Abstract
The effective use of larvae of the greenbottle fly, Lucilia sericata, in wound debridement requires a working knowledge of how feeding changes over time. Using a laboratory assay and bagged larval dressings, the effect of incubation time on larval feeding rates and body mass was investigated for up to 120 hours at 32°C. The mass of tissue digested increased significantly in incremental 24‐hour periods up to 72 hours, with no significant consumption occurring afterwards. Larval mass increased only up to 48 hours. A further test comparing the efficacy of a single 96‐hour application of larvae against two consecutive 48‐hour applications found that the mass of tissue digested in the latter was 14.3% higher than the former, a difference that was statistically significant. Current clinical guidance suggests a 4‐day application period for bagged larvae. Based on these results, an incubation time of 72 hours (3 days) for bagged larvae would be the most effective at the study temperature. However, it is acknowledged that wound temperature can vary, whereby feeding rates would likely differ. In view of this, we conclude that a period of 3 to 4 days is optimum for the application of larvae, and current guidelines should be adhered to.
Keywords: debridement, development time, larval therapy, Lucilia sericata, maggots
1. INTRODUCTION
Larval therapy is the therapeutic use of blowfly larvae to treat chronic, non‐healing wounds. Delays in wound healing can be caused by a variety of factors, including chronic disease; vascular insufficiencies; advanced age; neurological defects; nutritional deficiencies; or local factors such as infection, pressure, and oedema, and chronic wounds are characterised by a prolonged and self‐perpetuating inflammatory response that can be difficult to manage.1, 2 The failure to progress past this chronic state of inflammation can cause a cascade of abnormal tissue responses, which can generate and amplify a hostile microenvironment inside the wound, resulting in the accumulation of cellular debris on the surface. Unrestrained proteolytic activity and a disturbed oxidant/antioxidant balance can also cause further damage to the surrounding tissue, leading to infection and necrosis.3 To aid the process of wound healing and allow it to progress past the inflammatory stage, it is vital that this necrotic tissue is quickly and effectively debrided.4, 5 In larval therapy, medicinal maggots are introduced into the chronic wound to undertake this process of debridement. Primarily, larvae of the greenbottle fly, Lucilia sericata (Diptera: Calliphoridae), are used.
The efficacy of larval therapy in debridement has been proven clinically. Several clinical studies have been conducted to compare the efficacy of larval therapy with conventional treatment methods in debriding chronic wounds. A systematic review of the clinical studies of larval therapy noted 12 comparative studies, including six randomised controlled trials, from the years 2000 to 2014.6 Based on the analysis of these 12 studies, the authors concluded that larval therapy is both more effective and more efficient in the debridement of chronic ulcers when compared with conventional treatments. They also associated larval therapy with other benefits, including quicker healing rate of chronic wounds, a shortened time to healing in ulcers, a longer antibiotic‐free time period, decreased amputation risk, and similar antibiotic usage compared with conventional therapies.6 A different meta‐analysis of seven clinical studies from 1995 to 2009, including three randomised controlled trials and four non‐randomised trials, suggested that, whilst larvae were effective in debridement, there was not enough evidence to show that they were more effective than conventional treatments.7
In addition to debridement, the therapy is also associated with numerous secondary benefits. The larvae of L. sericata have been shown to possess significant antibacterial capabilities, not only by the removal of necrotic tissue but also through the antimicrobial action of their secretions.8, 9, 10 Larval therapy has also been implicated in promoting the growth of and appearance of extracellular matrix or granulation tissue, which may help to enhance new tissue formation.11, 12 In addition, other noted benefits of larval therapy have been suggested, including reperfusion, reduction in inflammation, and antifungal properties.13, 14
The ability of medicinal maggots to debride is primarily attributed to the way in which they feed. Being necrophagous by nature, the larvae break down and consume necrotic tissue enzymatically by a process of extracorporeal digestion. This is achieved by the release of excretions/secretions containing a mixture of proteolytic, glycolytic, lipolytic, and nuclease enzymes onto the tissue surface, causing liquefaction and digestion of the necrotic tissue, which is subsequently ingested.15, 16, 17, 18, 19 However, as effective as this feeding mechanism may be, the larvae cannot feed indefinitely. Newly hatched larvae will feed through three larval instar stages before reaching a pre‐pupal wandering stage.20 This wandering stage is characterised by the cessation of feeding and migration away from the feeding site as the larva begins to search for a suitable location to begin pupation.21 Because of the ability of the larvae to digest tissue being confined to these stages of development, a single treatment of larval therapy will only be effective in debridement for this limited timespan.
The current investigation focussed on the use of bagged larvae. In this method, the larvae are sealed inside a porous polymer bag, which allows for the flow of larval excretions and secretions and the liquefied necrotic tissue and wound exudate out of and into the bag whilst keeping the larvae contained.22, 23 This facilitates an ease of use in the application and removal of the larvae.24
Recommended application times for bagged larvae are variable. Guidance from medicinal larvae producer BioMonde (Bridgend, UK) recommends an application period of a maximum of 4 days for their bagged larvae products,25 although Thomas21 suggests that treatment times using bagged larvae can last 2 to 5 days. Using a wound model, Blake et al26 found that significantly more tissue was metabolised after 4 days than after 3 and, therefore, recommended a 4‐day application period. More recently, Čičková et al27 found that larval growth ceased after 48 hours when using a similar model and recommended an application period of 48 to 72 hours for bagged larvae.24, 27 Controlled trials using bagged larvae have also used application times of 2 to 3 days24, 28 and 3 to 4 days.29 Some research has also previously been undertaken in the area of larval development duration, although much of it relates to their usefulness as a forensic tool. These studies tend to focus on minimum development times and give little information about how the larvae feed over time,30, 31, 32, 33 so attempting to extrapolate useful larval therapy application times from these is only of limited value.
Debridement is often an imperative of wound treatment, and any delay in the time to debridement can, in turn, delay the wound‐healing process.34 A working knowledge of the feeding activity of medicinal larvae over time is, therefore, essential for the effective application of this treatment. Considering the significance of debridement to the wound treatment method and a current lack of consensus regarding a recommended application time of bagged larval therapy products, a clearer understanding of feeding and digestion processes of medicinal larvae over time is needed.
The aims of this study were, first, to investigate the activity of bagged medicinal grade L. sericata larvae over the course of an application period of up to 120 hours in a laboratory assay and, second, to determine the efficacy of larval feeding processes over this time. In addition, we sought to investigate whether two treatments of bagged larvae applied for 48 hours, one being replaced by the other, would result in greater total consumption than a single treatment applied for 96 hours. Such information would prove significant in understanding the digestive processes of larvae over time and in formulating an optimal application period of this larval therapy treatment.
2. METHODS
This study made use of a “larval activity assay” as described previously.35 The assay uses an aerated container fitted with a sponge, containing a mass of minced pork loin upon which the larvae feed. Efficacy in digestion is then indicated by the mass of tissue consumed by the larvae over time. Medicinal‐grade larvae were supplied by BioMonde in vials, each containing approximately 200 individuals, along with bagged larval dressings, measuring 25 × 40 mm, and foam spacers, measuring 8 × 8 × 10 mm. Upon set‐up of the tests, the larvae were second instar.
For every test iteration, 50 larvae were counted into each BioBag50, along with a foam spacer, before being heat sealed. The assay was constructed as described previously,35 but with some modifications. A mass of 15 g of minced pork tissue was used as the feeding substrate in this instance as this was found to be enough to ensure that there was feeding material available throughout the 120 hours. The construction of the assay was also modified to include an absorbent cotton pad placed over the BioBag, which absorbed excess fluid produced by the larval action, reducing the risk of larvae suffocating inside the dressing.
2.1. Time series test
The assays were incubated at 32°C ± 1°C for five time periods: 24, 48, 72, 96, and 120 hours, with 12 repeats conducted for each experiment, along with three control repeats that contained no larvae. This temperature was used as previous research has indicated this to be the approximate average wound surface temperature.26, 36, 37 After the designated time period, the assay container was removed and the BioBag opened. The larvae were counted out of the bag, the total mass of larvae was recorded, and the mean mass per larva was calculated. The dry mass of pork tissue remaining was also recorded, which was obtained by placing it in a drying incubator at 70°C ± 2°C for 48 hours. Dry mass was recorded to remove inconsistencies in tissue mass caused by water loss over the 5‐day test period. An estimated initial dry mass was obtained by drying separate 15 g portions of minced pork. Five samples were dried from each batch of pork loin used, and the mean dry mass calculated from these samples was used as the estimated initial dry mass.
2.2. Comparison test
A comparison test was also conducted to assess the feeding efficacies of two separate treatments over 96 hours. In one treatment, a single BioBag was used for the duration of the 96‐hour test, whilst in the other, the BioBag was replaced with a fresh bag containing a fresh batch of larvae after the first 48 hours of feeding. Results were then collected at the end of 96 hours, recording the same parameters as those in the time series test. Larval mass data were also recorded for both instances of the 48‐hour treatment. Nine repeats were conducted for each treatment. The process of replacing the BioBags was carried out by removing the assay containers from the incubator after 48 hours, opening the lid, and then removing the bag with thumb forceps. The new BioBag was then placed on top of the pork tissue with the same orientation as the previous bag, before being resealed and placed back in the incubator. In tests where the same bag was used for the duration of the 96 hours, these actions were mimicked but with the same bag being removed and then replaced.
2.3. Statistical analysis
Data analysis in the time series test consisted of separate analysis of variance (anova) tests to determine an overall effect of incubation time on both the mass of tissue lost and the mean mass per larva. To determine more specifically the effect of each successive 24‐hour increment, post‐hoc Tukey multiple comparison tests were subsequently performed to compare the mean of each increment with those of every other. Significant differences between means were indicated at the 0.05 level of significance. These were conducted for both the mean mass of tissue lost and the mean mass per larva. For the comparison test, data were analysed using unpaired t‐tests. These were conducted to compare the mean masses of tissue lost in the two treatments. Separate t‐tests were also conducted comparing the mean mass per larva of both the first 48‐hour application of larvae with the mass per larvae from the 96‐hour application of larvae and also for the second 48‐hour application of larvae against the 96‐hour application. One final t‐test was conducted comparing the mean mass per larvae of both 48‐hour applications.
3. RESULTS
3.1. Time series test
Overall, incubation with larvae for over 120 hours was found to have a significant impact on the mass of tissue lost, F(5, 66) = 331.8, P < 0.001. The mass of tissue removed over time increased steadily over the first 72 hours (Figure 1(A)). The multiple comparison tests demonstrated that the differences between these time periods were statistically significant at the 0.05 level of significance. After 72 hours, the increase in the mass of tissue loss was much less pronounced, and at 120 hours, tissue loss did not change at all (Figure 1(A)). Of the total mass of tissue removed over the 5‐day period, 92.1% was removed in the first 72 hours. No significant differences were observed after the 72‐hour time point (ie, the masses of tissue lost at the 72‐, 96‐, and 120‐hour time periods were not significantly different from each other). Control tests containing no larvae saw a relatively small loss of mass over time with (mean ± SEM) 0.31 ± 0.06 g lost after 24 hours, and rising in 24‐hour increments to 0.57 ± 0.05 g after 120 hours.
Figure 1.
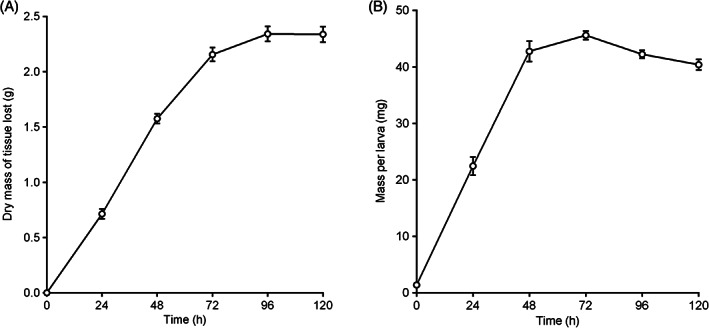
Mean ± SEM (A) total dry mass of pork tissue lost, and (B) mass per larva from artificial assay tests incubated for different time periods at 32 ± 1 °C using BioBags containing 5 larvae/cm2. n = 12 per time period
The trend in mean mass per larva saw an increase over time up to 72 hours, then gradually decreasing afterwards (Figure 1(B)). Incubation time over the whole 120‐hour test period was found to have a significant impact on the mass per larva, F(5, 66) = 228.4, P < 0.001. Mass per larva increased steadily over the first 48 hours, with the multiple comparison tests showing significant differences between the mean masses at 0, 24, and 48 hours. Mean larval mass increased again between 48 and 72 hours, but to a lesser degree, and the difference was not statistically significant. Mean mass then decreased after the 72‐hour point. Although the difference from the 72‐hour time point was not significantly lower at 96 hours, the decrease was statistically significant at 120 hours.
3.2. Comparison test
A total (mean ± SEM) 2.44 ± 0.05 g dry mass of tissue lost in the 96 hours’ treatment, compared with 2.79 ± 0.07 g of tissue lost in the two successive 48‐hour treatments, showing an increase of 14.34% (Figure 2(A)). Using an unpaired t‐test, the difference was found to be statistically significant, t(16) = 4.097, P < 0.001. The mean mass per larva was 40.38 ± 0.53 mg after the 96‐hour treatment. In the separate successive 48‐hour treatments, the mean larval mass was 37.59 ± 1.92 mg after the first 48‐hour application, and the replacement larvae were 38.44 ± 2.23 mg after the second 48‐hour application (Figure 2(B)). The mean larval mass of the 96‐hour treatment was not significantly different from either the first 48‐hour treatment, t(16) = 1.401, P = 0.180, or the second 48‐hour treatment, t(16) = 0.842, P = 0.412. There was also no statistically significant difference between the mean larval masses of the two 48‐hour applications, t(16) = 0.291, P = 0.775.
Figure 2.
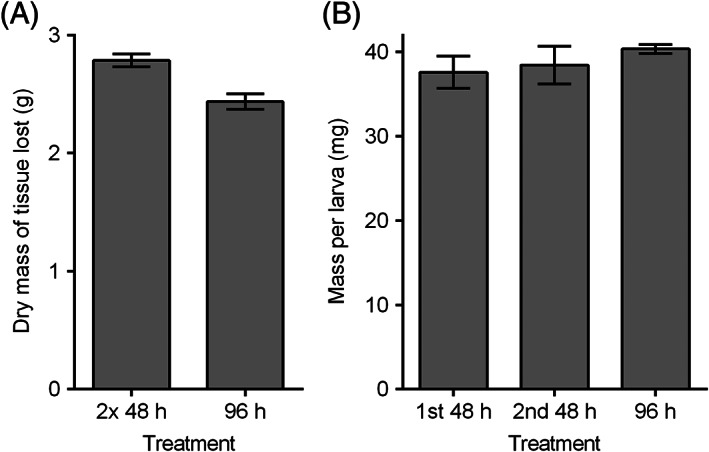
(A) Mean ± SEM of dry mass of pork tissue lost in artificial assay tests after 96 hours’ feeding, comparing a treatment where the BioBag was replaced with a fresh bag after 48 hours (“2 × 48 hours” treatment) and a treatment where the same BioBag was used for the full 96 hours (“96 hours” treatment). (B) Mean ± SEM mass per larva from assay tests after different periods of feeding, including larvae from the initially applied bag in the “2 × 48 hours” treatment (“1st 48 hours”), larvae from the bag applied second in the “2 × 48 hours” treatment (“2nd 48 hours”), and larvae from the from the bag applied in the “96 hours” treatment. All tests were incubated at 32°C ± 1°C, with BioBags containing 5.0 larvae/cm2. n = 9 per treatment
4. DISCUSSION
4.1. Mass of tissue lost
Debridement is the primary function of larval therapy, and it relies on the ability of the larvae to consume dead tissue. Maximising the efficacy of larval therapy treatments, therefore, requires a good knowledge of the length of time for which the larvae are active. In the present study, larval activity was determined, first, by the mass of tissue consumed over time. The results of this study indicate an active feeding period of up to 72 hours at the study temperature of 32°C. The primary explanation for the change in consumption rates over time is because of the developmental processes of the larvae. The active feeding period of larval development has been described as a “race against time” where, in response to the limited availability of food, larvae must obtain resources as quickly as possible to gain the critical weight required to successfully complete metamorphosis.38, 39, 40 It is likely for this reason that the rates of consumption are the highest in the earlier incubation period. The subsequent slowing in the rate of tissue removal indicates the point at which the larvae cease feeding and begin their wandering behaviour, a process that is largely dictated by temperature.31, 33, 41 In the present study, it appears that the cessation of feeding occurred in most individuals between 48 and 72 hours as the subsequent 24‐hour period showed no significant increase in tissue removal. Control tests containing no larvae saw a relatively small mass of tissue loss over time, possibly because of bacterial and autolytic degradation.
Larval development has been investigated in several ways in past studies. A majority looked either at the time taken to develop certain characteristics or at larval size/mass over time.31, 33, 41, 42 Few, however, have considered consumption rates, with only one previous study noted, which found that significantly more tissue was digested after 4 days than after 3.26 Those results differ somewhat from these in the present study. Although, in the present study, the mean dry mass of tissue lost after 96 hours was greater than at 72 hours, the rate of loss was far lower than that seen in previous 24‐hour intervals, and the difference in tissue loss between 72 and 96 hours was found not to be statistically significant. Differences between the results may be because of wet mass measurements being recorded by Blake et al,26 which may have caused inconsistencies in the measurements because of desiccation of the feeding material over time—a limitation that has been suggested previously.27 The impact of water loss was addressed in the present study by the use of dry mass measurements. In this case, there appeared to be little benefit of incubating the larvae for 96 hours rather than 72 hours in terms of tissue digestion.
4.2. Mass per larva
Larval mass is closely linked to activity as any changes will be correlated with food intake. In the present study, larval mass increased significantly up to 48 hours but showed no significant growth afterwards and began to decrease after 72 hours. The lack of growth after 48 hours was also observed in the comparison test where there was no significant difference observed in the mass per larva between the larvae that were incubated for 96 hours and either of those incubated for the 48‐hour periods. These results are also supported by those from a previous study, which found that larval growth ceased after 40 to 48 hours when incubated in simulated wound conditions.27
In the present study, it was found that larval growth did not always coincide with feeding. Larvae were found to still be effective in consuming tissue up to 24 hours after they had stopped showing any significant increase in mass. After the significant increases in growth up to 48 hours, it appears that rates of feeding in the following 24 hours were enough only to maintain body mass. Increases in body mass may also have been offset by emptying of the larval crop as they began to enter the wandering stage of development. Using only growth data to determine larval therapy application times, therefore, could lead to underutilising the larvae as this would result in their being removed at a time where they are still feeding and capable of debriding tissue. This is significant as previous studies have used larval growth as a parameter to justify recommendations of larval therapy application times. For example, Čičková et al27 noted that larval growth ceased after 40 to 48 hours and went on to recommend this time period as the most appropriate for larval therapy applications using the free‐range larvae method. The recommendation given for bagged larvae was longer, at 48 to 72 hours, but only as it was noted in a previous study that bagged larvae appeared to grow more slowly in certain types of wounds.24 As debridement is a key aim of larval therapy, it is important to also consider consumption data when considering optimal application times.
Based on the results of this investigation, the application of bagged larvae for 48 hours would result in an underutilisation of the larvae as they are capable of consuming significantly more tissue for a further 24 hours. This is also somewhat exemplified by data from the comparison test. In this test, the two consecutive 48‐hour treatments resulted in an increase in the mass of tissue removed over a single 96‐hour application of 14.3%, which was statistically significant. From a statistical point of view, one can conclude that the use of consecutive 48‐hour applications was more efficacious than a single 96‐hour application as it resulted in a greater mass of tissue removal. From a practical perspective, however, one could question whether the relatively modest increase in tissue removal justifies the costs that would be associated with applying two rounds of the treatment as opposed to one. Considering the consumption data both here and in the full 120‐hour test, it appears that a 72‐hour application would be a more effective use of the larvae at this temperature.
4.3. Impact of temperature
Temperature is a primary factor determining larval development rates, with higher temperatures resulting in faster development times regardless of food availability.30, 31, 33, 40, 41 The temperature that the larvae experience determines for how long they will be actively feeding. Therefore, in a larval therapy context, wound temperature plays a key role in determining for how long the debridement process will likely be effective.
A temperature of 32°C was chosen in this study as previous research has indicated this to be around the average wound surface temperature.26, 36, 37 However, it is acknowledged that the wound bed can exhibit a range of temperatures that can vary depending on body location, coverings, or dressings used, and levels of inflammation or vascularity, with one study measuring the wound surface temperature of 266 wounds, finding a range of 25.3°C to 37.3°C.37
Whilst this investigation gives an insight into the duration of larval feeding at average wound temperatures, further investigation into the effect of the range of temperatures that can be exhibited by chronic wounds on larval consumption rates would also be useful to better determine the optimal application times of larval therapy treatments. These differences in temperature could significantly impact for how long the larvae will be effective.
Although the present investigation found an active feeding period of up to 72 hours at the study temperature of 32°C, the current clinical recommendation of up to 4 days25 can still be considered sensible as wounds that present lower temperatures may cause slower larval development and a longer period of active feeding.
4.4. Future studies
Efforts were made for the assay to mimic aspects of a chronic wound so that results were as representative as possible to the clinical situation. However, the assay is not a substitute for clinical trials, and the limitations of this in vitro study should be acknowledged. There is no guarantee of a direct link between results found using this assay and those in the clinical situation, and although the findings of this study can aid in influencing future protocols for clinical application, further clinical investigation would be necessary to confirm optimal application times of medicinal larvae. Additional work may also look to consider the secondary benefits of larval therapy, such as antimicrobial action, in‐vitro by the inoculation of the meat substrate with pathogenic bacteria, and then examining the performance of the larvae in the presence of these bacteria.
This study investigated only the use of bagged larvae. Recommended application times vary depending on the larval therapy product, with loose larvae products generally being recommended shorter application times than bagged larvae.21, 27 A similar future study considering the optimal application times of loose larvae would, therefore, also be useful.
ACKNOWLEDGEMENTS
The authors thank BioMonde for supplying the dressings and medicinal larvae and the Prince of Wales Innovation Scholarship for financial support. Special thanks are extended to Dr Willi Jung for his contribution to this research.
Wilson MR, Nigam Y, Knight J, Pritchard DI. What is the optimal treatment time for larval therapy? A study on incubation time and tissue debridement by bagged maggots of the greenbottle fly, Lucilia sericata . Int Wound J. 2019;16:219–225. 10.1111/iwj.13015
Funding information Prince of Wales Innovation Scholarship
REFERENCES
- 1. Fonder MA, Lazarus GS, Cowan DA, Aronson‐Cook B, Kohli AR, Mamelak AJ. Treating the chronic wound: a practical approach to the care of nonhealing wounds and wound care dressings. J Am Acad Dermatol. 2008;58(2):185‐206. [DOI] [PubMed] [Google Scholar]
- 2. Zhao R, Liang H, Clarke E, Jackson C, Xue M. Inflammation in chronic wounds. Int J Mol Sci. 2016;17(12):2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Eming SA, Krieg T, Davidson JM. Inflammation in wound repair: molecular and cellular mechanisms. J Invest Dermatol. 2007;127(3):514‐525. [DOI] [PubMed] [Google Scholar]
- 4. Wolcott RD, Kennedy JP, Dowd SE. Regular debridement is the main tool for maintaining a healthy wound bed in most chronic wounds. J Wound Care. 2009;18(2):54‐56. [DOI] [PubMed] [Google Scholar]
- 5. Wilcox JR, Carter MJ, Covington S. Frequency of debridements and time to heal: a retrospective cohort study of 312,744 wounds. JAMA Dermatol. 2013;149(9):1050‐1058. [DOI] [PubMed] [Google Scholar]
- 6. Sun X, Jiang K, Chen J, et al. A systematic review of maggot debridement therapy for chronically infected wounds and ulcers. Int J Infect Dis. 2014;25:32‐37. [DOI] [PubMed] [Google Scholar]
- 7. Zarchi K, Jemec GBE. The efficacy of maggot debridement therapy—a review of comparative clinical trials. Int Wound J. 2012;9(5):469‐477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cazander G, Pritchard DI, Nigam Y, Jung W, Nibbering PH. Multiple actions of Lucilia sericata larvae in hard‐to‐heal wounds: larval secretions contain molecules that accelerate wound healing, reduce chronic inflammation and inhibit bacterial infection. Bioessays. 2013;35(12):1083‐1092. [DOI] [PubMed] [Google Scholar]
- 9. Valachová I, Bohová J, Kozánek M, Takáč P, Majtán J. Lucilia sericata medicinal maggots: a new source of antimicrobial compounds. In: Méndez‐Vilas A, ed. Microbial Pathogens and Strategies for Combating Them: Science, Technology and Education. Badajoz, Spain: Formatex Research Center; 2013;1745–1753. [Google Scholar]
- 10. Pöppel AK, Vogel H, Wiesner J, Vilcinskas A. Antimicrobial peptides expressed in medicinal maggots of the blow fly Lucilia sericata show combinatorial activity against bacteria. Antimicrob Agents Chemother. 2015;59(5):2508‐2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Horobin AJ, Shakesheff KM, Woodrow S, Robinson C, Pritchard DI. Maggots and wound healing: an investigation of the effects of secretions from Lucilia sericata larvae upon interactions between human dermal fibroblasts and extracellular matrix components. Br J Dermatol. 2003;148(5):923‐933. [DOI] [PubMed] [Google Scholar]
- 12. Horobin AJ, Shakesheff KM, Pritchard DI. Promotion of human dermal fibroblast migration, matrix remodelling and modification of fibroblast morphology within a novel 3D model by Lucilia sericata larval secretions. J Invest Dermatol. 2006;126(6):1410‐1418. [DOI] [PubMed] [Google Scholar]
- 13. Pritchard DI, Nigam Y. Maximising the secondary beneficial effects of larval debridement therapy. J Wound Care. 2013;22(11):610‐616. [DOI] [PubMed] [Google Scholar]
- 14. Evans R, Dudley E, Nigam Y. Detection and partial characterization of antifungal bioactivity from the secretions of the medicinal maggot, Lucilia sericata . Wound Repair Regen. 2015;23(3):361‐368. [DOI] [PubMed] [Google Scholar]
- 15. Telford G, Brown AP, Seabra RAM, et al. Degradation of eschar from venous leg ulcers using a recombinant chymotrypsin from Lucilia sericata . Br J Dermatol. 2010;163(3):523‐531. [DOI] [PubMed] [Google Scholar]
- 16. Telford G, Brown AP, Kind A, English JSC, Pritchard DI. Maggot chymotrypsin I from Lucilia sericata is resistant to endogenous wound protease inhibitors. Br J Dermatol. 2011;164(1):192‐196. [DOI] [PubMed] [Google Scholar]
- 17. Brown A, Horobin A, Blount DG, et al. Blow fly Lucilia sericata nuclease digests DNA associated with wound slough/eschar and with Pseudomonas aeruginosa biofilm. Med Vet Entomol. 2012;26(4):432‐439. [DOI] [PubMed] [Google Scholar]
- 18. Telford G, Brown AP, Rich A, English JSC, Pritchard DI. Wound debridement potential of glycosidases of the wound‐healing maggot, Lucilia sericata . Med Vet Entomol. 2012;26(3):291‐299. [DOI] [PubMed] [Google Scholar]
- 19. Pritchard DI, Čeřovský V, Nigam Y, et al. TIME management by medicinal larvae. Int Wound J. 2016;13(4):475‐484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Greenberg B, Kunich JC. Entomology and the Law: Flies as Forensic Indicators. Cambridge, UK: Cambridge University Press; 2002. [Google Scholar]
- 21. Thomas S. Maggot therapy. Surgical Dressings and Wound Management. Cardiff, UK: Medetec; 2010:563‐632. [Google Scholar]
- 22. Grassberger M, Fleischmann W. The biobag—a new device for the application of medicinal maggots. Dermatology. 2002;204(4):306. [DOI] [PubMed] [Google Scholar]
- 23. Fleischmann W, Grassberger M, Sherman R. Maggot Therapy: A Handbook of Maggot‐Assisted Wound Healing. Stuttgart, Germany: Thieme; 2004. [Google Scholar]
- 24. Čičková H, Čambal M, Kozánek M, Takáč P. Growth and survival of bagged Lucilia sericata maggots in wounds of patients undergoing maggot debridement therapy. Evid Based Complement Alternat Med. 2013;2013:1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. BioMonde . BioBag instructions for use. June 12, 2015.
- 26. Blake FAS, Abromeit N, Bubenheim M, Li L, Schmelzle R. The biosurgical wound debridement: experimental investigation of efficiency and practicability. Wound Repair Regen. 2007;15(5):756‐761. [DOI] [PubMed] [Google Scholar]
- 27. Čičková H, Kozánek M, Takáč P. Growth and survival of blowfly Lucilia sericata larvae under simulated wound conditions: implications for maggot debridement therapy. Med Vet Entomol. 2015;29(4):416‐424. [DOI] [PubMed] [Google Scholar]
- 28. Gilead L, Mumcuoglu KY, Ingber A. The use of maggot debridement therapy in the treatment of chronic wounds in hospitalised and ambulatory patients. J Wound Care. 2012;21(2):78‐85. [DOI] [PubMed] [Google Scholar]
- 29. Dumville JC, Worthy G, Bland JM, et al. Larval therapy for leg ulcers (VenUS II): randomised controlled trial. BMJ. 2009;338(May):b773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Anderson GS. Minimum and maximum development rates of some forensically important Calliphoridae (Diptera). J Forensic Sci. 2000;45(4):14778J. [PubMed] [Google Scholar]
- 31. Grassberger M, Reiter C. Effect of temperature on Lucilia sericata (Diptera: Calliphoridae) development with special reference to the isomegalen‐ and isomorphen‐diagram. Forensic Sci Int. 2001;120(1–2):32‐36. [DOI] [PubMed] [Google Scholar]
- 32. Wells JD, LaMotte LR. Estimating the postmortem interval. In: Byrd JH, Castner JL, eds. Forensic Entomology: The Utility of Arthropods in Legal Investigations. 2nd ed. Boca Raton, FL: CRC Press; 2009:367‐388. [Google Scholar]
- 33. Tarone AM, Picard CJ, Spiegelman C, Foran DR. Population and temperature effects on Lucilia sericata (Diptera: Calliphoridae) body size and minimum development time. J Med Entomol. 2011;48(5):1062‐1068. [DOI] [PubMed] [Google Scholar]
- 34. Schultz GS, Sibbald RG, Falanga V, et al. Wound bed preparation: a systematic approach to wound management. Wound Repair Regen. 2003;11(s1):S1‐S28. [DOI] [PubMed] [Google Scholar]
- 35. Wilson MR, Nigam Y, Jung W, Knight J, Pritchard DI. The impacts of larval density and protease inhibition on feeding in medicinal larvae of the greenbottle fly Lucilia sericata . Med Vet Entomol. 2016;30(1):1‐7. [DOI] [PubMed] [Google Scholar]
- 36. Xia Z, Sato A, Hughes MA, Cherry GW. Stimulation of fibroblast growth in vitro by intermittent radiant warming. Wound Repair Regen. 2000;8(2):138‐144. [DOI] [PubMed] [Google Scholar]
- 37. McGuiness W, Vella E, Harrison D. Influence of dressing changes on wound temperature. J Wound Care. 2004;13(9):383‐385. [DOI] [PubMed] [Google Scholar]
- 38. Ullyett GC. Competition for food and allied phenomena in sheep‐blowfly populations. Philos Trans R Soc B Biol Sci. 1950;234(610):77‐174. [Google Scholar]
- 39. Charabidze D, Hedouin V, Gosset D. Discontinuous foraging behavior of necrophagous Lucilia sericata (Meigen 1826) (Diptera Calliphoridae) larvae. J Insect Physiol. 2013;59(3):325‐331. [DOI] [PubMed] [Google Scholar]
- 40. Singh D, Bala M. The effect of starvation on the larval behavior of two forensically important species of blow flies (Diptera: Calliphoridae). Forensic Sci Int. 2009;193(1–3):118‐121. [DOI] [PubMed] [Google Scholar]
- 41. Wall R, French N, Morgan KL. Effects of temperature on the development and abundance of the sheep blowfly Lucilia sericata (Diptera: Calliphoridae). Bull Entomol Res. 1992;82(1):125‐131. [Google Scholar]
- 42. Clark K, Evans L, Wall R. Growth rates of the blowfly, Lucilia sericata, on different body tissues. Forensic Sci Int. 2006;156(2–3):145‐149. [DOI] [PubMed] [Google Scholar]


