Abstract
Pain is a serious problem for patients with leg ulcers. Research mainly focuses on dressing‐related pain; however, chronic background pain may be just as devastating. Our main objective was to describe the prevalence and characteristics of wound‐related background pain in persons with chronic venous leg ulcers. We performed a systematic review to synthesise data from quantitative studies. Studies were eligible if they reported original baseline‐ or cross‐sectional data on background pain in chronic venous leg ulcers. The initial search identified 2454 publications. We included 36 descriptive and effect studies. The pooled prevalence of wound‐related background pain (from 10 studies) was 80% (95% CI 65‐92%). The mean pain intensity score (from 27 studies) was 4 (0‐10 numeric rating scale) (95% CI 3.4‐4.5). Other pain characteristics could not be synthesised. We identified few sufficiently high‐quality studies on prevalence and intensity of wound‐related background pain in patients with chronic venous leg ulcers. Four of five persons experience mild to moderate pain. Because of poor quality of pain assessment and report, we believe that the available research does not provide a sufficiently nuanced understanding of background pain in this patient group.
Keywords: pain, systematic review, venous leg ulcer
1. INTRODUCTION
Pain experienced by people with chronic venous leg ulcers (CVLUs) is complex. In a consensus document, the World Union of Wound Healing Society (2004) proposed the terms background, incident, procedural, and operative pain to describe both the types and causes of wound‐related pain. The background pain is caused by the underlying pathology of the leg ulceration and the wound itself. Various daily activities can cause incident pain. The wound treatment causes procedural or operative pain, as well as complications such as skin irritation.1 Furthermore, wound‐related pain can be classified as acute or chronic, nociceptive or neuropathic.2, 3, 4 Wound‐related pain is a complex symptom, and patients with persistent leg ulcers often experience multiple types of pain from their ulcer, making this type of pain particularly complex.5
Approximately 1% to 2% of the population in western countries suffer from chronic leg ulcers,6, 7, 8 and CVLUs account for 70% to 90% of lower leg ulcers.9 CVLUs are defined as an open lesion between the knee and the ankle joint that remains unhealed for at least 30 days and occurs in the presence of venous disease.5 Peak prevalence of CVLUs occurs in the age group 60 to 80 years,9 and the prevalence rate is expected to increase as the population ages. These ulcers are a particular threat to older individuals as increased age is a major risk factor for impaired wound healing.10 CVLUs may take months or years to heal and are prone to recurrence because the underlying and wound‐provoking factors have not been, or cannot be, adequately addressed.11 Research shows that CVLUs have a major negative impact on the persons living with them. The ulcers cause pain, social isolation, sleep disturbance, depression, loss of time from work, and financial costs. These biopsychosocial factors can have a major negative impact on the patients' perception of pain.5 Both the wound itself and the wound‐related pain are significant causes of impaired function and quality of life (QoL).12
Literature searches identify a limited number of high‐quality studies specifically investigating wound‐related pain. The existing literature mainly focuses on pain at dressing change, and little attention is paid to chronic background pain. Background pain, sometimes called basal or baseline pain, is related to the underlying cause of the wound, local wound factors such as inflammation, and other related pathologies such as skin irritation. The pain is felt at rest, when there is no tissue manipulation or sudden changes in the patient physical condition, and it may be continuous or intermittent.5 Persistent background pain at rest and between wound‐related procedures might be just as devastating as the procedure‐related pain.13 In this systematic review, we focus on wound‐related background pain. Studies reporting on procedural or operative pain are not included.
Several studies describe the prevalence and characteristics of pain in relation to CVLUs. However, with prevalence rates varying from 46% up to 100%,14, 15, 16 it is difficult to evaluate the relative impact of pain associated with CVLUs. The most frequent pain characteristic described is pain intensity. Other pain characteristics, such as location of pain, temporal fluctuations of pain intensity, pain interference, and pain quality descriptors, are less frequently described. In the wound‐healing literature, pain related to CVLUs is described as constant or intermittent, and pain intensity varies from mild pain to intense pain.17, 18 Pain characteristics are important and necessary factors to assess when considering pain management and when evaluating treatments.
To date, little effort has been made to systematically review these studies to determine the overall prevalence and characteristics of wound‐related background pain. A lack of information and knowledge about this type of pain may have negative consequences for wound treatment as it is likely that adequate pain recognition and management are important in improving both QoL and adherence to treatment.19 Hence, the purpose of this review was to synthesise existing studies reporting the prevalence and characteristics of wound‐related background pain in order to provide a much needed and accurate estimate.
2. METHODS
2.1. Aim
Our main objectives were to (a) systematically review the literature to describe the prevalence of wound‐related background pain in published studies that focus specifically on CVLUs in samples from both community and hospital care settings and (b) describe characteristics of this wound‐related background pain (eg, intensity, qualities, location, and temporal fluctuations).
The secondary objectives were to perform a meta‐analysis on pain prevalence and pain characteristics and to identify factors associated with pain intensity.
2.2. Design
A systematic review was conducted to synthesise data from both descriptive and effect studies.20, 21 We used a systematic review methodology, using the guidance of PRISMA.22 A review protocol was created a priori and was registered on PROSPERO international prospective register of systematic reviews (CRD42017056027).
2.3. Search strategy
A systematic search was performed in the following electronic bibliographic databases: MEDLINE, EMBASE, CINAHL, The Cochrane Library (the Cochrane Database of Systematic Reviews, the Cochrane Central Register of Controlled Trials [CENTRAL], the Cochrane Methodology Register), and The British Nursing Index.
The search strategy included terms relating to different wound diagnoses and to pain. A detailed list of search terms was prepared and adapted to each database. The list consisted of a combination of medical subject headings (eg, MeSH) and free terms related to pain and ulcers. Data on pain as a subdomain of QoL were not included. The complete search strategy is described in Appendix.
We identified additional studies by manually searching relevant conference proceedings and specialist journals. The reference lists of all relevant studies and systematic reviews were hand searched for additional relevant studies.
The search was restricted to studies published between 1st January, 1990 and 31st October, 2017. Searches were re‐run before the final analyses were completed (1st February, 2019) in order to retrieve and include the latest relevant studies in the review.
2.4. Changes in protocol
To begin with, we chose a systematic mixed‐studies review method to synthesise data from studies with diverse research designs.20, 21, 23 In the present study, we present the quantitative data obtained from the literature search.24
Furthermore, we initially set out to establish the prevalence and characteristics of wound‐related pain in persons living with various diagnoses of chronic leg and foot ulcers. We found a great deal of heterogeneity in the diagnosis criteria, which made the synthesis of data complex. This led us to narrow the scope further and include only data on wound‐related background pain in persons with CVLUs. This decision was based on the argument that CVLUs make up the largest group of patients with chronic wounds and that the majority of studies focused on these patients.9
We initially also set out to include studies of all languages, but we realised that it was too resource‐demanding to translate all abstracts in other languages. As a result, we decided to include only studies published in English or Scandinavian languages.
2.5. Study selection
Two of the authors (L.L. and T.M.L.) independently assessed titles and abstracts of all potentially relevant publications identified from the literature search.
Inclusion and exclusion criteria are presented in Table 1. Pain measures included in generic health‐related QoL instruments were not included as the scope of this review was wound‐related pain. If the same data were analysed in multiple publications, the publication with the more complete or more extensive data was included.
Table 1.
Inclusion and exclusion criteria
| Inclusion criteria | Exclusion criteria | |
|---|---|---|
| Publication year | Published between January 1990 and February 2019 | |
| Language | English, Scandinavian | |
| Study design | Effect studies and descriptive studies (with cross‐sectional, longitudinal, prospective/retrospective design) |
Qualitative studies Case studies/series Reviews Conference papers Discussion papers Editorials Consensus documents Expert opinions Other non‐research publications |
| Study sample | Adult persons >18 years with active venous leg ulcer, duration >4 weeks | Not reporting on pain in persons with active CVLUs separately |
| Pain data | Original self‐reported data on pain prevalence or pain characteristics |
Pain assessment/instrument not defined/described Insufficient pain report (eg, only changes in pain score reported) Pain as an inclusion criterion in the study Procedure‐related pain reports only |
The initial search identified 2454 unique publications. The abstracts were screened, and the inclusion and exclusion criteria were applied. This left 556 articles, of which another 514 were excluded, resulting in a total of 43 articles. The updated search identified 164 publications, which was reduced to 118 after removing duplicates. After applying inclusion and exclusion criteria, three articles were included. In the quality assessment, 45 studies were evaluated, and 9 studies were excluded. A total of 36 studies were included in the synthesis, and 33 of these studies presented data eligible for the meta‐analyses. The PRISMA flow diagram in Figure 1 depicts the flow of information through the different phases of a systematic review.
Figure 1.
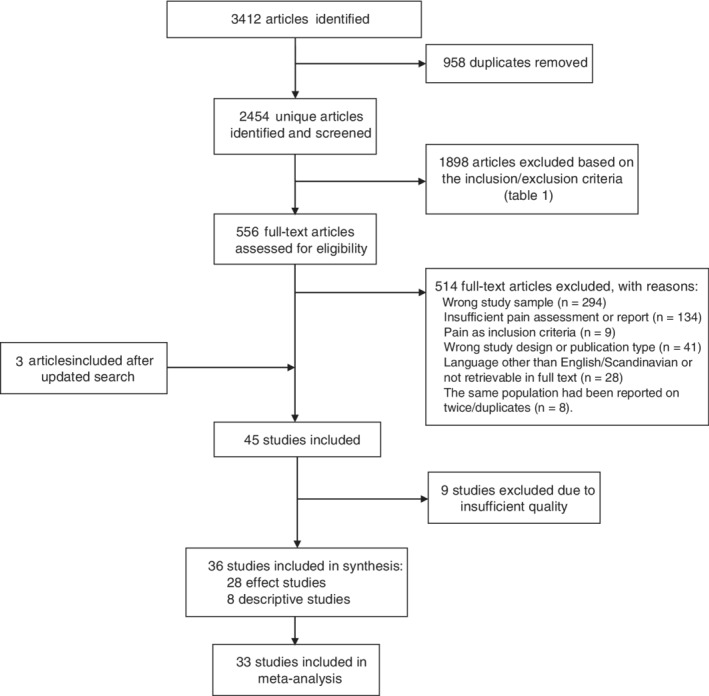
PRISMA flow diagram
2.6. Quality assessment
Six authors paired up and evaluated the quality of eligible studies using the Mixed Method Appraisal Tool MMAT‐v2011.25 The tool permits appraisal of studies across a range of designs, where different sections of the tools are used for the appraisal of the different study types.21, 26 Hence, each study design is judged within its methodological domain. The MMAT scores range from 0% (no criterion is met) to 100% (all four criteria are met). Disagreement on the score of the MMAT‐v2011 was resolved by discussion among the authors.
The quality score of nine studies was 0%, and these studies were thus excluded because of poor methodological quality or inadequate report of pain prevalence and characteristics.
2.7. Data extraction
One author (L.L.) summarised the study characteristics and pain findings in a table, and a second author (T.M.L.) verified this extracted data. The extracted data included information on author(s), year of publication, country, study purpose, design, sample characteristics (eg, sample size, age, gender, and wound duration), wound diagnostic criteria, data collection methods/recruitment, and type of pain assessment/report, as well as pain prevalence and characteristics (ie, intensity, duration and frequency, location, and quality). In addition, the respondents' use of pain medication and compression therapy was extracted.
2.8. Synthesis of data and analysis
There was a large diversity among the included studies regarding aim and focus of research. All studies assessed pain in persons with CVLUs and were considered sufficiently homogenous to provide a meaningful summary. Only baseline data were extracted from the studies with repeated measures. The inclusion criteria in effect studies were generally more detailed than in descriptive studies, typically resulting in more selected samples with fewer comorbidities. Therefore, we stratified the analysis by study design.
In the studies reporting pain intensity, a great variation of tools (ie, different numeric rating scales [NRS], different verbal rating scales [VRS], various anchor points) were used to assess pain intensity. Data were synthesised using standardised methods for converting different pain rating scales,27, 28, 29 providing an NRS of pain ranging from 0 to 10. In studies only providing information about median pain score and range, mean pain score and SD were calculated as described by Hozo et al.30
We performed a random‐effects meta‐analysis overall and stratified by study design for pain prevalence and pain intensity. Summary estimates were calculated to provide pooled estimates of proportion of pain and mean pain intensities in the included studies31 and were presented with accompanying 95% confidence intervals (CI). Heterogeneity between the studies was assessed with the Q‐test, and its magnitude was quantified using the I‐squared measure. This describes the proportion of the total variation because of heterogeneity rather than chance. We interpreted a value ≥75% as high heterogeneity.32
To explore sources of heterogeneity in pain intensity, we performed univariable random‐effects meta‐regression analyses. We considered the following variables: study design; year of publication; and participant's age, gender, and wound duration. Meta‐regression analyses were not performed for pain prevalence because of the small number of publications (n = 10).
We used the forest plot to present the summary estimates overall and stratified by study design. Publication bias was assessed by Egger's test.
All analyses were conducted using Stata 15.0 (State College, Texas). The metaprop and the metan commands were used to perform meta‐analysis of the prevalence data and the intensity data, respectively.
3. RESULTS
3.1. Selected studies
The included studies contained original data on pain prevalence, intensity, and/or characteristics. All these studies met the minimum quality score criteria of 25% on the MMAT. Publication year ranged from 1993 to 2018. A total of 36 studies were included. Ten studies were descriptive (ie, descriptive survey, registry study), and 26 were effect studies (ie, randomised controlled trials, non‐randomised efficacy studies, prospective uncontrolled trials). Detailed descriptions of the included studies are presented in Table 2.
Table 2.
Included studies in the meta‐analysis sorted by study design and publication year
| Author(s), year | Country | Study design | No. of participants | Mean age (years) | Tool used to assess pain prevalence | Pain assessment tools | Prevalence of pain | Pain intensity | MMAT Quality score (%) |
|---|---|---|---|---|---|---|---|---|---|
| Effect studies | |||||||||
| Teepe et al, 199333 | No info | Randomised controlled trial | 43 | 71.6 | VAS (0‐5) | ✓ | 25 | ||
| Morrell et al, 199834 | UK | Randomised controlled trial | 223 | 73.5 | SF‐MPQ | ✓ | 75c | ||
| Santilli et al, 199935 | No info | Non‐randomised efficacy study | 17 | 67.7 | NRS (0‐10) | ✓ | 25 | ||
| Walters et al, 199936 | UK | Randomised controlled trial | 233 | 74.6 | Not reported (“… 25% said they had no leg ulcer pain”) | SF‐MPQ | ✓ | ✓ | 50c |
| Wilson et al, 200237 | UK | Non‐randomised efficacy study | 21 | 74 | Verbal rating scale (0‐5), 0 = no pain | VRS | ✓ | 50 | |
| Navratilova et al, 200438 | Czech republic | Randomised controlled trial | 50 | 62.9 | VAS (0‐10) | ✓ | 50 | ||
| Mermet et al, 200739 | France | Prospective uncontrolled study | 15 | 79 | VAS (0‐100) | ✓ | 25 | ||
| Cameron et al, 200540 | UK | Randomised controlled trial | 35 | 73.2 | Verbal rating scale (0‐5), 0 = none | VRS | ✓ | 50 | |
| Benigni et al, 200741 | France | Non‐randomised efficacy study | 42 | 70.5 | Pain during previous compression system (y/n) | VRS | ✓ | 25 | |
| Szewczyk et al, 200716 | Poland | Non‐randomised efficacy study | 20 | 62.2 | Numeric pain scale (0‐10), 0 = no pain | NRS (0‐10) | ✓ | ✓ | 50 |
| Jünger et al, 200842 | No info | Prospective controlled trial | 39 | 67.2 | Pain scale (0‐10) | ✓ | 50 | ||
| Edwards et al, 200943 | Australia | Randomised controlled trial | 67 | MOS pain measures (0‐10) | ✓ | 50 | |||
| Brizzio et al, 201044 | No info | Randomised controlled trial | 55 | 61.7 | NRS (0‐100) | ✓ | 75c | ||
| Schumann et al, 201145 | Germany | Randomised controlled trial | 51 | 74 | VAS (0‐100) | ✓ | 75 | ||
| Escadon et al, 201246 | No info | Non‐randomised efficacy study | 10 | VAS (0‐10) | ✓ | 50c | |||
| Wong et al, 201247 | China | Randomised controlled trial | 321 | 71.7 | BPI (0‐10) | ✓ | 50c | ||
| Olyaie et al, 201348 | Iran | Randomised controlled trial | 90 | 38 | NRS (0‐20) | ✓ | 100 | ||
| Park et al, 201349 | Korea | Non‐randomised efficacy study | 16 | 50.3 | Subjective (0‐4) | ✓ | 50 | ||
| Behesthi et al, 201450 | Iran | Randomised controlled trial | 90 | 58.5 | NRS (0‐20) | ✓ | 75 | ||
| Brown et al. 201451 | Germany and USA | Randomised controlled trial | 120 | 68.3 | NRS (0‐100) | ✓ | 75 | ||
| Bradbury et al. 201552 | No info | Non‐randomised controlled trial | 15 | 64.4 | NRS (0‐10) | ✓ | 50 | ||
| Brehmer et al, 201553 | Germany | Randomised controlled trial | 14 | 67 | VAS (0‐100) | ✓ | 25 | ||
| Gibbons et al, 201554 | USA | Randomised controlled trial | 80 | 60.1 | VAS (150 mm) | ✓ | 75 | ||
| White et al, 201555 | UK | Randomised controlled trial | 36 | 69 | VAS (0–5) | ✓ | 100 | ||
| Harding et al, 201656 | UK and Poland | Non‐randomised efficacy study | 42 | 71.4 | VAS (0–10) | ✓ | 50 | ||
| Vitse et al, 201757 | France | Prospective randomised controlled trial | 71 | 67 | VAS (0–100) | ✓ | 100 | ||
| Salome & Ferreira, 201815 | Brazil | Randomised controlled trial | 90 | Numeric pain scale (0–10), 0 = no pain | ✓ | 100 | |||
| Domingues et al, 201858 | Brazil | Randomised controlled trial | 71 | 66.5 | NRS (0–10) | ✓ | 50 | ||
| Descriptive studies | |||||||||
| Jones et al, 200659 | UK | Explorative survey | 190 | 69 | Verbal rating scale (0‐4), 0 = no pain | VRS | ✓ | 50 | |
| Cwajda‐Bialasik et al, 201260 | Poland | Descriptive questionnaire survey | 101 | 66.2 | NRS (0‐10) | ✓ | 50 | ||
| Paul, 201314 | US | Descriptive survey | 41a | 67b | Wound‐related pain (y/n) | NRS (0‐10) | ✓ | 100 | |
| Goto et al, 201661 | Japan | Observational survey | 13 | 67.5 | Numeric rating scale (0–10), 0 = no pain | NRS (0‐10) | ✓ | ✓ | 25 |
| Hellström et al, 201662 | Sweden | Register study | 763a | Experienced pain (y/n) | NRS (0‐10) | ✓ | ✓ | 25 | |
Note: Ticked boxes indicate that the data (prevalence and/or intensity) are included in the meta‐analyses.
Abbreviations: BPI, Brief Pain Inventory, MOS, Medical Outcomes Study; NRS, Numeric Rating Scale, SF‐MPQ2, Short Form McGill Pain Questionnaire‐2, VRS, Verbal Rating Scale.
Venous leg ulcer only.
Total sample.
One criterion not applicable.
3.2. Pain prevalence
Pain prevalence was reported in 10 of the 36 studies. Six were effect studies (two randomised controlled trials, three non‐randomised efficacy studies, one controlled randomised prospective study), and four were descriptive studies (one registry study, three surveys). Four of these studies were conducted in the United Kingdom, and one was conducted in each of Czech Republic, Sweden, Poland, United States, Japan, and Brazil.
Prevalence of pain was determined by self‐report in all included studies, and various tools and descriptions were used to assess and report pain. “No pain” was used as a reference point to determine pain prevalence. The prevalence ranged from 46.3% to 100%. The pooled estimated proportion was 80% (ES 0.80 [95% CI 0.65‐0.92]), with high heterogeneity (I‐squared 96.5%). Subgroup analysis by design demonstrated a higher proportion in effect studies (90%) compared with descriptive studies (60%), both with high heterogeneity. The meta‐analysis of the prevalence of background wound‐related pain is illustrated in the forest plot in Figure 2.
Figure 2.
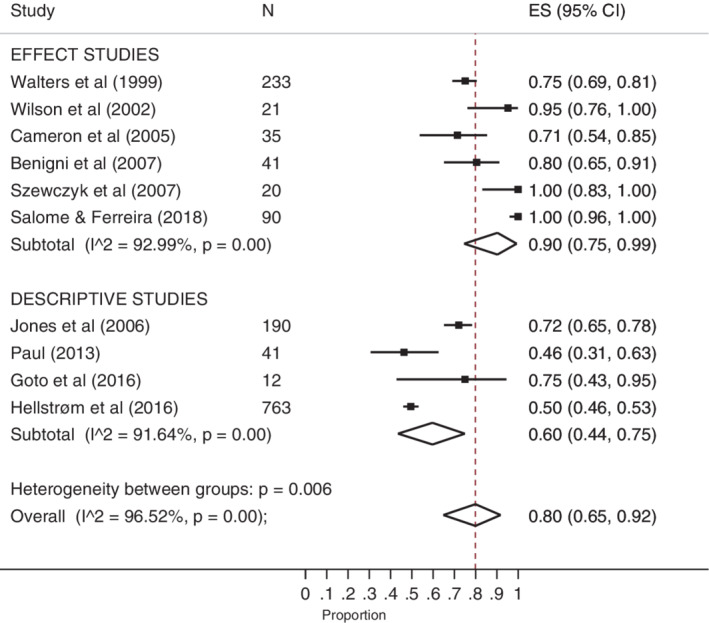
Forest plot of prevalence of wound‐related background pain
3.3. Pain intensity
Pain intensity was reported in 27 of the included studies. Three of these studies were descriptive studies, while 24 were effect studies (ie, randomised controlled trials and non‐randomised efficacy studies). The studies were conducted in 13 different countries spread over four continents (Table 1). The mean age of most of the patient samples ranged from 50.3 to 74.6 years. One study48 reported on a noticeably younger sample with a mean age of 38 years.
The mean pain intensity scores ranged from 2.3 to 6.6 (all converted to NRS 0–10). The overall pooled estimate of mean pain intensity was 4.0 (CI 95% 3.5, 4.5), with high heterogeneity. Subgroup analysis showed similar pooled estimates of mean pain intensity in effect studies (4.0) and in descriptive studies (3.8), both with high heterogeneity. The meta‐analysis of the intensity of background wound‐related pain is illustrated in the forest plot in Figure 3.
Figure 3.
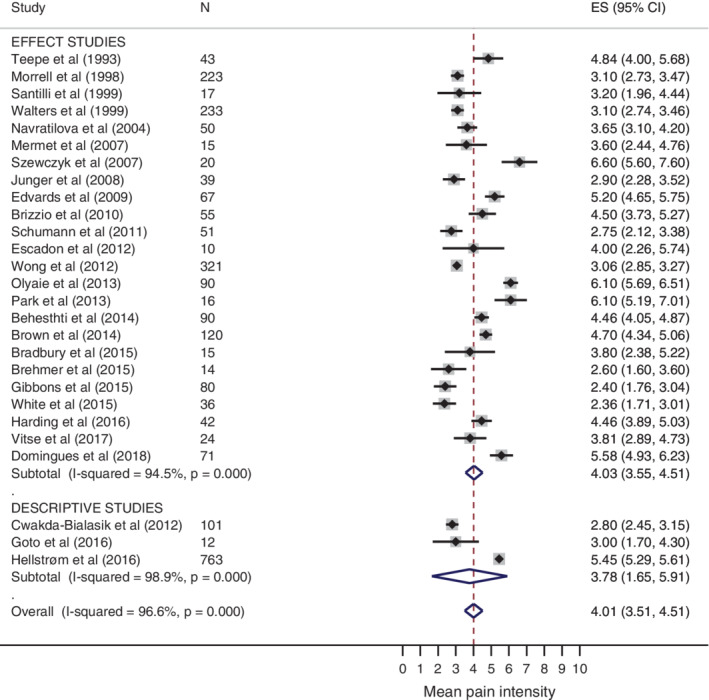
Forest plot of intensity of wound‐related background pain
The meta‐regression showed that there was an association between the observed effect size and the mean age of participants in the studies; for each year increase in age, the mean pain intensity decreased by 0.09 (P = .005). Year of publication, gender distribution, and mean ulcer duration were not statistically significantly associated with mean pain intensity.
We found no significant indication of publication bias (Egger's test P = .34).
3.4. Other pain characteristics
Information on pain characteristics was diverse and sparse. Therefore, a synthesis of wound pain characteristics (other than pain intensity) was not plausible. We present these studies' findings in Table 3 and describe them in a narrative way in the following. Seven studies reported on pain characteristics other than pain intensity. Three studies reported on characteristics assessed with the McGill Pain Questionnaire (MPQ), and one study used the neuropathic pain questionnaire Douleur Neuropathique 4 (DN4). One study reported on pain interference from the Brief Pain Inventory (BPI), and one reported on pain interference with sleep. One study reported on the temporal pattern of pain, without describing the means of assessment. Some studies reported pain intensity in a way that made it improper to include the data in the meta‐analysis. Most of these excluded studies reported pain intensity corresponding to mild to moderate,40, 63 which is similar to results found in the studies included in the meta‐analysis. Note that one excluded study41 report a high prevalence (40%) of intense pain.
Table 3.
Pain characteristics not included in the meta‐analysis
| Author(s), Year Sample size | Measures | Pain intensity | Pain quality | Pain pattern | Pain interference |
|---|---|---|---|---|---|
| Morell et al, 199834 N = 233 | SF‐MPQ |
Pain rating index (mean): Sensory (0‐33): 9.1 Affective (0‐12): 2.0 Number of words chosen (0‐15): 5.5 |
|||
| Cameron et al, 200540 N = 35 | VRS (6 items) MPQ modified version |
Percentage of sample reporting graded pain intensity: None: 14.3% Mild: 36.4% Uncomfortable: 20.2% Distressing: 11.3% Horrible: 14.5% Excruciating: 3.3% |
|||
| Benigni et al, 200741 N = 33 | VRS (minor, moderate, intense, very intense) |
Percentage of sample reporting graded pain intensity: Minor: 18% (n = 6) Moderate: 37% (n = 12) Intense: 40% (n = 13) Very intense: 6% (n = 2) |
Percentage of sample reporting various degrees of spontaneous pain at baseline: Absent: 10% (n = 4) Minor: 19% (n = 4) Moderate: 38% (n = 16) Intense: 33% (n = 14) Experience of previous treatment with compression bandages: 81% (n = 33) had experienced pain, which was “intense” in 40% (n = 13) |
||
| Closs et al, 200863 N = 79 | NRS (0‐5) MPQ |
Average rating of pain intensity (median): Average pain = 1 Worst pain = 2 Least pain = 0,5 Pain now = 0 |
MPQ pain rating index (mean ± SD): 17 ± 5 Number of words chosen (mean ± SD): 7 ± 5 Percentage of patients using the following pain sensory pain descriptors: Itchy: 50% Tender: 43% Throbbing 37% Burning: 33% Stinging: 33% |
||
| Wong et al, 201247 N = 321 | BPI | Pain interference (mean ± SD): 3.3 ± 2.5 | |||
| Eusen et al, 201664 N = 81 | DN4 | Percentage of sample having neuropathic pain: 57.1% | |||
| Goto et al, 201661 N = 13 | SF‐MPQ2 |
Scores on the SF‐MPQ‐2 scale (median and interquartile range): Continuous pain: 18.5 (6.5–30.0) Intermittent pain: 11.5 (0.0–28.5) Neuropathic pain: 13.5 (3.0–22.3) Affective descriptors: 0.0 (0.0–10.0) Total pain score: 54.0 (13.3–78.5) |
|||
| Hellström et al, 201662 N = 763 | 38% of those reporting pain also reported pain interference with sleep | ||||
| Salome and Ferreira (2018)15 N = 90 |
MPQ NPS classified as: absence (0), mild (0–3), moderate (4–6), intense (7–10), pain |
Moderate pain: 53.3% Intense pain: 46.7% Percentages of descriptors used (MPQ): None: 8.9% Sensory: 60% Affective: 51.1% Evaluative: 27.8% Miscellaneous: 32.2% |
Abbreviations: BPI, Brief pain inventory, DN4, DN4 Questionnaire (neuropathic pain questionnaire); MPQ, McGill Pain; NRS, Numeric Rating Scale, SF‐MPQ2, Short form McGill Pain Questionnaire‐2, VRS, Verbal Rating Scale.
4. DISCUSSION
To our knowledge, this is the first study attempting to synthesise prevalence and characteristics of wound‐related background pain in persons with CVLU. The main findings in the present study suggest that as many as 80% of persons with CVLU have wound‐related background pain. These patients report having mild to moderate pain intensity on average. Other characteristics of wound‐related pain were not possible to synthesise from available research.
4.1. Prevalence of wound‐related pain
The prevalence of wound‐related background pain in the studies included in this meta‐analysis varies from 46% to 100%.14, 15, 65 Possible reasons for this large variety of pain prevalence may be related to different research methods (eg, assess, report, define, and classify pain) that provide either lower or higher prevalence rates among studies. In the present meta‐analysis, we excluded a number of studies because of such methodological inconsistencies that we believe contribute to an even greater variety of pain prevalence rates.
On one hand, lower estimates may occur as a result of the chosen pain assessment method. For instance, research shows that staff‐administered instruments provide lower pain prevalence rates compared with oral or written self‐report instruments.66 Lower estimates may also occur because studies apply different definitions of pain. In some studies, no pain was defined as 3 or less (NRS 0–10), providing lower and faulty pain prevalence rates as they excluded patients with mild pain. Furthermore, studies only reporting on one type of pain, such as neuropathic pain,64 likely under‐report pain prevalence because they do not account for the fact that the patients may just as well have nociceptive or inflammatory pain, without a neuropathic component.
On the other hand, falsified high pain prevalence rates may occur when researchers do not explicitly assess wound‐related pain. With general pain questions, the respondents may report pain caused by other pathologies. Therefore, studies that assessed pain with health‐related QoL measures (eg, EQ‐5d, SF‐36) were excluded in this meta‐analysis as it is likely that the patients report pain other than wound‐related pain. However, even though all included studies imply that the pain reported is wound‐related pain, it is not always clear if the patients have been explicitly asked about wound‐related pain, and the patients do not report other frequent conditions such as musculoskeletal pain.
An additional interesting finding related to the prevalence of wound‐related pain is the significantly larger prevalence rates reported by the effect studies compared with the descriptive studies. This was a somewhat surprising finding as the inclusion and exclusion criteria in the effect studies are generally stricter. This rigour may result in study samples that are healthier with fewer comorbidities, possibly leading to a lower pain prevalence. However, the strict selection of participants in the effect studies may also lead to study samples with more severe or larger wounds, potentially increasing the pain prevalence rates. Note that the number of studies included in this meta‐analysis was small, with just six effect studies and four descriptive studies. Therefore, the variation of pain prevalence might also be the result of chance.
4.2. Intensity of wound‐related pain
The second main finding in this meta‐analysis is the mean pain intensity of background wound‐related pain of 4 (NRS 0–10), which is equivalent to mild to moderate pain.67 Note that persons with mild to moderate pain usually do not need pain management. The finding of low pain intensity levels in this meta‐analysis is surprising, considering that QoL studies show that pain may be the most bothersome symptom of having a CVLU.68, 69, 70 A number of methodological factors may explain the discrepancies reported in the research literature. First, it is important to recognise the great variation in pain intensity (ie, standard deviations, interquartile ranges, etc.) reported within the different studies. We believe it is more relevant to look at the percentage share of the patient samples experiencing moderate to severe pain rather than mean values of pain intensities. Future studies should rather report pain characteristics and evaluate treatment effects based on pain intensity in these subgroups.
Second, another main concern regarding the low pain intensity reported in the literature is that few studies report pain prevalence. Furthermore, many studies do not inform whether they calculate the mean pain intensity score of only those patients who have pain (ie, score greater than 0 on the NRS) or include all patients in their intensity calculations regardless the pain score (ie, NRS 0 to the highest score). If the latter scenario is the case, the inclusion of scores of no pain (NRS 0) leads to a lower mean pain intensity score for the sample. In future studies, more detailed information on the assessment and calculation of pain intensity is needed.
It is challenging to extract an estimate of wound pain intensity because of the great variations of pain assessment tools used in the research literature. Ideally, researchers should strive towards using the recommended common standard of 0 to 10 NRS of pain intensity.71 In 32 studies, we found eight different NRSs and three different VRSs for assessing pain intensity, with even more variation in anchors related to the different scales. Not all studies used 0 as the lowest anchor point of their pain scale, but all stated that the lowest point equals no pain. The highest anchor point, however, had various descriptions, including intense pain, extreme pain, unbearable pain, worst pain ever experienced, worst pain imaginable, extremely painful, severe pain, never felt such pain before, excruciating pain, most pain, and maximum pain. As all the included studies applied “no pain” as the lowest point and a descriptor of extreme pain as the highest point, as well as including enough options for participants to choose between different levels of pain intensity, we do believe that the studies included in this meta‐analysis are comparable in terms of pain intensity measures.
The most frequent pain intensity assessment methods used in the included studies were visual analogue scales (VAS), NRS, and VRS. Note that most of the pain intensity measures in the included studies are crude and do not state whether the pain assessed is of maximum or average intensity or at rest vs in activity. Furthermore, all of these assessment methods have potential problems in this population of older persons. They all require some degree of abstract thinking and fine discrimination among the response alternatives. Such tasks may be difficult for the elderly because of cognitive changes, as well as lack of experience with psychometric tests. In addition, the VAS may be especially difficult to administer to frail patients or those with limited vision.72 We assume that the studies have strived towards including persons with normal cognitive abilities. However, frailty and limited vision is still a potential risk of bias given the samples' high mean age and the limited methodology description in the included studies. In future research, and especially in clinical settings, it is important to carefully consider the assessment tool used and make sure to use methods suitable for the individual abilities of the person of interest. In general, research indicates that the NRS is proper to use in older people.73
4.3. Wound pain characteristics
In this meta‐analysis, we also aimed at describing a broad range of wound pain characteristics. However, we did not identify enough studies to synthesise any results regarding pain quality descriptors, temporal fluctuations, and pain interference with function. This is in itself an important finding, suggesting a knowledge gap in pain experienced by persons with CVLUs. It is of utmost importance that future studies apply validated and similar pain measures. Using common and standardised instruments provides the opportunity to investigate wound pain in subgroups of patients, as well as compare pain measures in persons with CVLUs with other patient groups. Therefore, we recommend using the NRS (0‐10) for pain intensity. The BPI should be used for variations in pain intensity (ie, pain now, worst, least, average), pain localisation, and pain interference with physical and psychosocial functions. MPQ should preferably be used to assess pain qualities.45, 74 All of these instruments are frequently used to assess pain in numerous patient groups. For clinical purpose, these instruments can be applied in wound pain assessment; however, the clinical assessment must be individually customised to the patient's resources and needs.
An understanding of these pain characteristics may in fact be of great clinical benefit in the treatment of CVLUs. The location of pain can help identify the cause of the pain (eg, pain caused by oedema, fixation of bandages, tissue damage, and inflammation). Temporal fluctuations of pain can guide the clinicians in choosing the appropriate pain management (eg, patients with no pain during night time should not receive analgesics around the clock). In addition, pain interference with both physical, emotional, and social function is a better metric of suffering than pain intensity75 and can help the clinician to tailor non‐pharmacological pain management according to the patients' individual needs. Hypothetically, the wound‐related background pain impact on function may be associated with the findings of pain as the most bothersome symptom of CVLUs in QoL studies. In future studies, the assessment of characteristics and consequences of wound‐related pain are needed.
4.4. Methodological discussion
Some methodological aspects of the existing research literature and the present meta‐analysis need to be elucidated and discussed. First, the concept and definition of chronic wound‐related background pain is often not clearly conceptualised and defined in research studies and the wound literature. While procedure‐related and operative pain is often differentiated from other wound‐related pain, we find no differentiation of background pain from incidence pain (eg, pain caused by activities3, 76). From a clinical point of view, different types of pain experienced in everyday life (other than procedure related pain) is likely difficult for the person with CVLUs to segregate into different categories. As a result, in this systematic review, wound‐related pain not caused by dressing change or other procedures are referred to as background pain.
We also need to be aware of the fact that we cannot state, based on the findings in this review and meta‐analysis, that the pain reported by persons with chronic CVLUs is a type of chronic pain. However, it is plausible that wound‐related background pain because of chronic wounds (ie, duration >4 weeks) is, in its nature, more similar to chronic pain than acute pain. When pain is chronic, the measure of mean pain intensity alone is not sufficient.75 A meaningful assessment of chronic pain is more demanding than assessing acute pain in both clinical practice and in research.71 Taking into account that persons with CVLUs often experience acute procedure‐related pain in addition to the more long‐lasting background pain, they must be recognised as a patient group that is highly exposed to pain. The complex pain picture in persons with CVLUs needs to be recognised in both future studies and in clinical practice.
In the present meta‐analysis, we used a broad search strategy in order to capture as many relevant studies as possible. In order to strengthen our results, we limited the impact of clinical heterogeneity by imposing strict selection criteria. The studies had to clearly identify wound diagnosis of CVLU, specify wound duration of at least 4 weeks, describe the pain measurement method used, and sufficiently report on relevant pain outcomes. In addition, eligible studies had to meet strict methodological quality criteria of the MMAT tool. This procedure left us with 36 sufficiently high‐quality articles reporting on pain prevalence and pain characteristics. Several identified studies were excluded because of poor quality, and a vast number was excluded because of insufficient report of wound‐related pain. Still, the tests for statistical heterogeneity in the included studies demonstrated substantial heterogeneity among the studies (I‐squared >90%). Because of the small number of included studies, as well as limited data obtained from the studies, further statistical analyses of study variance were not possible to perform. However, we can speculate that the diversity of clinical and methodological factors may lead to the great differences of pain prevalence and intensity among the included studies. For instance, the use of compression therapy and pain medication may relieve pain and thereby influence both pain prevalence and pain characteristics. In addition, differences in when pain was assessed (ie, at rest, with activity, at its worst or average) may influence the results. Furthermore, studies that include patients with larger ulcer size likely report higher prevalence and intensity of wound pain. Note, however, that the size of the tissue injury is not always linearly related to the level of pain.77 Likewise, patients recruited from hospital wards rather than outpatient clinics or community settings may have more serious disease and wound prognosis, thus experiencing wound‐related pain more often and with higher intensity. The random sampling method used in effect studies may also lead to greater variability of wound pain among patient rather than convenience sampling from a more homogenous patient group often used in descriptive studies. Finally, the inconsistent use of different pain assessment tools, as well as the fact that these tools are not well suited for the older patient group, might have an impact on the variability of pain prevalence and intensity reported in the studies included in this meta‐analysis.
It is crucial to be aware that only 10 studies on pain prevalence were included in the meta‐analysis. Considering the vast number of studies assessed for eligibility, this is a small number of studies. This finding demonstrates a lack of high‐quality research reporting pain prevalence in persons with CVLUs. One should therefore be careful to draw firm conclusions on wound‐related pain prevalence based on this small number of studies but should also be aware that this is the best available current estimate.
Finally, despite setting strict inclusion and exclusion criteria, we found it complicated to synthesise the data obtained on wound‐related pain in people with CVLUs. We argue that the lack of standardised methods for defining aetiology of wounds, as well as conceptualising, defining, and assessing core outcome measures such as pain, in combination with a lack of descriptions of the study samples, result in a heterogeneity that makes the synthesis of data very difficult. In general, there is a lack of high‐quality evidence in the field of wound management. Researchers and clinicians do not use standardised methods for assessing wounds and core outcome measures such as pain.77, 79, 80 Studies often have methodological flaws such as inadequate sample sizes, and they test pain‐relieving interventions without focusing particularly on wound‐related pain.78, 79, 80 Our review of the literature demonstrates a need for valid and standardised assessment methods and tools of this important patient‐reported outcome measure and more in‐depth research on characteristics of background pain related to CVLUs.
In conclusion, we have obtained the best available research data to demonstrate that the majority of persons with CVLU experience wound‐related background pain, reporting mild to moderate pain intensity. Because of the poor quality of the assessment and reporting of pain, it is likely that the research available underestimates the severity of wound pain and provides an inaccurate and simplified clinical picture. In the interest of improving the quality and reporting of data on pain prevalence and pain characteristics, we would encourage future studies to adhere to standardised methods for collecting and presenting data on wound and pain characteristics. Furthermore, the findings of pain intensity in this meta‐analysis indicate a need for a shift in focus from mean values to variations and subgroups in order to provide a person‐centred approach to clinical care and pain management for persons with CVLUs.
With the growing number of individuals living with CVLUs, it is crucial that we develop a better understanding of the pain that accompanies the ulcers. More importantly, it is imperative that clinicians are aware of the great extent and impact of background wound‐related pain and know how to accurately assess, evaluate, and initiate an individualised pain management regimen to meet the patient's needs.
CONFLICT OF INTEREST
We declare that we have no competing interests.
ACKNOWLEDGEMENTS
We thank academic librarian Marit Gjone Sandsleth at the department of Research and Internationalization, Library Unit at University of South‐Eastern Norway (USN) who provided professional assistance in the literature‐search process in this study. USN has funded the project based on funding from Norwegian Ministry of Education and Research. None of these institutions has had any roles in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Appendix 1: Complete search strategy 1.
| # | Search |
| 1 | exp Leg ulcer/ |
| 2 | (”chronic wound*” or “chronic ulcer*” or “leg wound*” or “leg ulcer” or “foot ulcer*” or “foot wound*” or “venous ulcer*” or “venous wound*” or “venous foot” or “varicose ulcer*” or "varicose wound*” or “diabetic foot” or “diabetic wound*” or “diabetic ulcer*” or “diabetic foot ulcer*” or “stasis wound*” or “stasis ulcer*”).tw. |
| 3 | 1 or 2 |
| 4 | (MH "Pain") OR (MM "Acute Pain") OR (MM "Neuralgia") OR (MM "Nociceptive Pain") OR (MM "Chronic Pain") OR (MM "Breakthrough Pain") |
| 5 | exp Hyperalgesia/ |
| 6 | exp Pain Perception |
| 7 | exp Pain measurement/ |
| 8 | (“pain prevalence” or “pain intensit*” or “pain qualit*” or “pain characteristic*” or “nociceptive pain” or “nociception” or “chronic pain” or “neuropathic pain” or “hyperalgesia” or “pain perception” or “neuralgia” or “acute pain” or “allodynia” or “pain assessment” or “pain measurement” or “breakthrough pain” or “background pain” or “persistent pain” or “inflammatory pain”).tw. |
| 9 | 4 or 5 or 6 or 7 or 8 |
| 10 | 2 and 9 |
| 11 | Limit 10 to (yr="1990 ‐ 2016" and (classical article or clinical study or clinical trial, all or comparative study or controlled clinical trial or "corrected and republished article" or evaluation studies or journal article or meta‐analysis or multicenter study or observational study or pragmatic clinical trial or randomized controlled trial or "review" or "scientific integrity review" or systematic reviews or validation studies)) |
Medline search strategy
| # | Search |
| 1 | exp leg ulcer/ |
| 2 | exp wounds, chronic/ |
| 3 | (“chronic wound*” or “chronic ulcer*” or “leg wound*” or “leg ulcer*” or “foot ulcer*” or “foot wound*” or “venous ulcer*” or “venous wound*” or “varicose ulcer*” or “varicose wound*” or “venous foot” or “diabetic foot” or “diabetic wound*” or “diabetic ulcer*” or “diabetic foot ulcer*” or “stasis wound*” or “stasis ulcer*”).tw. |
| 4 | 1 or 2 or 3 |
| 5 | (MH "Pain") OR (MM "Breakthrough Pain") OR (MM "Neuralgia") OR (MM "Nociceptive Pain") |
| 6 | exp allodynia/ |
| 7 | exp chronic pain/ |
| 8 | exp hyperalgesia/ |
| 9 | exp pain measurement/ |
| 10 | (“pain prevalence” or “pain intensit*” or “pain qualit*” or “pain characteristic*” or “nociceptive pain” or “nociception” or “chronic pain” or “neuropathic pain” or “hyperalgesia” or “pain perception” or “neuralgia” or “acute pain” or “allodynia” or “pain assessment” or “pain measurement” or “breakthrough pain” or “background pain” or “persistent pain” or “inflammatory pain”).tw. |
| 11 | 5 or 6 or 7 or 8 or 9 or 10 |
| 12 | 4 and 11 |
| 13 | Limit 12 to: Published Date: 19900101‐20161231; Publication Type: Brief Item, Clinical Trial, Corrected Article, Journal Article, Meta Analysis, Meta Synthesis, Questionnaire/Scale, Randomized Controlled Trial, Research, Research Instrument, Review, Statistics, Systematic Review |
Cinahl search strategy
Embase search strategy
| # | Search |
| 1 | exp leg ulcer/ |
| 2 | exp foot ulcer/ |
| 3 | exp diabetic foot/ |
| 4 | exp chronic wound/ |
| 5 | (“chronic wound*” or “chronic ulcer*” or “leg wound*” or “leg ulcer*” or “foot ulcer*” or “foot wound*” or “venous ulcer*” or “venous wound*” or “varicose ulcer*” or “varicose wound*” or “venous foot” or “diabetic foot” or “diabetic wound*” or “diabetic ulcer*” or “diabetic foot ulcer*” or “stasis wound*” or “stasis ulcer*”).tw. |
| 6 | 1 or 2 or 3 or 4 or 5 |
| 7 | Pain (ink. Acute pain)/ or allodynia/ or breakthrough pain/ or chronic pain/ or hyperalgesia/ or neuralgia/ or nociceptive pain/ |
| 8 | nociception/ |
| 9 | pain assessment/ or brief pain inventory/ or mcgill pain questionnaire/ or visual analog scale/ |
| 10 | exp pain measurement/ |
| 11 | Pain intensity/ |
| 12 | (“pain prevalence” or “pain intensit*” or “pain qualit*” or “pain characteristic*” or “nociceptive pain” or “nociception” or “chronic pain” or “neuropathic pain” or “hyperalgesia” or “pain perception” or “neuralgia” or “acute pain” or “allodynia” or “pain assessment” or “pain measurement” or “breakthrough pain” or “background pain” or “persistent pain” or “inflammatory pain”).tw. |
| 13 | 7 or 8 or 9 or 10 or 11 or 12 |
| 14 | 6 and 13 |
| 15 | Limit 14 to (yr="1990 ‐ 2016") and (article or report or "review" or short survey) |
Cochrane search strategy
| # | Search |
| 1 | exp Leg ulcer/ |
| 2 | (”chronic wound*” or “chronic ulcer*” or “leg wound*” or “leg ulcer” or “foot ulcee*” or “foot wound*” or “venous ulcer*” or “venous wound*” or “venous foot” or “varicose ulcer*” or "varicose wound*” or “diabetic foot” or “diabetic wound*” or “diabetic ulcer*” or “diabetic foot ulcer*” or “stasis wound*” or “stasis ulcer*”).tw. |
| 3 | 1 or 2 |
| 4 | (MH "Pain") OR (MM "Acute Pain") OR (MM "Neuralgia") OR (MM "Nociceptive Pain") OR (MM "Chronic Pain") OR (MM "Breakthrough Pain") |
| 5 | exp Hyperalgesia/ |
| 6 | exp Pain Perception |
| 7 | exp Pain measurement/ |
| 8 | (“pain prevalence” or “pain intensit*” or “pain qualit*” or “pain characteristic*” or “nociceptive pain” or “nociception” or “chronic pain” or “neuropathic pain” or “hyperalgesia” or “pain perception” or “neuralgia” or “acute pain” or “allodynia” or “pain assessment” or “pain measurement” or “breakthrough pain” or “background pain” or “persistent pain” or “inflammatory pain”).tw. |
| 9 | 4 or 5 or 6 or 7 or 8 |
| 10 | 2 and 9 |
British Nursing Index search strategy
| # | Search |
| 1 | SU.EXACT("Wounds") OR SU.EXACT("Leg Ulcers") OR "leg ulcer*" OR "foot ulcer*" OR "diabetic foot" OR "varicose ulcer*" OR "chronic wound*" OR "chronic ulcer*" OR "leg wound*" OR "leg ulcer*" OR "foot ulcer*" OR "foot wound*" OR "venous ulcer*" OR "venous wound*" OR "venous foot*" OR "varicose ulcer*" OR "varicose wound*" OR "diabetic foot" OR "diabetic wound*" OR "diabetic ulcer*" OR "diabetic foot ulcer*" OR "stasis wound*" OR "stasis ulcer*" |
| 2 | SU.EXACT("Pain: Measurement") OR SU.EXACT("Pain and Pain Management") OR "pain prevalence" OR "pain qualit*" OR "pain intensit*" OR "pain characteristic*" OR "Nociceptive pain" OR Nociception OR "Chronic pain" or "Neuropathic pain" OR Hyperalgesia OR "pain perception" OR Neuralgia OR "Acute pain" OR Allodynia OR "Pain assessment" OR "Pain measurement" OR "background pain" OR "breakthrough pain" OR "persistent pain" OR "inflammatory pain" |
| 3 | 1 AND 2 |
| 4 | Limit 3 to (Date: After January 01 1990) and (Document type: Article, Case Study, Evidence Based Healthcare, Interview, Literature Review, Review) |
Leren L, Johansen E, Eide H, Falk RS, Juvet LK, Ljoså TM. Pain in persons with chronic venous leg ulcers: A systematic review and meta‐analysis. Int Wound J. 2020;17:466–484. 10.1111/iwj.13296
REFERENCES
- 1. Renner R, Seikowski K, Simon JC. Association of pain level, health and wound status in patients with chronic leg ulcers. Acta Derm Venereol. 2014;94(1):50‐53. [DOI] [PubMed] [Google Scholar]
- 2. Woo KY, Sibbald RG. Chronic wound pain: a conceptual model. Adv Skin Wound Care. 2008;21(4):175‐188. [DOI] [PubMed] [Google Scholar]
- 3. WUWHS . Principles of best practice: minimising pain at wound dressing‐related procedures. A Consensus Document: World Union of Wound Healing Societies; 2004. London: Medical Education Partnership. Retrieved from: http://www.woundsinternational.com/media/issues/79/files/content_39.pdf. [Google Scholar]
- 4. White R. Pain assessment and management in patients with chronic wounds. Nurs Stand. 2008;22(32):62‐68. [DOI] [PubMed] [Google Scholar]
- 5. Stechmiller JK, Lyon D, Schultz G, et al. Biobehavioral mechanisms associated with nonhealing wounds and Psychoneurologic symptoms (pain, cognitive dysfunction, fatigue, depression, and anxiety) in older individuals with chronic venous leg ulcers. Biol Res Nurs. 2019;407‐419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nussbaum SR, Carter MJ, Fife CE, et al. An economic evaluation of the impact, cost, and medicare policy implications of chronic nonhealing wounds. Value Health. 2018;21(1):27‐32. [DOI] [PubMed] [Google Scholar]
- 7. Heyer K, Herberger K, Protz K, Glaeske G, Augustin M. Epidemiology of chronic wounds in Germany: analysis of statutory health insurance data. Wound Repair and Regen. 2016;24(2):434‐442. [DOI] [PubMed] [Google Scholar]
- 8. Guest JF, Ayoub N, McIlwraith T, et al. Health economic burden that wounds impose on the National Health Service in the UK. BMJ Open. 2015;5(12):e009283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Margolisa DJ, Bilkerb W, Santannab J, d Baumgartenc M. Venous leg ulcer: incidence and prevalence in the elderly. J Am Acad Dermatol. 2002;46(3):381‐386. [DOI] [PubMed] [Google Scholar]
- 10. Guo S, DiPietro LA. Factors affecting wound healing. J Dent Res. 2010;89(3):219‐229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Werdin F, Tennenhaus M, Schaller H‐E, Rennekampff H‐O. Evidence‐based management strategies for treatment of chronic wounds. Eplasty. 2009;9:169‐179. [PMC free article] [PubMed] [Google Scholar]
- 12. Vishwanath V. Quality of life: venous leg ulcers. Indian Dermatol Online J. 2014;5(3):397‐399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Woo KY, Sibbald RG, Fogh K, et al. Assessment and management of persistent (chronic) and total wound pain—Reprinted courtesy of Blackwell publishing, who retains the copyright. It first appeared in the international wound J 2008;5(2). W Council Enter Ther J. 2009;29(2):8‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Paul J. A cross‐sectional study of chronic wound‐related pain and itching. Ostomy Wound Manage. 2013;59(7):28‐34. [PubMed] [Google Scholar]
- 15. Salome G, Ferreira L. The impact of decongestive physical therapy and elastic bandaging on the control of pain in patients with venous ulcers. Rev Col Bra Cir. 2018;45(2):e1385. [DOI] [PubMed] [Google Scholar]
- 16. Szewczyk MT, Moscicka P, Cwajda J, Piotrowicz R, Jawien A. Evaluation of the effectiveness of new polyurethane foam dressings in the treatment of heavily exudative venous ulcers. [polish, English]. Acta Angiologica. 2007;13(2):85‐93. [Google Scholar]
- 17. Sussman C, Bates‐Jensen BM. Management of wound pain. Wound care: a collaborative practice manual: Lippincott Williams & Wilkins; 2007.
- 18. Charles H. Venous leg ulcer pain and its characteristics. J of Tissue Viability. 2002;12(4):154‐158. [DOI] [PubMed] [Google Scholar]
- 19. McMullen M. The relationship between pain and leg ulcers: a critical review. Br J Nurs. 2004;13(Sup4):S30‐S36. [DOI] [PubMed] [Google Scholar]
- 20. Grant MJ, Booth A. A typology of reviews: an analysis of 14 review types and associated methodologies. Health Info Libr J. 2009;26(2):91‐108. [DOI] [PubMed] [Google Scholar]
- 21. Pluye P, Hong QN. Combining the power of stories and the power of numbers: mixed methods research and mixed studies reviews. Annu Rev Public Health. 2014;35:29‐45. [DOI] [PubMed] [Google Scholar]
- 22. Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta‐analysis protocols (PRISMA‐P) 2015 statement. Syst Rev. 2015;4(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pope C, Mays N, Popay J. Synthesising Qualitative and Quantitative Health Evidence: A Guide to Methods. UK: McGraw‐Hill Education; 2007. [Google Scholar]
- 24. Pearson A, White H, Bath‐Hextall F, et al. Methodology for JBI mixed methods systematic reviews. Adelaide: The Joanna Briggs Institute. 2014;2014:5‐34. [Google Scholar]
- 25. Pluye P, Robert E, Cargo M, Bartlett G, O'Cathain A, Griffiths F, et al. Proposal: A mixed methods appraisal tool for systematic mixed studies reviews 2011 29.05.2018.
- 26. Pace R, Pluye P, Bartlett G, et al. Testing the reliability and efficiency of the pilot mixed methods appraisal tool (MMAT) for systematic mixed studies review. Int J Nurs Stud. 2012;49(1):47‐53. [DOI] [PubMed] [Google Scholar]
- 27. Collins SL, Moore RA, McQuay HJ. The visual analogue pain intensity scale: what is moderate pain in millimetres? Pain. 1997;72(1–2):95‐97. [DOI] [PubMed] [Google Scholar]
- 28. Kahl C, Cleland JA. Visual analogue scale, numeric pain rating scale and the McGill pain questionnaire: an overview of psychometric properties. Phys Ther Rev. 2005;10(2):123‐128. [Google Scholar]
- 29. Williamson A, Hoggart B. Pain: a review of three commonly used pain rating scales. J Clin Nurs. 2005;14(7):798‐804. [DOI] [PubMed] [Google Scholar]
- 30. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nyaga VN, Arbyn M. Aerts M. Stata module to perform fixed and random effects meta‐analysis of proportions: METAPROP; 2017. [Google Scholar]
- 32. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ. 2003;327(7414):557‐560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Teepe RG, Roseeuw DI, Hermans J, et al. Randomized trial comparing cryopreserved cultured epidermal allografts with hydrocolloid dressings in healing chronic venous ulcers. J Am Acad Dermatol. 1993;29(6):982‐988. [DOI] [PubMed] [Google Scholar]
- 34. Morrell CJ, Walters SJ, Dixon S, et al. Cost effectiveness of community leg ulcer clinics: randomised controlled trial. BMJ. 1998;316(7143):1487‐1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Santilli SM, Valusek PA, Robinson C. Use of a noncontact radiant heat bandage for the treatment of chronic venous stasis ulcers. Adv Wound Care. 1999;12(2):89‐93. [PubMed] [Google Scholar]
- 36. Walters SJ, Morrell CJ, Dixon S. Measuring health‐related quality of life in patients with venous leg ulcers. Qual Life Res. 1999;8(4):327‐336. [DOI] [PubMed] [Google Scholar]
- 37. Wilson J, Arseculeratne Y, Yang Y, Cherry G. Healing venous ulcers with cycloidal multidirectional vibration therapy. J Wound Care. 2002;11(10):395‐398. [DOI] [PubMed] [Google Scholar]
- 38. Navratilova Z, Slonkova V, Semradova V, Adler J. Cryopreserved and lyophilized cultured epidermal allografts in the treatment of leg ulcers: a pilot study. J Eur Acad Dermatol Venereol. 2004;18(2):173‐179. [DOI] [PubMed] [Google Scholar]
- 39. Mermet I, Pottier N, Sainthillier JM, et al. Use of amniotic membrane transplantation in the treatment of venous leg ulcers. Wound Repair Regen. 2007;15(4):459‐464. [DOI] [PubMed] [Google Scholar]
- 40. Cameron J, Hoffman D, Wilson J, Cherry G. Comparison of two peri‐wound skin protectants in venous leg ulcers: a randomised controlled trial. J Wound Care. 2005;14(5):233‐236. [DOI] [PubMed] [Google Scholar]
- 41. Benigni J, Lazareth I, Parpex P, et al. Efficacy, safety and acceptability of a new two‐layer bandage system for venous leg ulcers. J Wound Care. 2007;16(9):385‐390. [DOI] [PubMed] [Google Scholar]
- 42. Jünger M, Arnold A, Zuder D, Stahl HW, Heising S. Local therapy and treatment costs of chronic, venous leg ulcers with electrical stimulation (Dermapulse®): a prospective, placebo controlled, double blind trial. Wound Repair Regen. 2008;16(4):480‐487. [DOI] [PubMed] [Google Scholar]
- 43. Edwards H, Courtney M, Finlayson K, Shuter P, Lindsay E. A randomised controlled trial of a community nursing intervention: improved quality of life and healing for clients with chronic leg ulcers. J Clin Nurs. 2009;18(11):1541‐1549. [DOI] [PubMed] [Google Scholar]
- 44. Brizzio E, Amsler F, Lun B, Blättler W. Comparison of low‐strength compression stockings with bandages for the treatment of recalcitrant venous ulcers. J Vasc Surg. 2010;51(2):410‐416. [DOI] [PubMed] [Google Scholar]
- 45. Schumann H, Calow T, Weckesser S, Müller M, Hoffmann G. Water‐filtered infrared a for the treatment of chronic venous stasis ulcers of the lower legs at home: a randomized controlled blinded study. Br J Dermatol. 2011;165(3):541‐551. [DOI] [PubMed] [Google Scholar]
- 46. Escandon J, Vivas AC, Perez R, Kirsner R, Davis S. A prospective pilot study of ultrasound therapy effectiveness in refractory venous leg ulcers. Int Wound J. 2012;9(5):570‐578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wong IK, Andriessen A, Lee DT, et al. Randomized controlled trial comparing treatment outcome of two compression bandaging systems and standard care without compression in patients with venous leg ulcers. J Eur Acad Dermatol Venereol. 2012;26(1):102‐110. [DOI] [PubMed] [Google Scholar]
- 48. Olyaie M, Rad FS, Elahifar M‐A, Garkaz A, Mahsa G. High‐frequency and noncontact low‐frequency ultrasound therapy for venous leg ulcer treatment: a randomized, controlled study. Ostomy Wound Manage. 2013;59(8):14‐20. [PubMed] [Google Scholar]
- 49. Park KY, Kim IS, Yeo IK, Kim BJ, Kim MN. Treatment of refractory venous stasis ulcers with autologous platelet‐rich plasma and light‐emitting diodes: a pilot study. Journal of Dermatological Treatment. 2013;24(5):332‐335. [DOI] [PubMed] [Google Scholar]
- 50. Beheshti A, Shafigh Y, Parsa H, Zangivand AA. Comparison of high‐frequency and MIST ultrasound therapy for the healing of venous leg ulcers. Advances in Clinical and Experimental Medicine: Official Organ Wroclaw Medical University. 2014;23(6):969‐975. [DOI] [PubMed] [Google Scholar]
- 51. Brown A, Augustin M, Jünger M, et al. Randomized standard‐of‐care‐controlled trial of a silica gel fibre matrix in the treatment of chronic venous leg ulcers. Eur J Dermatol. 2014;24(2):210‐216. [DOI] [PubMed] [Google Scholar]
- 52. Bradbury S, Walkley N, Ivins N, Harding K. Clinical evaluation of a novel topical negative pressure device in promoting healing in chronic wounds. Adv Wound Care. 2015;4(6):346‐357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Brehmer F, Haenssle H, Daeschlein G, et al. Alleviation of chronic venous leg ulcers with a hand‐held dielectric barrier discharge plasma generator (PlasmaDerm® VU‐2010): results of a monocentric, two‐armed, open, prospective, randomized and controlled trial (NCT 01415622). J Eur Acad Dermatol Venereol. 2015;29(1):148‐155. [DOI] [PubMed] [Google Scholar]
- 54. Gibbons GW, Orgill DP, Serena TE, et al. A prospective, randomized, controlled trial comparing the effects of noncontact, low‐frequency ultrasound to standard care in healing venous leg ulcers. Ostomy Wound Manage. 2015;61(1):16‐29. [PubMed] [Google Scholar]
- 55. White J, Ivins N, Wilkes A, Carolan‐Rees G, Harding KG. Non‐contact low‐frequency ultrasound therapy compared with UKstandard of care for venous leg ulcers: a single‐Centre, assessor‐blinded, randomised controlled trial. Int Wound J. 2015;13(5):833‐842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Harding KG, Szczepkowski M, Mikosiński J, et al. Safety and performance evaluation of a next‐generation antimicrobial dressing in patients with chronic venous leg ulcers. Int Wound J. 2016;13(4):442‐448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Vitse J, Bekara F, Byun S, Herlin C, Teot L. A double‐blind, placebo‐controlled randomized evaluation of the effect of low‐level laser therapy on venous leg ulcers. Int J Low Extrem Wounds. 2017;16(1):29‐35. [DOI] [PubMed] [Google Scholar]
- 58. Domingues EAR, Kaizer UAO, Lima MHM. Effectiveness of the strategies of an orientation programme for the lifestyle and wound‐healing process in patients with venous ulcer: a randomised controlled trial. Int Wound J. 2018;15(5):798‐806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Jones J, Barr W, Robinson J, Carlisle C. Depression in patients with chronic venous ulceration. Br J Nurs. 2006;15(Sup2):S17‐S23. [DOI] [PubMed] [Google Scholar]
- 60. Cwajda‐Białasik J, Szewczyk MT, Mościcka P, Cierzniakowska K. The locus of pain control in patients with lower limb ulcerations. J Clin Nurs. 2012;21(23–24):3346‐3351. [DOI] [PubMed] [Google Scholar]
- 61. Goto T, Tamai N, Nakagami G, et al. Can wound exudate from venous leg ulcers measure wound pain status?: a pilot study. PLoS One. 2016;11(12):e0167478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hellström A, Nilsson C, Nilsson A, Fagerström C. Leg ulcers in older people: a national study addressing variation in diagnosis, pain and sleep disturbance. BMC Geriatr. 2016;16(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Closs SJ, Nelson EA, Briggs M. Can venous and arterial leg ulcers be differentiated by the characteristics of the pain they produce? J Clin Nurs. 2008;17(5):637‐645. [DOI] [PubMed] [Google Scholar]
- 64. Eusen M, Brenaut E, Schoenlaub P, Saliou P, Misery L. Neuropathic pain in patients with chronic leg ulcers. J Eur Acad Dermatol Venereol. 2016;30(9):1603‐1605. [DOI] [PubMed] [Google Scholar]
- 65. Szewczyk MT, Mościcka P, Cwajda J, Piotrowicz R, Jawień A. Evaluation of the effectiveness of new polyurethane foam dressings in the treatment of heavily exudative venous ulcers. Acta Angiologica. 2007;13(2):85‐93. [Google Scholar]
- 66. Kappesser J. Williams ACdC. Pain estimation: asking the right questions. Pain. 2010;148(2):184‐187. [DOI] [PubMed] [Google Scholar]
- 67. Woo A, Lechner B, Fu T, et al. Cut points for mild, moderate, and severe pain among cancer and non‐cancer patients: a literature review. An Pall Med. 2015;4(4):176‐183. [DOI] [PubMed] [Google Scholar]
- 68. Parker K. Psychosocial effects of living with a leg ulcer. Nurs Stand. 2012;26(45):50‐6,60,2. [Google Scholar]
- 69. Briggs M, Flemming K. Living with leg ulceration: a synthesis of qualitative research. J Adv Nurs. 2007;59(4):319‐328. [DOI] [PubMed] [Google Scholar]
- 70. Persoon A, Heinen MM, Van Der Vleuten CJ, De Rooij MJ, Van De Kerkhof P, Van Achterberg T. Leg ulcers: a review of their impact on daily life. J Clin Nurs. 2004;13(3):341‐354. [DOI] [PubMed] [Google Scholar]
- 71. Breivik H, Borchgrevink P, Allen S, et al. Assessment of pain. BJA: British Journal of Anaesthesia. 2008;101(1):17‐24. [DOI] [PubMed] [Google Scholar]
- 72. Woo K, Sibbald G, Fogh K, et al. Assessment and management of persistent (chronic) and total wound pain. Int Wound J. 2008;5(2):205‐215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Hjermstad MJ, Fayers PM, Haugen DF, et al. Studies comparing numerical rating scales, verbal rating scales, and visual analogue scales for assessment of pain intensity in adults: a systematic literature review. J Pain Symptom Manage. 2011;41(6):1073‐1093. [DOI] [PubMed] [Google Scholar]
- 74. The British Pain Society (2019) Outcome measures. London: Faculty of Pain Medicine of the Royal College of Anaesthetists. Retrieved from: https://www.britishpainsociety.org/static/uploads/resources/files/Outcome_Measures_January_2019.pdf. 2011;41(6):1073‐1093. [Google Scholar]
- 75. Ballantyne JC, Sullivan MD. Intensity of chronic pain—the wrong metric? New Eng J Med. 2015;373(22):2098‐2099. [DOI] [PubMed] [Google Scholar]
- 76. EWMA . Pain at Wound Dressing Changes. Medical Education Partnership: Position Document; 2002. http://ewma.org/fileadmin/user_upload/EWMA/pdf/Position_Documents/2002/Spring_2002__English_.pdf. [Google Scholar]
- 77. Pain IAFTSo. IASP terminology . https://www.iasp-pain.org/Education/Content.aspx?ItemNumber=1698#Pain2017
- 78. Gottrup F, Apelqvist J, Price P. Outcomes in controlled and comparative studies on non‐healing wounds: recommendations to improve the quality of evidence in wound management. J Wound Care. 2010;19(6):237‐268. [DOI] [PubMed] [Google Scholar]
- 79. Gethin G, Killeen F, Devane D. Heterogeneity of wound outcome measures in RCTs of treatments for VLUs: a systematic review. J Wound Care. 2015;24(5):211‐226. [DOI] [PubMed] [Google Scholar]
- 80. Lazarus G, Valle MF, Malas M, et al. Chronic venous leg ulcer treatment: future research needs. Wound Repair Regen. 2014;22(1):34‐42. [DOI] [PubMed] [Google Scholar]


