 | 97.93 | < 0.001 | Q = 10.99, p = 0.001 | |
| Health professionals | 3 | 402 | 3327 | 11.6 | 9.2 - 14.6 | 83.06 | < 0.001 | |||
Note: Only the first visit of longitudinal studies was included in order to avoid data duplication.
Studies involving stress, distress, insomnia were not compared between different populations because their numbers of studies in at least one population did not reach the minimum number of studies to synthesize data.
Table 5.
Prevalence of psychiatric comorbidities during the COVID-19 epidemic by sex, education level and marital status.
| Psychiatric outcomes | Categories | Number of studies | Events | Sample size | Prevalence (%) | 95% CI (%) | I2 (%) | p (within subgroup) | Q (p across subgroups) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Depression | Male | 5 | 1770 | 5892 | 32.4 | 20.1 - 47.6 | 99.00 | < 0.001 | Q = 0.02, p = 0.90 | |
| Female | 5 | 3234 | 9478 | 33.7 | 20.1 - 50.7 | 99.53 | < 0.001 | |||
| Anxiety | Male | 8 | 2748 | 9663 | 25.7 | 21.0 - 31.1 | 96.25 | < 0.001 | Q = 0.64, p = 0.42 | |
| Female | 8 | 4928 | 17907 | 28.7 | 23.8 - 34.1 | 98.07 | < 0.001 | |||
| Insomnia | Male | 5 | 848 | 4089 | 25.2 | 19.7 - 31.6 | 87.08 | < 0.001 | Q = 1.07, p = 0.30 | |
| Female | 5 | 1818 | 7048 | 31.7 | 21.6 - 43.9 | 98.72 | < 0.001 | |||
| Senior high school or below | 3 | 62 | 147 | 43.3 | 28.5 - 59.5 | 52.96 | 0.12 | Q = 1.15, p = 0.28 | ||
| University or above | 3 | 860 | 2486 | 34.6 | 31.4 - 38.1 | 56.12 | 0.10 | |||
| Married | 3 | 606 | 1775 | 34.6 | 31.0 - 38.3 | 47.55 | 0.15 | Q = 0.17, p = 0.68 | ||
| Unmarried | 3 | 316 | 859 | 35.8 | 31.140.9 | 43.56 | 0.17 | |||
| PTSS | Male | 4 | 235 | 993 | 19.1 | 4.2 - 56.3 | 98.65 | < 0.001 | Q = 0.08, p = 0.78 | |
| Female | 4 | 907 | 2199 | 25.4 | 5.1 - 68.3 | 99.56 | < 0.001 | |||
Note: Only studies reported all categories of sex and education level were included.
The minimum number of studies required to synthesize data is 3.
3.5. Meta-regression analyses
Meta-regression analyses revealed that the prevalence estimates of depression (r=2.31), stress (r=4.54) and insomnia (r=3.97) were positively and significantly associated with proportion of female participants. Studies with higher quality scores reported higher prevalence of depression (r=0.64), anxiety (r=0.40) and PTSS (r=2.08). Details of meta-regression analyses are shown in Supplementary Table 2.
3.6. Prevalence of psychiatric comorbidities in special subpopulations
A case-control study in Hong Kong reported that the prevalence of depression in pregnant women during the SARS epidemic was 12.3% (Lee et al., 2006), while another cross-sectional study in mainland China reported that the prevalence of depression in pregnant women during the COVID-19 epidemic was 29.6% (Wu et al., 2020). Two cross-sectional studies conducted in mainland China reported that the prevalence of depression in children and adolescents during the COVID-19 epidemic ranged from 22.6% to 43.7%, and the prevalence of anxiety in children and adolescents during the COVID-19 epidemic ranged from 18.9% to 37.4% (Xie et al., 2020, Zhou et al., 2020). A cross-sectional study conducted in mainland China reported that during the COVID-19 epidemic, parents of children hospitalized for any reason had significantly more severe depression and anxiety than parents of non-hospitalized children (48.0% vs. 8.0% in depression; 42.0% vs. 8.0% in anxiety) (Yuan et al., 2020).
A longitudinal study in mainland China reported that inpatients with COVID-19 or suspected COVID-19 had high levels of anxiety (86.1% before psychological intervention vs. 58.3% after psychological intervention; p<0.05) (Han et al., 2020), while a cross-sectional study in Vietnam reported that outpatients with suspected COVID-19 symptoms had significantly higher prevalence of depression than those without (64.3% vs. 35.7%; p<0.001) (Nguyen et al., 2020). A cross-sectional study in Hong Kong reported that during the SARS epidemic mental health problems were common in patients on a waiting list for thoracic surgeries, of whom 26.3% had depression, and 42.1% had anxiety (Wan et al., 2004).
3.7. Quality assessment and publication bias
Of the 82 included studies, the mean quality assessment score was 4.9, ranging from 3 to 7. Eighty studies are rated as “moderate quality”, while one study was rated as “low quality” and one study was rated as “high quality” (Supplementary Table 1). Egger's test found marginal publication bias in studies on PTSS during the COVID-19 epidemic (t=2.26, p=0.04; shown in Table 2). Funnel plots are shown in Supplementary Figures 8-15. A sensitivity analysis using the trim-and-fill method was performed with one imputed study, producing an approximately symmetrical funnel plot (Supplementary Figure 14). Using the trim-and-fill method, the adjusted pooled prevalence of PTSS was 53.1% (95% CI: 30.2% - 74.7%).
4. Discussion
To the best of our knowledge, this was the first systematic review that compared the prevalence of psychiatric comorbidities between the SARS and COVID-19 epidemics in all sub-populations. We found that psychiatric comorbidities were common in different subpopulations in both epidemics, and the prevalence estimates of psychiatric comorbidities were similar between both epidemics.
The overall prevalence of depression in all subpopulations studied during the COVID-19 epidemic was 23.9% (95% CI: 18.4%-30.3%) in this systematic review, which is similar to the findings of an earlier meta-analysis (18.9%; 95% CI: 13.0% - 26.6%) of depression during the COVID-19 epidemic (Li et al., 2020). We found the overall prevalence of anxiety in all subpopulations studied during the COVID-19 epidemic was 23.4% (95% CI: 19.9% - 27.3%), which is significantly lower than the corresponding figure in an earlier meta-analysis (44.5%; 95% CI: 29.8% - 60.1%) (Li et al., 2020). The reasons might be that the previous meta-analysis included studies published on or before 6 March 2020 (early stage of the COVID-19 epidemic), and conducted specifically on frontline health professionals, confirmed cases and quarantined populations. Another meta-analysis on COVID-19 patients also found higher prevalence of depression (45%; 95% CI 37% - 54%) and anxiety (47%; 95% CI 37% - 57%) (Deng et al., 2020), probably due to uncertainty about the novel virus, lack of specific treatments and fear of transmission to vulnerable populations (Xiang et al., 2020). The pooled prevalence of insomnia in this systematic review was 26.5% (95% CI: 19.1% - 35.5%), which is comparable with the findings of two earlier meta-analyses (49.8%, 95% CI: 18.6% - 81.1% (Li et al., 2020); and 34%, 95% CI: 19% - 50% (Deng et al., 2020)). The overall prevalence of stress and PTSS in this systematic review was 14.2% (95% CI: 8.4% - 22.9%) and 24.9% (95% CI: 11.0% - 46.8%), respectively, both of which are comparable with the corresponding figure in the previous meta-analysis (21.6%; 95% CI: 3.4%-68.1%) conducted in early stage of the COVID-19 epidemic (Li et al., 2020).
We found that the prevalence of depression and anxiety in all subpopulations studied between the SARS and COVID-19 epidemics were similar (Q=0.34, p=0.85 for depression; Q=0.59, p=0.44 for anxiety), which is also consistent with the findings in health professionals (Q=1.153, p=0.283 for depression; Q=0.557, p=0.456 for anxiety) (Salazar de et al., 2020). We found that the prevalence of PTSS in all subpopulations studied between the SARS and COVID-19 epidemics were similar (Q=0.89, p=0.35). However, in an earlier meta-analysis the prevalence of PTSD features in health professionals during the SARS, MERS and COVID-19 epidemics were different (16.7% in SARS, 40.7% in MERS, and 7.7% in COVID-19 epidemics; Q=22.74, p<0.001) (Salazar de et al., 2020). This may be because only one COVID-19 study with very low prevalence of PTSD features was included (Salazar de et al., 2020).
Subgroup analyses revealed that compared with health professionals, the general population was more likely to have poor general mental health status during the COVID-19 epidemic. This could be due to several reasons. Widespread misinformation on social mass media may have resulted in panic, fear and other mental health problems at the early phase of COVID-19 epidemic (Apuke and Omar, 2020, Pennycook et al., 2020, Brennen et al., 2020). Compared to health professionals, the general population may have less relevant medical knowledge to appraise the appropriate level of risks (O'Connor and Murphy, 2020), and may be more likely to suffer from mental health problems. In addition, substantial mental health services and psychological assistances were specifically developed for health professionals during the COVID-19 epidemic, which reduced the risk of adverse mental health effects (Liu et al., 2020, Li et al., 2020).
The prevalence of depression and anxiety between the general population and health professionals during the COVID-19 epidemic are comparable, consistent with previous findings (Li et al., 2020) in which the prevalence of depression was 12.6% (95% CI: 7.2%-21.2%) in the general population and 13.4% (95% CI: 5.3% - 29.7%) in health professionals during the COVID-19 epidemic, while the corresponding figures of anxiety was 40.6% (95% CI: 15.1% - 72.4%) and 41.1% (95% CI: 28.4% - 55.1%), respectively (Li et al., 2020). In contrast to the previous study, no significant difference in the prevalence of PTSS between the general population and health professionals was found in this meta-analysis. In the previous study, the prevalence of stress-related symptoms in health professionals (73.4%, 95% CI: 71.1% - 75.5%) was higher than in the general population (2.3%, 95% CI: 0.6% - 8.7%) (Li et al., 2020). However, the previous study only had one survey each on stress-related symptoms in the general population and in health professionals respectively (Li et al., 2020), which could lead to unreliable results.
Subgroup analyses revealed that no gender difference was found in the prevalence of depression, anxiety, insomnia and PTSS in all subpopulations studied during the COVID-19 epidemic in this meta-analysis, which is consistent with earlier meta-analyses conducted in COVID-19 patients (Deng et al., 2020) and health professionals (Pappa et al., 2020). However, meta-regression analysis found that female gender was positively associated with higher risk of depression, stress and insomnia. An earlier meta-analysis found that female health professionals were more likely to suffer from distress in coronavirus disease epidemics (Salazar de et al., 2020). This may be attributed to hormonal and cultural differences in females, for instance, socially sanctioned expression of emotions is encouraged in females more than males (Burt and Stein, 2002, Albert, 2015, Zhang and Wing, 2006, Barsky et al., 2001, Jayaratne et al., 1983). Marital status and education level did not moderate the prevalence of insomnia in this meta-analysis. As no other meta-analysis examined this potential association, direct comparisons could not be made. We also found that higher quality studies were associated with higher prevalence of depression, anxiety and PTSS. Due to random sampling, large sample size, strict study design and better trained interviewers that were adopted in high quality studies, mental health problems were more likely to be identified compared to lower quality studies (Rao et al., 2020, Xu et al., 2018, Wang et al., 2018).
The strengths of this systematic review included first, psychiatric comorbidities of all subpopulations studied during the COVID-19 and SARS epidemics were included, while previous meta-analyses focused either on COVID-19 or SARS alone (Deng et al., 2020, Li et al., 2020, Salari et al., 2020), and only on certain subpopulations (Rogers et al., 2020, Deng et al., 2020, Salazar de et al., 2020, Kisely et al., 2020). Second, the number of included studies and the total sample size were large, which enabled us to perform sophisticated analyses, such as subgroup and meta-regression analyses and test publication bias. However, several methodological limitations should be noted when interpreting the results. First, only studies published in English and Chinese languages were included. Second, even after subgroup analyses were performed, significant between-study heterogeneity was found. Such heterogeneity is unavoidable in the meta-analyses of epidemiological studies (Rotenstein et al., 2016, Wang et al., 2017). Third, some factors related to psychiatric comorbidities, such as pre-existing psychiatric disorders, social support, and severity and treatments of SARS and COVID-19, were not examined due to insufficient data.
In conclusion, psychiatric comorbidities were common in different subpopulations during both the SARS and COVID-19 epidemics. Although clinical features of both diseases are different, their prevalence of psychiatric comorbidities were almost similar. Considering the negative impact of psychiatric comorbidities on health and wellbeing during serious epidemics, timely screening and appropriate interventions for psychiatric comorbidities should be conducted for vulnerable subpopulations. Further public mental health education and psychological assistance hotlines should also be provided for the affected populations.
Contributors
Study design: Qing-E Zhang, Yu-Tao Xiang.
Data collection, analysis and interpretation: Yan-Jie Zhao, Yu Jin, Wen-Wang Rao, Wen Li, Na Zhao, Yuan-Yuan Wang.
Drafting of the manuscript: Yan-Jie Zhao, Yu Jin, Teris Cheung, Yu-Tao Xiang.
Critical revision of the manuscript: Chee H. Ng.
Approval of the final version for publication: all co-authors.
Declaration of Competing Interest
There is no conflict of interest related to the topic of this manuscript.
Acknowledgments
Acknowledgments
None.
Role of funding
The study was supported by the National Science and Technology Major Project for investigational new drug (2018ZX09201-014), the Beijing Municipal Science & Technology Commission (No. Z181100001518005), and the University of Macau (MYRG2019-00066-FHS).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jad.2021.03.016.
Appendix. Supplementary materials
References
- World Health Organization, 2020. Novel Coronavirus – China. https://www.who.int/csr/don/12-january-2020-novel-coronavirus-china/en/ (access 12 January 2020).
- World Health Organization, 2020. Naming the coronavirus disease (COVID-19) and the virus that causes it. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-)-and-the-virus-that-causes-it (assess 11 February 2020).
- World Health Organization, 2020. Novel Coronavirus(2019-nCoV): Situation Report-10. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200130-sitrep-10-ncov.pdf?sfvrsn=d0b2e480_2 (access 30 January 2020).
- World Health Organization, 2020. Statement on the second meeting of the International Health Regulations (2005) Emergency Committee regarding the outbreak of novel coronavirus (2019-nCoV). https://www.who.int/news-room/detail/30-01-2020-statement-on-the-second-meeting-of-the-international-health-regulations-(05)-emergency-committee-regarding-the-outbreak-of-novel-coronavirus-(19-ncov) (access 30 January 2020).
- Johns Hopkins University, 2021. COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). https://gisanddata.maps.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6 (access 28 February 2021).
- World Health Organization, 2004. Severe Acute Respiratory Syndrome (SARS). https://www.who.int/health-topics/severe-acute-respiratory-syndrome#tab=tab_1 (access 9 October 2020).
- World Health Organization, 2004. Situation Updates - SARS. https://www.who.int/csr/sars/archive/en/ (access 9 October 2020).
- World Health Organization, 2003. Summary of probable SARS cases with onset of illness from 1 November 2002 to 31 July 2003. https://www.who.int/csr/sars/country/table2004_04_21/en/ (access 31 December 2003).
- Grant MC, Geoghegan L, Arbyn M, et al. The prevalence of symptoms in 24,410 adults infected by the novel coronavirus (SARS-CoV-2; COVID-19): A systematic review and meta-analysis of 148 studies from 9 countries. PLoS One. 2020;15(6) doi: 10.1371/journal.pone.0234765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Diseases Control and Prevention, 2017. Severe acute respiratory syndrome (SARS). https://www.cdc.gov/sars/index.html (access 18 October 2020).
- Centers for Diseases Control and Prevention, 2020. Symptoms of Coronavirus. https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html (access 13 May 2020).
- Liu Y, Gayle AA, Wilder-Smith A, Rocklov J. The reproductive number of COVID-19 is higher compared to SARS coronavirus. J Travel Med. 2020;27(2) doi: 10.1093/jtm/taaa021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, Wang Z, Liao H, Marley G, Wu D, Tang W. Epidemiologic, clinical, and laboratory findings of the COVID-19 in the current pandemic: systematic review and meta-analysis. BMC Infect Dis. 2020;20(1):640. doi: 10.1186/s12879-020-05371-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley S, Fraser C, Donnelly CA, et al. Transmission dynamics of the etiological agent of SARS in Hong Kong: impact of public health interventions. Science. 2003;300(5627):1961–1966. doi: 10.1126/science.1086478. [DOI] [PubMed] [Google Scholar]
- Lipsitch M, Cohen T, Cooper B, et al. Transmission dynamics and control of severe acute respiratory syndrome. Science. 2003;300(5627):1966–1970. doi: 10.1126/science.1086616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization, 2020. Coronavirus. https://www.who.int/health-topics/coronavirus#tab=tab_3 (access 4 May 2020).
- Rogers JP, Chesney E, Oliver D, et al. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry. 2020 doi: 10.1016/S2215-0366(20)30203-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawryluck L, Gold WL, Robinson S, Pogorski S, Galea S, Styra R. SARS control and psychological effects of quarantine, Toronto, Canada. Emerging Infectious Diseases. 2004;10(7):1206–1212. doi: 10.3201/eid1007.030703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma JY. Silent SARS survivors (in Chinese) Southern People Weekly. 2009 http://www.infzm.com/content/31372 (access 13 June 2009) [Google Scholar]
- Cong Z, Lv QY, Yan J, Huang XB. Mental stress and crisis intervention in the patients with SARS and the people related (in Chinese) Journal of Peking University (Health Sciences) 2003;35(S1):47–50. [PubMed] [Google Scholar]
- Chinese Ministry of Health Chinese Ministry of Civil Affairs, Chinese Ministry of Public Security, China Disabled Persons Federation. China Mental Health Work Plan: from 2002 to 2010 (in Chinese) Shanghai Archives of Psychiatry. 2003;15(2):125–128. [Google Scholar]
- Deng J, Zhou F, Hou W, et al. The prevalence of depression, anxiety, and sleep disturbances in COVID-19 patients: a meta-analysis. Ann N Y Acad Sci. 2020 doi: 10.1111/nyas.14506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar de Pablo G, Vaquerizo-Serrano J, Catalan A, et al. Impact of coronavirus syndromes on physical and mental health of health care workers: Systematic review and meta-analysis. J Affect Disord. 2020;275:48–57. doi: 10.1016/j.jad.2020.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Zhang CD, Luo JJ, et al. Psychological status among different populations during COVID-19 epidemic: a systematic review and Meta-analysis (in Chinese) Journal of Tongji University (Medical Science) 2020;41(2):147–154. [Google Scholar]
- Kisely S, Warren N, McMahon L, Dalais C, Henry I, Siskind D. Occurrence, prevention, and management of the psychological effects of emerging virus outbreaks on healthcare workers: rapid review and meta-analysis. BMJ (Clinical research ed) 2020;369:m1642. doi: 10.1136/bmj.m1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappa S, Ntella V, Giannakas T, Giannakoulis VG, Papoutsi E, Katsaounou P. Prevalence of depression, anxiety, and insomnia among healthcare workers during the COVID-19 pandemic: A systematic review and meta-analysis. Brain Behav Immun. 2020;88:901–907. doi: 10.1016/j.bbi.2020.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salari N, Hosseinian-Far A, Jalali R, et al. Prevalence of stress, anxiety, depression among the general population during the COVID-19 pandemic: a systematic review and meta-analysis. Global Health. 2020;16(1):57. doi: 10.1186/s12992-020-00589-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Bmj. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loney PL, Chambers LW, Bennett KJ, Roberts JG, Stratford PW. Critical Appraisal of the Health Research Literature: Prevalence or Incidence of a Health Problem. Chronic Diseases in Canada. 1998;19(4):170–176. [PubMed] [Google Scholar]
- Boyle MH. Guidelines for evaluating prevalence studies. Evidence Based Mental Health. 1998;1(2):37–39. [Google Scholar]
- Yang C, Zhang L, Zhu P, Zhu C, Guo Q. The prevalence of tic disorders for children in China: A systematic review and meta-analysis. Medicine. 2016;95(30):e4354. doi: 10.1097/MD.0000000000004354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DTS, Sahota D, Leung TN, Yip ASK, Lee FFY, Chung TKH. Psychological responses of pregnant women to an infectious outbreak: A case-control study of the 2003 SARS outbreak in Hong Kong. Journal of Psychosomatic Research. 2006;61(5):707–713. doi: 10.1016/j.jpsychores.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Zhang C, Liu H, et al. Perinatal depressive and anxiety symptoms of pregnant women along with COVID-19 outbreak in China. American Journal of Obstetrics and Gynecology. 2020 doi: 10.1016/j.ajog.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, Xue Q, Zhou Y, et al. Mental Health Status Among Children in Home Confinement During the Coronavirus Disease 2019 Outbreak in Hubei Province, China. JAMA Pediatrics. 2020 doi: 10.1001/jamapediatrics.2020.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S-J, Zhang L-G, Wang L-L, et al. Prevalence and socio-demographic correlates of psychological health problems in chinese adolescents during the outbreak of covid-19. European Child & Adolescent Psychiatry. 2020 doi: 10.1007/s00787-020-01541-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan R, Xu QH, Xia CC, et al. Psychological status of parents of hospitalized children during the COVID-19 epidemic in China. Psychiatry Research. 2020;288 doi: 10.1016/j.psychres.2020.112953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han ZH, Chen XJ, Zhang L, qian MM. Brief talk on the psychological intervention in suspected or confirmed COVID-19 patients (in Chinese) Medical Dietary Therapy and Health. 2020;18(06):108–109. [Google Scholar]
- Nguyen HC, Nguyen MH, Do BN, et al. People with suspected covid-19 symptoms were more likely depressed and had lower health-related quality of life: The potential benefit of health literacy. Journal of Clinical Medicine. 2020;9(4) doi: 10.3390/jcm9040965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan IYP, Wat KHY, Ng CSH, et al. Evaluation of the emotional status of patients on a waiting list for thoracic surgery during the outbreak of Severe Acute Respiratory Syndrome (SARS) Stress and Health. 2004;20(4):209–212. [Google Scholar]
- Xiang YT, Yang Y, Li W, et al. Timely mental health care for the 2019 novel coronavirus outbreak is urgently needed. Lancet Psychiatry. 2020;7(3):228–229. doi: 10.1016/S2215-0366(20)30046-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apuke OD, Omar B. Fake news and COVID-19: modelling the predictors of fake news sharing among social media users. Telematics and Informatics. 2020 doi: 10.1016/j.tele.2020.101475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennycook G, McPhetres J, Zhang Y, Lu JG, Rand DG. Fighting COVID-19 Misinformation on Social Media: Experimental Evidence for a Scalable Accuracy-Nudge Intervention. Psychol Sci. 2020;31(7):770–780. doi: 10.1177/0956797620939054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennen JS, Simon F, Howard PN, Nielsen RK. Types, sources, and claims of COVID-19 misinformation. Reuters Institute. 2020;7(3):1. [Google Scholar]
- O'Connor C, Murphy M. Going viral: doctors must tackle fake news in the covid-19 pandemic. BMJ. 2020;369:m1587. doi: 10.1136/bmj.m1587. [DOI] [PubMed] [Google Scholar]
- Liu S, Yang L, Zhang C, et al. Online mental health services in China during the COVID-19 outbreak. The Lancet Psychiatry. 2020;7(4) doi: 10.1016/S2215-0366(20)30077-8. e17-e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Yang Y, Liu ZH, et al. Progression of Mental Health Services during the COVID-19 Outbreak in China. Int J Biol Sci. 2020;16(10):1732–1738. doi: 10.7150/ijbs.45120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt VK, Stein K. Epidemiology of depression throughout the female life cycle. J Clin Psychiatry. 2002;63(Suppl 7):9–15. [PubMed] [Google Scholar]
- Albert PR. Why is depression more prevalent in women? J Psychiatry Neurosci. 2015;40(4):219–221. doi: 10.1503/jpn.150205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Wing YK. Sex differences in insomnia: a meta-analysis. Sleep. 2006;29(1):85–93. doi: 10.1093/sleep/29.1.85. [DOI] [PubMed] [Google Scholar]
- Barsky AJ, Peekna HM, Borus JF. Somatic symptom reporting in women and men. J Gen Intern Med. 2001;16(4):266–275. doi: 10.1046/j.1525-1497.2001.00229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaratne S, Tripodi T, Chess WA. Perceptions of emotional support, stress, and strain by male and female social workers. Social Work Research and Abstracts; 1983. 1983:19–27. [Google Scholar]
- Rao WW, Zhu XM, Zong QQ, et al. Prevalence of prenatal and postpartum depression in fathers: A comprehensive meta-analysis of observational surveys. J Affect Disord. 2020;263:491–499. doi: 10.1016/j.jad.2019.10.030. [DOI] [PubMed] [Google Scholar]
- Xu DD, Rao WW, Cao XL, et al. Prevalence of major depressive disorder in children and adolescents in China: A systematic review and meta-analysis. J Affect Disord. 2018;241:592–598. doi: 10.1016/j.jad.2018.07.083. [DOI] [PubMed] [Google Scholar]
- Wang F, Zhang QE, Zhang L, et al. Prevalence of major depressive disorder in older adults in China: A systematic review and meta-analysis. J Affect Disord. 2018;241:297–304. doi: 10.1016/j.jad.2018.07.061. [DOI] [PubMed] [Google Scholar]
- Rotenstein LS, Ramos MA, Torre M, et al. Prevalence of Depression, Depressive Symptoms, and Suicidal Ideation Among Medical Students: A Systematic Review and Meta-Analysis. Jama. 2016;316(21):2214–2236. doi: 10.1001/jama.2016.17324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YH, Shi ZT, Luo QY. Association of depressive symptoms and suicidal ideation among university students in China: A systematic review and meta-analysis. Medicine (Baltimore) 2017;96(13):e6476. doi: 10.1097/MD.0000000000006476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed MZ, Ahmed O, Aibao Z, Hanbin S, Siyu L, Ahmad A. Epidemic of COVID-19 in China and associated Psychological Problems. Asian Journal of Psychiatry. 2020:51. doi: 10.1016/j.ajp.2020.102092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bo HX, Li W, Yang Y, et al. Posttraumatic stress symptoms and attitude toward crisis mental health services among clinically stable patients with COVID-19 in China. Psychological Medicine. 2020:1–2. doi: 10.1017/S0033291720000999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai W, Lian B, Song X, Hou T, Deng G, Li H. A cross-sectional study on mental health among health care workers during the outbreak of Corona Virus Disease 2019. Asian Journal of Psychiatry. 2020:51. doi: 10.1016/j.ajp.2020.102111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao W, Fang Z, Hou G, et al. The psychological impact of the COVID-19 epidemic on college students in China. Psychiatry Research. 2020;287 doi: 10.1016/j.psychres.2020.112934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan AOM, Huak CY. Psychological impact of the 2003 severe acute respiratory syndrome outbreak on health care workers in a medium size regional general hospital in Singapore. Occupational Medicine. 2004;54(3):190–196. doi: 10.1093/occmed/kqh027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J, Yuan Y, Wang D. Mental health status and its influencing factors among college students during the epidemic of COVID-19 (in Chinese) Journal of Southern Medical University. 2020;40(2):171–176. doi: 10.12122/j.issn.1673-4254.2020.02.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CS, Wu HY, Yang P, Yen CF. Psychological distress of nurses in Taiwan who worked during the outbreak of SARS. Psychiatric Services. 2005;56(1):76–79. doi: 10.1176/appi.ps.56.1.76. [DOI] [PubMed] [Google Scholar]
- Chen Y, Zhou H, Zhou Y, Zhou F. Prevalence of self-reported depression and anxiety among pediatric medical staff members during the COVID-19 outbreak in Guiyang, China. Psychiatry Research. 2020;288 doi: 10.1016/j.psychres.2020.113005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng SK, Sheng B, Lau KK, et al. Adjustment outcomes in Chinese patients following one-month recovery from severe acute respiratory syndrome in Hong Kong. The Journal of Nervous and Mental Disease. 2004;192(12):868–871. doi: 10.1097/01.nmd.0000147169.03998.dc. [DOI] [PubMed] [Google Scholar]
- Chew NWS, Lee GKH, Tan BYQ, et al. A multinational, multicentre study on the psychological outcomes and associated physical symptoms amongst healthcare workers during COVID-19 outbreak. Brain, Behavior, and Immunity. 2020 doi: 10.1016/j.bbi.2020.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong MY, Wang WC, Hsieh WC, et al. Psychological impact of severe acute respiratory syndrome on health workers in a tertiary hospital. British Journal of Psychiatry. 2004;185(AUG):127–133. doi: 10.1192/bjp.185.2.127. [DOI] [PubMed] [Google Scholar]
- Consolo U, Bellini P, Bencivenni D, Iani C, Checchi V. Epidemiological Aspects and Psychological Reactions to COVID-19 of Dental Practitioners in the Northern Italy Districts of Modena and Reggio Emilia. International Journal of Environmental Research and Public Health. 2020;17(10) doi: 10.3390/ijerph17103459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Y, Zhe D, Shuran L. Survey on Mental Status of Subjects Recovered from SARS (in Chinese) Chinese Mental Health Journal. 2004;18(10):675–677. [Google Scholar]
- Gao J, Zheng P, Jia Y, et al. Mental health problems and social media exposure during COVID-19 outbreak. PLoS ONE. 2020;15(4) doi: 10.1371/journal.pone.0231924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong X, Currier GW, Zhao X, Jiang Y, Zhou W, Wei J. Posttraumatic stress disorder in convalescent severe acute respiratory syndrome patients: A 4-year follow-up study. General Hospital Psychiatry. 2009;31(6):546–554. doi: 10.1016/j.genhosppsych.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang JZ, Han MF, Luo TD, Ren AK, Zhou XP. Mental health survey of medical staff in a tertiary infectious disease hospital for COVID-19 (in Chinese) Chinese journal of industrial hygiene and occupational diseases. 2020;38(3):192–195. doi: 10.3760/cma.j.cn121094-20200219-00063. [DOI] [PubMed] [Google Scholar]
- Huang Y, Zhao N. Chinese mental health burden during the COVID-19 pandemic. Asian Journal of Psychiatry. 2020:51. doi: 10.1016/j.ajp.2020.102052. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Ko CH, Yen CF, Yen JY, Yang MJ. Psychosocial impact among the public of the severe acute respiratory syndrome epidemic in Taiwan. Psychiatry and Clinical Neurosciences. 2006;60(4):397–403. doi: 10.1111/j.1440-1819.2006.01522.x. [DOI] [PubMed] [Google Scholar]
- Kwek SK, Chew WM, Ong KC, et al. Quality of life and psychological status in survivors of severe acute respiratory syndrome at 3 months postdischarge. Journal of Psychosomatic Research. 2006;60(5):513–519. doi: 10.1016/j.jpsychores.2005.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai J, Ma S, Wang Y, et al. Factors Associated With Mental Health Outcomes Among Health Care Workers Exposed to Coronavirus Disease 2019. JAMA Netw Open. 2020;3(3) doi: 10.1001/jamanetworkopen.2020.3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam MHB, Wing YK, Yu MWM, et al. Mental morbidities and chronic fatigue in severe acute respiratory syndrome survivors long-term follow-up. Archives of Internal Medicine. 2009;169(22):2142–2147. doi: 10.1001/archinternmed.2009.384. [DOI] [PubMed] [Google Scholar]
- Lancee WJ, Maunder RG, Goldbloom DS. Prevalence of psychiatric disorders among Toronto hospital workers one to two years after the SARS outbreak. Psychiatric Services. 2008;59(1):91–95. doi: 10.1176/ps.2008.59.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau JTF, Yang X, Tsui HY, Pang E, Wing YK. Positive mental health-related impacts of the SARS epidemic on the general public in Hong Kong and their associations with other negative impacts. Journal of Infection. 2006;53(2):114–124. doi: 10.1016/j.jinf.2005.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AM, Wong JGWS, McAlonan GM, et al. Stress and psychological distress among SARS survivors 1 year after the outbreak. The Canadian Journal of Psychiatry. 2007;52(4):233–240. doi: 10.1177/070674370705200405. [DOI] [PubMed] [Google Scholar]
- Lei L, Huang XM, Zhang S, Yang JR, Yang L, Xu M. Comparison of Prevalence and Associated Factors of Anxiety and Depression Among People Affected by versus People Unaffected by Quarantine During the COVID-9 Epidemic in Southwestern China. Medical Science Monitor. 2020;26 doi: 10.12659/MSM.924609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Yu H, Bian G, et al. Prevalence, risk factors, and clinical correlates of insomnia in volunteer and at home medical staff during the COVID-19. Brain, behavior, and immunity. 2020 doi: 10.1016/j.bbi.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Wang Y, Jiang J, et al. Psychological distress among health professional students during the COVID-19 outbreak. Psychological medicine. 2020:1–12. doi: 10.1017/S0033291720001555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang LL, Ren H, Cao RL, et al. The Effect of COVID-19 on Youth Mental Health. Psychiatric Quarterly. 2020 doi: 10.1007/s11126-020-09744-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CY, Yang YZ, Zhang XM, et al. The prevalence and influencing factors in anxiety in medical workers fighting COVID-19 in China: A cross-sectional survey. Epidemiology and Infection. 2020:1–17. doi: 10.1017/S0950268820001107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N, Zhang F, Wei C, et al. Prevalence and predictors of PTSS during COVID-19 outbreak in China hardest-hit areas: Gender differences matter. Psychiatry Research. 2020;287 doi: 10.1016/j.psychres.2020.112921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Kakade M, Fuller CJ, et al. Depression after exposure to stressful events: Lessons learned from the severe acute respiratory syndrome epidemic. Comprehensive Psychiatry. 2012;53(1):15–23. doi: 10.1016/j.comppsych.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu ZR, Huang YQ, Dang WM, Liu M, Li SR. The mental health status of students from three universities in Beijing and its associative factors during SARS epidemic (in Chinese) Chinese Journal of Epidemiology. 2004;25(7):594–597. [Google Scholar]
- Lü SH, Tian BC, Yang TZ, Chen DW, Chi YH. Perceived stress in general public during prevalence of severe acute respiratory syndrome and its impact on health behavior (in Chinese) Chinese Journal of Preventive Medicine. 2010;44(2):128–133. [PubMed] [Google Scholar]
- Lu W, Wang H, Lin Y, Li L. Psychological status of medical workforce during the COVID-19 pandemic: A cross-sectional study. Psychiatry Research. 2020;288 doi: 10.1016/j.psychres.2020.112936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu YC, Shu BC, Chang YY, Lung FW. The mental health of hospital workers dealing with severe acute respiratory syndrome. Psychotherapy and Psychosomatics. 2006;75(6):370–375. doi: 10.1159/000095443. [DOI] [PubMed] [Google Scholar]
- Lung FW, Lu YC, Chang YY, Shu BC. Mental Symptoms in Different Health Professionals During the SARS Attack: A Follow-up Study. Psychiatr Q. 2009;80(2):107–116. doi: 10.1007/s11126-009-9095-5. [DOI] [PubMed] [Google Scholar]
- Mak IWC, Chu CM, Pan PC, Yiu MGC, Chan VL. Long-term psychiatric morbidities among SARS survivors. General Hospital Psychiatry. 2009;31(4):318–326. doi: 10.1016/j.genhosppsych.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maunder RG, Lancee WJ, Balderson KE, et al. Long-term psychological and occupational effects of providing hospital healthcare during SARS outbreak. Emerging Infectious Diseases. 2006;12(12):1924–1932. doi: 10.3201/eid1212.060584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazza C, Ricci E, Biondi S, et al. A Nationwide Survey of Psychological Distress among Italian People during the COVID-19 Pandemic: Immediate Psychological Responses and Associated Factors. International Journal of Environmental Research and Public Health. 2020;17(9) doi: 10.3390/ijerph17093165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihashi M, Otsubo Y, Yinjuan X, Nagatomi K, Hoshiko M, Ishitake T. Predictive factors of psychological disorder development during recovery following SARS outbreak. Health Psychology. 2009;28(1):91–100. doi: 10.1037/a0013674. [DOI] [PubMed] [Google Scholar]
- Ni MY, Yang L, Leung CMC, et al. Mental Health, Risk Factors, and Social Media Use During the COVID-19 Epidemic and Cordon Sanitaire Among the Community and Health Professionals in Wuhan, China: Cross-Sectional Survey. JMIR Mental Health. 2020;7(5):e19009. doi: 10.2196/19009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickell LA, Crighton EJ, Tracy CS, et al. Psychosocial effects of SARS on hospital staff: survey of a large tertiary care institution. CMAJ. 2004;170(5):793–798. doi: 10.1503/cmaj.1031077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozamiz-Etxebarria N, Dosil-Santamaria M, Picaza-Gorrochategui M, Idoiaga-Mondragon N. Stress, anxiety, and depression levels in the initial stage of the COVID-19 outbreak in a population sample in the northern Spain. Cadernos de Saude Publica. 2020;36(4) doi: 10.1590/0102-311X00054020. [DOI] [PubMed] [Google Scholar]
- Peng EYC, Lee MB, Tsai ST, et al. Population-based post-crisis psychological distress: An example from the SARS outbreak in Taiwan. Journal of the Formosan Medical Association. 2010;109(7):524–532. doi: 10.1016/S0929-6646(10)60087-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds DL, Garay JR, Deamond SL, Moran MK, Gold W, Styra R. Understanding, compliance and psychological impact of the SARS quarantine experience. Epidemiology and Infection. 2008;136(7):997–1007. doi: 10.1017/S0950268807009156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shacham M, Hamama-Raz Y, Kolerman R, Mijiritsky O, Ben-Ezra M, Mijiritsky E. COVID-19 Factors and Psychological Factors Associated with Elevated Psychological Distress among Dentists and Dental Hygienists in Israel. International Journal of Environmental Research and Public Health. 2020;17(8) doi: 10.3390/ijerph17082900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim K, Chong PN, Chan YH, Soon WS. Severe acute respiratory syndrome-related psychiatric and posttraumatic morbidities and coping responses in medical staff within a primary health care setting in Singapore. The Journal of Clinical Psychiatry. 2004;65(8):1120–1127. doi: 10.4088/jcp.v65n0815. [DOI] [PubMed] [Google Scholar]
- Sim K, Huak Chan Y, Chong PN, Chua HC. Wen Soon S. Psychosocial and coping responses within the community health care setting towards a national outbreak of an infectious disease. Journal of Psychosomatic Research. 2010;68(2):195–202. doi: 10.1016/j.jpsychores.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su TP, Lien TC, Yang CY, et al. Prevalence of psychiatric morbidity and psychological adaptation of the nurses in a structured SARS caring unit during outbreak: A prospective and periodic assessment study in Taiwan. Journal of Psychiatric Research. 2007;41(1-2):119–130. doi: 10.1016/j.jpsychires.2005.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan W, Hao F, McIntyre RS, et al. Is returning to work during the COVID-19 pandemic stressful? A study on immediate mental health status and psychoneuroimmunity prevention measures of Chinese workforce. Brain, Behavior, and Immunity. 2020 doi: 10.1016/j.bbi.2020.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W, Hu T, Hu B, et al. Prevalence and correlates of PTSD and depressive symptoms one month after the outbreak of the COVID-19 epidemic in a sample of home-quarantined Chinese university students. Journal of Affective Disorders. 2020 doi: 10.1016/j.jad.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tham KY, Tan YH, Loh OH, Tan WL, Ong MK, Tang HK. Psychiatric morbidity among emergency department doctors and nurses after the SARS outbreak. Annals of the Academy of Medicine, Singapore. 2004;33(5 Suppl):S78–S79. [PubMed] [Google Scholar]
- Tian BC, Yang TZ, Lü SH, Chen DW. The change of health related behavior during and after severe acute respiratory syndrome prevalence (in Chinese) Chinese Journal of Preventive Medicine. 2007;41(4):254–257. [PubMed] [Google Scholar]
- Tian F, Li H, Tian S, Yang J, Shao J, Tian C. Psychological symptoms of ordinary Chinese citizens based on SCL-90 during the level I emergency response to COVID-19. Psychiatry Research. 2020;288 doi: 10.1016/j.psychres.2020.112992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Pan R, Wan X, et al. Immediate Psychological Responses and Associated Factors during the Initial Stage of the 2019 Coronavirus Disease (COVID-19) Epidemic among the General Population in China. International Journal of Environmental Research and Public Health. 2020;17(5) doi: 10.3390/ijerph17051729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Xie L, Xu Y, Yu S, Yao B, Xiang D. Sleep disturbances among medical workers during the outbreak of COVID-2019. Occupational Medicine. 2020 doi: 10.1093/occmed/kqaa074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu K, Wei X. Analysis of Psychological and Sleep Status and Exercise Rehabilitation of Front-Line Clinical Staff in the Fight Against COVID-19 in China. Medical Science Monitor Basic Research. 2020;26 doi: 10.12659/MSMBR.924085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Q, Sun Z, Liu T, et al. Posttraumatic Stress Symptoms of Health Care Workers during the Corona Virus Disease 2019 (COVID-19) Clinical Psychology & Psychotherapy. 2020 doi: 10.1002/cpp.2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Yang L, Liu S, et al. Survey of Insomnia and Related Social Psychological Factors Among Medical Staff Involved in the 2019 Novel Coronavirus Disease Outbreak. Frontiers in Psychiatry. 2020;11 doi: 10.3389/fpsyt.2020.00306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang KR, Xu Y, Liu ZG, et al. Controlled study of posttraumatic stress disorder among patients with severe acute respiratory syndrome and first-line hospital staffs as well as public in prevalent areas (in Chinese) Chinese Journal of Clinical Rehabilitation. 2005;9(12):94–96. [Google Scholar]
- Zhang WR, Wang K, Yin L, et al. Mental Health and Psychosocial Problems of Medical Health Workers during the COVID-19 Epidemic in China. Psychotherapy and Psychosomatics. 2020 doi: 10.1159/000507639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Ma ZF. Impact of the COVID-19 Pandemic on Mental Health and Quality of Life among Local Residents in Liaoning Province, China: A Cross-Sectional Study. International Journal of Environmental Research and Public Health. 2020;17(7) doi: 10.3390/ijerph17072381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Sun L, Zhang L, et al. Prevalence and Influencing Factors of Anxiety and Depression Symptoms in the First-Line Medical Staff Fighting Against COVID-19 in Gansu. Frontiers in Psychiatry. 2020;11 doi: 10.3389/fpsyt.2020.00386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S, Wu Y, Zhu CY, et al. The immediate mental health impacts of the COVID-19 pandemic among people with or without quarantine managements. Brain, Behavior, and Immunity. 2020 doi: 10.1016/j.bbi.2020.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi TY, Jiang C, Jia SH, Liu QG, Zhang J, Qi XY. Analysis on SARS-related post-traumatic stress disorder and its correlative factors (in Chinese) Chinese Journal of Clinical Rehabilitation. 2005;9(44):9–13. [Google Scholar]
- Zhang XJ, Sun YH, Ye DQ, et al. The mental health status of community population in Hefei city during SARS epidemic (in Chinese) Chinese Journal of Disease Control and Prevention. 2003;7(4):280–282. [Google Scholar]
- He LP, Guo Y, Chen XY, et al. SARS- related cognitive behavior and mental health survey in residents from Wuhan city (in Chinese) Chinese Journal of Public Health. 2004;20(4):387. [Google Scholar]
- Zhao Q, Hu CH, Feng RJ, Yang Y. The depressive, anxious and somatic symptoms in COVID-19 patients (in Chinese) Chinese Journal of Neurology. 2020;53(06):432–436. [Google Scholar]
- Gao HS, Wu HL, Lan XX, et al. A follow-up study on post-traumatic stress disorder in SARS patients (in Chinese) Chinese Journal of Rehabilitation Medicine. 2006;21(11):1003–1004. 26. [Google Scholar]
- Gao HS, Hu YL, Hui WL, et al. A follow-up study on depressive and anxious symptoms of convalescent SARS patients in one hospital (in Chinese) Acta Academiae Medicinae CPAPF. 2006;15(6):525–527. [Google Scholar]
- Wei LP, Zhou BR, Deng YH, Chen AW, Zhu QP, Cai YC. The correlation analysis on illness state, steroids application and convalescent mental status in SARS-infected health professionals (in Chinese) Guangzhou Medical Journal. 2005;36(1):33–35. [Google Scholar]
- Cheng SK, Wong CW, Tsang J, Wong KC. Psychological distress and negative appraisals in survivors of severe acute respiratory syndrome (SARS) Psychological Medicine. 2004;34(7):1187–1195. doi: 10.1017/s0033291704002272. [DOI] [PubMed] [Google Scholar]
- Wu KK, Chan SK, Ma TM. Posttraumatic stress after SARS. Emerging Infectious Diseases. 2005;11(8):1297–1300. doi: 10.3201/eid1108.041083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns Hopkins University. COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). 2020: https://gisanddata.maps.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6 (access 18 October 2020).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.