Abstract
Bacterial infection is a common wound complication that can significantly delay healing. Classical local therapies for infected wounds are expensive and are frequently ineffective. One alternative therapy is photodynamic therapy (PDT). We conducted a systematic review to clarify whether PDT is useful for bacteria‐infected wounds in animal models. PubMed and Medline were searched for articles on PDT in infected skin wounds in animals. The language was limited to English. Nineteen articles met the inclusion criteria. The overall study methodological quality was moderate, with a low‐moderate risk of bias. The animal models were mice and rats. The wounds were excisional, burn, and abrasion wounds. Wound size ranged from 6 mm in diameter to 1.5 × 1.5 cm2. Most studies inoculated the wounds with Pseudomonas aeruginosa or methicillin‐resistant Staphylococcus aureus. Eleven and 17 studies showed that the PDT of infected wounds significantly decreased wound size and bacterial counts, respectively. Six, four, and two studies examined the effect of PDT on infected wound‐cytokine levels, wound‐healing time, and body weight, respectively. Most indicated that PDT had beneficial effects on these variables. PDT accelerated bacteria‐infected wound healing in animals by promoting wound closure and killing bacteria.
1. INTRODUCTION
Wounds are mainly caused by trauma, surgery, and diseases, including diabetes mellitus and vascular diseases. They can have a profound deleterious effect on patient quality of life and are common. For example, it is estimated that, at any given time point, 1.5 to 2 million people in Europe have acute or chronic wounds that require medical care.1 Moreover, several studies have shown that patients with wounds occupy 27% to 50% of acute hospital beds in Europe on any given day2 and cost the United Kingdom approximately £5 billion annually in 2012/2013: the latter accounted for 3% of all British health care expenditure in 2012/2013.3
Infection is one of the most common complications of wounds that could delay wound healing. Bacterial infections are particularly frequent. The most common culprit is Staphylococcus aureus. The most common therapy for infected wounds is antibiotics. However, much to the alarm of health care workers all over the world, antibiotics have become increasingly less effective for bacterial wound infections because of the abuse of antibiotics and the subsequent emergence of increasing numbers of multi‐resistant bacterial species such as Pseudomonas aeruginosa and methicillin‐resistant S. aureus.4 Other classical local therapies for infected wounds include dressings, topical products, and—more recently—negative pressure therapy. However, all of these classical therapies, including antibiotics, are expensive and not consistently effective.5 Thus, there is a great need for alternative therapies for infected wounds.
One possibility is photodynamic therapy (PDT).6 This is not a new treatment as it has been used for about 100 years to topically treat various skin lesions, starting with superficial non‐melanoma skin cancers.7, 8 PDT involves three key ingredients, namely, a photosensitiser,9 light, and oxygen. The process starts with the topical application of the photosensitiser onto the target lesion. The application is followed by an incubation period that allows the abnormally proliferating cells in the lesion to take up the photosensitiser and convert it to a component in the heme biosynthetic pathway, called protoporphyrin‐IX. When the lesion is illuminated with light of the appropriate wavelength, photoporphyrin‐IX becomes activated and converts molecular oxygen into reactive oxygen species. This in turn causes the cells to die via necrosis or apoptosis. The preferential uptake of the photosensitiser by proliferating cells means that this therapy does not affect the surrounding normal tissue.10
Recently, several researchers showed that PDT improves wound healing in mice and humans,11, 12 has antimicrobial effects on bacteria isolated from infected human burn wounds,13 and improves the healing of leg ulcers.14 Human studies also showed that PDT is well tolerated by the patients.12, 14 Thus, PDT may be a suitable alternative to standard therapies for infected wounds.
In 2018, Nesi‐Reis et al conducted a systematic review on the ability of PDT to improve (non‐infected) chronic ulcers and superficial non‐melanoma skin cancers in humans.15 However, a systematic review on the antimicrobial effects of PDT and its ability to promote the healing of infected wounds has not been reported. Here, we conducted a systematic review to clarify whether PDT is useful for bacteria‐infected wounds in animal models. We expect that this review will promote further research and ultimately aid the clinical treatment of wounds.
2. MATERIALS AND METHODS
This study followed the guidelines for systematic reviews of animal studies that were suggested by Vries et al.16
2.1. Data sources and searches
The PubMed and Medline databases (from inception to May 2019) were searched for all PDT‐related articles. The language was limited to English. The Medical Subject Headings (MeSH) terms were as follows: (“Wound Healing” OR “Re‐Epithelialization” OR “Regeneration”) AND (“Photochemotherapy” OR “Photochemistry” OR “Photosensitizing Agents”). The bibliographic references of the articles that were captured by this electronic database search were also searched manually to identify additional potential studies.
2.2. Study selection
All articles on animals that reported the antimicrobial effects of PDT in skin wound healing and that had a PDT‐untreated control group were eligible for inclusion. The titles and abstracts of all captured articles were screened for possible inclusion. Reviews, duplicates, human studies, studies on wounds that did not involve the skin, and studies irrelevant to our topic were excluded. After reading the full text of the remaining articles, all studies that compared animals treated with PDT alone to untreated control animals were included in the systematic review. Articles in which the PDT was only delivered in combination with another treatment were excluded. Articles that involved wounds that had not been inoculated with bacteria were also excluded. Two independent reviewers (Yan Sun and Bi‐Huan Xiao) conducted this article selection procedure. Disagreements were resolved by discussions between these reviewers or by asking a third reviewer (Liang‐Hong Chen) to help achieve consensus.
2.3. Data extraction and quality assessment
Two reviewers (Yan Sun and Bi‐Huan Xiao) independently extracted the following data from the included articles: first author, year of publication, country of research, animal species, type of wound, bacterial species, the photosensitiser and light parameters used for PDT, the primary and secondary study outcome measures, and the main results.
Two reviewers (Yan Sun and Bi‐Huan Xiao) also independently assessed the methodological quality of the included studies. Disagreements about quality were resolved by discussions or by asking for help from a third reviewer (Liang‐Hong Chen). Methodological quality was assessed by using the risk‐of‐bias tool of the Systematic Review Center for Laboratory animal Experimentation (SYRCLE), which involves 10 items: (a) animal allocation sequence generation, (b) comparison of baseline characteristics of the comparator animal groups, (c) allocation concealment, (d) random housing of the animals in the animal room, (e) investigator blinding, (f) random outcome assessment, (g) assessor blinding, (h) incomplete outcome data addressed, (i) free of selective outcome reporting, and (j) free of other sources of bias.17 For each item, “yes” indicated a “low risk of bias,” while “no” represented a “high risk of bias.” If the information provided was not sufficient, the item was judged as “unclear,” which was considered an “unclear risk of bias.”
2.4. Data synthesis
A meta‐analysis was performed by using Review Manager 5.3 (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014). To determine the homogeneity of the outcome (body weight) of the included studies, χ² test was performed, and the I 2 statistic were calculated. The study outcomes were considered to be heterogenous if the χ² test P value was <.1 and the I 2 statistic was >50%. If heterogeneity was detected, the random‐effects model was used. If homogeneity was observed, the fixed‐effects model was used. The difference between the PDT‐treated and untreated groups in terms of outcome was expressed relative to the outcome variability in the study by calculating the standard mean difference with 95% confidence intervals. P values of .05 were considered to indicate statistically significant differences between PDT‐treated and untreated groups. To ensure the reliability and accuracy of the results, two independent reviewers (Yan Sun and Bi‐Huan Xiao) synthesised the data separately.
3. RESULTS
3.1. Identification of eligible studies
A total of 779 articles were identified by the preliminary bibliographic search. After screening the title and abstract of each article, 738 were excluded because they were reviews, duplicates, human studies, involved non‐skin wounds, or were irrelevant to our topic. The full text of the remaining 41 articles underwent detailed evaluation. Twenty‐two articles with combined interventions or wounds not inoculated with bacteria were excluded. Finally, 19 articles that related to antimicrobial PDT in skin wound healing in animal models were included. The selection process is illustrated in Figure 1.
Figure 1.
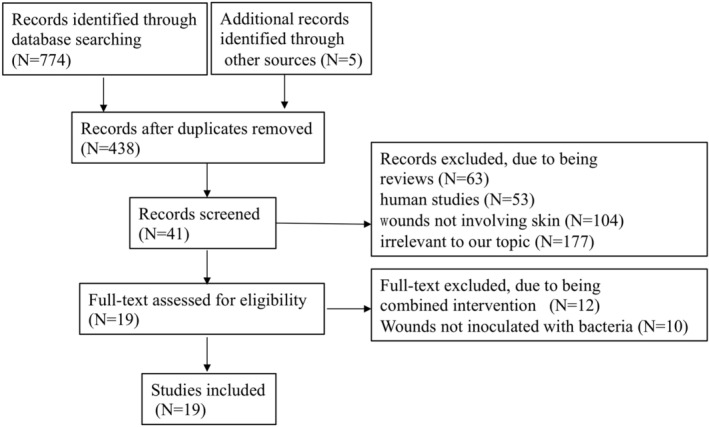
Flow diagram
3.2. Characteristics of the included studies
All articles were written in English and were published between 2002 and 2018. The country in which the study was performed was the United States,18, 19, 20, 21, 22, 23, 24 India,25, 26, 27, 28 China,29, 30, 31 Japan,32, 33 Turkey,34 Egypt,35 and Italy.36 The baseline characteristics of the studies are listed in Table 1.
Table 1.
The baseline characteristics of included studies
| Author | Animal | Wound | Bacteria | Photosensitiser | Light |
|---|---|---|---|---|---|
| Katayama et al32 | Male C57BL/ksj db/db mice | Excisional wounds | PA | 0.1% or 0.5% ALA; topical | 410 nm LED, 164.5 mW/cm2, 6 or 9 J/cm2 |
| Mai et al29 | Female BALB/c mice | Burn wounds | S. aureus or MRSA | 10, 5, or 2 μM DVDMS, injected subcutaneously, 75 min | 635 nm semiconductor laser, 50 J/cm2, 300 mW/cm2 |
| Chen et al30 | Sprague–Dawley rats | Excisional wounds | S. aureus | 1 mM ZnPc‐(Lys)5 or ZnPc‐S4, 25 μL, 30 min | 680 nm, 100 mW, 15 J/cm2, 5 min |
| Sahu et al25 | Female Swiss albino mice | Excisional wounds | PA | 200 μM pl‐cp6, topical, 30 min | (660 ± 25) nm red light, 100 mW/cm2, 60 J/cm2, 10 min |
| Sahu et al26 | Male and female diabetic swiss albino mice | Excisional wounds | MRSA | 200 μM pl‐cp6, 20 μL, topical, 30 min | (660 ± 25) nm red light, 100 mW/cm2, 60 or 120 J/cm2, 10 or 20 min |
| Topaloglu et al34 | Female Wistar albino rats | Abrasion wounds | MRSA | 500, 1000, 2000 μg/mL ICG, 50 μL, 30 min | 808 nm diode laser, 450 J/cm2, 15 min |
| Morimoto et al33 | Male C57BL/ksj db/db mice | Excisional wounds | MRSA | 50 or 200 mg/kg 5‐ALA, injected intraperitoneally | 410 nm LED, 164.5 mW/cm2, 10 or 50 J/cm2 |
| Fu et al31 | Male BALB/c mice | Excisional wounds | MRSA | 200 μM LAEtNBS or EtNBS‐COOH, 50 μL, topical, 90 min | (640 ± 10) nm LED, 90 J/cm2, 30 min |
| Sahu et al24 | Female Swiss albino mice | Excisional wounds | MRSA or PA | 100 or 200 μM pl‐cp6, 20 μL, topical, 30 min | (660 ± 25) nm, ~100 mW/cm2, 60 or 120 J/cm2, 10 or 20 min |
| Nafee et al35 | Female Wistar rat | Excisional wounds | S. sureus | 0.124 μM HY‐DMSO or HY‐NPs, 50 μL | 23.5 J/cm2 |
| Sahu et al28 | Female Swiss albino mice | Excisional wounds | PA | 200 μM pl‐cp6, 25 μL, topical, 30 min | (660 ± 25) nm red light, ~100 mW/cm2, 60 or 120 J/cm2, 10 or 20 min |
| Vecchio et al18 | Immunosuppressed female BALB/c mice | Abrasion wounds | MRSA | 75 μM RLP068/Cl or TBO, 40 μL, topical, 30 min | (690 ± 15) nm or (630 ± 15) nm noncoherent light, 100 mW/cm2, 84 J/cm2, 14 min |
| Simonetti et al36 | CD1 mice or BALB/c mice | Excisional wounds | MRSA or S. aureus | 0.01%, 0.1%, 0.3%, or 0.5% RLP068/Cl, 25 μL, topical, 1 h | 698 nm diode laser, 120 mW/cm2,60 J/cm2 |
| Dai et al19 | Immunosuppressed female BALB/c mice | Abrasion wounds | MRSA | 400 μM PEI‐ce6, 50 μL, topical | (660 ± 15) nm non‐coherent light, 100 mW/cm2, 360 J/cm2 |
| Dai et al20 | Female BALB/c mice | Burn | A. baumannii | PEI‐ce6, 50 μL, topical, 15 min | (660 ± 15) nm non‐coherent light, 100 mW/cm2, 240 J/cm2 |
| Demidova et al22 | Mice | Excisional wounds | E. coli or PA | pl‐ce6, 50 μL, topical, 30 min | 660 nm diode laser, 100 mW/cm2, 165 or 240 J/cm2 |
| Lambrechts et al21 | Male BALB/c mice | Burn | S. aureus | 500 μM PTMPP, 100 μL, topical and injected | (635 ± 15)nm, 84 mW/cm2, 211 or 423 J/cm2, 42 or 82 min |
| Hamblin et al23 | Male BALB/c | Excisional wounds | PA | 200 μM pl–ce6, 50 μL, topical, ≥30 min | 665 nm diode laser, 100 mW/cm2, 240 J/cm2 |
| Hamblin et al24 | Male BALB/c | Excisional wounds | E. coli | 100 μM pl–ce6, 50 μL, topical,30 min | 665 nm diode laser, 100 mW/cm2, 160 J/cm2, 27 min |
Abbreviations: A. baumaninii, Acinetobacter baumannii; ALA, aminolevulinic acid; DVDMS, sinoporphyrin sodium; E. coli, Escherichia coli; EtNBS‐COOH, 5‐(30 ‐Carboxypropylamino)‐9‐diethylaminobenzogurepheno‐thiazinium chloride; ICG, indocyanine green; LAEtNBS, b‐lactamase Activated EtNBS; HY‐DMSO, hypericin‐dimethyl sulfoxide; HY‐NPs, hypericin‐laden nanoparticles; LED, light‐emitting diode; MRSA, methicillin‐resistant Staphylococcus aureus; PA, Pseudomonas aeruginosa; PEI‐ ce6, polyethylenimine‐chlorin e6; pl‐ce6, poly‐L‐lysine‐chlorin(e6); pl‐cp6, poly‐L‐lysine conjugate of chlorine p6; PTMPP, 5‐Phenyl‐10,15,20‐tris(N‐methyl‐4‐pyridyl)porphyrin chloride; S. aureus, Staphylococcus aureus; TBO, toluidine blue O; ZnPc‐(Lys)5, pentalysine β‐carbonylphthalocyanine zinc; ZnPc‐S4, Zinc phthalocyanine tetrasulfonate.
3.3. Methodological characteristics of the included studies
The quality of each study was assessed by applying the risk‐of‐bias tool from SYRCLE. Of the 19 studies, 26.3% generated an adequate allocation sequence (item 1),29, 31, 34, 35, 36 89.5% reported the baseline characteristics of the PDT‐treated and untreated groups (including gender, age, and weight of the subjects) (item 2),18, 19, 20, 21, 23, 24, 25, 26, 27, 28, 29, 31, 32, 33, 34, 35, 36 5.3% adequately concealed allocation (item 3),35 36.8% reported that each animal was housed in a cage of its own in the animal room (item 4),19, 20, 27, 32, 33, 35, 36 73.7% addressed incomplete outcome data (item 8),18, 19, 20, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35 and all but one study23 (94.7%) were free of selective outcome reporting (item 9). None of the studies indicated whether the investigators were blinded (item 5), the outcome assessments were random (item 6), or the assessors were blinded (item 7). All of the studies were free of other sources of bias. Figure 2 presents a summary of the methodological quality of the 19 studies.
Figure 2.
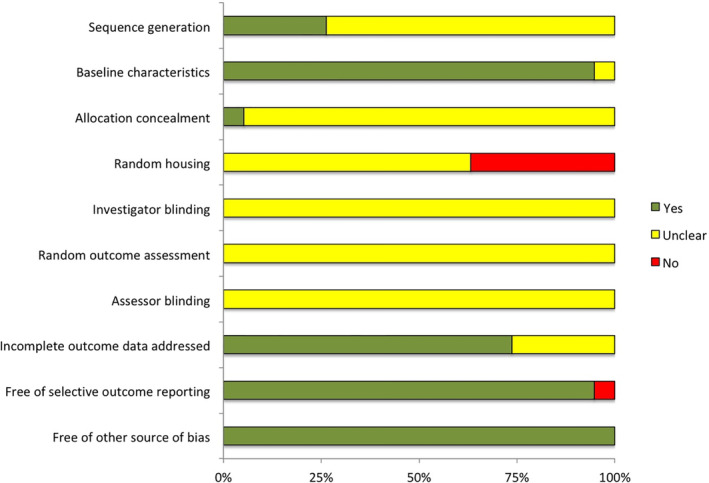
The methodological quality of included studies
3.4. Animal models
The animal species used in the 19 studies were mice (n = 16)18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 31, 32, 33, 36 and rats (n = 3).30, 34, 35 Of the 16 mouse studies, nine used BALB/c mice,18, 19, 20, 21, 23, 24, 29, 31, 36 4 utilised Swiss albino mice,25, 26, 27, 28 two used C57BL/ksj db/db mice,32, 33 and one used CD1 mice.36 One study22 did not report the mouse strain that was used. Notably, three of these studies used diabetic mice to produced chronic wound models, namely, the two studies using C57BL/ksj db/db mice32, 33 and one study using Streptozotocin‐injected Swiss albino mice.25 Two studies used immunosuppressed mice; the low peripheral blood neutrophils of these mice created an environment that was more vulnerable to infection.18, 19 Of the rat studies, two used Wistar rats34, 35 and one used Sprague‐Dawley rats.30
3.5. Wounds
All wounds were generated on the dorsum of the animals. They were excisional wounds,22, 23, 24, 25, 26, 27, 28, 30, 31, 32, 33, 35, 36 burn wounds,20, 21, 29 and abrasion wounds.18, 19, 34 The wound sizes ranged from 6 mm in diameter32 to 1.5 × 1.5 cm2.31
3.6. Bacteria
To investigate the antimicrobial effect of PDT, the wounds in all studies were inoculated with one or two species of bacteria. The majority were P. aeruginosa 22, 23, 26, 27, 28, 32 and methicillin‐resistant S. aureus.18, 19, 25, 27, 29, 31, 33, 34, 36 Others were unresistant S. aureus strains,21, 29, 30, 35, 36 Escherichia coli,22, 24 and Acinetobacter baumannii.20
3.7. Photosensitizers
The photosensitisers that are used for PDT can be classified biochemically as first‐, second‐, and third‐generation photosensitisers.37 The photosensitisers that were used in the studies were from the second generation with the exception of hypericin‐laden nanoparticles, which was used in the study by Nafee et al35 and which belong to the third‐generation photosensitiser class. The second‐generation photosensitiser that is most commonly used in recent times is 5‐aminolevulinic acid. It was used in two studies.32, 33 Other used photosensitisers were 5‐phenyl‐10; 15, 20‐tris (N‐methyl‐4‐pyridyl) porphyrin chloride (PTMPP)21; sinoporphyrin sodium (also known as DVDMS)29; indocyanine green (ICG)34; pentalysine β‐carbonylphthalocyanine zinc (ZnPc‐[Lys]5)30; zinc phthalocyanine tetrasulfonate (ZnPc‐S4)30; poly‐L‐lysine conjugate of chlorine p6 (pl‐cp6),25, 26, 27, 28 β‐lactamase‐activated EtNBS (LAEtNBS)31; RLP068/Cl,18, 36 toluidine blue O (TBO)18; polyethylenimine‐chlorin(e6) (PEI‐ce6)19, 20; and poly‐l‐lysine‐chlorin e6 (pL‐ce6).22, 23, 24
The photosensitisers were administered topically in 13 studies,18, 19, 20, 22, 23, 24, 25, 26, 27, 28, 31, 32, 36 by subcutaneous injection in one study,29 and by intraperitoneal injection in another study.33 In one study, burn wounds were simultaneously treated by both topical application and injection under the wound.21 The application route was not described in three studies.30, 34, 35 The incubation time ranged from 15 minutes20 to 90 minutes.31
3.8. Light
The light wavelengths that were used in most studies belonged to the red light range. Two studies used 410‐nm light‐emitting diodes (LEDs),32, 33, 38 one study used an 808‐nm diode laser,34 and one study did not mention the wavelength of the light source.35 When visible light was used, the fluence ranged from 6 32 to 450 J/cm2,34 and the energy density ranged from 8421 to 300 mW/cm2.29 For the 808‐nm diode laser, the fluence was 450 J/cm2.34
3.9. Treatment methods, outcome measures, and main results
The treatment methods, outcome measures, and the main results are listed in Table 2. The outcomes after PDT that were measured were wound size, bacterial numbers in the wound, wound cytokine levels, wound‐healing time, and body weight.
Table 2.
The treatment methods, outcome measures, and the main results of included studies
| Author | Treatment methods | Outcome measures | Main results | ||
|---|---|---|---|---|---|
| T | C | ||||
| Cb | Cu | ||||
| Katayama et al32 |
T1:0.1%ALA + 0.005% EDTA‐2Na + 9 J/cm2 (N = 5); T2:0.5%ALA+0.005% EDTA‐2Na + 9 J/cm2 (N = 5); T3:0.5%ALA+0.001% EDTA‐2Na + 9 J/cm2 (N = 5); T4:0.5%ALA+0.005% EDTA‐2Na + 6 J/cm2 (N = 5) |
Cb1:NT (N = 5); Cb2:piperacillin‐tazobactam (N = 5) | Cu:NT (N = 5) | 1, 2 | 1:T2 < T1 < Cb1, T2 < T4; T2 vs Cu, T3 vs Cb1 (P > .05); 2:T2 (2 log10 units) < Cb2 (7 log10 units), Cb1 vs Cb2 (P > .05) |
| Mai et al29 | T1:10 μMDVDMS + 50 J/cm2 (once per day, 2 days) (N = 8); T2:5 μMDVDMS + 50 J/cm2 (once per day, 2 days) (N = 8); T3: 2 μM DVDMS+50 J/cm2 (once per day, 2 days) (N = 8) | Cb: 0.1mlPBS (N = 8) | 2, 3, 4(a), 5, 6, 7, 8, 9, 20 | 2:T < Cb; 3:T > Cb, highest in T1 on Day 7; 4(a): T < Cb, lowest in T1 on Day 3; 5: T < Cb, lowest in T1; 6: T > Cb, increased gradually; 7: T > Cb, highest in T1; 8: T > C on Day7, 14 and 21; 9: T < C on Day 7, 14, and 21; 20. T1 vs T2 vs T3 vs Cb (P > .05) | |
| Chen et al30 | T1:1μMZnPc‐(Lys)5 + 15 J/cm2 ; T2: ZnPc‐S4 + 15 J/cm2 | Cb1:1μMZnPc‐ (Lys)5 ; Cb2: ZnPc‐S4 | 1, 2, 10 | 1: smallest in T1; 2: lowest in T1; 10: T1 (1.4‐fold) < Cb1 (2.2‐fold) < T2/Cb2 (2.8‐fold) | |
| Sahu et al25 | T: 200 μM pl‐cp6 + 60 J/cm2 | Cb: NT | Cu: NT | 2, 3, 4(b), 4(c), 4(d), 11, 12(a), 12(b), 13 | 2: T < Cb; 3: T > Cb; 4(b), 4(c), 4(d): Cb > Cu, T < Cb on Day 5; 11: Cb > Cu, T < Cb on Day 2; T < Cb, Cb > Cu (2‐fold) on Day 5; 12(a): T > Cb > Cu on Day 2; Cb > T, T vs Cu (P > .05) on Day 5; 12(b): Cb > Cu, 12(b) < 12(a) on Day 2; 13: Cb < Cu (1.5‐fold on Day 2 and 4‐fold on Day 5), T > Cb |
| Sahu et al26 | T: 20 μL 200 μM pl‐cp6 + 60 J/cm2 (once per day, 3 days) | Cb1: NT; Cb2: AgNO3; Cb3: AG | Cu1: 20 μl 200 μM pl‐cp6 + 60 J/cm2 on Day 2; Cu2: 20 μl 200 μM pl‐cp6 + 120 J/cm2 on Day 2 | 6(b), 12(c), 12(d), 12(e), 14, 15 | 6(b): increased in Cu1, decreased in Cu2, increased in T by 1.75‐fold, lower in Cb2 and Cb3 than T; 12(c), 12(d), 12(e), 15: T < Cb2 < Cb3, Cb2 > Cb1; 14: increased in Cu1 by 1.5‐fold, decreased in Cu2 by 1.5‐fold, increased in T, Cb1 vs Cb2 (P > .05) |
| Topaloglu et al34 | T1‐1: 500 μg/mL ICG + 450 J/cm2 (N ≥ 8) T1‐2: 500 μg/mL ICG + 450 J/cm2 (N = 5); T2: 1000 μg/mL ICG + 450 J/cm2 (N ≥ 8); T3: 2000 μg/mL ICG + 450 J/cm2 (N ≥ 8) | Cb1:NT (N ≥ 8); Cb2:450 J/cm2 (N ≥ 8); Cb3: ICG (N ≥ 8); Cb4: mupirocin (N = 5) | 1, 2, | 1: T1‐2 < Cb4; 2: reduced 90% in T1‐1/T3/T4, T1‐1 vs T2 vs T3, Cb2 vs Cb1 (P > .05) | |
| Morimoto et al33 | T1: 50 mg/kg 5‐ALA+50 J/cm2 (N = 5); T2‐1: 200 mg/kg 5‐ALA+50 J/cm2 (N = 5); T2‐2: 200 mg/kg 5‐ALA+50 J/cm2 (N = 3); T3: 200 mg/kg 5‐ALA+10 J/cm2 (N = 5) | Cb1‐1:NT (N = 5); Cb1‐2: NT (N = 3); Cb2: 50 J/cm2 (N = 5); Cb3: 200 mg/kg 5‐ALA (N = 5); Cb4‐1: VCM (N = 5); Cb4‐2: VCM (N = 3) | Cu: NT (N = 5) | 1, 2 | 1: Cb1‐1 > Cu, Cb2 > T1 > T2‐1, T3 > T2‐1, Cb4‐1 > Cu; T2‐1 vs Cu, Cb3 vs Cb1‐1, Cb4‐1 vs Cb1‐1(P > .05) 2: T2‐2 (2 log10 units of reduction) < Cb1‐2 on Day 7 |
| Fu et al34 | T1: 200 μM LAEtNBS+90 J/cm2; T2: 200 μM EtBS‐COOH+90 J/cm2 | Cb: PBS + 90 J/cm2 | 1, 2, 16 | 1, 2: T1/T2 < Cb; T1 vs T2 (P > .05); 16: T1 (14.8 ± 1.5)/T2 (14.5 ± 1.7) < Cb (21.0 ± 2.3), T1 vs T2 (P > .05) | |
| Sahu et al24 | T1: pl‐cp6 + 60 J/cm2 (MRSA); T2: pl‐cp6 + 120 J/cm2 (MRSA); T3: pl‐cp6 + 60 J/cm2 (PA) | Cb1: NT(MRSA); Cb2: pl‐cp6 (MRSA); Cb3: NT(PA) | Cu | 8, 17(a), 17(b) | 8:T1/T2 > Cb1/Cb2 (3‐fold), Cb1/Cb2 < Cu on Day 18; 17(a), 17(b): Cb3 > Cu,Cb3 > T3 |
| Nafee et al35 | T1: HY‐DMSO+23.5 J/cm2 (once per day, 5 days) (N = 4); T2: HY‐NPs + 23.5 J/cm2 (once per day, 5 days) (N = 4) | Cb: NT (N = 4) | 1, 2, 5, 6(a), 18, 19 | 1: T2 < T1 < Cb on Day 10; 2: T2 < T1 < Cb; 5: T2 < T1 6(a), 18, 19: T1/T2 > Cb | |
| Sahu et al28 | T1: 200 μM pl‐cp6 + 60 J/cm2 (N = 6); T2: 200 μM pl‐cp6 + 120 J/cm2 (N = 6) | Cb1: NT (N = 6); Cb2: 200 μM pl‐cp6 (N = 6) | Cu (N = 6) | 1, 2, 4(a), 5, 16 | 1: Cb1 > Cu on Day 2 and decreased gradually; T1/T2 reduced continuously, significant different till Day 10; 2: Cb1/Cb2 increased by 30% at 24 h and decreased by 80% at 72 h, T1/T2 reduced by 1.5/2.0 log at 24 h, T1/T2 < Cb1/Cb2 at 240 h; 4(a), 5: Cb1 > Cu (1.9 times and 3 times higher, separately), T1/T2 < Cb1 at 24 and 96 h (4–5 times lower); 16: T1/T2 < Cb1 (4–5 days lesser) |
| Vecchio et al18 | T1:75 μM PLP068/Cl + 84 J/cm2 (N = 6); T2:75 μM TBO + 84 J/cm2 (N = 6) | Cb1 (N = 6); Cb2: 84 J/cm2 (N = 6); Cb3: 75 μM PLP068/Cl (N = 6); Cb4: 75 μM TBO (N = 6) | 1, 2 | 1: T1 (22%) < T2 (76%) < Cb3 (78%) < Cb4 (89%) < Cb2 (98%) < Cb1 (100%) on Day 4; 2: T1 decreased 2.9 log unit, while Cb3 decreased 0.85 log unit, T2 decreased 1.0 log unit, while Cb4 negligible reduced; T1 was the lowest | |
| Simonetti et al36 | T1: 0.01% PLP68/Cl + 60 J/cm2 (N = 6); T2: 0.1% PLP68/Cl + 60 J/cm2 (N = 6); T3‐1: 0.3% PLP68/Cl + 60 J/cm2 (N = 6); T3‐2: 0.3% PLP68/Cl + 60 J/cm2 (N = 12); T4: 0.5% PLP68/Cl + 60 J/cm2 (N = 6) | Cb1: NT (N = 12); Cb2‐1: placebo gel (N = 6); Cb2‐2: placebo gel (N = 12); Cb3: teicoplanin(N = 12) | 2 | 2: Day 2: RLP068 / Cl gave a dose‐related reduction, T1 (no change) > T2(83% reduction) > T3‐1, T2 (3.3 × 106 ± 4.0 × 106 CFU/mL) < Cb1(1.0 × 109 ± 9.6 × 108 CFU/mL), T3‐1 vs T4, Cb1 vs Cb2‐2 Day 9: Cb1 (2.1 × 109 ± 2.5 × 109 CFU/mL)/Cb2‐2 (9.5 × 108 ± 8.0 × 108 CFU/mL) > Cb3 (4.7 × 107 ± 3.9 × 107 CFU/mL) > T3‐2 (2.6 × 107 ± 2.0 × 107 CFU/mL), Cb1 vs Cb2‐2(P > .05) | |
| Dai et al19 | T: 400μMPEI‐ce6 + 360 J/cm2 (N = 10) | Cb: NT (N = 12) | 2, 16, 20 | 2: T reduced 2.7 log10 reduction, T1 < Cb(1.3 log10 lower); 16: T (5.6 ± 5.1) < Cb (14.2 ± 2.6); 20: T(6.1 ± 3.4%) < Cb (11.6 ± 4.0%) on Day 2 | |
| Dai et al20 | T1: PEI‐ce6 + 240 J/cm2 1 day after infection; T2: PEI‐ce6 + 240 J/cm2 on Day 0 (N = 7); T3: PEI‐ce6 + 240 J/cm2 on Day 1 (N = 11); T4: PEI‐ce6 + 240 J/cm2 on Day 2 (N = 6); T5: PEI‐ce6 + 240 J/cm2 on Day 1 and Day 2(N = 9) | Cb1: NT; Cb2: PEI‐ce6; Cb3: PBS + 240 J/cm2 | 2 | 2: T1 reduced 1.8 log unit, Cb2 reduced less than 0.9 log unit, Cb1 (1.02 × 106RLU) > T1(2.73 × 106RLU); T2 decreased 3.6 log units in a light exposure‐dependent manner, T3/T4 decreased 1.7 long unit, T5 decreased 1.7/2.7 log unit on Day1/2 | |
| Demidova et al22 | T: 200 μM pl‐ce6 + 240 J/cm2 (N = 10) | Cb1: NT (N = 10); Cb2: 200 μM pl‐ce6 (N = 10) Cb3: 240 J/cm2 (N = 10) | 2 | 2: T < Cb2 < Cb1, Cb1 vs Cb3 (P > .05) | |
| Lambrechts et al21 | T1: 500 μM PTMPP+ PBS + 211 J/cm2 (N = 3); T2‐1: 500 μM PTMPP+25% DMSO/PBS + 211 J/cm2 (N = 5); T2‐2: 500 μM PTMPP+25% DMSO/PBS + 211 J/cm2 (N = 3); T3: 500 μM PTMPP+25% DMSO/PBS + 423 J/cm2 (N = 4); T4: 500 μM PTMPP+25% DMSO/PBS + 211 J/cm2 + AgSD (N = 3); T5: 500 μM PTMPP+25% DMSO/PBS +211/423 J/cm2) (N = 5) | Cb1: NT (N = 3); Cb2: 500 μM PTMPP (N = 3); Cb3: light (N = 3); Cb4: AgSD (N = 3) | Cu (N = 3) | 1, 2, 16, 21 | 1: T5 < Cb2 on Day 18, T5 < Cb1 on Day 12, 16, and 18, T5 < Cb4 on Day 12 and 18; Cb2 < Cb1 on Day 6 and 14; Cb2 < Cb4 on Day 6, Cb2 < T4 from Day 8 to 16; Cb1 > Cb3 on Day 12, 14 and 16; Cb3 < Cb4 on Day 6, Cb3 < T4 on Day 12, 16 and 18; Cb2 < T4 on Day 12 2: T1 (70% reduction) > T2 (2 log10 unit reduction); Cb1 < Cb3 on Day 12, Cb1 > Cb2 on Day 2, T3 < Cb4/T4 on Day 3; 16: Cb4 (19 ± 4.6) < Cb3 (27.7 ± 2.9), Cb4 < T5 (28.8 ± 3.6), Cb1 (22.7 ± 1.2) < T5, Cb2 (22.7 ± 2.3) < T5, T4 (26.7 ± 2.3); 21: T2‐2 vs T3, Cb4 (13.0 ± 5.57 days) vs T2‐1/T2‐2 (18.5 ± 5.65) vs T4 (21.3 ± 4.16) (P > .05) |
| Hamblin et al23 | T1: 200 μM pl‐ce6 + 240 J/cm2 (N = 10); T2: 200 μM pl‐ce6 + 240 J/cm2 (half PA) (N = 6) | Cb1: NT (N = 10); Cb2: 240 J/cm2 (N = 10); Cb3: 200 μM pl‐ce6 (N = 10); Cb4: AgNO3 (N = 10); Cb5: AgNO3 (half PA) (N = 6) | Cu1: NT (N = 6); Cu2: 200 μM pl‐ce6 + 240 J/cm2 (N = 6); Cu3: AgNO3(N = 6) | 1, 2 | 1: T1 < Cb4, T2 < Cb5; Cu3 vs Cu2, Cu3 vs Cu1 (P > .05) 2: T1 produced a fluence‐dependent loss of luminescence, T1 < Cb3 < Cb1/Cb2, T1 < Cb4; Cb1 vs Cb2 (P > .05) |
| Hamblin et al24 | T: 100 μM pl‐ce6 + 160 J/cm2 (N = 6) | C1‐1: NT (N = 6); C1‐2: 100 μM pl–ce6 (N = 6); C1‐3: 160 J/cm2 (N = 6) | 1, 2 | 1: T was the smallest (P > .05); 2: T was the lowest and showed a semilogarithmic light dose‐dependent reduction | |
Abbreviations: AG, aminoguanidine; AgNO3, silver nitrate; AgSD, silver sulfadiazine;C, control group; Cb, bacteria‐infected group; Cu, un‐infected group; NT, no treatment; T, treatment group; VCM,vancomycin; 1, wound size; 2, bacteria measurement; 3, β‐fibroblast growth factor (FGF); 4(a), Interleukin (IL)‐6; 4(b),IL‐1α; 4(c), IL‐1β; 4(d), IL‐2; 5, tumour necrosis factor (TNF)‐α; 6(a), vascular endothelial growth factor (VEGF); 6(b), VEGF‐A; 7, transforming growth factor (TGF)‐β1; 8, hydroxyproline (Hyp); 9, malondialdehyde (MDA); 10, blood flow; 11, toll‐like receptor (TLR‐4); 12(a), nuclear factor kappa B (NF‐κB)‐p50; 12(b), NF‐κB‐p105; 12(c), phospho‐IKB‐α, 12(d), NF‐κB p65; 12(e), phospho‐NF‐κB p‐65; 13, alkaline phosphatase (ALP); 14, nitric oxide (NO); 15, phospho‐p38 MAPK; 16, wound‐healing time; 17(a), metalloproteinase (MMP)‐8; 17(b), MMP‐9; 18, platelet‐derived growth factor (PDGF); 19, cyclooxygenase (COX)‐2; 20, body weight; 21. infection time.
3.10. Wound size
Eleven studies examined the effect of PDT on infected wounds at various times after treatment.18, 21, 23, 24, 28, 30, 31, 32, 33, 34, 35 As expected, several studies showed that bacterial infections delayed wound healing: as a result, the untreated infected wounds had significantly greater wound sizes at various assessment time points than the uninfected wounds.28, 33 With regard to the effect of PDT on wound size, 1 of the 11 studies showed that PDT did not differ from various controls in terms of reducing wound size.24 However, the remaining 10 studies all found that the PDT‐treated bacterially infected wounds were significantly smaller at various time points than the infected wounds that had not been treated18, 21, 28, 32, 35 or had been treated with photosensitiser only18, 21, 30, 31, 33 or light only.18
In one study, PDT was combined with topical 1% silver sulfadiazine cream and compared with the cream on its own: the combination treatment performed significantly worse in terms of reducing wound size than the cream.21 In two other studies, topical 0.5% AgNO3 23 or 2% mupirocin34 on their own served as positive controls for PDT: in both cases, PDT reduced wound size better than these drugs.
Two studies showed that PDT reduced infected ulcer size so effectively that it was not only significantly smaller than the size of untreated bacteria‐infected ulcers, but it was similar to the size of uninfected control wounds at most time points.32, 33 Both studies also showed that altering PDT parameters affected efficacy in terms of wound size: PDT with higher photosensitiser concentrations or light fluences reduced infected ulcer size better than when these parameters were set at lower levels.32, 33 Moreover, Nafee et al. showed that, when a second‐generation photosensitiser (hypericin) was loaded onto nanoparticles (thus creating a third‐generation photosensitiser preparation), it yielded smaller wound sizes than when the wound was treated with the same concentration of the photosensitiser diluted in dimethyl sulfoxide (DMSO).35
3.11. Bacterial measurements
Seventeen studies measured the effect of PDT on wound bacteria counts or bioluminescence.18, 19, 20, 21, 22, 23, 24, 26, 28, 29, 30, 31, 32, 33, 34, 35, 36 All showed that PDT reduced the infection. Specifically, the PDT‐treated infected wounds had significantly (P < .05) lower bacterial counts at various time points than infected control wounds that had not been treated19, 20, 22, 23, 24, 26, 28, 29, 35, 36 or had been treated with photosensitiser only18, 22, 23, 24, 28, 30, 34 or light only.23, 24, 31, 34
In several studies, topical or injected antibacterial drugs served as positive controls for PDT: in all cases, PDT performed similarly or better in terms of reducing bacterial levels.23, 32, 33, 36 In one study, PDT + silver sulfadiazine cream was not as effective as the cream alone in terms of reducing the bacterial infection.21
Several studies examined the effect of altering PDT parameters on bacterial counts. One study showed that PDT with 0.01% of the photosensitiser RLP68/Cl did not reduce bacterial counts, but PDT with 0.1% or 0.3% RLP68/Cl reduced it by ≥83% (P < .05). PDT with 0.5% RLP68/Cl did not reduce the counts further.36 However, another study showed that PDT with the photosensitiser ICG at concentrations of 500, 1000, and 2000 μg/mL reduced the bacterial viability equally well (P > .05).34 Furthermore, Nafee et al. showed that hypericin‐loaded nanoparticles (a third‐generation photosynthesiser preparation) lowered the bacterial counts better (P < .05) than the same concentration of hypericin diluted in DMSO.35
3.12. Cytokine levels
Six studies evaluated the effect of PDT on the levels of various cytokines in infected wounds.25, 26, 27, 28, 29, 35 They showed that, compared with no treatment, PDT significantly increased the wound levels of β‐fibroblast growth factor,26, 29 transforming growth factor (TGF)‐β1,29 vascular endothelial growth factor (VEGF),29, 35 VEGF‐A,25 hydroxyproline,27, 29 alkaline phosphatase,26 fibroblast growth factor‐2,26 nitric oxide,25 platelet‐derived growth factor,35 and cyclooxygenase‐235 while significantly (P < .05) decreasing the wound levels of interleukin (IL)‐6,28, 29 IL‐1α,26 IL‐1ß,26 IL‐2,26 malondialdehyde,29 toll‐like receptor‐4,26 nuclear factor kappa B (NF‐κB)‐p65,25 phosphorylated NF‐κB‐p65,25 phosphorylated‐IKB‐α,25 phosphorylated p38 mitogen‐activated protein kinase,25 metalloproteinase‐8 and ‐9,27 and tumour necrosis factor‐α.28, 29 Nafee et al. showed that that, when the photosynthesiser hypericin was loaded onto nanoparticles, it reduced TNF‐α expression more markedly (P < .05) than when the wound was treated with the same concentration of hypericin diluted in DMSO.35
3.13. Wound‐healing time
Four studies compared PDT‐treated and untreated infected wounds in terms of wound‐healing time.19, 21, 28, 31 Three showed that PDT significantly accelerated wound healing.19, 28, 31 In contrast, Lambrechts et al. showed that PDT increased the wound‐healing time of infected wounds compared with no treatment and photosensitiser alone. Notably, light by itself tended to slow infected wound healing as well, albeit not as much as PDT. It was suggested that the slow infected wound‐healing time of PDT reflected damage caused by the light treatment, which involved high radiation energy (84 mW/cm2, 42/82 minutes).21
We were able to perform a meta‐analysis of the studies by Lambrechts et al.21 and Dai et al.19 because the data were available. We had to use the random‐effects model because the I 2 value was 88% and the χ² P value was .003. There was no significant difference between the two studies in terms of wound‐healing time (SMD = −0.29 [95% CI −4.13, 3.54], P = .88).
3.14. Body weight
Two studies examined the effect of PDT on the body weight of mice with infected wounds.19, 29 Dai et al. found that, over the first 4 days of infection, the untreated control mice exhibited a rapid loss of weight that troughed on day 2. The PDT‐treated mice exhibited a similar pattern, but the weight loss was significantly less. For example, on day 2, the mean body weights of the PDT‐treated and untreated mice were 11.6 ± 4.0% and 6.1 ± 3.4%, respectively (P = .0009).19 In contrast, Mai et al. found that mice with untreated infected wounds exhibited progressive increases in body weight over the first 5 days. Moreover, the body weight of mice with PDT‐treated infected wounds exhibited similar patterns, regardless of the concentration of the photosensitiser. This suggests that PDT does not have any observable side effects.29
4. DISCUSSION
Physiological wound healing is a process that repairs damaged tissues. It involves three somewhat overlapping sequential phases, namely, the inflammatory, proliferative, and remodelling phases. Many cells, molecules, and biochemical events are involved in this process.39, 40 During the inflammatory phase, immune cells in the wound bed (eg, neutrophils and macrophages) release a variety of growth factors and chemokines to remove contaminating microbes.41 During the proliferative phase, fibroblasts proliferate in the wound bed, and keratinocytes migrate inward from wound margins. Both cell types release a variety of cytokines that promote reepithelisation and angiogenesis. Finally, during the remodelling phase, collagen is deposited, and water is reabsorbed. This increases the strength of the scar and reduces its thickness.42
If any step of this physiological repair process is hampered, or aberrant activities occur, wound healing can be delayed. This can lead to the formation of chronic wounds.9, 43 Many factors contribute to delayed wound healing, including diabetes mellitus, vascular insufficiency, local pressure, protease deregulation, reduced growth factor activity, inflammation, and concurrent infection.44 Infections with bacteria have a particularly deleterious effect on wound healing, especially when the wound already exhibits delayed wound healing.45 The four most common bacteria in wounds are Enterobacteriaceae family members, Enterococcus species, P. aeruginosa, and Staphylococcus species.46 Mixed infections with these bacteria are often observed. Bacterial infections slow down wound healing because they prolong the inflammatory phase47 and produce virulence factors, such as enterotoxins, haemolysins, matrix metalloproteinases, and hyaluronidase, that overcome host defences, promote bacterial proliferation, and aggravate local tissue destruction.48 The studies included in our systematic review used one or more of the bacterial species listed above to create wound infections. In all cases, PDT decreased the bacterial counts in the wound.18, 19, 20, 21, 22, 23, 24, 26, 28, 29, 30, 31, 32, 33, 34, 35, 36 This effect was particularly notable when the photosensitiser was loaded onto nanoparticle carriers.35 Thus, PDT has marked microbicidal effects on bacterial infections in wounds.
The photosensitiser is one of the three essential elements in PDT. These molecules are classified as first‐, second‐, and third‐generation photosensitisers. The first‐generation photosensitisers include hematoporphyrin derivatives and photofrin II and are somewhat effective in some tumours.49 However, these photosensitisers have low cell selectivity and high cutaneous phototoxicity. These limitations led to the development of the second‐generation photosensitisers, which have high photosensitivity, a narrow absorption spectrum, and good tissue selectivity. The structure of most second‐generation photosensitisers are based on porphyrin and include benzoporphyrins, purpurins, phthalocyanines, chlorines, and protoporphyrin‐IX. Recently, the third‐generation photosensitisers were generated by gene engineering or nanotechnology to further improve PDT outcomes. All but one of the studies included in our systemic review used second‐generation photosensitisers. All of these studies showed that PDT accelerated the closure and healing of bacteria‐infected wounds. The remaining study by Nafee et al. used a third‐generation photosensitiser, namely, hypericin loaded onto nanoparticles. They showed that it killed wound bacteria and promoted the closure and healing of the infected wounds significantly better than the second‐generation formulation of the photosensitiser (hypericin diluted in DMSO).35
After incubation, pro‐drug photosensitisers are converted into protoporphyrin‐IX. The absorption spectra of protoporphyrin‐IX include a maximal peak at 410 nm (Soret band) and four smaller peaks (Q bands) between 500 and 630 nm.50 Several kinds of lasers51, 52 and LEDs12, 53 have been used to excite the photosensitiser in PDT. Lasers can emit monochromatic light with high fluency and target lesions more precisely, thereby inducing little damage to adjacent tissues. LEDs are made of electronic components and provide a narrower spectrum of light irradiation. Compared with lasers, LEDs are smaller, cheaper, and easier to operate and have a larger irradiation field. All of the studies that were included in this systematic review used lasers or LEDs as the light source. The majority of the light wavelengths were in the range of the absorption peaks of protoporphyrin‐IX.
Several studies have shown that PDT can change the immune status of the target tissue.54, 55 First, it has a significant impact on neutrophil activation: after PDT, neutrophils gather in the target lesions. This reaction is due in part to upregulated TNF‐α expression after PDT.56 PDT also induces the release by various cells of pro‐inflammatory cytokines, which activate other cells of the innate immune system57 and induce monocytes, macrophages, and mast cells to accumulate in the PDT‐treated lesion. This in turn activates CD8+ T cells and eliminates damaged cells and tissues.58, 59 One of the most important cytokines in wound healing is TGF‐β. It participates in the entire wound‐healing process. Specifically, it promotes the epithelial‐mesenchymal transition and induces keratinocytes to migrate from the wound margins towards the centre. It also stimulates the chemotaxis of fibroblasts in the wound bed, promotes their synthesis of collagen, and induces them to differentiate into myofibroblasts.55, 60 The studies included in this systematic review showed that PDT of infected wounds increased their β‐fibroblast growth factor, TGF‐β1, and VEGF levels while decreasing their IL‐6, IL‐1α, IL‐1β, IL‐2, malondialdehyde, toll‐like receptor‐4, NF‐κB, and TNF‐α. Thus, PDT effectively alters the inflammatory environment of infected wounds; these changes may directly or indirectly increase bacterial killing and promote wound closure.
Several human studies have shown that PDT is excellent at accelerating uninfected wound healing.55, 59 Our systematic review suggests that this effect may be due in part to the antimicrobial activity of PDT, which may prevent infections from taking hold and preventing wound closure. It also shows that PDT is effective in even heavily infected wounds. In addition, PDT is non‐invasive and safe61 because the photosensitiser is absorbed selectively by the proliferating target cells or tissues. Indeed, it has been shown that, after a photosensitiser is applied to an infected lesion, the resulting protoporphyrin‐IX accumulates specifically in the bacteria; other parts of the lesion have low levels of this molecule.33 The safety of PDT is also shown by the fact that PDT does not influence the body weight of animal models with infected wounds. Indeed, PDT exerted protective effects on the body weight of mice with P. aureus‐infected wounds.19, 29
5. CONCLUSIONS
In conclusion, this systematic review demonstrated that PDT could accelerate the healing of bacteria‐infected wounds in animals. However, there were some limitations in this study. First, the language was limited to English, and only two databases were included. This may have caused us to overlook other relevant publications. Second, some of the included studies were of low quality and/or did not describe the details of the treatment. Third, all included studies were in vivo experiments with mice and rats. Additional high‐quality studies that examine the antimicrobial effect of PDT on skin wound healing in other species (including humans) are warranted.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
ACKNOWLEDGEMENT
This work was supported by the National Key Research and Development Program of China (No.2016YFC0901504) and the 111 Project (D18011).
Sun Y, Ogawa R, Xiao B‐H, et al. Antimicrobial photodynamic therapy in skin wound healing: A systematic review of animal studies. Int Wound J. 2020;17:285–299. 10.1111/iwj.13269
Funding information 111 Project, Grant/Award Number: D18011; National Key Research and Development Program of China, Grant/Award Number: 2016YFC0901504
Contributor Information
Yan Wu, Email: jlwuyan@126.com.
Liang‐Hong Chen, Email: skill002@163.com.
REFERENCES
- 1. Lindholm C, Searle R. Wound management for the 21st century: combining effectiveness and efficiency. Int Wound J. 2016;13:5‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Posnett J, Gottrup F, Lundgren H, Saal G. The resource impact of wounds on health‐care providers in Europe. J Wound Care. 2009;18(4):154‐161. [DOI] [PubMed] [Google Scholar]
- 3. Guest JF, Ayoub N, Mcilwraith T, et al. Health economic burden that wounds impose on the National Health Service in the UK. BMJ Open. 2015;5(12):e009283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kardas P, Devine S, Golembesky A, Roberts C. A systematic review and meta‐analysis of misuse of antibiotic therapies in the community. Int J Antimicrob ag. 2005;26(2):106‐113. [DOI] [PubMed] [Google Scholar]
- 5. Duque APD, Pinto NDC, Mendes RD, et al. In vivo wound healing activity of gels containing Cecropia pachystachya leaves. J Pharm Pharmacol. 2016;68(1):128‐138. [DOI] [PubMed] [Google Scholar]
- 6. Pariser DM, Eichenfield LF, Bukhalo M, Waterman G, Jarratt M, Grp PS. Photodynamic therapy with methyl aminolaevulinate 80 mg g(−1) for severe facial acne vulgaris: a randomized vehicle‐controlled study. Brit J Dermatol. 2016;174(4):770‐777. [DOI] [PubMed] [Google Scholar]
- 7. Jerjes W, Hamdoon Z, Hopper C. Photodynamic therapy in the management of basal cell carcinoma: retrospective evaluation of outcome. Photodiagnosis Photodyn Ther. 2017;19:22‐27. [DOI] [PubMed] [Google Scholar]
- 8. Ding C, Ma YY, Wang P, Liu J. Multiple Bowen's diseases and basal cell carcinomas in a patient with acute promyelocytic leukemia treated with arsenic trioxide: a case report and effective treatment with photodynamic therapy. Dermatol Ther. 2018;31(6):e12718. [DOI] [PubMed] [Google Scholar]
- 9. Morton LM, Phillips TJ. Wound healing and treating wounds: differential diagnosis and evaluation of chronic wounds. J Am Acad Dermatol. 2016;74(4):589‐605. [DOI] [PubMed] [Google Scholar]
- 10. Ozog DM, Rkein AM, Fabi SG, et al. Photodynamic therapy: a clinical consensus guide. Dermatol Surg. 2016;42(7):804‐827. [DOI] [PubMed] [Google Scholar]
- 11. Garrier J, Bezdetnaya L, Barlier C, Grafe S, Guillemin F, D'Hallewin MA. Foslip (R)‐based photodynamic therapy as a means to improve wound healing. Photodiagn Photodyn. 2011;8(4):321‐327. [DOI] [PubMed] [Google Scholar]
- 12. Rosa LP, da Silva FC, Vieira RL, et al. Application of photodynamic therapy, laser therapy, and a cellulose membrane for calcaneal pressure ulcer treatment in a diabetic patient: a case report. Photodiagnosis Photodyn Ther. 2017;19:235‐238. [DOI] [PubMed] [Google Scholar]
- 13. Mahmoudi H, Pourhajibagher M, Alikhani MY, Bahador A. The effect of antimicrobial photodynamic therapy on the expression of biofilm associated genes in Staphylococcus aureus strains isolated from wound infections in burn patients. Photodiagnosis Photodyn Ther. 2019;25:406‐413. [DOI] [PubMed] [Google Scholar]
- 14. Mosti G, Picerni P, Licau M, Mattaliano V. Photodynamic therapy in infected venous and mixed leg ulcers: a pilot experience. J Wound Care. 2018;27(12):816‐821. [DOI] [PubMed] [Google Scholar]
- 15. Nesi‐Reis V, Lera‐Nonose DSSL, Oyama J, et al. Contribution of photodynamic therapy in wound healing: a systematic review. Photodiagn Photodyn. 2018;21:294‐305. [DOI] [PubMed] [Google Scholar]
- 16. de Rob BM, Vries CRH, Langendam MW, et al. A protocol format for the preparation, registration and publication ofsystematic reviews of animal intervention studies. Evidence‐Based Preclinical Med. 2015;1(1):1‐9. [Google Scholar]
- 17. Hooijmans CR, Rovers MM, de Vries RB, Leenaars M, Ritskes‐Hoitinga M, Langendam MW. SYRCLE's risk of bias tool for animal studies. BMC Med Res Methodol. 2014;14:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vecchio D, Dai TH, Huang LY, Fantetti L, Roncucci G, Hamblin MR. Antimicrobial photodynamic therapy with RLP068 kills methicillin‐resistant Staphylococcus aureus and improves wound healing in a mouse model of infected skin abrasion. J Biophotonics. 2013;6(9):733‐742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dai TH, Tegos GP, Zhiyentayev T, Mylonakis E, Hamblin MR. Photodynamic therapy for methicillin‐resistant Staphylococcus aureus infection in a mouse skin abrasion model. Laser Surg Med. 2010;42(1):38‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dai TH, Tegos GP, Lu ZS, et al. Photodynamic therapy for Acinetobacter baumannii burn infections in mice. Antimicrob Agents Ch. 2009;53(9):3929‐3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lambrechts SAG, Demidova TN, Aalders MCG, Hasan T, Hamblin MR. Photodynamic therapy for Staphylococcus aureus infected burn wounds in mice. Photoch Photobio Sci. 2005;4(7):503‐509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Demidova TN, Gad F, Zahra T, Francis KP, Hamblin MR. Monitoring photodynamic therapy of localized infections by bioluminescence imaging of genetically engineered bacteria. J Photoch Photobio B. 2005;81(1):15‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hamblin MR, Zahra T, Contag CH, McManus AT, Hasan T. Optical monitoring and treatment of potentially lethal wound infections in vivo. J Infect Dis. 2003;187(11):1717‐1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hamblin MR, O'Donnell DA, Murthy N, Contag CH, Hasan T. Rapid control of wound infections by targeted photodynamic therapy monitored by in vivo bioluminescence imaging. Photochem Photobiol. 2002;75(1):51‐57. [DOI] [PubMed] [Google Scholar]
- 25. Sahu K, Sharma M, Dube A, Gupta PK. Topical antimicrobial photodynamic therapy improves angiogenesis in wounds of diabetic mice. Laser Med Sci. 2015;30(7):1923‐1929. [DOI] [PubMed] [Google Scholar]
- 26. Sahu K, Sharma M, Gupta PK. Modulation of inflammatory response of wounds by antimicrobial photodynamic therapy. Laser Ther. 2015;24(3):201‐208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sahu K, Sharma M, Sharma P, et al. Effect of poly‐L‐lysine‐chlorin P6‐mediated antimicrobial photodynamic treatment on collagen restoration in bacteria‐infected wounds. Photomed Laser Surg. 2014;32(1):23‐29. [DOI] [PubMed] [Google Scholar]
- 28. Sahu K, Sharma M, Bansal H, Dube A, Gupta PK. Topical photodynamic treatment with poly‐L‐lysine‐chlorin p6 conjugate improves wound healing by reducing hyperinflammatory response in Pseudomonas aeruginosa‐infected wounds of mice. Lasers Med Sci. 2013;28(2):465‐471. [DOI] [PubMed] [Google Scholar]
- 29. Mai B, Gao Y, Li M, et al. Photodynamic antimicrobial chemotherapy for Staphylococcus aureus and multidrug‐resistant bacterial burn infection in vitro and in vivo. Int J Nanomedicine. 2017;12:5915‐5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chen Z, Zhang YX, Wang D, et al. Photodynamic antimicrobial chemotherapy using zinc phthalocyanine derivatives in treatment of bacterial skin infection. J Biomed Opt. 2016;21(1):18001. [DOI] [PubMed] [Google Scholar]
- 31. Fu XJ, Zhu YQ, Peng YB, et al. Enzyme activated photodynamic therapy for methicillin‐resistant Staphylococcus aureus infection both inv itro and in vivo. J Photoch Photobio B. 2014;136:72‐80. [DOI] [PubMed] [Google Scholar]
- 32. Katayama B, Ozawa T, Morimoto K, et al. Enhanced sterilization and healing of cutaneous pseudomonas infection using 5‐aminolevulinic acid as a photosensitizer with 410‐nm LED light. J Dermatol Sci. 2018;90(3):323‐331. [DOI] [PubMed] [Google Scholar]
- 33. Morimoto K, Ozawa T, Awazu K, et al. Photodynamic therapy using systemic administration of 5‐Aminolevulinic acid and a 410‐nm wavelength light‐emitting diode for methicillin‐resistant Staphylococcus aureus‐infected ulcers in mice. Plos One. 2014;9(8):e105173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Topaloglu N, Guney M, Yuksel S, Gulsoy M. Antibacterial photodynamic therapy with 808‐nm laser and indocyanine green on abrasion wound models. J Biomed Opt. 2015;20(2):28003. [DOI] [PubMed] [Google Scholar]
- 35. Nafee N, Youssef A, El‐Gowelli H, Asem H, Kandil S. Antibiotic‐free nanotherapeutics: hypericin nanoparticles thereof for improved in vitro and in vivo antimicrobial photodynamic therapy and wound healing. Int J Pharm. 2013;454(1):249‐258. [DOI] [PubMed] [Google Scholar]
- 36. Simonetti O, Cirioni O, Orlando F, et al. Effectiveness of antimicrobial photodynamic therapy with a single treatment of RLP068/cl in an experimental model of Staphylococcus aureus wound infection. Brit J Dermatol. 2011;164(5):987‐995. [DOI] [PubMed] [Google Scholar]
- 37. Kou J, Dou D, Yang L. Porphyrin photosensitizers in photodynamic therapy and its applications. Oncotarget. 2017;8(46):81591‐81603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Alam M, Voravutinon N, Warycha M, et al. Comparative effectiveness of nonpurpuragenic 595‐nm pulsed dye laser and microsecond 1064‐nm neodymium:yttrium‐aluminum‐garnet laser for treatment of diffuse facial erythema: a double‐blind randomized controlled trial. J Am Acad Dermatol. 2013;69(3):438‐443. [DOI] [PubMed] [Google Scholar]
- 39. Kasuya A, Tokura Y. Attempts to accelerate wound healing. J Dermatol Sci. 2014;76(3):169‐172. [DOI] [PubMed] [Google Scholar]
- 40. Rahim K, Saleha S, Zhu X, Huo L, Basit A, Franco OL. Bacterial contribution in chronicity of wounds. Microb Ecol. 2017;73(3):710‐721. [DOI] [PubMed] [Google Scholar]
- 41. Eming SA, Martin P, Tomic‐Canic M. Wound repair and regeneration: mechanisms, signaling, and translation. Sci Transl Med. 2014;6(265):265sr6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lindley LE, Stojadinovic O, Pastar I, Tomic‐Canic M. Biology and biomarkers for wound healing. Plast Reconstr Surg. 2016;138(3 Suppl):18S‐28S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lazarus GS, Cooper DM, Knighton DR, et al. Definitions and guidelines for assessment of wounds and evaluation of healing. Arch Dermatol. 1994;130(4):489‐493. [PubMed] [Google Scholar]
- 44. Stojadinovic O, Pastar I, Nusbaum AG, Vukelic S, Krzyzanowska A, Tomic‐Canic M. Deregulation of epidermal stem cell niche contributes to pathogenesis of nonhealing venous ulcers. Wound Repair Regen. 2014;22(2):220‐227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gompelman M, van Asten SA, Peters EJ. Update on the role of infection and biofilms in wound healing: pathophysiology and treatment. Plast Reconstr Surg. 2016;138(3 Suppl):61S‐70S. [DOI] [PubMed] [Google Scholar]
- 46. Nolff MC, Reese S, Fehr M, Dening R, Meyer‐Lindenberg A. Assessment of wound bio‐burden and prevalence of multi‐drug resistant bacteria during open wound management. J Small Anim Pract. 2016;57(5):255‐259. [DOI] [PubMed] [Google Scholar]
- 47. Wolcott RD, Rhoads DD, Dowd SE. Biofilms and chronic wound inflammation. J Wound Care. 2008;17(8):333‐341. [DOI] [PubMed] [Google Scholar]
- 48. Serra R, Grande R, Butrico L, et al. Chronic wound infections: the role of Pseudomonas aeruginosa and Staphylococcus aureus . Expert Rev Anti Infect Ther. 2015;13(5):605‐613. [DOI] [PubMed] [Google Scholar]
- 49. Gao H, Shi L, Yin H, et al. Evaluation of the effect of photodynamic therapy with hematoporphyrin monomethyl ether on VX2 tumors implanted in the rectal submucosa of rabbits. J Photochem Photobiol B. 2016;163:162‐169. [DOI] [PubMed] [Google Scholar]
- 50. Pottier RH, Chow YF, LaPlante JP, Truscott TG, Kennedy JC, Beiner LA. Non‐invasive technique for obtaining fluorescence excitation and emission spectra in vivo. Photochem Photobiol. 1986;44(5):679‐687. [DOI] [PubMed] [Google Scholar]
- 51. Ricatto LG, Conrado LA, Turssi CP, Franca FM, Basting RT, Amaral FL. Comparative evaluation of photodynamic therapy using LASER or light emitting diode on cariogenic bacteria: an in vitro study. Eur J Dent. 2014;8(4):509‐514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Azizi A, Shohrati P, Goudarzi M, Lawaf S, Rahimi A. Comparison of the effect of photodynamic therapy with curcumin and methylene blue on Streptococcus mutans bacterial colonies. Photodiagnosis Photodyn Ther. 2019;27:203‐209. [DOI] [PubMed] [Google Scholar]
- 53. Sun Y, Chen L, Zhang Y, Gao X, Wu Y, Chen H. Topical photodynamic therapy with 5‐aminolevulinic acid in Chinese patients with rosacea. J Cosmet Laser Ther. 2018;21(4):196‐200. [DOI] [PubMed] [Google Scholar]
- 54. Reginato E, Wolf P, Hamblin MR. Immune response after photodynamic therapy increases anti‐cancer and anti‐bacterial effects. World J Immunol. 2014;4(1):1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Grandi V, Bacci S, Corsi A, et al. ALA‐PDT exerts beneficial effects on chronic venous ulcers by inducing changes in inflammatory microenvironment, especially through increased TGF‐beta release: a pilot clinical and translational study. Photodiagnosis Photodyn Ther. 2018;21:252‐256. [DOI] [PubMed] [Google Scholar]
- 56. Anzengruber F, Avci P, de Freitasa LF, Hamblin MR. T‐cell mediated anti‐tumor immunity after photodynamic therapy: why does it not always work and how can we improve it? Photoch Photobio Sci. 2015;14(8):1492‐1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Gollnick SO, Evans SS, Baumann H, et al. Role of cytokines in photodynamic therapy‐induced local and systemic inflammation. Br J Cancer. 2003;88(11):1772‐1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Corsi A, Lecci PP, Bacci S, Cappugi P, Pimpinelli N. Early activation of fibroblasts during PDT treatment in leg ulcers. G Ital Dermatol Venereol. 2016;151(3):223‐229. [PubMed] [Google Scholar]
- 59. Agostinis P, Berg K, Cengel KA, et al. Photodynamic therapy of cancer: an update. CA Cancer J Clin. 2011;61(4):250‐281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Weber CE, Li NY, Wai PY, Kuo PC. Epithelial‐mesenchymal transition, TGF‐beta, and osteopontin in wound healing and tissue remodeling after injury. J Burn Care Res. 2012;33(3):311‐318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Nestor MS, Berman B, Patel J, Lawson A. Safety and efficacy of Aminolevulinic acid 10% topical gel versus Aminolevulinic acid 20% topical solution followed by blue‐light photodynamic therapy for the treatment of actinic keratosis on the face and scalp: a randomized. Double‐Blind Study J Clin Aesthet Dermatol. 2019;12(3):32‐38. [PMC free article] [PubMed] [Google Scholar]


