Abstract
This study compares two vs six weeks of topical antimicrobial therapy with Cadexomer Iodine in patients with diabetic foot ulcers (DFUs) complicated by chronic biofilm infections. Patients with non‐healing DFUs with suspected chronic biofilm infections were eligible for enrolment. Patients were randomised to receive either two or six weeks of treatment with topical Cadexomer Iodine. Tissue biopsies from the ulcers were obtained pre‐and‐post treatment and underwent DNA sequencing and real‐time quantitative polymerase chain reaction (PCR) to determine the total microbial load, community composition, and diversity of bacteria. Scanning electron microscopy confirmed biofilm in all 18 ulcers with suspected chronic biofilm infections. Cadexomer Iodine resulted in 14 of 18 (78%) samples achieving a mean 0.5 log10 reduction in microbial load. Regardless of treatment duration, there was no statistical difference in the reduction of total microbial loads. No difference in the rate of wound healing in the two groups was seen at 6 weeks. Cadexomer Iodine reduces the total microbial load in DFUs with chronic biofilm infections and affects microbial community composition and diversity. All ulcers in both groups showed an initial reduction in wound size with application of Cadexomer Iodine, which might reflect its effect on biofilms.
Keywords: biofilms, Cadexomer Iodine, diabetic foot ulcer, microbiome
1. INTRODUCTION
Chronic non‐healing wounds represent a substantial increase to health care expenditure, are associated with reduction in a patient's quality of life, and can precede serious events such as lower extremity amputation and mortality.1 The presence of microbial biofilms has been identified as a driver in a wide range of chronic and persistent infections, from those related to implanted medical devices,2, 3 to chronic infections such as those occurring in tissues4, 5 and bone.6 The presence of bacteria in a biofilm phenotype has been proposed as a cause of impaired skin wound healing, contributing to the chronicity of a wound.7, 8, 9, 10, 11
Biofilms possess multiple tolerance mechanisms to antimicrobial treatments, which aids their persistence after exposure.12 In the treatment of chronic biofilm infections, physical removal of the infected tissue or device when possible often represents the optimal therapeutic approach.13, 14 In chronic wounds, physical removal of biofilm typically occurs through debridement of non‐viable and infected tissue and represents one of the most important treatment strategies.14, 15 The method of debridement can be via a surgical approach undertaken in a sterile theatre setting, or alternatively by regular sharp conservative debridement performed in the out‐patient setting.15 Regular debridement is an important facet of care, as biofilm re‐formation and maturation occurs rapidly within two to three days.4, 14, 15 Debridement and physical removal of tissue alone may not be successful in removing all biofilm aggregates because of the macro‐spatial variation within tissues4, 16 and the inability of the treating clinician to visually identify and target biofilm aggregates accurately. As adequate debridement is often not possible, augmentation with multi‐faceted approaches is recommended.14
Some clinicians use topical antimicrobials on wounds complicated by chronic biofilm infections.14 However, in vivo evidence of efficacy of these agents against biofilm is lacking. The ability of Cadexomer Iodine to reduce the total microbial load in diabetic foot ulcers (DFUs) over a seven‐day period has been demonstrated.17 The study design, however, did not correlate reductions in total microbial loads with wound metrics, nor did it establish an optimal treatment duration. It is possible that a short duration of exposure may not allow sufficient time to suppress or reduce biofilm microorganisms to a level that can be managed by the host.18 In this pilot study, DNA sequencing, real‐time quantitative polymerase chain reaction (quantitative PCR [qPCR]), and scanning electron microscopy (SEM) are used to determine the effects of two different treatment durations with Cadexomer Iodine on non‐healing DFUs with suspected chronic biofilm infections.
2. METHODS
2.1. Study design
A pilot prospective cohort study in Sydney, Australia was conducted at an acute tertiary referral hospital High‐Risk Foot Service. Consecutive patients aged over 18 years presenting with a DFU were recruited over an 18‐month period. Patients were eligible for the study if they had a non‐healing DFU with suspected chronic biofilm infection.19 Non‐healing was defined as a failure to respond to standard wound care, in the presence of appropriate offloading or compression therapy and adequate peripheral perfusion, with no reduction in ulcer size since onset. Exclusion criteria were the presence of clinical signs of acute infection as per the International Working Group on the Diabetic Foot—Diabetic Foot Infection Guidelines20 and Infectious Disease Society of America—Diabetic Foot Infection Guidelines,21 osteomyelitis that was associated with the DFU, or use of topical or systemic antimicrobial therapy two weeks prior to enrolment. The reason for excluding patients with acute clinical infection was the assumption that the behaviour of these wounds would be driven predominantly by planktonic microorganisms.22 Participants with contraindications to the use of Cadexomer Iodine as per the manufacturers guidelines were also excluded.23 Participants were randomised by coin toss on a 1:1 allocation basis to receive either two‐weeks or six‐weeks of topical antimicrobial therapy once recruited. Topical Cadexomer Iodine (Iodosorb, Smith and Nephew) was applied to wounds every second day. All patients received standard woundcare including any required offloading, compression therapy, wound bed cleansing with normal saline solution and debridement of non‐viable tissue. Tissue biopsies according to a previously described method17 were obtained from the wound edge for each participant pre‐and‐post treatment (Week 0 and End of Treatment: Week 2 or 6).
The presence or absence of biofilms in DFUs was confirmed through SEM. The total microbial load of DFUs was determined through real‐time qPCR. Microbial communities and any shifts in diversity were explored through next generation DNA sequencing (16S rRNA).
Wound metrics were recorded using an eKare inSight 3D wound camera (eKare inc, Merrifield, Virginia) and clinical metadata were collected from electronic patient medical records. Because of the high variation in ulcer size and shape, each ulcer surface area was transformed into a linear measure of advancing wound edge.24 Changes in this linear measure were calculated at two and six weeks to produce a healing rate for each ulcer. In addition to measuring the size of wounds, we collected observational data using a combination of proposed markers specific to chronic wounds complicated by biofilm.25, 26 This included observations such as the presence of malodour, poor quality wound bed tissue, and gelatin material covering the wound.
2.2. Topical antimicrobial agent Cadexomer iodine (Iodosorb, Smith, and Nephew)
Iodosorb ointment is designed as a carrier system enabling the delivery of iodine, which can penetrate the cell wall of microorganisms and disrupt protein and nucleic acid structure and synthesis.27 Iodosorb consists of small polysaccharide beads (Cadexomer) containing 0.9% iodine, which, in the presence of wound exudate, swell and allow a slow sustained release of iodine into the wound.
2.3. Tissue processing and analysis workflow for DNA sequencing, qPCR and SEM
The tissue processing and analysis workflows have been described previously.17 Sample collection and storage, DNA extraction, next generation DNA sequencing, sequence analysis and quality control, qPCR, and the characterisation and visualisation of biofilms in DFUs using SEM are described in full in S1. All tissue samples were stored until the end of the study recruitment period and processed in bulk. Participant identifiers and group allocation were not disclosed to the scientist undertaking the analyses.
2.4. Outcomes
The primary outcome was a difference in microbial load calculated as pre‐and‐post Log10 reduction (16S copies per mg of tissue) between the two cohorts. All patient outcomes were followed until the study end of 12‐weeks. Secondary outcomes included changes to microbial diversity and communities pre‐treatment and post‐treatment, confirmation of biofilm within wound samples, changes in the linear measure of the ulcer size, and healing of the ulcer.
2.5. Statistics
Wound metrics and microbiome data were analysed through Statistical Package for Social Sciences Version 23 (SPSS Inc., Chicago, Illinois). Statistical analysis was undertaken by an investigator not involved with study recruitment, and unaware of participant allocation. 16S copy numbers per mg of tissue were log transformed to create normally distributed data. Student's t test for independent samples was used to analyse pre‐treatment and post‐treatment Log10 reductions. As the sample size is small, ulcer healing rates are not normally distributed. Independent‐samples median test was used to analyse changes in both the linear measure of ulcer size (LMUS) and the healing rate of ulcers between groups after 2 and 6 weeks of treatment. Fisher's Exact test was used to test for differences between the groups in the proportion of patients achieving full healing. For all comparisons and modelling, the level of significance was set at P < .05.
2.6. Human Research Ethics
Ethics approval for this study was granted by the South West Sydney Local Health District Research and Ethics Committee (HREC/16/LPOOL/491). Informed written consent was obtained prior to enrolment in keeping with ethics compliance.
3. RESULTS
A total of 18 patients with DFUs were enrolled and completed the full study duration. No adverse events were reported. Patient demographics and wound metrics are shown in S2. The demographic differences between the treatment groups that demonstrated significance was; duration of DFU prior to enrolment (Treatment Group 2 Weeks = 15.8 ± 11.3; Treatment Group 6 Weeks = 32.3 ± 31.1, P = .26, confidence interval [CI] −41 to 8.2). The presence of biofilm was confirmed in all 18 participants with non‐healing DFUs with suspected chronic biofilm infections using SEM (Figure 1). The tissue samples from two patients contained low numbers of 16S rRNA gene sequence reads and were removed from the microbial community analysis, leaving sixteen samples available for analysis.
Figure 1.
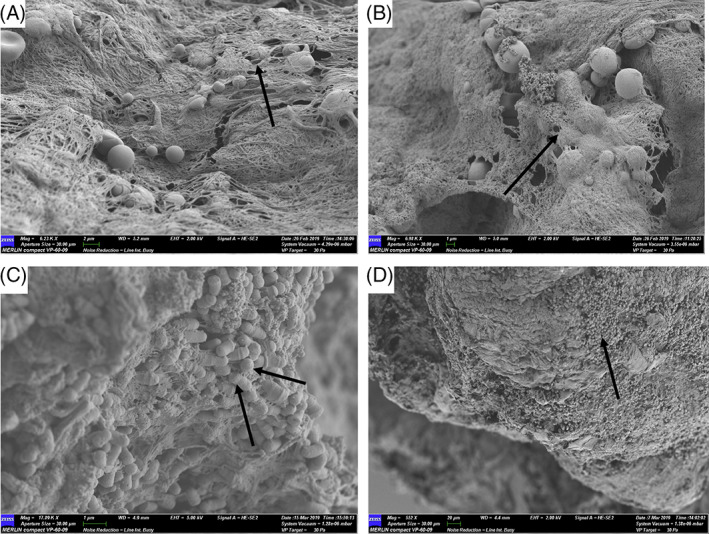
Scanning electron microscopy of select patient samples to illustrate the confirmed presence of biofilm. A, P18, arrow demonstrating coccoid microorganisms under a dense extracellular polymeric substance (EPS). B, P8, arrow demonstrates a small coccoid shaped microbial aggregate under a dense EPS. C, P9 demonstrates aggregates of both rod and coccoid microorganisms, DNA sequencing identifies the presence of Pseudomonas spp., Enterococcus spp., Streptococcus spp., and Staphylococcus spp. D, P5 illustrates a large dense aggregate of coccoid microorganisms interspersed with planktonic cells. P = patient
3.1. Total microbial load of DFUs complicated by chronic biofilm infections
At baseline, the total microbial loads between cohorts were not statistically different (two weeks treatment cohort = 4.3 Log10 (±0.34) 16S copies/mg of tissue vs six weeks treatment cohort = 4.5 Log10 (±0.73) 16S copies/mg of tissue, P = .36 95% CI −0.7 to 0.4 Log10). There was no statistical difference between cohorts with respect to the mean total microbial loads (Figure 2) (two weeks treatment cohort total microbial load = 4.1 Log10 16S copies/mg of tissue and 0.35 Log10 (±0.36) reduction vs six weeks treatment cohort total microbial load = 4.1 Log10 (±0.54) 16S copies/mg of tissue and 0.5 Log10 (±0.71) reduction 16S copies/mg of tissue, P = .71 95% CI −0.6 to 0.45 Log10). When patients were grouped regardless of treatment duration, the application of Cadexomer Iodine resulted in 14 of 18 samples achieving a mean 0.5 log10 reduction in microbial load (mean microbial load pre‐treatment = 4.5 log10 [± 0.6] 16S copies/mg of tissue vs mean microbial load post‐treatment 4.0 log10 16S copies/mg of tissue, range 0‐2.1 Log10, P = .04, 95% CI 0 to 0.7 log10).
Figure 2.
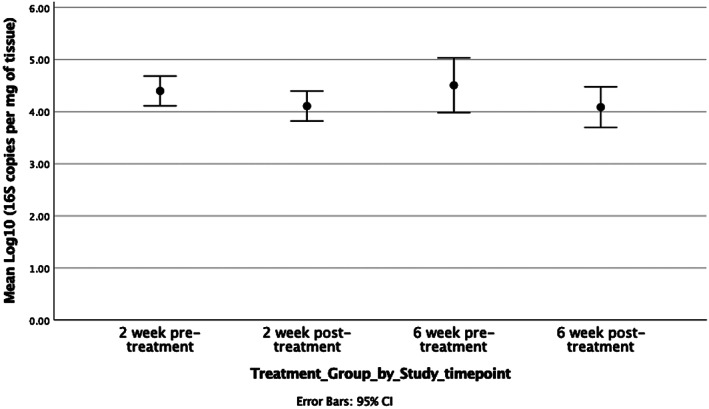
Pre‐treatment and post‐treatment time points for each respective cohorts' mean total microbial loads and corresponding Log10 reductions. CI, confidence interval
3.2. Microbial community composition and diversity of DFUs treated with Cadexomer Iodine
Next‐generation DNA sequencing generated 9 111 558 sequences, which were denoised to show 1227 sub‐operational‐taxonomic‐unit (sOTUs). The denoising of sOTUs at the genera level is noted for individual patients at timepoint 0 (week 0 to initial presentation) in Figure 3. The most commonly sequenced and abundant microorganisms from patients at initial presentation (week 0 to pre‐treatment) were Corynebacterium spp., Staphylococcus spp., Morganella spp., Enterobacteriaceae, Prevotella spp., and Pseudomonas spp.
Figure 3.
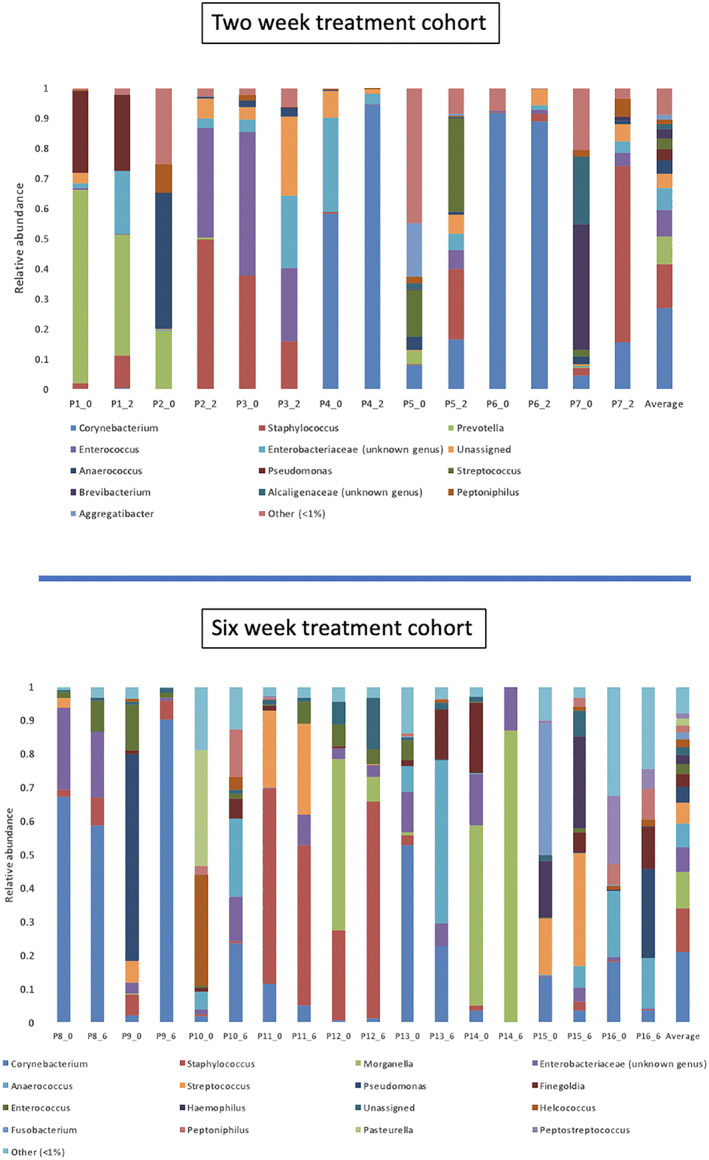
Bar chart of relative abundance at baselines (_0) and end of treatment (EOT) for each treatment group (EOT two week treatment group = _2 or EOT six week treatment group = _6)
The application of Cadexomer Iodine resulted in shifts to microbial community composition, richness, and diversity between pre‐treatment and post‐treatment samples in both the two‐week and six‐week treatment cohorts. These shifts were only evident when viewing the patient‐specific data owing to the large heterogeneity in the data set. Shifts in community composition were generally underpinned by alterations in the relative abundance of key potentially pathogenic genera (Figure 3). This can be demonstrated in patient samples such as; Patient nine (six‐week treatment cohort) in whom pre‐treatment genera of Pseudomonas spp., Enterococcus spp., and Streptococcus spp. were high in relative abundance but were absent post‐treatment. Patient three (two‐week treatment cohort) demonstrated post‐treatment reductions in relative abundance of both Staphylococcus spp. and Enterococcus spp. by 20%. Conversely, some patients had minor or no shifts in community composition; Patient six (two‐week treatment cohort) in whom pre‐treatment Corynebacterium spp. relative abundance was 92% and post‐treatment Corynebacterium spp. was 88%.
Analysis of the pooled data for pre‐treatment and post‐treatment samples in each treatment cohort identified no differences in microbial composition (S4), species richness (sOTUs) and diversity (Shannon index) (S5). However, analysis of individual samples identified trends in alpha diversity, which were patient specific (Figure 4). Additionally, these trends did not correlate with patients experiencing either lower or higher total microbial loads or percentage reductions in ulcer surface area. Microbial community shifts were also reflected in the Bray‐Curtis dissimilarity between samples (Figure 5). This measure to quantify the differences in species populations between pre‐treatment and post‐treatment samples varied significantly but did not appear to be related to the degree of percentage reduction in the ulcer surface area. For example, patient eight (41% reduction in ulcer surface area) demonstrated a highly similar community between their pre‐treatment and post‐treatment samples, whereas patient ten (32% reduction in ulcer surface area) had a highly dissimilar community.
Figure 4.
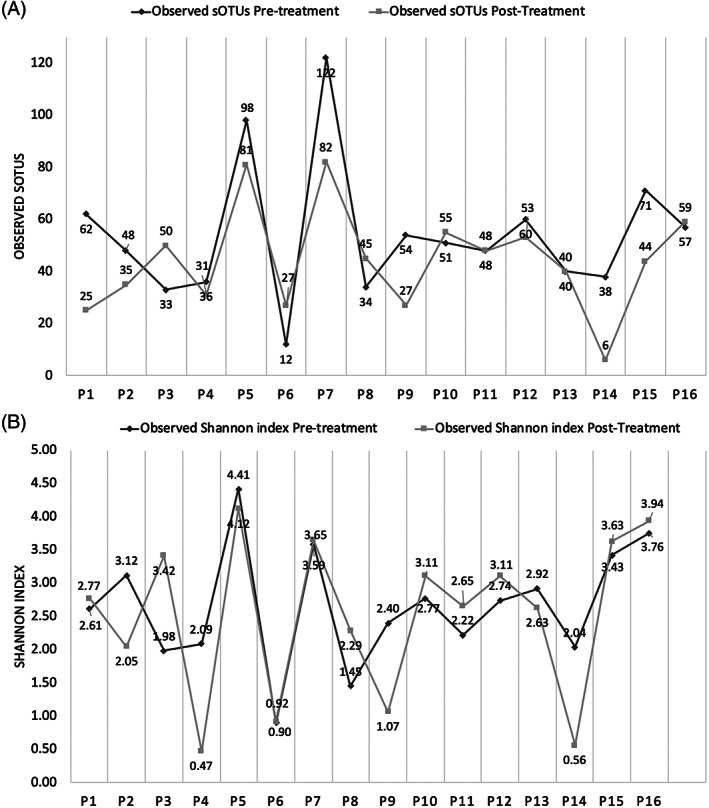
Individual patient level data for A, Species richness (the number of sub‐operational‐taxonomic‐unit) and B, Shannon index (the number of unique microbial taxa and their relative evenness). The graphs demonstrate no trends for richness or diversity to either increase or decrease after treatment with Cadexomer Iodine. Patients 1–7 represent the two‐week treatment cohort and patients 8–16 represent the six‐week treatment cohort
Figure 5.
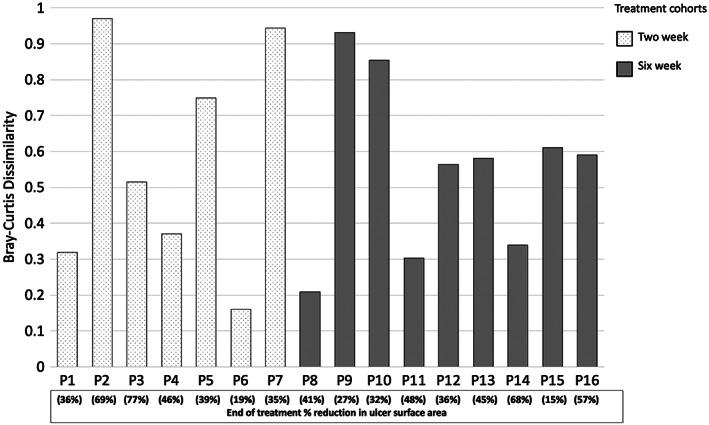
Bray‐Curtis is a dissimilarity measure based on the difference in rank‐abundances of taxa between samples. The closer the metric reaches to number one, the more dissimilar the community structure. The closer to zero denotes the community structure is highly similar. Patients 1–7 represent the two‐week treatment cohort and patients 8–16 represent the six‐week treatment cohort
3.3. Wound metrics
Wound metrics were recorded for all patients at each time‐point. There was no significant difference between patients with regard to the duration of ulcer (S6) or median LMUS at baseline for the two‐week treatment group (Group 1) and the 6‐week treatment group (Group 2) (Group 1 median LMUS 12.2 mm, range 8–39 mm, Group 2 median LMUS 16.3 mm, range 9.5–28.4 mm) (S7). At timepoint week two, there was no significant difference between the median LMUS of the two groups (Group 1 median LMUS 9.4 mm, range 6.4–22.6 mm, Group 2 median LMUS 14.1 mm, range 7.4–24.4 mm). Similarly, at timepoint week 6, there was no significant difference between the median LMUS of the two groups (Group 1 median LMUS 6 mm, range 0–20.5 mm, Group 2 median LMUS 12.2 mm, range 6.2–22.6 mm), and again at timepoint 12 weeks (Group 1 median LMUS 0 mm, range 0–18 mm, Group 2 median LMUS 8.8 mm, range 0–17.8 mm). At the end of the study (Week 12), five of eight patients in the two‐week treatment cohort had healed compared with two of ten patients in the six‐week treatment cohort but this was non‐significant (P = .145). There was no significant difference in the baseline median LMUS for those DFUs that had healed at 12 weeks and those that had not (Healed median LMUS 16.5 mm, range 8–28.4 mm, Non‐healed median LMUS 15.3 mm, range 9.5–30.9 mm). Additional data on arbitrary wound observations identified improvements from baseline (pre‐treatment) to post‐treatment in other areas of wound metrics (S9). Patients experienced a reduction in; exudate levels, malodour, poor quality wound bed tissue (ie, improvements in granulation tissue formation), chronic infective symptoms, pyocyanin, and fibrin slough formation.
4. DISCUSSION
Using a combination of DNA sequencing and microscopy approaches, we have previously illustrated that Cadexomer Iodine can reduce the total microbial load (range 0‐2 Log10) of DFUs complicated by biofilm over a seven‐day treatment period.17 However, from a clinical perspective the actual duration of treatment of topical antimicrobials that patients receive typically extends beyond that of a one‐week period. Despite using topical antimicrobials in wounds complicated by biofilm, there is little to no evidence on their in vivo performance against biofilms/microbial communities.17, 28
In this study, we demonstrate that Cadexomer Iodine reduces the total microbial load of DFUs complicated by biofilm and causes a shift in microbial composition, richness and diversity. However, in the design of this study, we hypothesised that biofilms in chronic wounds are extremely tolerant to treatment, and as such may require longer treatment durations to effect biofilm microorganisms to a level that can be managed by the host.18 The results from this study suggest this null hypothesis may not be reflective of what occurs clinically. Regardless of the treatment duration, there was no statistical difference in the reduction of total microbial loads in DFUs treated for two weeks (0.35 Log10, range = 0.1 to 0.9 Log10) compared with those treated for six weeks (0.5 Log10, range = 0.1 to 2.1 Log10, P = .71), nor did treating for a longer duration causes any differences to microbial composition, richness or diversity when compared with a shorter treatment duration. However, with an adequately powered study, a statistically significant difference may be identified. These findings are in alignment with a previous study by Schwartz et al who used conventional culture to illustrate treatment with Cadexomer Iodine resulted in a one‐two Log10 reduction (median 0.3 Log10 reduction) in DFU bioburden.23
The application of Cadexomer Iodine treatment resulted in shifts to microbial community composition (as defined by shifts in relative abundance), richness and diversity between time points for both two‐week and six‐week treatment cohorts. Of note, patient specific shifts were underpinned by changes (typically reductions) in the relative abundance of key potentially pathogenic genera. This can be demonstrated in patient samples such as; Patient nine (six‐week treatment cohort), where pre‐treatment genera including the high relative abundance of Pseudomonas spp., Enterococcus spp., and Streptococcus spp. were absent post‐treatment. Patient 3 (two‐week treatment cohort) with demonstrated post‐treatment reductions in Staphylococcus spp. and Enterococcus spp. Conversely, in Patient six (two‐week treatment cohort), we observed no reductions in the total microbial load (pre‐treatment Log10 = 4.1 vs post‐treatment Log10 = 4.2) and a minor shift in community composition (pre‐treatment = Corynebacterium spp. relative abundance 92% and post‐treatment Corynebacterium spp. 88%) and diversity (pre‐treatment Shannon index = 0.9 vs post‐treatment Shannon Index = 0.92). The shifts in microbial communities and reductions in potentially key microorganisms identified in this study are similar to findings reported by Loesche et al, who undertook an analysis of the temporal dynamics of chronic wound microbiomes using 16S amplicon sequencing.29 Loesche et al reported that shifts in microbial communities were associated with faster healing and improved outcomes. Exposure to systemic antibiotics also destabilised wound microbiota, rather than altering overall diversity or relative abundance of specific taxa.
The primary goals in managing infected ulcers are to eliminate the source of infection and then obtain skin coverage. The latter is a key marker of success and resolution of any infective process.30 If skin coverage is not achieved, then risk of re‐infection is increased. Cadexomer Iodine may disrupt a pathogenic biofilm through reducing the total microbial load and by affecting the community composition and diversity. Disruption to a chronic infective process may allow the host to restore the normal reparative process of wound healing. In small ulcers, it is attractive to hypothesise that there is less tissue to repair and therefore the duration of treatment required to disrupt any pathogenic process could be shorter in contrast to that required for larger ulcers which have more tissue to repair, and in which an early discontinuation of topical Cadexomer Iodine may allow a pathogenic process to re‐establish itself. Larger ulcers may therefore require longer treatment durations to maintain microbial load reduction (which this study shows does not occur), to continue in disrupting community cohesion of the biofilm to allow healing. However, there was no significant difference at baseline between the median LMUS of those ulcers that healed and those that did not, which argues against this hypothesis.
While application of Cadexomer Iodine disrupts susceptible biofilms, and this might allow healing to occur through removal of this barrier, there might be other factors such as off‐loading, change of footwear, regular cleaning of the DFU, differences in arterial and venous microcirculation, change of daily routines of the patient, and so on that could affect healing of the DFUs in the sample. All patients had improved in LMUS at week six, but two patients in each group had deteriorated when assessed again at week twelve. In this instance, it would seem beneficial that once a barrier to wound healing is removed/managed, that advanced therapies (such as negative‐pressure wound therapy, or skin substitutes) may be useful to support faster wound closure.14 This would be a possible direction for future studies.
In conclusion, topical antimicrobial agents can directly impact the microbial load and composition of wounds, but this does not necessarily translate into greater rates of wound healing, particularly if the quality of studies for a topical antimicrobial agent and its performance against biofilm is low. Cadexomer Iodine has previously been shown to support higher rates of healing in venous leg ulcers,31 however, a recent systematic review suggests that there is only low‐certainty evidence that shows that antimicrobial dressings may increase the number of DFUs healed in the medium‐term.32 Therefore, the primary rationale for clinicians to initiate the use of a topical antimicrobial agent should be to aid in the removal of microorganism/biofilm barrier for healing to progress and not as a direct measure to improve wound healing.
4.1. Study limitations
The numbers in each group are small, and the power of the study to detect a difference in healing rates is low. The study was designed to assess the effect size of two different durations of application of Cadexomer Iodine, and not the effect on healing.
In this study, we obtained single‐tissue biopsies located near the edges of the wound, with subsequent post‐treatment biopsies obtained as near to the index biopsy as technically possible. Microorganisms may not form in a homogenous distribution across the wound surface and thus a single biopsy may not be representative of the species present. It would not, however, be ethically feasible to excise an entire wound in vivo for analysis. Other studies exploring the microbiome of wounds have opted for use of a superficial swab technique to sample over a larger surface area,7 but this technique may not detect microorganisms that have invaded deeper into tissue. Both techniques have their strengths and weakness. There are limitations of qPCR (based on 16 seconds rRNA gene) with its inability to distinguish from viable and non‐viable cells in samples predominantly composed of biofilm phenotype cells with low metabolism.33 The log reductions noted in this study, therefore, represent a minimal response. Some of the bacteria detected by qPCR could be dead, resulting in a lower calculable efficacy for Cadexomer Iodine.
ACKNOWLEDGEMENTS
We would like to acknowledge the support of South West Sydney LHD who presented the lead author with an early career research award, allowing the undertaking of this project. This work was supported by a research grant awarded by Smith and Nephew that contributed to the analysis of tissue samples using DNA sequencing and microscopy techniques. M.M. received a research grant from Smith and Nephew for this study. All other authors have nothing to declare.
Malone M, Schwarzer S, Radzieta M, et al. Effect on total microbial load and community composition with two vs six‐week topical Cadexomer Iodine for treating chronic biofilm infections in diabetic foot ulcers. Int Wound J. 2019;16:1477–1486. 10.1111/iwj.13219
Funding information Smith and Nephew
REFERENCES
- 1. McCosker L, Tulleners R, Cheng Q, et al. Chronic wounds in Australia: a systematic review of key epidemiological and clinical parameters. Int Wound J. 2019;16:84‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vickery K, Hu H, Jacombs AS, Bradshaw AB, Deva AK. A review of bacterial biofilms and their role in device‐associated infection. Healthcare Infect. 2013;18:61‐66. [Google Scholar]
- 3. Stoodley P, Kathju S, Hu FZ, et al. Molecular and imaging techniques for bacterial biofilms in joint arthroplasty infections. Clin Orthop Relat Res. 2005;437:31‐40. [DOI] [PubMed] [Google Scholar]
- 4. Johani K, Malone M, Jensen S, et al. Microscopy visualisation confirms multi‐species biofilms are ubiquitous in diabetic foot ulcers. Int Wound J. 2017;14(6):1160‐1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Malone M, Bjarnsholt T, McBain AJ, et al. The prevalence of biofilms in chronic wounds: a systematic review and meta‐analysis of published data. J Wound Care. 2017;26:20‐25. [DOI] [PubMed] [Google Scholar]
- 6. Malone M, Johani K, Jensen SO, Gosbell IB, Dickson HG, Vickery K. Next generation DNA sequencing of tissues from infected diabetic foot ulcers. EBioMedicine. 2017;21:142‐149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kalan L, Loesche M, Hodkinson BP, et al. Redefining the chronic‐wound microbiome: fungal communities are prevalent, dynamic, and associated with delayed healing. mBio. 2016;7:e01058‐e01016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhao G, Hochwalt PC, Usui ML, et al. Delayed wound healing in diabetic (db/db) mice with Pseudomonas aeruginosa biofilm challenge – a model for the study of chronic wounds. Wound Repair Regen. 2010;18:467‐477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhao G, Usui ML, Underwood RA, et al. Time course study of delayed wound healing in a biofilm‐challenged diabetic mouse model. Wound Repair Regen. 2012;20:342‐352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Trøstrup H, Lerche CJ, Christophersen LJ, et al. Chronic Pseudomonas aeruginosa biofilm infection impairs murine S100A8/A9 and neutrophil effector cytokines—implications for delayed wound closure? Pathog Dis. 2017;75:1‐10. [DOI] [PubMed] [Google Scholar]
- 11. Marano RJ, Wallace HJ, Wijeratne D, Fear MW, Wong HS, O'Handley R. Secreted biofilm factors adversely affect cellular wound healing responses in vitro. Sci Rep. 2015;5:13296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lewis K. Persister cells and the riddle of biofilm survival. Biochemistry (Mosc). 2005;70:267‐274. [DOI] [PubMed] [Google Scholar]
- 13. Høiby N, Bjarnsholt T, Moser C, et al. ESCMID* guideline for the diagnosis and treatment of biofilm infections 2014. Clin Microbiol Infect. 2015;21:S1‐S25. [DOI] [PubMed] [Google Scholar]
- 14. Gregory S, Thomas B, James GA, et al. Consensus guidelines for the identification and treatment of biofilms in chronic nonhealing wounds. Wound Repair Regen. 2017;25:744‐757. [DOI] [PubMed] [Google Scholar]
- 15. Wolcott RD, Rumbaugh KP, James G, et al. Biofilm maturity studies indicate sharp debridement opens a time‐dependent therapeutic window. J Wound Care. 2010;19:320‐328. [DOI] [PubMed] [Google Scholar]
- 16. Price LB, Liu CM, Frankel YM, et al. Macro‐scale spatial variation in chronic wound microbiota: a cross‐sectional study. Wound Repair Regen. 2011;19:80‐88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Malone M, Johani K, Jensen SO, et al. Effect of cadexomer iodine on the microbial load and diversity of chronic non‐healing diabetic foot ulcers complicated by biofilm in vivo. J Antimicrob Chemother. 2017;72:2093‐2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Castaneda P, McLaren A, Tavaziva G, Overstreet D. Biofilm antimicrobial susceptibility increases with antimicrobial exposure time. Clin Orthop Relat Res. 2016;474:1659‐1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Frykberg RG, Banks J. Challenges in the treatment of chronic wounds. Adv Wound Care. 2015;4:560‐582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lipsky BA, Aragón‐Sánchez J, Diggle M, et al. IWGDF guidance on the diagnosis and management of foot infections in persons with diabetes. Diabetes Metab Res Rev. 2016;32:45‐74. [DOI] [PubMed] [Google Scholar]
- 21. Lipsky BA, Berendt AR, Cornia PB, et al. Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis. 2012;54:e132‐e173. [DOI] [PubMed] [Google Scholar]
- 22. Wolcott R, Costerton JW, Raoult D, Cutler SJ. The polymicrobial nature of biofilm infection. Clin Microbiol Infect. 2013;19:107‐112. [DOI] [PubMed] [Google Scholar]
- 23. Schwartz JA, Lantis JC, Gendics C, Fuller AM, Payne W, Ochs D. A prospective, non comparative, multicenter study to investigate the effect of cadexomer iodine on bioburden load and other wound characteristics in diabetic foot ulcers. Int Wound J. 2013;10:193‐199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cukjati D, Reberšek S, Miklavčič D. A reliable method of determining wound healing rate. Med Biol Eng Comput. 2001;39:263‐271. [DOI] [PubMed] [Google Scholar]
- 25. Metcalf DG, Bowler PG, Hurlow J. A clinical algorithm for wound biofilm identification. J Wound Care. 2014;23:137‐142. [DOI] [PubMed] [Google Scholar]
- 26. Gardner SE, Hillis SL, Frantz RA. Clinical signs of infection in diabetic foot ulcers with high microbial load. Biol Res Nurs. 2009;11:119‐128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Edwards‐Jones V. Essential Microbiology for Wound Care. Manchester, UK: Oxford University Press; 2016. [Google Scholar]
- 28. Johani K, Malone M, Jensen SO, et al. Evaluation of short exposure times of antimicrobial wound solutions against microbial biofilms: from in vitro to in vivo. J Antimicrob Chemother. 2018;73:494‐502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Loesche M, Gardner SE, Kalan L, et al. Temporal stability in chronic wound microbiota is associated with poor healing. J Invest Dermatol. 2017;137:237‐244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jia L, Parker CN, Parker TJ, et al. Incidence and risk factors for developing infection in patients presenting with uninfected diabetic foot ulcers. PLoS One. 2017;12:e0177916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. O'Meara S, Al‐Kurdi D, Ologun Y, Ovington LGR. Antibiotics and antiseptics for venous leg ulcers. Cochrane Database Syst Rev. 2014;1:CD003557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dumville JC, Lipsky BA, Hoey C, Cruciani M, Fiscon M, Xia J. Topical antimicrobial agents for treating foot ulcers in people with diabetes. Cochrane Database Syst Rev. 2017;14:CDC011038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Weigel KM, Jones KL, Do JS, et al. Molecular viability testing of bacterial pathogens from a complex human sample matrix. PLoS One. 2013;8:e54886. [DOI] [PMC free article] [PubMed] [Google Scholar]


