Abstract
We aimed to report the clinical features of squamous cell carcinoma (SCC) occurring on scalp scar tissue among a Chinese population, demonstrate its pathological progress, analyse the prognosis‐related factors, and share our clinical experience of managing this rare disease in practice. A retrospective study was conducted at West China Hospital from January 2013 to January 2018 aiming to identify patients with a diagnosis of SCC or squamous atypical hyperplasia arising from scalp scars. Their medical records were reviewed, and related data were retrieved. Follow up was conducted, and informed consent was obtained by phone calls in June 2018. Of the 31 scalp Marjolin's ulcer (MU) patients, the average latency period and post‐ulceration period were 42.9 years and 37.5 months, respectively. Among them, 30 patients (97%) were diagnosed with cancer more than 5 years after initial injury, and 25 patients (80.7%) experienced a pre‐ulceration period longer than 20 years. A negative correlation between scalp MU's post‐ulceration period and its pre‐ulceration period was identified. Only burn scars caused post‐ulceration periods of more than 24 months (7/19). Incomplete healing wounds experienced a significantly shorter latency period (P = 0.004) and longer post‐ulceration period than others (P < 0.0001). However, the depth of tumour infiltration and complete tumour resection were the only two independent factors that significantly dictated patients' survival in this study. In conclusion, the scalp scaring tissue experienced a long‐term stable period but would transform to malignancy rapidly and progressively once ulceration formed. The underlying malignant transformation mechanism remains unclear. Thus, we recommend scalp scarring tissue to be radically removed.
Keywords: Marjolin's ulcer, malignant transformation, squamous cell carcinoma, scalp scar
1. INTRODUCTION
Being the second most common form of skin cancer, squamous cell carcinoma (SCC) was mainly caused by cumulative ultraviolet exposure in white populations, while the most predisposing conditions of SCC were scarring processes and chronic inflammation in other populations.1, 2 This malignant transformation of chronic wounds or scar tissues (mostly post‐burn scars) is known as Marjolin's ulcer (MU), with a reported incidence rate from 0.77% to 2%.3, 4, 5 Once the malignancy develops, it tends to be more aggressive and is associated with a higher risk of metastasis compared with sun‐induced SCC in Caucasians.2, 6, 7
“Marjolin's ulcer” was first named and published when the French surgeon Jean‐Nicholas Marjolin demonstrated the cellular changes of ulcerated lesions in scar tissue in the 19th century. In 1850, its pathology was described in detail by Robert Smith. Since then, MU has been reported to be a rare and aggressive cutaneous malignant transformation after inflammatory or traumatic insult to the skin.
Despite emphasis on early surgical management, including scar excision and grafting, to prevent scar formation and malignant transformation of deep burns,4, 8 such preventive strategies were poorly achieved in a clinical context9 because of the low incidence of MU and high cost of surgery or because of the lack of healthy flap tissue. Because of the financial limitations and low expectations of medical care in developing countries, plastic surgeons tend to be conservative, and early excision with grafting is often thought to be a heroic treatment.10 Even unstable scars with suspected masses or ulcers that occur secondary to burn injury are inadequately managed in most post‐burn patients, especially in developing countries, resulting in cases advancing to the stage of malignant disease.11 As reported, the suffering of burned patients in China is partially contributed by poor access to ideal management of post‐burn complications, which is a universal challenge in all developing countries.12
MU cases have been previously reported, and literature was reviewed to demonstrate the aggressive nature of the disease, its treatment options, and prognosis.11, 13, 14, 15 MU in scalp, among ulcers all over the body, attracts our attention the most as it may aggressively invade beyond scalp layers, and once the cranium is invaded, tumour resection and scalp reconstruction become intractable,16, 17 and this means an unfavourable prognosis for patients. However, the limited case volume of MU made it inadequate to exhibit the unique nature of scalp‐involved disease or to signify the importance of properly managing scalp MU.
Here, with a volume of favourable clinical cases, we aimed to report on the clinical features of scalp SCC arising in scar tissue among the Chinese population, demonstrate its pathological progress, analyse the prognosis‐related factors, and share our clinical experience of managing this rare disease in practice.
2. METHODS
We included patients who had primary SCC or squamous atypical hyperplasia (SAH) occurring on the scalp. Patients whose lesion never experienced an ulceration period before the malignancy occurred, patients who had organ transplantation and committed to immunosuppressive agents, patients who have severe systemic diseases, and patients who had metastatic cancer arising from other parts of the body were excluded.
We retrieved medical records of patients with diagnostic key words such as: “scalp OR head OR occipital OR frontal OR temporal OR parietal” AND “carcinoma OR tumor OR malignancy OR cancer” from the medical database of West China Hospital between January 2013 and January 2018. Pathological diagnosis was made by experienced pathologists in West China Hospital. Head magnetic resonance imaging (MRI) scans were performed preoperatively to evaluate the lesion evasion and intracranial metastasis. Tissue infiltration depth was determined by both imaging and intraoperative biopsy. The surgical decision was made following the principle of resecting the lesion with safe boundaries and preserving function as much as possible. Postoperative management strategies were comprehensively made based on patients' clinical features, available guidelines, and consultation from oncologists.
Data review included demographic information (age and gender), primary lesion cause, latency period (time from initial lesion to carcinomatous change), pre‐ulceration period and post‐ulceration period, ulceration cause, scar characteristics (location, scar contracture, repeated rupture), pathological diagnosis, surgery records (tumour infiltration information, reconstructive strategy, surgical result), and treatment history. Follow‐up was conducted by phone call in June, 2018.
Pearson correlation analysis was performed to evaluate the relationship between pre‐ulceration period (years) and post‐ulceration period (months). An ordinary one‐way anova test was performed to analyse the difference between multiple groups, and an unpaired t test was performed to analyse the difference between the two groups. Chi‐square test was performed to evaluate the distribution of patients with or without complete tumour resection in the post‐ulceration period based on groups. For the survival analysis, we conducted a log‐rank test to compare the survival distribution of indicated groups. A P‐value less than 0.05 was defined as the cut‐off value of statistical significance in this study. Mean values were presented with lower and upper 95%CI, and median values were presented with 75% percentile. For the analysis related to latency‐based groups, we defined the cut‐off values of pre‐ulceration and post‐ulceration period as 50 years and 6 months, respectively, in order to stratify patients with the most meaningful factors.
The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in approval by West China Hospital of Sichuan University Biomedical Research Ethics Committee. Oral informed consent was obtained by a phone call to each patient involved in this study.
3. RESULTS
A total of 289 medical files were initially identified and reviewed further. Thirty‐one patients were ultimately included after the removal of duplicates, non‐squamous cell carcinoma, and cancer that did not arise from scalp scar and ulceration. The average follow‐up time after diagnosis was 4.1 (0.5‐8) years. Demographic and relevant clinical information is presented in Table 1, including age, gender, aetiology of the primary lesion, latency period, pre‐ and post‐ulceration periods, cause for ulceration, location, and the occurrence of scar contracture and repeated rupture.
Table 1.
Demographic and relevant clinical information
| Age (years) |
Mean 52.2 (46.2–58.2) |
Median 49 (40–63) |
||
| Gender |
Female 15 (48.4%) |
Male 16 (51.6%) |
||
| Primary lesion causes |
Burn 19 (61.3%) |
Trauma/surgery 7 (22.6%) |
Infection 5 (16.1%) |
|
| Latency period (years) |
Mean 42.9 (35.3–50.5) |
Median 46 (33–60) |
||
| Pre‐ulceration period (years) |
>20 years 25 (80.7%) |
≤20 years 6 (19.3%) |
||
| Post‐ulceration period (months) |
Mean 37.5 (7.0–67.9) |
Median 6 (2–24) |
||
| Ulceration causes |
Spontaneous 12 (38.7%) |
Trauma 10 (32.3%) |
Scratching 5 (16.1%) |
Incomplete healing 4 (12.9%) |
| Scalp areas involved |
Top 24 (77.4%) |
Occiput 13 (41.9%) |
Temple 7 (22.6%) |
|
| Scar contracture |
Yes 6 (19.4%) |
No 25 (80.6%) |
||
| Repeated rupture |
Yes 7 (22.6%) |
No 24 (77.4%) |
||
4. CLINICAL FEATURES OF SCALP MU
Sixteen male and 15 female patients were diagnosed with MU on the scalp, with a mean age of 52.2 (46.2‐58.2) years. Among them, 30 patients (97%) were diagnosed with cancer more than 5 years after initial injury, and the average latency period is 42.9 (35.3‐50.5) years. Causes of primary wounds/scars included burn in 19 cases (61.3%), trauma (surgery) in 7 cases (22.6%), and infection in 5 cases (16.1%). For the characteristics of the lesions, the top of the head was found to be involved in 24 cases (77.4%), which was the most often involved area. Scar contracture occurred in six patients (19.4%).
To demonstrate the characteristics of scalp MU's malignant transformation, we performed a thorough analysis of scalp MU's latency period. In all the included scalp MU cases, 25 of 31 cases (80.7%) experienced a pre‐ulceration period longer than 20 years. We found a negative correlation (R 2 = 0.2697) between MU's post‐ulceration period and its pre‐ulceration period, indicating the longer the scalp scar stays stable, the faster the malignant transformation happens (Figure 1A). In an analysis of the primary causes of lesions, we found that both the latency period and pre‐ulceration period of infection‐caused lesions are significantly longer than those of lesion caused by burns (P = 0.008; 0.011) or trauma/surgery (P = 0.041; 0.043) (Figure 1B,C). However, patients with and without scar contracture did not show any significant difference between their latency periods (P = 0.906), nor did patients with and without repeated rupture (P = 0.957) (Figure 1E,F).
Figure 1.
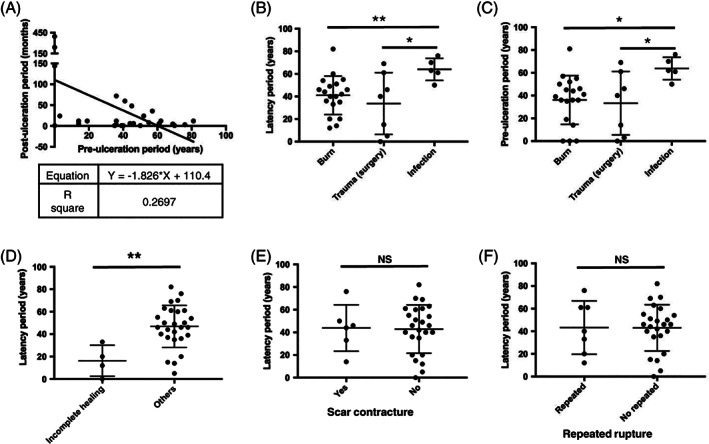
Latency period of malignant transformation analysis. A, MU's post‐ulceration period is negatively correlated with its pre‐ulceration period. B, C, The latency period and pre‐ulceration period of primary lesion caused by infection is significantly longer than that by burn or trauma/surgery. D, Incomplete healing wounds experienced a significantly shorter latency period than other ulcerations occurring on scar. E, F, No significant difference of latency period was found between patients with and without scar contracture, nor between patients with and without repeated rupture. *P < 0.05; **P < 0.01; NS, P > 0.05
After a relatively long stable period, most wounds/scars became ulcers through a secondary irritating factor, which included 12 cases of spontaneous ulcer (38.7%), 10 cases of trauma (32.3%), 5 cases of scratching (16.1%), and 4 cases of incomplete healing (12.8%). The average post‐ulceration period of all the included patients is 37.5 (7.0‐67.9) months. We analysed factors that might affect the post‐ulceration period and found only burn scars caused in post‐ulceration periods of more than 24 months, and this happened in 7 of 19 cases (Figure 2A). Considering patients with original wounds that never healed as a separate group, we found that patients with incomplete healing ulcerations experienced a significantly shorter latency period (P = 0.004) and longer post‐ulceration period than others (P < 0.0001) (Figures 1D and 2B), indicating that this group of wounds is more chronic than others, and the pathophysiological malignant transformation of chronic wounds is different from scar tissues.
Figure 2.
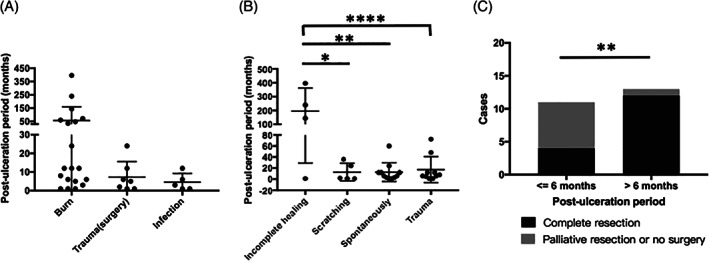
Post‐ulceration period and its related factors. A, Only burn scar was found to cause post‐ulceration periods of more than 24 months, and it happened in 7 out of 19 cases. B, Incomplete healing caused a significantly longer post‐ulceration period than other ulceration causes. C, Longer post‐ulceration period (>6 months) is positively correlated with complete tumour resection. *P < 0.05; **P < 0.01; ****P < 0.0001; NS, P > 0.05
5. TREATMENTS AND OUTCOMES
To demonstrate the prognostic information of included MU patients in detail, we reported the tumour invasion and pathological types, treatment details, and clinical outcomes of each patient included in Table 2.
Table 2.
Treatment and outcomes
| Infiltration depth |
At or above the skull 17 (54.8%) |
Under the skull 10 (32.3%) |
Unclear 4 (12.9%) |
||
| Pathological diagnosis |
SCC 27 (87.1%) |
SAH 4 (12.9%) |
|||
| Reconstructive strategies |
Free flap 12 (38.7%) |
Local flap 2 (6.5%) |
Skin grafting 6 (19.3%) |
No surgery 4 (12.9%) |
Unclear 7 (22.6%) |
| Surgical results |
Complete resection 16 (51.6%) |
Palliative resection or no surgery 8 (25.8%) |
Unclear 7 (22.6%) |
||
| Other treatments |
Radiotherapy 12 (38.7%) |
No 14 (45.2%) |
Unclear 5 (16.1%) |
||
| Survival outcomes |
Tumour free 16 (51.6%) |
Death 7 (22.6%) |
Lost follow‐up 8 (25.8%) |
||
Abbreviations: SCC, squamous cell carcinoma; SAH, squamous atypical hyperplasia.
To identify potential risk factors of the scalp MU patients' survival, we performed a survival analysis based on tumour infiltration depth, surgical resection, pathological type, adjunctive radiotherapy, and pre‐ and post‐ulceration period. Finding out whether the tumour infiltrated beyond the skull and whether it was fully resected were two independent factors that significantly dictated patients' survival (P < 0.0001; P = 0.0018) (Figure 3A,B). In our study, all the patients whose tumour did not go beyond the skull or patients who received completed tumour resection were alive without tumour till the completion of our follow up. However, for those with the diagnosis of SCC and intracranial metastasis who lost the opportunity to fully resect the tumour subsequently, whether they had the palliative surgery or not did not help them to live free of tumour for 5 years, and four of eight patients (50%) died within 2 years after diagnosis. Although no significant difference was found between the survival of SAH and SCC group (P = 0.2241), we reported deaths of 5 of 17 (29.4%) SCC patients within 2 years after their diagnosis, while all the SAH patients lived tumour‐free for more than 2 years (Figure 3C). We also found that radiotherapy does not affect MU patients' survival (P = 0.8251) (Figure 3D), which corresponds with previous opinions that MU does not respond to radiotherapy. Interestingly, we noticed that patients with a longer post‐ulceration period (more than 6 months) were more likely to have a better survival outcome (P = 0.0843) (Figure 3E), which corresponded well with the result that a longer post‐ulceration period meant more complete tumour resection (P = 0.0078) (Figure 2C). We expected to see a shorter pre‐ulceration period dictating worse survival as pre‐ and post‐ulceration periods were negatively correlated as we demonstrated before (Figure 1A). However, we failed to identify a significant survival difference between subgroups based the on pre‐ulceration period (P = 0.2767) (Figure 3F). Interestingly, when we took a closer look at the data, we noticed that only 1of 8 (12.5%) patients died within 2 years post‐diagnosis in the longer pre‐ulceration period group, while 4 of 13 (30.8%) patients died within 2 years in the shorter group (Figure 3F). We further analysed the possible survival‐related factors, including the primary lesion cause, ulceration causes and scar characteristics, but failed to identify any significant difference between these groups.
Figure 3.
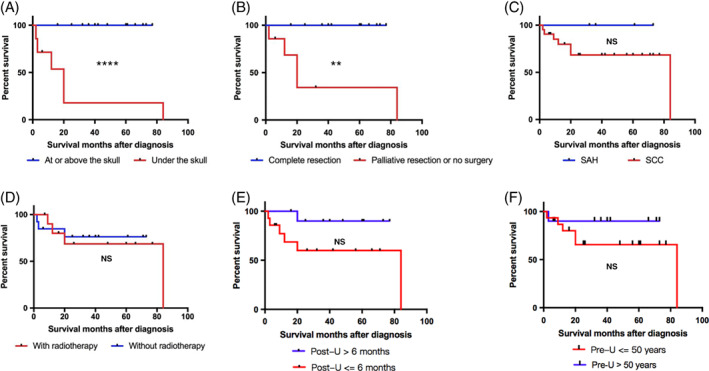
Prognostic factors analysis. Survival curve of patients grouped by: A, Lesion infiltration depth. B, Tumour resection outcome. C, Pathological type. D, Radiotherapy. E, Post‐ulceration period. F, Latency periods. **P < 0.01; ****P < 0.0001; NS, P > 0.05
6. DISCUSSION
In this study, we found that scalp MU's post‐ulceration period was negatively correlated with its pre‐ulceration period. A scalp scar experienced a long‐term stable period but would then transform to malignancy rapidly and progressively once the ulceration was formed. Incompletely healed ulcerations or chronic wounds without formed scar experienced a shorter latency period before the malignancy was formed but a longer post‐ulceration period compared with other scalp MUs. However, chronic inflammatory irritating factors did not dictate worse survival of scalp MU patients. Tumour infiltrate depth and complete tumour resection were the only two independent factors that significantly affected patients' survival in this study.
A typical latency period of MU including pre‐ and post‐ulceration period was described to be an important pathophysiological character of the disease, and it helps demonstrate the nature of the disease.18 Previous studies reported two types of MU, those with short pre‐ulceration period and long duration of ulceration and the others with long pre‐ulceration period and sudden onset of malignancy after ulceration happened. Skin breakdown on chronic scars and chronically unhealed ulcers are two main sources of MU. MU was suggested to be preventable with a close surveillance of the ulcer during the latency period.18 Along with our practice, we realised that the malignant transformation that occurred secondary to a scalp scar tended to experience a long pre‐ulceration period, during which the scar tissue is stable. As a result, both patients and health care providers would regard radical surgery as an unnecessary option. However, once the ulceration takes place, no matter what the irritating factor is, malignancy will progress so rapidly that surgeons are always left with a late‐stage disease to fight with.
Based on theories that attributed malignant transformation to repeated ulceration and prolonged stimulus for cellular proliferation provided by subsequent initiation of reepithelialisation and possible spontaneous mutations,19, 20, 21 we analysed the irritating factors during the course of disease. Surprisingly, we found that repeated rupture or the formation of scar contracture did not make any difference to the latency period. In addition, incomplete healing or an infection‐irritated scar experienced longer post‐ulceration period, which subsequently meant better survival. It indicated that the malignancy transformation of scalp MU was hard to be solely explained by chronic irritation theories and might be completely different from MUs that were mostly reported in limbs before. The underlying mechanisms of the progress of scalp MU need to be further elucidated.
We failed to identify significant survival differences between groups based on incomplete wound healing, pathological types, adjunctive radiotherapy, and pre‐ and post‐ulceration period. However, we do observe that patients with SCC and shorter pre‐ and post‐ulceration period are more likely to die within 2 years post‐diagnosis. We expect to see statistically significant differences within these groups with larger case volume or prospective studies in the future.
In China, the rehabilitation measures or any prosthetic care given for scar is not yet covered by public health insurance. As a result, patients and health providers constantly face the dilemma of when the proper time to be radical is and how to actively manage the scalp scar during its pathological progression. One strategy of saving MU patients could be to prolong the latency period to exceed their lifespans. However, as our results demonstrated that longer pre‐ulceration period means shorter post‐ulceration time, it could be extremely risky to live with a stable scalp scar for years as the scar would progress rapidly once it was formed, leaving us little time to conduct complete resection. Considering the dreadful aftermath of scalp malignancy occurring secondary to wounds healed by secondary intention, wounds healed inappropriately, and fragile burn scars that ulcerate easily, we strongly believe that early excision and grafting of scalp scar is sensible and cost‐effective as a preventive measure for MU's occurrence.
There were several limitations within this retrospective study. First, we had eight cases lost to follow‐up as demonstrated in Table 2. As a result, all the survival analysis was constructed with survival data of the other 23 patients. This led to a higher risk of selective bias and minimised the sample size as well. Second, it is impossible for us to report the survival rate of either all included patients as a whole or each defined group because the follow‐up time of each patient were undefined. Third, in order to find the most distinguished differences between groups to stratify patients, we discarded the average value or mean as the cut‐off values and defined pre‐ulceration and post‐ulceration period as 50 years and 6 months, respectively, instead. Although it is not a statistical routine to do so, we present case data in the most meaningful way. Considering the limitations of a retrospective study and the relatively small sample size, larger prospective multicentre studies across several hospitals should be further performed to better address the clinical characteristics, analyse prognostic factors, and explore optimised treatment strategies for scalp MU.
In conclusion, the scalp scaring tissue experienced a long‐term stable period but transformed to malignancy rapidly and progressively once the ulceration formed. As a result, the rapidly invasive and lethal disease could be easily overlooked at an early stage. The underling malignant transformation mechanism could be something other than chronic inflammatory irritation. Thus, we strongly recommend scalp scarring tissue to be preventively removed.
CONFLICTS OF INTEREST
The authors have no conflicts of interest to report.
ACKNOWLEDGEMENTS
This study was supported by a grant from the National Natural Science Foundation of China (81772079).
Xiao H, Deng K, Liu R, et al. A review of 31 cases of Marjolin's ulcer on scalp: Is it necessary to preventively remove the scar? Int Wound J. 2019;16:479–485. 10.1111/iwj.13058
Haitao Xiao, Ke Deng and Ruolin Liu contributed equally to this study.
Funding information National Natural Science Foundation of China
Contributor Information
Ying Cen, Email: ycen7116@gmail.com.
Xuewen Xu, Email: xxw_0826@163.com.
REFERENCES
- 1. Kabir S, Schmults CD, Ruiz ES. A review of cutaneous squamous cell carcinoma epidemiology, diagnosis, and management. Int J Cancer Manag. 2018;11(1): e60846. [Google Scholar]
- 2. Bradford PT. Skin cancer in skin of color. Dermatol Nurs. 2009;21:170‐177. [PMC free article] [PubMed] [Google Scholar]
- 3. Bazaliński D, Przybek‐Mita J, Barańska B, Więch P. Marjolin's ulcer in chronic wounds – review of available literature. Contemp Oncol (Poznan, Poland). 2017;21:197‐202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Copcu E. Marjolin's ulcer: a preventable complication of burns? Plast Reconstr Surg. 2009;124:156e‐164e. [DOI] [PubMed] [Google Scholar]
- 5. Al‐Zacko SM. Malignancy in chronic burn scar: a 20 year experience in Mosul – Iraq. Burns. 2013;39:1488‐1491. [DOI] [PubMed] [Google Scholar]
- 6. Mora RG, Perniciaro C. Cancer of the skin in blacks. I. A review of 163 black patients with cutaneous squamous cell carcinoma. J Am Acad Dermatol. 1981;5:535‐543. [DOI] [PubMed] [Google Scholar]
- 7. Kim GK, Del Rosso JQ, Bellew S. Skin cancer in Asians: part 1: nonmelanoma skin cancer. J Clin Aesthet Dermatol. 2009;2:39‐42. [PMC free article] [PubMed] [Google Scholar]
- 8. Das KK, Chakaraborty A, Rahman A, Khandkar S. Incidences of malignancy in chronic burn scar ulcers: experience from Bangladesh. Burns. 2015;41:1315‐1321. [DOI] [PubMed] [Google Scholar]
- 9. Kowal‐Vern A, Criswell BK. Burn scar neoplasms: a literature review and statistical analysis. Burns. 2005;31:403‐413. [DOI] [PubMed] [Google Scholar]
- 10. Das KK. Response to letter to the editor: “Marjolin”s ulcers: a dreaded aftermath of inadequately managed deep burns. Burns. 2016;42:1143. [DOI] [PubMed] [Google Scholar]
- 11. Liu Z, Zhou Y, Zhang P, et al. Analysis of clinical characteristics of 187 patients with Marjolin's ulcers. Chin J Burns. 2016;32:293‐298. [DOI] [PubMed] [Google Scholar]
- 12. Saaiq M, Siddiqui S. Marjolin's ulcers: a dreaded aftermath of inadequately managed deep burns. Burns. 2016;42:1143. [DOI] [PubMed] [Google Scholar]
- 13. Aköz T, Erdoğan B, Görgü M, Aslan G. The necessity for aggressive treatment with Marjolin's ulcers of the scalp. Plast Reconstr Surg. 1997;100:805‐806. [PubMed] [Google Scholar]
- 14. Ozek C, Celik N, Bilkay U, Akalin T, Erdem O, Cagdas A. Marjolin's ulcer of the scalp: report of 5 cases and review of the literature. J Burn Care Rehabil. 2001;22:65‐69. [DOI] [PubMed] [Google Scholar]
- 15. Calikapan GT, Akan M, Karaca M, Aköz T. Marjolin ulcer of the scalp. J Craniofac Surg. 2008;19:1020‐1025. [DOI] [PubMed] [Google Scholar]
- 16. Tenekeci G, Sari A, Hamzaoglu V, Ozalp H. Reconstruction of a Marjolin ulcer defect of the scalp invading brain and causing brain abscess formation using free latissimus Dorsi flap. J Craniofac Surg. 2017;28:e510‐e512. [DOI] [PubMed] [Google Scholar]
- 17. Desai SC, Sand JP, Sharon JD, Branham G, Nussenbaum B. Scalp reconstruction. JAMA Facial Plast Surg. 2015;17:56‐66. [DOI] [PubMed] [Google Scholar]
- 18. Yu N, Long X, Lujan‐Hernandez JR, et al. Marjolin's ulcer: a preventable malignancy arising from scars. World J Surg Oncol. 2013;11:313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gül U, Kiliç A. Squamous cell carcinoma developing on burn scar. Ann Plast Surg. 2006;56:406‐408. [DOI] [PubMed] [Google Scholar]
- 20. Hill BB, Sloan DA, Lee EY, McGrath PC, Kenady DE. Marjolin's ulcer of the foot caused by nonburn trauma. South Med J. 1996;89:707‐710. [DOI] [PubMed] [Google Scholar]
- 21. Fairbairn NG, Hamilton SA. Management of Marjolin's ulcer in a chronic pressure sore secondary to paraplegia: a radical surgical solution. Int Wound J. 2011;8:533‐536. [DOI] [PMC free article] [PubMed] [Google Scholar]


