Abstract
The objective was to systematically review monetary data related to management of incontinence‐associated dermatitis (IAD) in an adult population. Six electronic databases were searched: MEDLINE, CINAHL, Web of Science, EMBASE, The Cochrane Library and EconLit. The search string combined index terms and text words related to IAD and monetary data. The quality of the articles was assessed using the consensus on Health Economic Criteria. Results were synthesised narratively because of methodological heterogeneity. Nine studies were included. Only direct medical costs were reported. The product cost per application for prevention ranged between $0.05 and $0.52, and for treatment between $0.20 and $0.35. The product cost per patient/day for prevention ranged between $0.23 and $20.17. The product cost of IAD prevention and treatment per patient/day ranged between $0.57 and $1.08. The cost to treat IAD did not consider the treatment of secondary infection. The calculation of labour cost and total cost differed considerably between studies. Summarising monetary data is a challenge because of heterogeneity in currencies, settings, samples, time horizons, health‐ and cost outcome valuation, IAD definition and measurements, and included costs. Procedures for health economic evaluations are to be clarified to guarantee valid interpretation and comparison with other studies.
Keywords: health economic evaluation, incontinence‐associated dermatitis, monetary data, prevention, systematic review
1. INTRODUCTION
1.1. Background
1.1.1. Incontinence‐associated dermatitis
Incontinence‐associated dermatitis (IAD) is an irritant contact dermatitis resulting from prolonged contact of the skin with urine and/or faeces. 1 IAD is clinically observed as erythema and edema of the skin, sometimes accompanied by bullae with serous exudate, erosion, or secondary cutaneous infection. IAD falls under the umbrella term of moisture‐associated skin damage (MASD). Other types of MASD are intertriginous dermatitis (ITD), peri‐wound skin damage, and peri‐stomal MASD. 2 IAD is an underreported health condition. 3 , 4 Depending on the type of setting and population studied, prevalence ranges between 7.6% and 45.7%. 5 , 6 , 7 , 8 IAD is not only a painful condition, it also increases the susceptibility to develop secondary skin infections (such as candidiasis and Pseudomonas aeruginosa), and deep wounds such as pressure ulcers. 9 , 10 Skin assessment, cleansing, restoring, and protection of the barrier function are key strategies to prevent and treat IAD. Skin care products for IAD prevention/intervention can be divided into cleansers, moisturisers, and skin protectants or a combination of those products. 9 , 11
1.1.2. Health economic evaluations
Consequences of IAD such as pressure ulcer development, fungal infections and extra caregiver time needed for skin care, impose a financial burden on organisations and individuals. Economic and budget limitations, such as nurse scarcities and a high staff turnover, 12 , 13 urge policy‐makers to decide how to wisely spend appropriate resources for maximum benefit. As a result of the increased costs associated with IAD, insight in the monetary data related to the prevention and treatment regimens to best improve the condition is essential. 14 A distinction can be made between full economic and partial economic evaluations. Full economic evaluations (cost–benefit analysis, cost‐utility analysis, cost‐effectiveness analysis, and cost‐minimization analysis) compare both costs and consequences (effectiveness; benefits) of two or more interventions. Partial evaluations (costs analysis, cost‐outcome description, cost description, outcome description, and cost‐of‐illness studies) consider costs and/or consequences, but do not involve a comparison between alternative interventions or do not relate costs to benefits. Only full economic evaluations can help answer questions related to efficiency, however, partial evaluations can be useful because they can deliver elements of information for a full evaluation. 15 , 16
Health economic evaluations need to be clear about the included costs; if it is direct‐ or indirect costs, medical‐ or non‐medical costs and how they are collected, to guarantee valid interpretation and comparison with other studies. 16 In economic evaluations, costs refer to the (monetary) value of anything that has to be sacrificed in order to acquire something. In health economics, costs usually reflect the expenditure of the healthcare system on resources such as interventions, products, staff time, and other consumables. The expenditures are quantified in monetary terms, such as product cost and labour cost. 17 Total costs include all costs incurred in the production of a set quantity of service. Which cost components are incorporated depends on the objectives and context of the evaluation. 18 Key considerations are the economic perspective (societal, institutional, insurer, or patient), reflecting who is paying for the costs and the time horizon (the duration over which health outcomes and costs are calculated). 19 , 20 The chosen time horizon is determined by the nature of the disease, the intervention under consideration, and the purpose of the analysis. Chronic conditions with a long‐term treatment and follow‐up require longer time horizons opposed to acute conditions requiring a shorter time horizon. 16
Knowledge about the financial impact related to management of IAD can inform clinicians, individuals, and policy‐makers to make decisions regarding the correct allocation of scarce healthcare resources and funding; and can help health care workers focus on the timely implementation of a cost‐effective preventative/treatment protocol to enhance patient quality care. 20 , 21 A systematic review on monetary data related to the management of incontinence‐associated dermatitis is however lacking.
1.1.3. Aim
To systematically review monetary data related to management of IAD in an adult population.
2. METHODS
2.1. Design
A systematic review was conducted using the Cochrane Collaboration guidelines for systematic reviews of interventions integrating economic evidence (The Cochrane Collaboration, 2019). A review protocol was registered in the PROSPERO database (number CRD42020163167).
2.2. Search methods for identification of studies
A four‐step search strategy was applied to identify relevant literature. Six electronic databases were systematically searched: MEDLINE (using the PubMed interface), Cumulative Index to Nursing and Allied Health Literature (CINAHL) (using the EBSCO interface), Web of Science, EMBASE (using the Embase.com interface), The Cochrane Library and EconLit (using the EBSCO interface). Thereafter, conference proceedings of the annual European Pressure Ulcer Advisory Panel (EPUAP) meetings (2003–2018) and the European Wound Management Association (EWMA) meetings (2001–2018) were screened. Next, ClinicalTrials.gov and WHO International Clinical Trials Registry Platform (ICTRP) were searched. Lastly, the reference lists of all included trials and other relevant literature were screened to identify additional papers.
The search string included a combination of index terms and text words using Boolean operators and was optimised with the support of a librarian technician. The search filter for cost was based on the search filter of Neyt and Chalon (2013) and was adapted to each database. 22 When appropriate Medical Subject Headings or comparable alternatives were used. The search strategy for MEDLINE (using the PubMed interface) is represented in Table 1. Retrieved records were imported in Endnote and duplicates were removed using the duplicate search function and by manually reviewing the list.
TABLE 1.
The search strategy for MEDLINE (using the PubMed interface)
| Concept | Line | Search strategy |
|---|---|---|
| Incontinence‐associated dermatitis | 1 | “diaper rash”[MeSH] OR “diaper dermatitis”[TIAB] OR “diaper erythema”[TIAB] OR “diaper rash*”[TIAB] OR “diaper wetness”[TIAB] OR “napkin dermatitis”[TIAB] OR “napkin erythema”[TIAB] OR “napkin rash*”[TIAB] OR “napkin wetness”[TIAB] OR “nappy dermatitis”[TIAB] OR “nappy erythema”[TIAB] OR “nappy rash*”[TIAB] OR “nappy wetness”[TIAB] OR “perineal dermatitis”[TIAB] OR “perineal erythema”[TIAB] OR “perineal rash*”[TIAB] OR “perineal wetness”[TIAB] OR “incontinence‐associated dermatitis”[TIAB] OR “irritant contact dermatitis”[TIAB] OR “contact dermatitis”[TIAB] OR “incontinence dermatitis”[TIAB] OR “incontinenceassociated dermatitis”[TIAB] OR “IAD”[TIAB] OR “irritant dermatitis”[TIAB] OR “incontinence skin”[TIAB] OR “incontinence lesion*”[TIAB] OR “moisture lesion*”[TIAB] OR “moisture skin*”[TIAB] OR “moisture‐associated skin damage”[TIAB] OR “MASD”[TIAB] OR “moisture maceration injur*”[TIAB] OR “intertrigo”[TIAB] OR “heat rash”[TIAB] |
| Health economics | 2 |
“Economics”[MeSH] OR “cost analysis”[TIAB] OR “costs analyses”[TIAB] OR “COI”[TIAB] OR “cost–benefit analysis”[TIAB] OR “cost allocation”[TIAB] OR “cost control”[TIAB] OR “cost compar*”[TIAB] OR “cost sharing”[TIAB] OR “cost of illness”[TIAB] OR “cost effect*”[TIAB] OR “hospital cost*”[TIAB] OR “health care cost*”[TIAB] OR “cost*”[TIAB] OR “health economic*”[TIAB] OR “economic*”[TIAB] OR “hospital economic*”[TIAB] OR “economic advantage*”[TIAB] OR “nursing economic*”[TIAB] OR “economic impact*”[TIAB] OR “costing”[TIAB] OR “medical care cost*”[TIAB] OR “treatment cost*”[TIAB] OR “direct service cost*”[TIAB] OR “cost measure*”[TIAB] OR “health expenditure*”[TIAB] OR “financing”[TIAB] OR “public expenditure*”[TIAB] OR “health Insurance*”[TIAB] OR “price*”[TIAB] OR “pricing”[TIAB] OR “budget*”[TIAB] OR “insurance”[TIAB] OR “financial management”[TIAB] OR “economic analysis”[TIAB] OR “economic analyses”[TIAB] OR “affordabilit*” [TIAB] |
| Filter/search block | 3 | None. |
| Combination of concepts | 4 | #1 AND #2 |
Abbreviations: MeSH, medical subject headings; TIAB, title and abstract.
* = Truncation symbol, representing any group of characters, including no character.
2.3. Study eligibility
Title and abstract of all identified records were independently screened for eligibility by two researchers using Rayyan. 23 Disagreements were discussed until consensus was reached. Studies were included if the following criteria were met: (a) targeting an adult population, in any healthcare setting, with IAD (defined as erythema and edema of the skin, sometimes accompanied by bullae with serous exudate, erosion, or secondary cutaneous infection) or at risk for developing IAD; (b) providing monetary data of at least the direct medical cost of IAD prevention or treatment; (c) retrieved from the original research, or health economic modelling using data from international literature. There were no restrictions with respect to language, date of publication or study setting. Papers were excluded if (a) they only discussed other wounds and not IAD; (b) if they exclusively reported on caregiver time; (c) if they reported about interventions and products not being applied in health care.
2.4. Data extraction
A data extraction table was developed including author details, year of publication, intervention, prevention and/or treatment focus, country and currency, fiscal year of data collection, sample size, IAD classification, type of health economic study (cost‐effectiveness analysis, cost‐utility analysis, cost–benefit analysis, outcome description; cost description; cost outcome description; effectiveness evaluation; cost analysis), time horizon, economic perspective (societal, institution, patient, or insurance perspective), type of cost (direct medical, non‐direct medical, direct non‐medical, or non‐direct non‐medical), cost‐related measurements, outcomes, and converted costs. Data extraction was completed independently by two reviewers. Disagreements were discussed until consensus was reached.
2.5. Quality assessment
Included articles were assessed for methodical quality using the Consensus Health Economic Criteria (CHEC‐) list; a 19‐point checklist to assess economic evaluation studies. The CHEC‐list is suitable for systematic reviews including full economic evaluation studies and partial economic evaluation studies based on clinical trials (cohort studies, case–control studies, randomised controlled clinical trials). 24 A quality assurance was completed independently by a second reviewer. The level of agreement between both assessors was calculated using the Cohen's Kappa correlation. 25 Disagreements were discussed until consensus was reached. When necessary, a third reviewer was consulted. Because of the scope of this review, the methodological quality of the studies was no reason for exclusion.
2.6. Data collection and analysis
Monetary data were extracted from the included studies. Original data were converted to 2018 US$ using the Campbell and Cochrane Economics Methods Group (CCEMG)‐Evidence for Policy and Practice Information and Co‐ordinating Centre (EPPI‐centre)‐Converter. 26 If studies did not report the reference year of the data collection, the year before the article was published was used as reference. When studies did not report the country of data collection the USA and US$ were used to convert the original data. Because of methodological heterogeneity, results were synthesised narratively, and no meta‐analysis was performed.
3. RESULTS
In total, 1374 records were retrieved from systematic searches in databases (436 in PubMed, 69 in CINAHL, 223 Web of Science, 349 EMBASE, 2 in Econlit and 295 in the Cochrane Library). Removal of duplicates resulted in 1025 potentially relevant records. Of these, 964 records were excluded after screening of title and abstract, and 52 records were assessed for full text screening. After identifying records through other sources, nine articles were included in the review. The PRISMA flowchart 27 is summarised in Figure 1.
FIGURE 1.
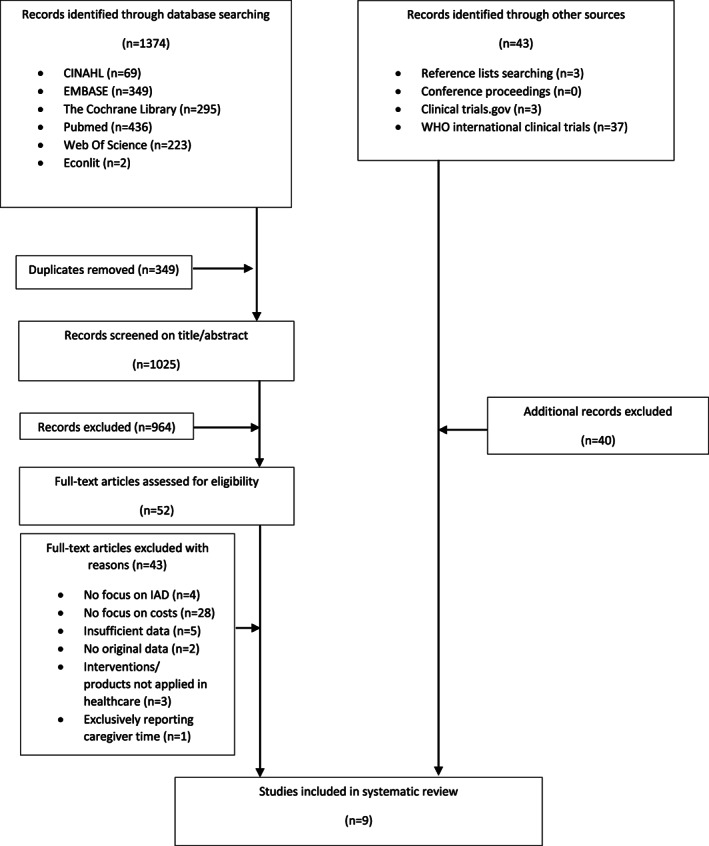
PRISMA flowchart
The level of agreement (Cohen's Kappa correlation) between the authors was 0.96. Disagreements were discussed until consensus was reached. An overview of the quality assessment is presented in Table 2. One article reported the chosen perspective. 28 In six studies, the time horizon was in agreement with the research question. Relevant costs related to the research question were reported in all studies. Expenditures were expressed in product costs, labour cost, and total cost. In one study, costs were not measured and valued appropriately in physical units. 29 Five studies used a validated scale to assess IAD. An incremental analysis was performed in one study, 30 and in none of the studies a sensitivity analysis was performed. Conclusions followed from the reported data in all studies. Generalizability of the study outcomes were discussed in eight studies.
TABLE 2.
Quality assessment
| Reference | Avşar et al. (2018) | Baatenburg de Jong et al. (2004) | Bale et al. (2004) | Brunner et al. (2012) | Bliss et al. (2007) | Byers et al. (1995) | Palese et al. (2011) | Warshaw et al. (2004) | Zehrer et al. (2004) |
|---|---|---|---|---|---|---|---|---|---|
| Study population clearly described | + | + | + | + | + | + | + | + | + |
| Competing alternatives clearly described | + | + | + | + | + | + | + | + | + |
| Well‐defined research question | + | + | + | + | + | + | + | + | + |
| Economic study design appropriate for stated objective | + | + | + | + | + | + | + | + | + |
| Chosen time horizon appropriate to include relevant costs and consequences | [+] | + | + | [+] | + | + | + | [+] | + |
| Actual perspective chosen appropriate | − | − | + | − | − | − | − | − | − |
| All important and relevant costs for each alternative identified | + | + | + | [+] | + | [+] | + | [+] | + |
| All costs measured appropriately in physical units | [+] | + | + | [+] | + | − | + | [+] | + |
| Costs valued appropriately | − | − | [+] | [+] | + | − | [+] | [+] | + |
| Important and relevant outcomes for each alternative | + | + | + | + | + | + | + | + | + |
| All health outcomes measured appropriately | + | [+] | [+] | [+] | [+] | [+] | [+] | + | [+] |
| All outcomes valued appropriately | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Incremental analysis of costs and outcomes alternatives performed | [+] | + | − | − | − | − | − | − | − |
| All future costs and outcomes discounted appropriately | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| All important variables whose values are uncertain, appropriately subjected to sensitivity analysis | − | − | − | − | − | − | − | − | − |
| Conclusions follow from the data reported | + | + | + | + | + | + | + | + | + |
| Does the study discuss generalizability of the results to other settings and patient/groups | + | + | − | + | + | [+] | [+] | [+] | [+] |
| Articles indicates there is no potential conflict of interest of study researcher and funder | − | + | − | + | + | + | + | + | + |
| Ethical and distributional issues discussed appropriately | + | − | + | + | + | − | − | + | + |
Note: +: present, [+]: partly present, NA: not applicable, −: absent.
3.1. Study characteristics
The included studies focused on IAD prevention (n = 7) 28 , 29 , 31 , 32 , 33 , 34 , 35 , IAD treatment (n = 1) 36 or both (n = 1) 30 . Studies were performed in nursing homes (n = 6) 28 , 29 , 30 , 32 , 33 , 34 , long‐term care facilities (n = 1) 36 , and acute and intensive care (n = 2) 31 , 35 . Countries of study were the United States of America (USA) (n = 5) 29 , 32 , 34 , 35 , 36 , Turkey (n = 1) 31 , Italy (n = 1) 33 , the Netherlands (n = 1) 30 , and the United Kingdom (UK) (n = 1) 28 . All studies used different skin assessment tools, of which five were validated. Sample sizes varied between 10 and 981. Time horizons varied between 4 days and 7 months. Only Bale et al. (2004) specified the economic perspective (societal perspective including the National Health Services). A full economic study design was performed in all studies. The main study characteristics are provided in Table 3.
TABLE 3.
Study characteristics
| Reference | Country currency fiscal year of data collection | Setting sample | P/T | IAD classification | Health economic study | Time horizon | Economic perspective |
|---|---|---|---|---|---|---|---|
| Avşar et al. (2018) |
Turkey US $ 2015 |
ICU N = 154 CG = 77 IG = 77 |
P |
IAD Severity (IADS) instrument |
Full economic study: cost‐effective analysis |
CG: 8.85 days ±5.19 days IG: 8.71 days ±4.28 days |
NR |
| Baatenburg de Jong et al. (2004) |
The Netherlands Euro NR |
Nursing home N = 40 CG = 20 IG = 20 |
P/T | Skin condition assessment form(non‐validated) |
Full economic study: cost‐effective analysis |
14 days | NR |
| Bale et al. (2004) |
UK Pound (£) 2002 |
Nursing home N = 164 Pre‐intervention = 79 Post‐intervention = 85 |
P | EPUAP grading system |
Full economic study: cost‐effective analysis |
7 months | National Health Services and social cost (private cost + external cost) |
| Bliss et al. (2007) |
USA US $ NR |
Nursing home N = 981 Regimen W = NR Regimen X = NR Regimen Y=NR Regimen Z = NR |
P | Notepad with poster on IAD (non‐validated) |
Full economic study: cost‐effective analysis |
6 weeks or till IAD was confirmed |
NR |
|
Brunner et al. (2012) |
USA US $NR |
ACU CCU N = 64 Product A = 33 Product B = 31 |
P | Skin breakdown grading tool | Full economic study: cost‐effective analysis | Duration of hospital stay (average 4 to 5 days) | NR |
| Byers et al. (1995) |
USA US $ NR |
Nursing home N = 10 |
P |
AB Evaporimeter; Courage and Khazaka 900‐PC Skin pH Meter; Diastrom ETm210 Erythema Meter |
Full economic study: cost‐effective analysis | 15 weeks | NR |
| Palese et al. (2011) |
Italy Euro NR |
Nursing home N = 63 |
P | NR | Full economic study: cost‐effective analysis | 59 days | NR |
|
Warshaw et al. (2002) |
USA US $ NR |
Long‐term facility acute care and Skilled facility N = 19 Site one = 10 Site two = 9 |
T | The Perineal Assessment Tool (PAT) and Erythema Grading Scale | Full economic study: cost‐effective analysis | 7 days | NR |
| Zehrer et al. (2004) |
USA US $ 2003 |
Nursing home N = 250 CG 1 = 67 CG 2 = 38 IG 1 = 67 IG 2 = 78 |
p |
Four‐point scale: Normal skin, mild skin damage, moderate skin damage or severe skin damage |
Full economic study: cost‐effective analysis | 6 months | NR |
Abbreviations: ACU, acute care unit; CG, control group; ICU, intensive care unit; IG, intervention group; NR, not reported; P, prevention; T, treatment.
3.2. The cost of IAD prevention
3.2.1. Skin cleansing
Three interventions included skin cleansing. Two interventions were tested in a nursing home and one in an acute care setting. The cost of the interventions is provided in Table 4. The product cost for one intervention was reported. The use of liquid antiseptic soap (Chlorhexidine 4,0%) and water in an acute care unit (ACU) resulted in a product cost of $20.03 per patient per day. The labour cost for the intervention per patient per day was $57.51, and the total cost was $142.92. The labour cost included repositioning, mobilisation, changing bed sheets, skin care, and documentation. No costs per application were reported.
TABLE 4.
Cost of the interventions
| Intervention | P/T | Endpoint | Product | Labour | Total | |||
|---|---|---|---|---|---|---|---|---|
| Cost per application | Cost per patient/day | Cost per application | Cost per patient/day | Cost per application | Cost per patient/day | |||
| Skin cleansing | ||||||||
| Water and soap 29 | P | NR | NR | NR |
Faeces and urine = $3.62 Urine = $1.29 |
NR | NR | NR |
| Liquid antiseptic soap (Chlorhexidine 4,0%) and lukewarm water with the aid of a gauze to rub the skin twice daily and following a faecal and/or urinary episode 31 | P |
31.2% a (n = 24/77) |
NR | $20.01 | NR | $57.51 | NR | $142.92 |
| No‐rinse skin cleanser 29 | P | NR | NR | NR |
Faeces and urine = $0.85 Urine = 0.30 |
NR | NR | NR |
| Skin cleansing and protection | ||||||||
| Water and soap | ||||||||
| + protective cosmetic creams 28 | P | 25.32% b (n = 20/79) | NR | $2.36 | NR | NR | NR | NR |
| + moisture barrier 29 | P | NR | NR | NR | NR | NR | NR | NR |
| pre‐moistened soft, disposable, non‐woven cleaning wipes + barrier cream and/or spray if IAD diagnosis, skin cleansing min. 1x/day and after any faecal and/or urinary episode 31 | P | 10.4% a (n = 8/77) | NR | $20.17 | NR | $72.94 | NR | $114.58 |
| Pre‐moistened soft, disposable, non‐woven cleaning wipes, dry wipes or wash cloths with soap from the wall dispenser or a no‐rinse perineal skin cleanser + antifungal cream 36 | T | NR | $0.35 | NR | $0.48 | NR | NR | NR |
| No‐rinse incontinence cleanser | ||||||||
| + moisture barrier 29 | P | NR | NR | NR | NR | NR | NR | NR |
| + pre‐moistened soft, disposable, non‐woven cleaning wipes or wash cloths+ skin barrier paste 36 | T | NR | $0.28 | NR | $0.91 | NR | NR | NR |
| +durable moisture barrier cream/film28 | P | 4.71% b (n = 4/85) | NR | $4.73 | NR | NR | NR | NR |
| + film polymeric solution spray 35 | P | 22.6% b (n = 7/31) | NR | $7.41 | NR | NR | NR | NR |
| One‐step procedure | ||||||||
| Cleanser, moisturiser, barrier washcloth impregnated with 3% dimethicone 35 | P | 27.3% b (n = 9/33) | NR | $3.00 | NR | NR | NR | NR |
|
One‐step barrier lotion spray 36 |
T | 47% a (n = 9/19) | $0.20 | NR | $0.50 | NR | NR | NR |
| Skin protection | ||||||||
| Acrylate terpolymer‐based barrier film (spray) | ||||||||
| 3x/week 32 , 34 | P | 3.5% b (n = 34/981) | $0.05 | NR | $0.01 | NR | $0.06 | NR |
| P | 3.9% b (n = NR) | $0.52 | $0.23 | $0.28 | $0.16 | $0.35 | $0.35 | |
| 1x/daily 34 | P | 3% b (n = NR) | $0.52 | $0.52 | $0.28 | $0.28 | $0.80 | $0.80 |
| Application frequency in accordance with skin score (every 24, 48 or 72 hours) 30 | P/T | NR | NR | $0.57 | NR | $5.24 | NR | $5.81 |
| Ointment | ||||||||
| 43% petrolatum | ||||||||
| After each episode of incontinence 32 | P | 2.1% b (n = 20/981) | $0.27 | $1.62 | $0.20 | $1.20 | $0.49 | $2.94 |
| Applied if needed 34 | P | 2.6% b (n = NR) | $0.21 | $0.98 | $0.18 | $0.83 | $0.39 | $1.83 |
| 98% petrolatum | ||||||||
| After each episode of incontinence 32 | P |
4.0% b (n = 39/981) |
$0.26 | $1.56 | $0.20 | $1.20 | $0.42 | $2.52 |
| Applied if needed 34 | P | NR | $0.23 | $1.02 | $0.18 | $0.83 | $0.41 | $1.87 |
| Cream | ||||||||
| Cream with 12% zinc oxide +1% dimethicone, after each episode of incontinence 32 | P | 4.1% b (n = 40/981) | $0.31 | $1.86 | $0.21 | $1.26 | $0.48 | $2.88 |
| Oil | ||||||||
| Zinc oxide oil 30 | P/T | NR | NR | $1.08 | NR | $6.74 | NR | $7.87 |
| Incontinence material + skin care regime | ||||||||
|
Polymer diapers/pads + every morning no‐rinse emulsion cleanser + in case of IAD: zinc‐based cream + during the day: pre‐moistened soft, disposable, non‐woven cleaning wipes (urine leakage) or mousse‐based no‐rinse cleanser (mild faecal incontinence) or liquid soap + skin protectant (no IAD) or zinc oxide base cream (IAD) if significant episode of incontinence 33 |
P | 31.7% b (n = 20/63) | NR | $3.41 | NR | $5.93 | NR | NR |
| Polymer diapers/pads + skin care regime + advice provided by continence nurses 33 | P | 3.1% b (n = 2/63) | NR | $1.92 | NR | $3.16 | NR | NR |
IAD prevalence.
IAD incidence; P: prevention; T; treatment; NA: not applicable; NR: not reported.
In nursing homes, the labour cost for cleansing the skin in case of an episode of urine incontinence ranged between $0.30 (no‐rinse cleanser) and $1.29 (water and soap), while the labour cost for cleansing the skin in case of an episode of faeces and urine incontinence ranged between $0.85 (no‐rinse cleanser) and $3.62 (water and soap). For both interventions, no labour costs per patient per day were reported. No total costs were provided for the interventions tested in the nursing homes.
3.2.2. Skin cleansing and protection
Seven interventions included skin cleansing and skin protection. Six interventions, of which four were tested in a nursing home, included a two‐step procedure to cleanse and protect the skin. One intervention included a one‐step procedure and was tested in an ACU. The cost of the interventions is provided in Table 4.
The product cost per patient per day in an ACU ranged between $7.41 (no‐rinse cleanser followed by a film polymeric solution spray) and $20.17 (cleansing wipes followed by a barrier cream/spray) for a two‐step procedure, while a product cost per patient per day of $3.00 was reported when using an one‐step cleanser, moisturiser, barrier washcloth (with 3% dimethicone) in an ACU. In nursing homes, the product cost per patient per day using a two‐step procedure ranged between $2.36 (water and soap followed by a protective cosmetic cream [Drapolene, Nivea]) and $4.73 (no‐rinse cleanser followed by a durable moisture barrier cream). For none of the interventions, product costs per application were reported.
Labour and total cost were reported for one intervention. The use of cleaning wipes followed by a barrier cream/spray in an ACU resulted in a labour cost per patient per day of $72.94, and a total cost per patient per day of $114.48. Labour cost included repositioning, mobilisation, changing bed sheets, skin care, and documentation.
3.2.3. Skin protection
Eight interventions included skin protection and were tested in a nursing home. The cost of the interventions is provided in Table 4.
Skin protectants included an acrylate terpolymer‐based barrier film (spray) (n = 3), petrolatum ointments (n = 4), and a protectant cream with 12% zinc oxide and 1% dimethicone (n = 1). The product cost per application ranged between $0.05 (acrylate terpolymer‐based barrier film 3x/week) and $0.52 (acrylate terpolymer‐based barrier film 1x/daily). The product cost per patient per day ranged between $0.23 (acrylate terpolymer‐based barrier film 3x/week) and $1.86 (12% zinc oxide and 1% dimethicone cream per incontinent episode).
The labour cost per application ranged between $0.01 (acrylate terpolymer‐based barrier film 3x/week) and $0.28 (acrylate terpolymer‐based barrier film 1x/daily), and ranged per patient per day between $0.16 (acrylate terpolymer‐based barrier film 3x/week) and $1.26 (12% zinc oxide and 1% dimethicone cream). The total cost per application ranged between $0.06 (acrylate terpolymer‐based barrier film 3x/week) and $0.80 (acrylate terpolymer‐based barrier film 1x/daily), while the total cost per patient per day ranged between $0.35 (acrylate terpolymer‐based barrier film 3x/week) and $2.94 (43% petrolatum ointment).
Two interventions introduced a new incontinence material and a structured skin care program in nursing homes. The cost of the interventions is provided in Table 4.
A product cost per patient per day of $3.41 was reported for a prevention protocol using polymer incontinence material and a skin care regime (depending on the type of incontinence). The product cost per patient per day for the same protocol followed by the advice provided by continence nurses was $1.92. The labour cost per patient per day for the intervention—without the advice provided by continence nurses—was $5.93, while with the advice, the labour cost per patient per day was $3.16. Labour cost, included changing of incontinence material and skin care. Total costs were not reported.
3.3. Cost of treatment
3.3.1. Skin cleansing and protection
Three interventions included skin cleansing and protection and were performed in a long‐term care setting. Two interventions contained a two‐step procedure and one intervention contained a one‐step barrier lotion spray. The cost of the interventions is provided in Table 4.
The product cost per application for a two‐step procedure ranged between $0.28 (no‐rinse cleanser and pre‐moistened soft, disposable, non‐woven cleaning wipes followed by a skin barrier paste) and $0.35 (no‐rinse cleanser or pre‐moistened soft, disposable, non‐woven cleaning wipes followed by an antifungal cream). For a one‐step procedure using a one‐step barrier lotion spray, product cost per application of $0.20 was reported. No product costs per patient per day were provided.
For the two‐step procedures, labour cost per application ranged between $0.48 (no‐rinse cleanser or pre‐moistened soft, disposable, non‐woven cleaning wipes followed by an antifungal cream) and $0.91 (no‐rinse cleanser and pre‐moistened soft, disposable, non‐woven cleaning wipes followed by a skin barrier paste). The application of a one‐step barrier lotion spray resulted in a labour cost per application of $0.50. Labour cost included cleansing the perineal area and the time spent applying the protectant. Labour costs per patient per day were not reported. For none of the interventions a total cost was provided.
3.4. Cost of combined prevention and treatment
3.4.1. Skin protection
Two interventions focused on the application of a skin protectant and were tested in nursing homes (Table 4). The product cost per patient per day ranged between $0.57 (acrylate terpolymer‐based barrier film, application frequency in accordance with skin score) and $1.08 (zinc oxide oil). The labour cost per patient per day ranged between $5.24 (acrylate terpolymer‐based barrier film) and $6.74 (zinc oxide oil).
Labour cost included removing the protectant, washing the perianal area, and the time spent applying the protectant. The total cost per patient per day ranged between $5.81 (acrylate terpolymer‐based barrier film) and $7.87 (zinc oxide oil). No product, labour, and total costs per application were provided.
4. DISCUSSION
The aim was to systematically review monetary data related to management of incontinence‐associated dermatitis in an adult population. Skin damage because of urine and/or faecal exposure can be reduced by applying protocols focusing on incontinence management and the application of a structured skin care regimen (cleansing, protecting, and restoring the skin at risk). 9 Nine articles were included in this review. Only direct medical costs were reported. The product cost per application for prevention ranged between $0.05 and $0.52. The product cost per application for treatment ranged between $0.20 and $0.35. The product cost per patient/day for prevention ranged between $0.23 and $20.17. The product cost of IAD prevention and treatment per patient/day ranged between $0.57 and $1.08. The cost to treat IAD did not fully consider the systemic treatment of IAD‐associated infection (bacterial/fungal). The calculation of labour cost and total cost differed considerably between studies. Therefore, an interpretation of the labour and total cost of IAD prevention and/or treatment is challenging.
Variation in reported costs are related to methodological differences between studies, such as cost value (e.g. cost per patient per day vs cost per episode of incontinence), items included in the labour and total cost calculations, types of skin care products studied, and different health systems and economic contexts (USA vs UK vs Europe). For that reason, directly compare findings between studies is challenging.
Adequate education of nursing staff and the use of effective interventions are the first concern to prevent and treat IAD. The next step is to evaluate the associated costs because of the high prevalence of IAD and the substantial number of persons being at risk for developing IAD. Water and soap are non‐expensive resources for skin cleansing. However, a no‐rinse cleanser is less expensive because of low labour cost ($0.85 versus $3.62 per episode of faecal and urine incontinence). 29 , 32 For skin protection the results indicate that, for both product and labour cost, the acrylate terpolymer‐based barrier film applied 3 times a week could be a cost‐effective skin protectant to prevent and treat IAD. 30 , 32 , 34 In the treatment of IAD, a one‐step barrier lotion spray (combined product) appears to be less expensive in terms of product and labour cost, but the average time to skin breakdown was longer for a two‐step procedure, using a no‐rinse cleanser in combination with a barrier film. 35 , 36 This review demonstrated that skin care is an area of nursing practice where savings can be made by introducing a structured approach (including a structured skin care program, education, and nursing advice). 31
4.1. Limitations of the study
Because of the methodological heterogeneity of the extracted data, only a narrative synthesis was possible. Heterogeneity was found in country, currency, setting, sample, time horizon, health‐ and cost outcome valuation, measurement, and total cost explanations. It is important to note that every country has its own specific guidelines for performing economic evaluations and a lack of detailed and transparent reporting hindered the interpretation of the cost of IAD prevention and/or treatment. Most studies in the review were published before 2011 28 , 29 , 30 , 32 , 34 , 36 and the interventions and knowledge on IAD development may have changed. Therefore, the results of this review need to be interpreted with caution.
Despite only one study reported the economic perspective, 28 it is a key consideration in economic evaluations. Health economics recommend the inclusion of societal perspective in economic evaluations because of the consistency and comparability. 37 The societal perspective includes indirect non‐medical costs (eg, absenteeism) and minimises the potential bias of narrow perspectives. 16 , 38 Even though the economic impact of indirect costs on the social expenditures may be negligible for IAD, it could be valuable to include indirect medical costs (future costs related to other diseases) in future studies. 16
Pressure ulcer development and associated costs can be minimised by decreasing IAD as a risk factor. Even though not all people who develop IAD will develop a pressure ulcer, a systematic review and meta‐analysis conducted by Beeckman et al. (2014) indicated an association between the etiologic factors and the pathogenesis of IAD and the development of pressure ulcers. 39 , 40 The exposure to moisture and irritants changes the mechanical skin properties and underlying tissue by diminishing the crosslinks between the collagen in the dermis and by softening the stratum corneum. 41 Additionally, moisture increases the skin's coefficient of friction and increases the exposure of underlying blood vessels to the effects of pressure.
By including a more comprehensive and inclusive set of direct medical costs (cost of linen changes, skin cleansers and protectants, cost of labour, …) and taking into account indirect medical costs, future studies can provide a view on the total impact of IAD prevention and treatment. Another key consideration is the applied time horizon. Among the studies, time horizons varied between 4 days and 7 months and none of the studies used identical time horizons. Guidelines suggest that time horizons should cover a lifetime to include all health and cost outcomes. Shorter time horizons are appropriate if justified. 42 The time horizon of incontinence‐associated dermatitis cannot be compared with the time horizon of a chronic disease where long time horizons are appropriate. No research is available on what the ideal time horizon should be for IAD development and/or treatment; therefore, future trials can explore this concept.
This review highlighted the lack of a standardised, valid, method to measure and report costs. There was a considerable lack of detail when describing labour and total costs. It was challenging to identify which cost components were included in labour and total cost. Additionally, cost values (e.g. cost per application, cost per patient per day) were not consistent and only Byers et al. (1995) separated costs for urine and faecal incontinence. 29 The results of this review encourage to incorporate monetary data into a Core Outcome Set (COS). A COS is a consensus‐derived set of outcomes that includes the minimum that should be reported in all clinical trials in a specific disease or population. It encompasses a core domain set (defining what core outcomes should be measured) and a core measurement set (defining measurement/assessment instruments for each core domain). 36 Using identical outcomes across trials allow comparability of results, increases the value of evidence synthesis, and decreases the risk of outcome reporting bias. 43 By incorporating monetary data into a COS, data collection can be standardised and improved, which will aid the process determining the cost‐effectiveness of products and interventions.
This review emphasised the importance to include more health economic analyses in research related to the prevention and treatment of IAD. It is well documented that many countries have a challenge with nurse scarcities and a high staff turnover. 12 , 13 By using evidence‐based prevention/interventions to avoid skin breakdown, cost can be saved in nursing time, and also free up nursing time to tend to other patient‐related tasks. Furthermore, new technologies such as cyanoacrylate‐based formulations have been developed to serve as an advanced skin protectant. 44 Therefore, future studies should not only focus on the cost‐effectiveness of the current best practices, but also on comparing new technologies.
5. CONCLUSION
Prevention and treatment of IAD are associated with a significant cost. Summarising monetary data is a challenge because of heterogeneity in currencies, settings, samples, time horizons, health‐ and cost outcome valuation, IAD definition and measurements and which costs are included (product costs with/without labour costs). Only direct medical costs are reported. The cost to treat IAD does not fully consider the systemic treatment of IAD associated infection (bacterial/fungal). Procedures for health economic evaluations are to be clarified to guarantee valid interpretation and comparison with other studies.
CONFLICT OF INTEREST
All authors declare: all authors had financial support from Essity Hygiene and Health AB, Gothenburg for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work. The funder had no role in the design of the review, data collection, analyses, interpretation of the results, or the development and submission of the manuscript.
ACKNOWLEDGEMENTS
We thank Dr Nele Pauwels for her support in the literature search and Amber Werbrouck for providing advice regarding health economic studies. This work was financial supported by Essity Hygiene and Health AB, Gothenburg.
Raepsaet C, Fourie A, Van Hecke A, Verhaeghe S, Beeckman D. Management of incontinence‐associated dermatitis: A systematic review of monetary data. Int Wound J. 2021;18:79–94. 10.1111/iwj.13496
Funding information Essity Hygiene and Health AB, Gothenburg
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.World Health Organization. International classification of diseases for mortality and morbidity statistics 2018. https://icd.who.int/browse11/l-m/en (accessed 25 March 2020)
- 2. Gray M, Black JM, Baharestani MM, et al. Moisture‐associated skin damage: overview and pathophysiology. J Wound Ostomy Continence Nurs. 2011;38(3):233‐241. 10.1097/WON.0b013e318215f798 [DOI] [PubMed] [Google Scholar]
- 3. McNichol LL, Ayello EA, Phearman LA, et al. Incontinence‐associated dermatitis: state of the science and knowledge translation. Adv Skin Wound Care. 2018;31(11):502‐513. 10.1097/01.Asw.0000546234.12260.61 [DOI] [PubMed] [Google Scholar]
- 4. Campbell J, Cook JL, Doubrovsky A, Vann A, McNamara G, Coyer F. Exploring Incontinence‐Associated Dermatitis in a single centre intensive care unit: a longitudinal point prevalence survey. J Wound Ostomy Continence Nurs. 2019;46(5):401‐407. 10.1097/won.0000000000000571 [DOI] [PubMed] [Google Scholar]
- 5. Johansen E, Bakken LN, Duvaland E, et al. Incontinence‐associated dermatitis (IAD): prevalence and associated factors in 4 hospitals in southeast Norway. J Wound Ostomy Continence Nurs. 2018;45(6):527‐531. 10.1097/won.0000000000000480 [DOI] [PubMed] [Google Scholar]
- 6. Campbell JL, Coyer FM, Osborne SR. Incontinence‐associated dermatitis: a cross‐sectional prevalence study in the Australian acute care hospital setting. Int Wound J. 2016;13(3):403‐411. 10.1111/iwj.12322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gray M, Incontinence‐Associated Dermatitis GKK. Characteristics and relationship to pressure injury: a multisite epidemiologic analysis. J Wound Ostomy Continence Nurs. 2018;45(1):63‐67. 10.1097/won.0000000000000390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kayser SA, Phipps L, VanGilder CA, et al. Examining prevalence and risk factors of Incontinence‐associated dermatitis using the international pressure ulcer prevalence survey. J Wound Ostomy Continence Nurs. 2019;46(4):285‐290. 10.1097/won.0000000000000548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Beeckman D, et al. Proceedings of the Global IAD expert panel. Incontinence‐associated dermatitis: moving prevention forward Wounds Int (2015), pp. 1‐21. Available to download from: www.woundsinternational.com.
- 10. Junkin J, Selekof JL. Prevalence of incontinence and associated skin injury in the acute care inpatient. J Wound Ostomy Continence Nurs. 2007;34(3):260‐269. 10.1097/01.WON.0000270820.91694.1f [DOI] [PubMed] [Google Scholar]
- 11. Beeckman D, Van Damme N, Schoonhoven L, et al. Interventions for preventing and treating incontinence‐associated dermatitis in adults. Cochrane Database Syst Rev. 2016;11(11):Cd011627. 10.1002/14651858.CD011627.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bargagliotti LA. Work engagement in nursing: a concept analysis. J Adv Nurs. 2012;68(6):1414‐1428. 10.1111/j.1365-2648.2011.05859.x [DOI] [PubMed] [Google Scholar]
- 13. Simpson K, Simpson R. What do we know about our agency nurse population? A scoping review. Nurs Forum. 2019;54(4):492‐498. 10.1111/nuf.12361 [DOI] [PubMed] [Google Scholar]
- 14. Browning P, Beeckman D, White R, et al. Report of the proceedings of a UK skin safety advisory group. Br J Nurs. 2018;27(20):S34‐s40. 10.12968/bjon.2018.27.Sup20.S34 [DOI] [PubMed] [Google Scholar]
- 15. Wounds International . International consensus: Making the case for cost‐effective wound management. Wounds International 2013. Available to download from www.woundsinternational.com.
- 16. Annemans L. Health Economics for Non‐Economists Principles. Kalmthout: Pelckmans Pro; 2018. [Google Scholar]
- 17. Consortium YHE . Cost 2016. http://www.yhec.co.uk/glossary/cost/
- 18. (NICHSR) NICoHSRaHCT . Health Economics Information Resources: A Self‐Study Course 2020. https://www.nlm.nih.gov/nichsr/edu/healthecon/glossary.html
- 19. Al‐Gharibi KA, Sharstha S, Al‐Faras MA. Cost‐effectiveness of wound care: a concept analysis. Sultan Qaboos Univ Med J. 2018;18(4):e433‐e439. 10.18295/squmj.2018.18.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Carter MJ. Economic evaluations of guideline‐based or strategic interventions for the prevention or treatment of chronic wounds. Appl Health Econ Health Policy. 2014;12(4):373‐389. 10.1007/s40258-014-0094-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Järbrink K, Ni G, Sönnergren H, et al. The humanistic and economic burden of chronic wounds: a protocol for a systematic review. Syst Rev. 2017;6(1):15. 10.1186/s13643-016-0400-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Neyt M, Chalon PX. Search MEDLINE for economic evaluations: tips to translate an OVID strategy into a PubMed one. PharmacoEconomics. 2013;31(12):1087‐1090. 10.1007/s40273-013-0103-0 [DOI] [PubMed] [Google Scholar]
- 23. Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan‐a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):210‐210. 10.1186/s13643-016-0384-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Evers S, Goossens M, De Vet H, et al. Criteria list for assessment of methodological quality of economic evaluations: consensus on health economic criteria. Int J Technol Assess Health Care. 2005;21:240‐245. [PubMed] [Google Scholar]
- 25. Rousson V, Gasser T, Seifert B. Assessing intrarater, interrater, and test–retest reliability of continuous measurements. Stat Med. 2002;21(22):3431‐3446. 10.1002/sim.1253 [DOI] [PubMed] [Google Scholar]
- 26. (EPPI‐Centre) hCaCEMGCatEfPaPIaCC . CCEMG‐EPPI‐Centre cost converter; Version 1.4. 2018.
- 27. Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta‐analysis protocols (PRISMA‐P) 2015 statement. Syst Rev. 2015;4(1):1–9. 10.1186/2046-4053-4-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bale S, Tebble N, Jones V, Price P. The benefits of implementing a new skin care protocol in nursing homes. J Tissue Viability. 2004;14(2):44‐50. [DOI] [PubMed] [Google Scholar]
- 29. Byers PH, Ryan PA, Regan MB, Shields A, Carta SG. Effects of incontinence care cleansing regimens on skin integrity. J Wound Ostomy Continence Nurs. 1995;22(4):187‐192. 10.1097/00152192-199507000-00010 [DOI] [PubMed] [Google Scholar]
- 30. Baatenburg de Jong H, Admiraal H. Comparing cost per use of 3M Cavilon no sting barrier film with zinc oxide oil in incontinent patients. J Wound Care. 2004;13(9):398‐400. [DOI] [PubMed] [Google Scholar]
- 31. Avşar P, Karadağ A. Efficacy and cost‐effectiveness analysis of evidence‐based nursing interventions to maintain tissue integrity to prevent pressure ulcers and Incontinence‐Associated Dermatitis. Worldviews Evid‐Based Nurs. 2018;15(1):54‐61. [DOI] [PubMed] [Google Scholar]
- 32. Bliss DZ, Zehrer C, Savik K, et al. An economic evaluation of four skin damage prevention regimens in nursing home residents with incontinence: economics of skin damage prevention. J Wound Ostomy Continence Nurs. 2007;34(2):143‐152. [DOI] [PubMed] [Google Scholar]
- 33. Palese A, Carniel G. The effects of a multi‐intervention incontinence care program on clinical, economic, and environmental outcomes. J Wound Ostomy Continence Nurs. 2011;38(2):177‐183. [DOI] [PubMed] [Google Scholar]
- 34. Zehrer CL, Lutz JB, Hedblom EC, et al. A comparison of cost and efficacy of three incontinence skin barrier products. Ostomy Wound Manage. 2004;50(12):51‐58. [PubMed] [Google Scholar]
- 35. Brunner M, Droegemueller C, Rivers S, et al. Prevention of incontinence‐related skin breakdown for acute and critical care patients: comparison of two products. Urol Nurs. 2012;32(4):214‐219. [PubMed] [Google Scholar]
- 36. Warshaw E, Nix D, Kula J, et al. Clinical and cost effectiveness of a cleanser protectant lotion for treatment of perineal skin breakdown in low‐risk patients with incontinence. Ostomy Wound Manage. 2002;48(6):44‐51. [PubMed] [Google Scholar]
- 37. van Lier LI, Bosmans JE, van Hout HPJ, et al. Consensus‐based cross‐European recommendations for the identification, measurement and valuation of costs in health economic evaluations: a European Delphi study. Eur J Health Econ. 2018;19(7):993‐1008. 10.1007/s10198-017-0947-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Larg A, Moss JR. Cost‐of‐illness studies: a guide to critical evaluation. PharmacoEconomics. 2011;29(8):653‐671. 10.2165/11588380-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 39. Gray M, Beeckman D, Bliss DZ, et al. Incontinence‐associated dermatitis: a comprehensive review and update. J Wound Ostomy Continence Nurs. 2012;39(1):61‐74. 10.1097/WON.0b013e31823fe246 [DOI] [PubMed] [Google Scholar]
- 40. Beeckman D, Van Lancker A, Van Hecke A, et al. A systematic review and meta‐analysis of incontinence‐associated dermatitis, incontinence, and moisture as risk factors for pressure ulcer development. Res Nurs Health. 2014;37(3):204‐218. 10.1002/nur.21593 [DOI] [PubMed] [Google Scholar]
- 41. Mayrovitz HN, Sims N. Biophysical effects of water and synthetic urine on skin. Adv Skin Wound Care. 2001;14(6):302‐308. 10.1097/00129334-200111000-00013 [DOI] [PubMed] [Google Scholar]
- 42. Philips Z, Bojke L, Sculpher M, et al. Good practice guidelines for decision‐analytic modelling in health technology assessment: a review and consolidation of quality assessment. PharmacoEconomics. 2006;24(4):355‐371. 10.2165/00019053-200624040-00006 [DOI] [PubMed] [Google Scholar]
- 43. Van den Bussche K, De Meyer D, Van Damme N, et al. CONSIDER ‐ Core outcome set in IAD research: study protocol for establishing a core set of outcomes and measurements in incontinence‐associated dermatitis research. J Adv Nurs. 2017;73(10):2473‐2483. 10.1111/jan.13165 [DOI] [PubMed] [Google Scholar]
- 44. Brennan MR, Milne CT, Agrell‐Kann M, Ekholm BP. Clinical evaluation of a skin protectant for the management of incontinence‐associated dermatitis: an open‐label, nonrandomized, prospective study. J Wound Ostomy Continence Nurs. 2017;44(2):172‐180. 10.1097/won.0000000000000307 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


