Abstract
Bacterial collagenase from the aerobic non‐pathogenic Vibrio alginolyticus chemovar iophagus is an extracellular metalloproteinase. This collagenase preparation is obtained through a fermentation process and is purified chromatographically, resulting in a highly purified 82‐kDa single‐band protein that does not contain non‐specific proteases or other microbial impurities. V. alginolyticus collagenase was added to a hyaluronan (HA)‐based device to develop a novel debriding agent to improve the treatment of ulcers, necrotic burns, and decubitus in the initial phase of wound bed preparation. In this study, an in vitro biochemical characterisation of V. alginolyticus collagenase versus a commercial preparation from a Clostridium histolyticum strain on various dermal extracellular matrix (ECM) substrates was performed. V. alginolyticus collagenase demonstrated its ability to carry out the enzymatic cleavage of the substrate, allowing a selective removal of necrotic tissues while sparing healthy tissue, as reported in clinical studies and through routine clinical experience. in vitro tests under physiological conditions (pH, presence of Ca++, etc.) have demonstrated that V. alginolyticus collagenase exhibits very poor/limited non‐specific proteolytic activity, whereas the collagenase preparation from C. histolyticum is highly active both on collagen and on non‐collagenic substrates. This finding implies that while the V. alginolyticus enzyme is fully active on the collagen filaments that anchor the necrotic tissue to the wound bed, it does not degrade other minor, but structurally important, components of the dermal ECM. This feature could explain why collagenase preparation from V. alginolyticus has been reported to be much gentler on perilesional, healthy skin.
Keywords: bacterial collagenase, clostridium histolyticum, hyaluronic acid, Vibrio alginolyticus, wound
1. INTRODUCTION
Collagenase belongs to a family of zinc‐dependent metalloproteinases that degrade collagen substrates that consist of a protein structure featured by a rigid left‐handed triple‐helical configuration1 rich in proline, hydroxyproline, and glycine.2, 3 In humans, collagenases (matrix metallo‐proteinases, MMPs) play an essential role in the modulation of various aspects of inflammation.4
Such enzymes are made not only by humans5, 6 but also by several bacteria, such as Clostridium histolyticum,7, 8 Nocardiopsis dassonvillei,9 and V. alginolyticus.10, 11
Bacterial collagenase preparations, such as ointments, have been successfully used in clinical practice for more than 25 years as debriding agents in chronic wounds.12 These agents allow cleansing to remove cellular “debris,” which often constitutes an obstacle for the migration of epidermal cells in wound re‐epithelialisation.13 In fact, bacterial collagenases perform lytic functions and selective removal of necrotic tissue,14, 15 with only minor involvement of the perilesional, healthy skin.16, 17
Historically, the most widely used collagenase preparation was derived from a highly pathogenic strain of C. histolyticum.18 This preparation represents the reference standard compared with other recently discovered collagenases. However, although C. histolyticum‐derived collagenase is regularly used, its production is generally reported to be limited because of low purity.19 The collagenase preparation from C. histolyticum (Santyl, Noruxol) is available as a hydrophobic ointment indicated for topical application of cutaneous lesions of various origins, such as chronic wounds, bedsores, and burns.
The bacterial collagenase (“Achromobacter” collagenase, EC 3.4.24.3) derived from the non‐pathogenic aerobic V. alginolyticus chemovar iophagus strain has also attracted much interest within the medical community and has been recently used in the debridement of wounds in a similar ointment formulation.20, 21, 22 V. alginolyticus collagenase is an 82‐kDa extracellular metalloproteinase highly specific for collagen; its amino acid sequence does not bear significant similarity to other collagenases.23
We have recently developed a process for the isolation of such a collagenase. The aerobic fermentation process allows for the production of a highly purified enzyme lacking in non‐specific proteases or other microbial contaminants, making this preparation particularly useful in clinical settings.24
Hyalo4 Start (Fidia Farmaceutici S.p.A, Abano Terme, Padova, Italy) is a semi‐solid preparation consisting of a hydrophobic ointment containing V. alginolyticus collagenase and low‐molecular weight hyaluronic acid (LMW‐HA, 100‐400 kDa). The product combines the collagenolytic activity of the novel collagenase with the wound‐healing properties of hyaluronic acid for the topical treatment of wounds, bedsores, burns, and ulcers.25, 26, 27 Furthermore, from a clinical standpoint, the product has been reported as a safer formulation compared with its competitors because it does not damage the perilesional skin and does not cause dermatitis, pain, or redness.21, 22 In fact, to avoid any damage to the perilesional skin, competitor products envisage confining the wound area where the product is applied with a zinc‐oxide paste to create a physical barrier that prevents the contact of the ointment preparation with the surrounding healthy skin.28, 29, 30, 31
To investigate findings that support clinical evidence of Hyalo4 Start in improving the wound‐healing process and the protection of perilesional skin, we evaluated the efficacy of available V. alginolyticus and C. histolyticum collagenase preparations.32 The working hypothesis is that both enzymes are equally active on the specific substrate (i.e., type I‐III collagen) but not as active on other minor components of the dermal extracellular matrix (ECM). Therefore, by preserving such structurally important elements because of the less‐aggressive behaviour of V. alginolyticus collagenase, a lower rate of clinical complications in the healing of chronic ulcers would be expected.
To test this hypothesis, under the same physiological conditions, we used in vitro testing to assess the purity, enzymatic activity, and specificity towards each selected substrate of the dermal ECM of purified soluble preparations/extracts of the two enzymes from V. alginolyticus and C. histolyticum. Then, we compared the outcomes of each enzymatic digestion.
2. MATERIALS AND METHODS
2.1. Bacterial strain and collagenases
Microbial collagenase (“Achromobacter” collagenase, EC 3.4.24.3) is produced by the non‐pathogenic aerobic bacterium V. alginolyticus chemovar iophagus (NCIMB Number: 11038, synonym LMG 3418, hereinafter referred to as Vibrio alginolyticus). The manufacturing process results in high yields of collagenase with a stable, reproducible, and economical fermentation.24
A standard soluble C. histolyticum collagenase preparation (NB4, cat. no. 17454, SERVA Electrophoresis GmbH, Heidelberg, Germany) was used in this study, and this preparation is assumed to be representative of the product currently available on the market.
Given that we are dealing with purified enzymes, the comparison of the two preparations has been made on a weight‐to‐weight basis (same mg of the enzyme/test sample).
2.2. V. alginolyticus collagenase manufacturing process
Production and purification of collagenase from V. alginolyticus cultures has been described previously24 and is briefly summarised in the following sections.
2.2.1. Preparation of inoculum
A 1.5‐mL ampoule of V. alginolyticus (from Working Cell Bank) is inoculated into a 5‐L Erlenmeyer flask containing 2 L of culture medium consisting of a solution of 1.21 g/L TRIS, 23.4 g/L NaCl, 0.29 g/L CaCl2, and 15 g/L peptone of non‐animal origin dissolved in distilled water (Millipore milliQ). The pH of the culture medium is adjusted to 7.1 with HCl and sterilised in the autoclave at a temperature of up to 122°C for 30 minutes. The culture is grown at 30°C under stirring at 150 rpm until the optical density at 600 nm (OD600) reaches a value of 3 (approximately 16 hours). The inoculum is then ready to be transferred to the main 1500 L fermenter (TECNinox s.r.l, Noceto [PR], Italy).
2.2.2. Fermentation
The fermentation broth is the same as that used for the preparation of inoculum with the addition of 0.25 g/L of antifoam 204 (Sigma, St. Louis, Missouri) and sterilised as previously noted. A volume of 2 L of inoculum is transferred into the fermenter containing 800 L of fermentation broth. The following fermentation parameters are employed: temperature 30 ± 1°C, stirring 100 rpm, air 10 to 80 Nm3/h, dissolved oxygen greater than 50%, pH 7.1 ± 0.1, and pressure 0.4 bar. Collagenase is an extracellular enzyme; therefore, it is secreted in the culture medium. Collagenase activity is determined spectrophotometrically using the modified Wunsch–Heidrich method.33 When the final enzymatic activity is greater than 25 000 nkat/L (on average after 16 hours), the fermentation is terminated, and CaCl2 is added to a final concentration of 1.47 g/L to stabilise the enzyme. Finally, the temperature is lowered to 8°C under stirring at 300 rpm for approx. 20 minutes.
2.3. V. alginolyticus collagenase purification
Briefly, 800 L of fermented culture broth is transferred to a Holder Sartocon II ultrafiltration system (Sartorius, Gottingen, Germany) equipped with five cassettes of modified PolyEtherSulphone (PES) membrane (Sartorius) with a MW cut‐off of 300 kDa. The clarified culture medium is concentrated by ultrafiltration using an UF‐A‐P0971 ultrafiltration system (PALL, Port Washington, New York) equipped with 10‐kDa cut‐off PES membrane (PALL). The 20‐fold concentrated medium (40 L) was dialysed against 10 mM CaCl2, 25 mM TRIS–HCl, and pH 7.1 buffer.
The solution from the previous step underwent a first purification by weak anion‐exchange chromatography using a column prefilled with DE‐52 resin (DEAE: diethylaminoethyl cellulose, Whatman, Maidstone, UK). Collagenase activity is recovered within 3 to 5 bed‐column volumes (BV) eluted with 700 mM NaCl, 10 mM CaCl2, 300 mM TRIS–HCl, and pH 7.1 buffer. The raw enzyme then undergoes a strong anion‐exchange chromatography by using a column pre‐filled with Source 15Q resin (GE Healthcare, Chicago, Illinois). The collagenase is eluted from the column with 10 mM CaCl2, 300 mM Tris–HCl, and pH 7.1 buffer. All chromatography runs were monitored using a UV–vis detector (GE Healthcare) at 280 nm. The individual fractions eluted from each chromatographic column were subjected to an enzyme activity assay33, 34 and analysed by Sodium Dodecyl Sulphate–PolyAcrylamide Gel Electrophoresis (SDS‐PAGE).
Pooled fractions exhibiting collagenolytic activity underwent ultrafiltration by the Cogent system (Millipore, Burlington, Massachusetts) equipped with 30 kDa cut‐off modified PES membranes (Millipore) against 10 mM CaCl2, 25 mM TRIS–HCl, and pH 7.1 buffer. Routinely, a purified V. alginolyticus collagenase preparation from an 8‐L solution exhibits 700 nKat/mL enzymatic activity.
2.4. Ultra‐high‐pressure fast size exclusion chromatography (UPLC‐SEC)
UPLC‐SEC (ACQUITY UPLC H‐Class Bio System equipped with ACQUITY UPLC BEH200 SEC 200 Å, 1.7‐μm column, Waters, Milford, Massachusetts) was employed to trace enzyme dimers, higher molecular weight products, and collagenase degradation products (altogether considered as impurities) using 99.5% purified V. alginolyticus collagenase as an internal standard. Separation was accomplished using isocratic elution in 150 mM NaCl, 20 mM Na‐Phosphate buffer pH 6.8. The following parameters were used: sample injection volume, 5 μL (0.5 mg/mL); flow rate, 0.5 mL/min; temperature, 30 ± 1°C; total run time, 8 minutes; and wavelength, 214 nm (TUV ACQUITY UPLC Detector, Waters).
2.5. Electrophoresis on polyacrylamide gel (SDS‐PAGE)
Protein content and purity were verified using the Laemmli method35 with 12% polyacrylamide gel electrophoresis in the presence of sodium dodecyl sulphate (SDS‐PAGE) using a Mini‐PROTEAN 3 (BIO‐RAD, Hercules, California) following the supplier's instructions. The molecular weight of the purified V. alginolyticus enzyme was estimated by comparison with high molecular weight standard proteins (250 to 10 kDa, All Blue Standards, BIO‐RAD). SDS‐PAGE gels were stained with Coomassie Brilliant Blue G‐250 (BIO‐RAD). Images were acquired by ImageQuant 300 TL (GE Healthcare), whereas qualitative and quantitative analyses were performed using the instrument's software.
2.6. Collagenase assay
2.6.1. Wunsch–Heidrich modified method
The collagenase activity is determined by the enzymatic reaction according to the Wunsch–Heidrich modified method33 against a synthetic peptide (Sigma). The reaction of aqueous solution of collagenase with the synthetic substrate PZ‐l‐prolyl‐l‐leucyl‐glicyl‐l‐prolyl‐d‐arginine (where PZ = 4‐phenyl azobenzyloxycarbonyl) under controlled conditions (pH, temperature, and time) produces two fragments: PZ‐L‐prolyl‐l‐leucine and glicyl‐l‐prolyl‐d‐arginine. The first fragment is determined spectrophotometrically at 320 nm after acidified ethyl acetate extraction. This reaction is specific for the collagenolytic activity, which is expressed as nanomoles of reacted substrate per second per mL of solution (nkat/mL). The specification of purified V. alginolyticus collagenase is 500 to 700 nKat per mL of solution.
2.6.2. Mandl's modified method
Collagenase activity is determined according to the Mandl's modified method.34 Briefly, 25 mg of bovine collagen (Sigma) were solubilised in 5.0 mL of 0.05 M TES ([Tris(hydroxymethyl)‐methyl‐2‐aminoethane sulphonate]), 0.36 mM CaCl2, and pH 7.5 buffer; the reaction mixture was pre‐warmed at 37°C for 15 minutes. Consequently, 0.1 mL of enzyme dilution was added to the pre‐warmed reaction buffer. After 5 hours, 0.2 mL of reaction mixture was transferred in a test tube containing 1.0 mL of ninhydrin‐citric acid mixture (50 mL of 4% ninhydrin in methyl cellosolve together with 50 mL of 0.2 M sodium citrate, 0.71 mM stannous chloride, and pH 5.0). This mixture was heated for 20 minutes in a boiling water bath. After cooling on ice, the test sample was diluted with 5 mL of 50% n‐propanol. After 15 minutes, the absorbance was read at 600 nm. The free l‐leucine micromoles produced by ninhydrin reaction from an l‐leucine standard curve were determined.
The collagenase activity in the sample is calculated using the following equation:
2.6.3. Caseinase activity
Caseinase activity in collagenase solution was determined as described by Anson, M.L.36 and by the Folin Ciocalteu Method37 using casein (Sigma) as a substrate and evaluating the production of tyrosine following substrate digestion. One caseinase unit definition is the amount of enzyme/protein able to hydrolyse casein to produce 1.0 μmole of tyrosine at pH 7.5 and 37°C for 5 hours (colour produced by Folin & Ciocalteu reagent). V. alginolyticus specifications are less than 1.0 U/mL of purified collagenase preparation.
2.6.4. Experimental conditions for specific enzymatic activity on selected dermal substrates
Bovine Achilles tendon collagen (Fluka), pig skin gelatine, human plasma fibronectin, and bovine decorin (all from Sigma) were each brought to a final concentration of 1 mg/mL with a buffer consisting of 10 mM CaCl2, 25 mM TRIS–HCl, and pH 7.1.
To 50 μg of each substrate, 0.8 μg of V. alginolyticus collagenase or 0.8 μg of C. histolyticum collagenase preparation was added to yield a final volume of 60 μL. The reaction mix was incubated at 37°C for 90 minutes. Control tubes containing substrates only or collagenase preparations only were run in the same final volume of 60 μL. Tubes in which pigskin gelatine was present were inactivated and used for the Mandl's34 modified colorimetric test. The tubes containing bovine Achilles tendon collagen were duplicated. One sample was diluted in loading buffer up to 120 μL, and 25 μL of sample/well were employed for SDS‐PAGE analysis. The other sample was inactivated and used for Mandl's34 modified colorimetric test.
A portion of the content of decorin and fibronectin tubes after 1:1 dilution in loading buffer was loaded on SDS‐PAGE wells.
3. RESULTS
3.1. Production and purification system of the soluble autologous enzyme
A process for collagenase isolation from a selected V. alginolyticus bacterial strain was developed. The process uses a culture medium free of animal components, so the purification process results in a highly pure and stable end product for clinical use, as demonstrated by the single peak in the UPLC‐SEC chromatogram (Figure 1). In addition, a 99% pure, 82‐kDa single band can be detected by SDS‐PAGE (Figure 2A). On average, the biological activity of each production batch is set as >1000 nkat/mg of protein.
Figure 1.
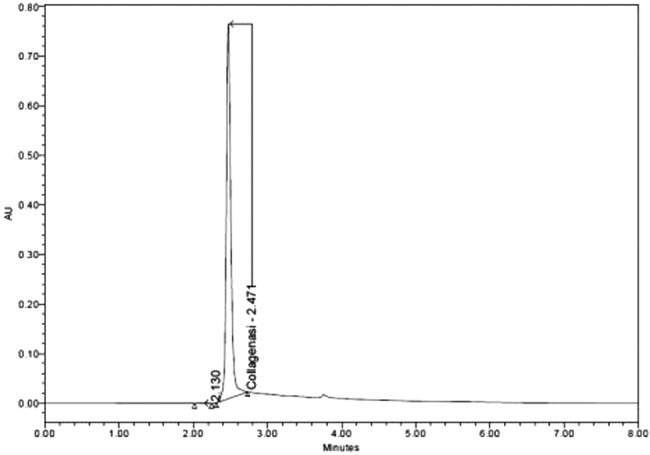
UPLC‐SEC chromatogram of the V. alginolyticus collagenase product after purification
Figure 2.
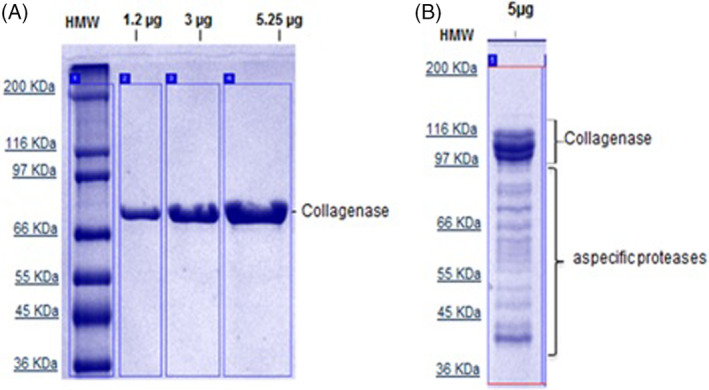
Purity by 12,5% SDS‐PAGE and detection in Coomassie Gel Blue stain. A, Electrophoretic profile of Vibrio alginolyticus collagenase; B, Electrophoretic profile of Clostridium histolyticum collagenase
3.2. Electrophoretic profile of V. alginolyticus versus C. histolyticum collagenase
The electrophoretic profile of V. alginolyticus collagenase has been proven to be highly pure (˃98%) and does not show contaminants even after heavy loading of the enzyme preparation in the 12,5% SDS‐PAGE gel (Figure 2A). In contrast, the electrophoretic profile of the C. histolyticum NB4 collagenase preparation shows a double band at 110 to 100 kDa, which is identified as the collagenase enzyme, in addition to several contaminant bands at lower MW (Figure 2B).
In fact, V. alginolyticus collagenase employed in this study shows caseinase activity less than 1.0 U/mL. In contrast, the preparation of C. histolyticum collagenase (NB4) exhibits caseinase activity of approximately 250 U/mL, confirming the aspecific proteolytic activity of the contaminant protein pool.
The comparative results demonstrate how V. alginolyticus collagenase exhibits a better safety profile in pharmaceutical/biomedical applications with a considerably higher purity grade compared with other commercially available collagenase from C. histolyticum.
3.3. Comparison of the specific collagenolytic activity on selected dermal substrates: collagen
In the electrophoretic profile of type I collagen in lane 1, two main bands are observed at 120 and 110 kDa. Such bands disappeared; in fact, the bands are completely digested separately by both V. alginolyticus and C. histolyticum collagenase preparations as shown in lines 2 and 4, respectively (Figure 3A).
Figure 3.
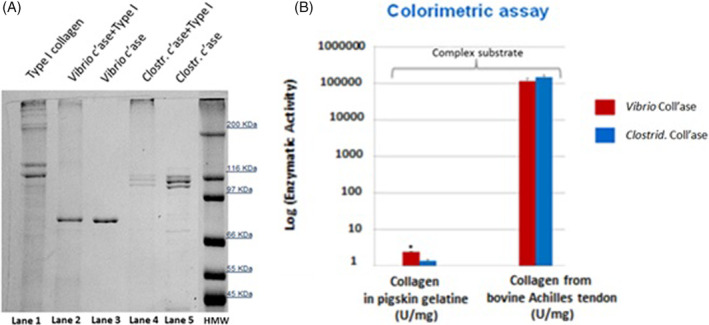
Comparison of the specific collagenolytic activity on collagen substrate. A, 7,5% SDS‐PAGE after 90 minutes of incubation with Type I collagen (bovine). Lane 1: Control test tubes run with 50 μg Type I collagen; Lane 2: 50 μg Type I collagen combined with 0.8 μg Vibrio alginolyticus collagenase; Lane 4: 50 μg Type I collagen combined with 0.8 μg Clostridium histolyticum collagenase; and Lanes 3 and 5: Control test tubes run with collagenase preparations only (0.8 μg). B, Specific activity by collagenase assay on pigskin gelatine and bovine Achilles tendon, which represents complex natural substrates, using the Mandl's colorimetric method. Data are expressed as mean (SD) from three different experiments (*P < 0.05, Student's t‐test, n = 3)
Of note, the NB4 collagenase preparation could undergo digestion of itself in the presence of the specific collagen substrate. In fact, the characteristic 110 to 100‐kDa double band is no longer detected at the end of the reaction (lane 4, Figure 3A). The test result obtained (line 4, Figure 3A) suggests the presence of an intermolecular pathway in the process of autolysis of the collagenase preparation NB4 in the presence of its digestible substrate at low concentrations, as previously reported for calcium‐activated neutral protease (CANP).38 In fact, various studies on the production of collagenase in Achromobacter iophagus have demonstrated how the synthesis of the enzyme itself is induced by its specific collagen substrate present in the medium. In particular, the maximum quantity of enzymatic activity that resulted was proportional to the final concentration of the inductor present in the culture medium.39, 40, 41 Furthermore, in neutral solutions, it was reported that the pure collagenase from bacteria produces partially degraded enzymatic forms through autodigestion that are still active towards the native collagen.42 In addition, this process of autodigestion of collagenase seems to be similar to that described for some proteins, which exhibit proteolytic activity accompanied by inherent autolytic serine protease‐like activities.43, 44, 45 On the other hand, the process of the autodigestion of the collagenase enzyme NB4 in the presence of collagen type I could also result in the enzymatic activity of aspecific proteases present in the analysed preparation.46
According to the colorimetric assay, both collagenase preparations digest type I collagen from bovine Achilles tendon and collagen in pig skin gelatine to the same extent (Figure. 3B). Therefore, such enzymes are equally active on the native substrate and on the denatured, more complex matrix of pigskin gelatine.
3.4. Comparison of the specific enzymatic activity on selected dermal substrates: decorin
Here, 4% to 15% SDS‐PAGE after decorin digestion with both collagenase preparations shows that V. alginolyticus collagenase degrades decorin only to a (very) minor extent (five main bands in the range 50‐15 kDa were produced), whereas the C. histolyticum collagenase preparation degrades decorin completely with no remnants of the original 75‐kDa smeared band (Figure 4).
Figure 4.
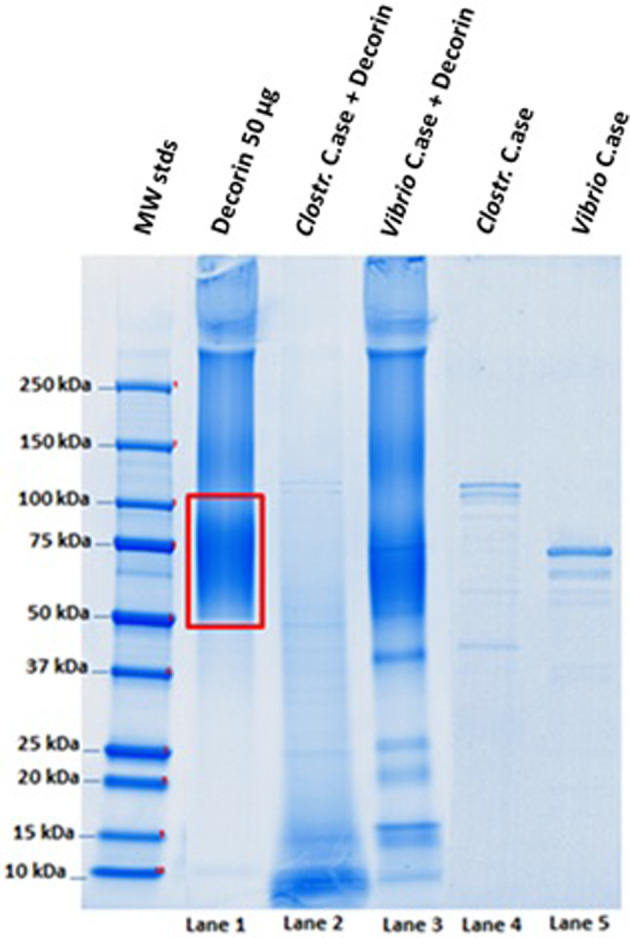
Electrophoresis by 4% to 15% SDS‐PAGE. Experimental conditions: 90 minutes incubation at 37°C, pH 7.1; Lane 1: Control test tubes run with 50 μg decorin; Lane 2: 50 μg decorin combined with 0.8 μg Clostridium histolyticum collagenase; Lane 3: 50 μg decorin combined with 0.8 μg Vibrio alginolyticus collagenase; and Lanes 4 and 5: Control test tubes run with collagenase preparations only (0.8 μg)
3.5. Comparison of the specific enzymatic activity on selected dermal substrates: fibronectin
Regarding fibronectin, 4% to 15% SDS‐PAGE shows only a partial degradation of the substrate by V. alginolyticus collagenase, resulting in the production of a main band at 220 kDa (58% of the total amount). Specifically, 42% of the starting material was still present (lane 2, Figure 5), and the lower bands collectively comprised <1%. This activity is evaluated using a camera‐based imaging system CCD where the quantitative analysis is achieved by applying ImageQuant TL image analysis software (GE Healthcare). In contrast, the Clostridium preparation degrades 100% of fibronectin, resulting in many bands at considerably lower MWs (lane 3, Figure 5).
Figure 5.
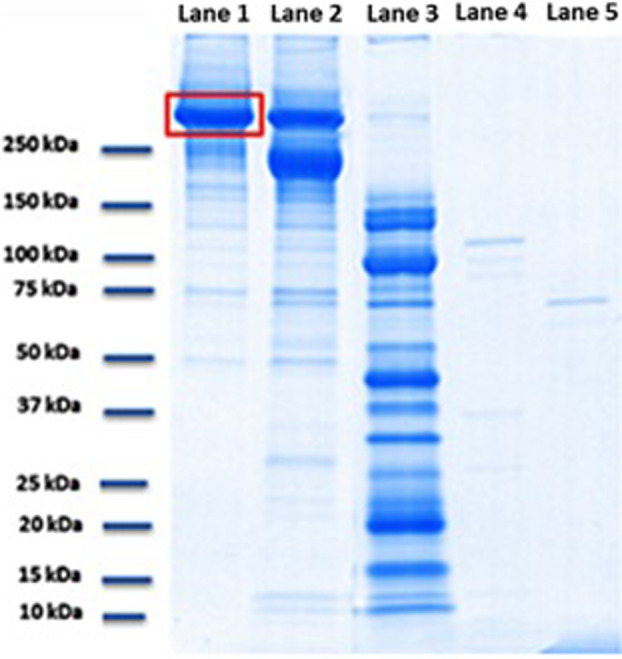
Electrophoresis by 4% to 15% SDS‐PAGE. Experimental conditions: 90 minutes incubation at 37°C, pH 7.1; Lane 1: Control sample lane run with 50 μg fibronectin; Lane 2: 50 μg fibronectin combined with 0.8 μg Vibrio alginolyticus collagenase; Lane 3: 50 μg fibronectin combined with 0.8 μg Clostridium histolyticum collagenase; Lane 4: Control sample lane run with Clostridium histolyticum collagenase preparation only (0.8 μg); and Lane 5: Control sample lane run with Vibrio alginolyticus collagenase preparation only (0.8 μg)
Therefore, the specific enzymatic activity of the two collagenases on selected dermal substrates was compared. Our findings demonstrate that with the same concentration of the analysed substrate (and equal protein concentrations of the two collagenases), V. alginolyticus collagenase completely degrades only the collagen substrate. Thus, V. alginolyticus collagenase exhibits not only a high catalytic activity but also a high specificity of action. In contrast, C. histolyticum collagenase completely degrades all the three analysed protein substrates.
3.6. Influence of pH on collagenase activity
In vitro collagenase activity was assessed at different pH values, which were characteristic of healthy skin and a healing wound environment. Pure collagenase preparations lose activity at acidic pH. This finding is particularly true for V. alginolyticus collagenase. At pH values of 5.6, V. alginolyticus collagenase exhibited 20/25% activity. However, at pH of 5.0, the activity was completely lost. In contrast, the C. histolyticum preparation still exhibits 60% activity at pH 5.6 and 30% activity at pH 5.0 (Figure 6).
Figure 6.
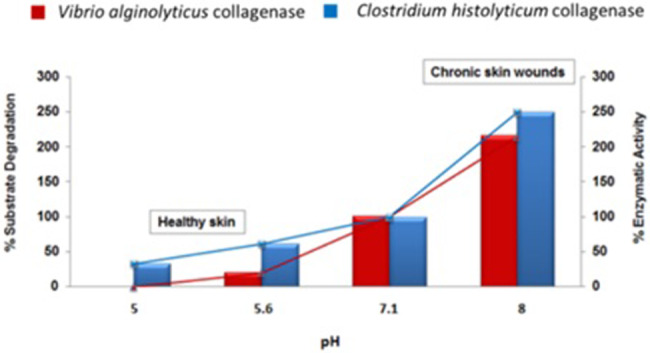
In vitro collagenase activity was determined at different pH values by quantifying the fragment produced by the enzymatic reaction according to the Wünsch method
4. DISCUSSION
Hyalo4 Start is a class III medical device based on 0.2% LMW‐HA and a novel collagenase preparation purified from a non‐pathogenic aerobic V. alginolyticus bacterial strain. This combination forms a novel debriding agent where the two components act in a synergistic manner to speed up the healing process by an effective wound bed preparation for ulcers, necrotic burns, and bed sores.47 The novel V. alginolyticus collagenolytic enzyme is characterised by a single, highly purified 82‐kDa protein. A key factor in achieving this result was the use of a culture medium for bacterial growth completely lacking protein inducers (either from animal or non‐animal sources). Typically, approximately 1000 nkat/mg (batch size approximately 5 g) of collagenase are recovered from each biosynthetic preparation.
Clinical experience has demonstrated the effectiveness of this device (Hyalo4 Start) along with a lower rate of perilesional skin complications compared with collagenase‐based products derived from C. histolyticum.22, 32 The latter preparation showed two major bands at 110 and 100 kDa via SDS‐PAGE in addition to other bands of lower MW. We assumed that a different substrate specificity, particularly in the case of minor structural components of the dermis, might explain such a lower complication rate. Therefore, in our in vitro study, a biochemical characterisation of V. alginolyticus collagenase versus a commercial preparation from C. histolyticum strain on collagen and various dermal ECM substrates was performed. The same amounts of enzyme preparations were used in the assays; therefore, such amounts reflect the quantity of product/cm2 commonly employed for wound management (activity).48, 49 in vitro studies of the enzymatic preparations were not performed regarding comparable enzyme activity of the collagenase given the presence of C. histolyticum proteases associated with the preparation. Although these proteases represented a minimal portion of the preparation, they reacted with the collagenase in the degradation of collagen.
In vitro studies showed that the V. alginolyticus enzyme is as effective as the commercial preparations of C. histolyticum collagenase on many types of collagen.50 Collagen digestion performed with each enzyme preparation was examined by SDS‐PAGE, probing type I collagen from bovine Achilles tendon, or a colorimetric assay probing either collagen type I or pig skin gelatine, which is mainly composed of denatured dermal collagen. In both cases, the two preparations were equally active in substrate digestion.
In contrast, when other minor components of the dermal ECM were probed with the V. alginolyticus or C. histolyticum collagenase, different behaviours were observed.
V. alginolyticus collagenase degrades fibronectin to a limited extent, mainly resulting in a major band at 220 kDa. In addition, 42% of the starting substrate was not digested. In the case of C. histolyticum digestion, the 250‐kDa fibronectin band completely disappeared, and only lower MW bands in the range 130 to 15 kDa were detected.
When probed with decorin, the same results were observed. Only partial degradation was observed with the V. alginolyticus preparation, and most of the original protein band remained undigested. In contrast, C. histolyticum enzyme completely digested the substrate. This evidence is crucial because decorin and fibronectin, although present in small amounts, are structurally fundamental components of the dermal ECM.51 In fact, decorin is a proteoglycan that plays a role in the control of fibrosis and scar formation as a tissue healing physiological regulator, and the lack of expression or the absence of this protein in skin tissues reduces the regenerative ability after skin damage.52, 53, 54 On the other hand, fibronectin, a high molecular weight glycoprotein of the ECM, facilitates dermal fibroblast migration during normal wound healing.55
The different substrate specificity can be explained taking into account that Vibrio collagenase cleaves the Y‐Gly bond of the collagen sequence –Pro‐Y‐Gly‐Pro–.56 Preparations of C. histolyticum collagenase are actually made of six enzymes with both collagenolytic and proteolytic activity.57 The latter enzyme preparation cleaves the collagen strand at the Y‐Gly bonds in the repeating Yaa‐Gly‐Xaa‐Yaa collagen sequence (MEROPS, Summary for family M9). It is important to recall that the collagenolytic activity refers to the cleavage of any collagen substrate measured in nkat (amount of a collagen peptide digested/time/mL),58 whereas the proteolytic activity refers to the cleavage of any protein present in the reaction mixture measured in mg protein digested/time. Therefore, proteases present in the C. histolyticum collagenase preparation are responsible for non‐collagenic substrate digestion, such as decorin or fibronectin, whereas pure collagenase preparations (such as the V. alginolyticus one) degrade non‐collagenic substrates to a minor extent. This finding is confirmed by the caseinase assay results. The C. histolyticum enzyme pool promptly degrades casein, whereas V. alginolyticus collagenase does not.
Normal healthy skin is characterised by pH values in the mildly acidic range (5.0‐5.6),59 and similar pH values are noted in spontaneously healing, acute wounds. Non‐healing, chronic wounds are characterised by higher pH values in the mild basic range (8.0‐9.5).59 V. alginolyticus collagenase loses 90% activity at pH 5.6 (compared with 100% activity at pH 7). In contrast, C. histolyticum collagenase preparation still retains 60% and 30% activity at pH values of 5.6 and 5.0, respectively, therefore implying that this enzyme pool is still able to degrade the ECM matrix in healthy skin. At mild basic pH values (7.4‐8.5), typical of chronic, non‐healing wounds, both preparations are equally effective on the collagenic substrate.
In conclusion, a difference in aggressiveness between the two products containing different collagenases that act on periwound skin was demonstrated.32 Actually, the wound edges in patients treated with V. alginolyticus‐derived collagenase were in better condition than the wounds treated with C. histolyticum‐derived enzyme. In fact, the V. alginolyticus enzyme is gentler on healthy tissue and allows an improvement of the surrounding skin. It can be speculated that the protective action is mediated by the presence of 0,2% hyaluronic acid in the product; hyaluronan (HA) allows the formation of the ECM matrix, promotes cell migration, modulates the inflammatory response, and improves collagen deposition.32, 60, 61 However, the purpose of this comparative study was to evaluate the effectiveness and safety of enzymatic debriders, V. alginolyticus‐derived collagenase, and C. histolyticum‐derived collagenase, by in vitro biochemical characterisation of various dermal ECM substrates.
Therefore, we demonstrated that the V. alginolyticus collagenolytic enzyme is fully active on the collagen filaments that anchor the necrotic tissue to the wound bed, whereas the same collagenase is inactive on other minor but structurally important components of the dermal ECM. In addition, regarding the influence of pH on V. alginolyticus collagenase activity, the analysis demonstrated that this enzyme performs its lytic activity exclusively on necrotic tissue. This could be the reason why collagenase preparation from V. alginolyticus has been reported to be much more gentle on perilesional, healthy skin.22, 32, 47, 62, 63
Finally, these findings support ever more the clinical evidence that the effect of Hyalo4 Start is not as aggressive on perilesional skin as the commercial C. histolyticum‐based products.32
Di Pasquale R, Vaccaro S, Caputo M, et al. Collagenase‐assisted wound bed preparation: An in vitro comparison between Vibrio alginolyticus and Clostridium histolyticum collagenases on substrate specificity. Int Wound J. 2019;16:1013–1023. 10.1111/iwj.13148
REFERENCES
- 1. Ramachandran GN. Stereochemistry of collagen. Int J Pept Protein Res. 1988;31:1‐16. [PubMed] [Google Scholar]
- 2. Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Biologia Molecolare Della Cellula. Bologna, Italy: Zanichelli; 2009:1172‐1177. [Google Scholar]
- 3. Watanabe K. Collagenolytic proteases from bacteria. Appl Microbiol Biotechnol. 2004;63:520‐526. [DOI] [PubMed] [Google Scholar]
- 4. Nissinen L, Kähäri VM. Matrix metalloproteinases in inflammation. Biochim Biophys Acta. 2014;1840:2571‐2580. [DOI] [PubMed] [Google Scholar]
- 5. Pendás AM, Matilla T, Estivill X, López‐Otín C. The human collagenase‐3 (CLG3) gene is located on chromosome 11q22.3 clustered to other members of the matrix metalloproteinase gene family. Genomics. 1995;26:615‐618. [DOI] [PubMed] [Google Scholar]
- 6. Springman EB, Angleton EL, Birkedal‐Hansen H, Van Wart HE. Multiple modes of activation of latent human fibroblast collagenase: evidence for the role of a Cys73 active‐site zinc complex in latency and a “cysteine switch” mechanism for activation. Proc Natl Acad Sci USA. 1990;87:364‐368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bond MD, Van Wart HE. Characterization of the individual collagenases from clostridium histolyticum. Biochemistry. 1984;23:3085‐3091. [DOI] [PubMed] [Google Scholar]
- 8. Bond MD, Van Wart HE. Purification and separation of individual collagenases of clostridium histolyticum using red dye ligand chromatography. Biochemistry. 1984;23:3077‐3085. [DOI] [PubMed] [Google Scholar]
- 9. Abood A, Salman AMM, Abdelfattah AM, El‐Hakim AE, Abdel‐Aty AM, Hashem AM. Purification and characterization of a new thermophilic collagenase from Nocardiopsis dassonvillei NRC2aza and its application in wound healing. Int J Biol Macromol. 2018. Sep;116:801‐810. [DOI] [PubMed] [Google Scholar]
- 10. Lim DV, Jackson RJ, Pull‐VonGruenigen CM. Purification and assay of bacterial collagenases. J Microbiol Methods. 1993;18:241‐253. [Google Scholar]
- 11. Supuran CT, Scozzafava A, Clare BW. Bacterial protease inhibitors. Med Res Rev. 2002;22:329‐372. [DOI] [PubMed] [Google Scholar]
- 12. Berger MM. Enzymatic debriding preparations. Ostomy Wound Manage. 1993;39:61‐62. 66‐69. [PubMed] [Google Scholar]
- 13. Rao DB, Sane PG, Georgiev EL. Collagenase in the treatment of dermal and decubitus ulcers. J am Geriatr Soc. 1975;23:22‐30. [DOI] [PubMed] [Google Scholar]
- 14. Westerhof W. Future prospects of proteolytic enzymes and wound healing. In: Westerhof W, Vanscheidt W, eds. Proteolytic Enzymes and Wound Healing. Berlin Heidelberg, Germany: Springer‐Verlag; 1994:99‐102. [Google Scholar]
- 15. Nano M, Ricci E, De Simone M, Lanfranco G. Collagenase therapy in the treatment of decubitus ulcers. In: Abatangelo G, Donati L, Vanscheidt W, eds. Proteolysis in Wound Repair. Berlin Heidelberg, Germany: Springer‐Verlag; 1996:61‐69. [Google Scholar]
- 16. Helaly P, Vogt E, Schneider G. Wound healing disorders and their enzymatic therapy: a multicenter double‐blind study. Schweiz Rundsch Med Prax. 1988;77:1428‐1434. [PubMed] [Google Scholar]
- 17. Riley KN, Herman IM. Collagenase promotes the cellular responses to injury and wound healing in vivo. J Burns Wounds. 2005;4:e8. [PMC free article] [PubMed] [Google Scholar]
- 18. Marazzi M, Stefani A, Chiaratti A, Ordanini MN, Falcone L, Rapisarda V. Effect of enzymatic debridement with collagenase on acute and chronic hard‐to‐heal wounds. J Wound Care. 2006;15:222‐227. [DOI] [PubMed] [Google Scholar]
- 19. Mandl I, Zipper H, Ferguson LT. Clostridium histolyticum collagenase: its purification and properties. Arch Biochem Biophys. 1958;74:465‐475. [DOI] [PubMed] [Google Scholar]
- 20. Vaccaro S, Gennari G, Callegaro L, Giannelli A, Caruso S. New Pharmaceutical Compositions Containing Hyaluronic Acid or its Derivatives and Collagenase for the Topical Treatment of Wounds, Burns and Ulcers. Patent number: EP1901755 B1; 2011.
- 21. Cortivo R, Abatangelo G, et al. Bionect Start: the biological synergy for the evolution of enzymatic debridement. J Wound Technol. 2011;13:1‐6. [Google Scholar]
- 22. Onesti MG, Fioramonti P, Carella S, Fino P, Sorvillo V, Scuderi N. A new association between hyaluronic acid and collagenase in wound repair: an open study. Eur Rev Med Pharmacol Sci. 2013;17:210‐216. [PubMed] [Google Scholar]
- 23. Takeuchi H, Shibano Y, Morihara K, et al. Structural gene and complete amino acid sequence of vibrio alginolyticus collagenase. Biochem J. 1992;281(pt 3):703‐708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vaccaro S, Caputo M, Cuppari C, Gennari G. New Process for the Production and Purification of the Collagenase Enzyme from Vibrio Alginolyticus. Patent number: WO 2013/156525 A1; 2013.
- 25. Ghazi K, Deng‐Pichon U, Warnet JM, Rat P. Hyaluronan fragments improve wound healing on in vitro cutaneous model through P2X7 purinoreceptor basal activation: role of molecular weight. PLoS One. 2012;7:e48351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tolg C, Telmer P, Turley E. Specific sizes of hyaluronan oligosaccharides stimulate fibroblast migration and excisional wound repair. PLoS One. 2014;9:e88479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gariboldi S, Palazzo M, Zanobbio L, et al. Low molecular weight hyaluronic acid increases the self‐defense of skin epithelium by induction of beta‐defensin 2 via TLR2 and TLR4. J Immunol. 2008;181:2103‐2110. [DOI] [PubMed] [Google Scholar]
- 28. Weller RB, Hunter JAA, Savin J, Dahl M. Clinical Dermatology. Hoboken, NJ: Wiley‐Blackwell; 2015. [Google Scholar]
- 29. Bradley M, Cullum N, Sheldon T. The debridement of chronic wounds: a systematic review. Health Technol Assess. 1999;3(17, pt 1):1‐78. [PubMed] [Google Scholar]
- 30. Zimmermann WE. The importance of collagenase for the local treatment of major burns. In: Mandl I, ed. Collagenase. New York, NY: Gordon and Breach; 1971:131‐141. [Google Scholar]
- 31. Gorinshteyn B. Compositions and Methods for Treating Surface Wounds. Patent number: WO 2014120700 A1; 2014.
- 32. Onesti MG, Fioramonti P, Fino P, Sorvillo V, Carella S, Scuderi N. Effect of enzymatic debridement with two different collagenases versus mechanical debridement on chronic hard‐to‐heal wounds. Int Wound J. 2015;13:1111‐1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wuensch E, Heidrich HG. On the quantitative determination of collagenase. Hoppe Seylers Z Physiol Chem. 1963;333:149‐151. [DOI] [PubMed] [Google Scholar]
- 34. Mandl I, MacLennan JD, Howes EL. Isolation and characterization of proteinase and collagenase from cl. Histolyticum. J Clin Invest. 1953;32:1323‐1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680‐685. [DOI] [PubMed] [Google Scholar]
- 36. Anson ML. The estimation of pepsin, trypsin, papain, and cathepsin with hemoglobin. J Gen Physiol. 1938;22:79‐89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Folin O, Ciocalteu V. On tyrosine and tryptophane determinations in proteins. J Biol Chem. 1927;73:627‐650. [Google Scholar]
- 38. Inomata M, Kasai Y, Nakamura M, Kawashima S. Activation mechanism of calcium‐activated neutral protease. Evidence for the existence of intramolecular and intermolecular autolyses. J Biol Chem. 1988;263(36):19783‐19787. [PubMed] [Google Scholar]
- 39. Keil‐Dlouha V, Misrahi R, Keil B. The induction of collagenase and a neutral proteinase by their high molecular weight substrates in Achromobacter iophagus. J Mol Biol. 1976;107(3):293‐305. [DOI] [PubMed] [Google Scholar]
- 40. Keil B. Some newly characterized collagenases from procaryotes and lower eucaryotes. Mol Cell Biochem. 1979;23(2):87‐108. [DOI] [PubMed] [Google Scholar]
- 41. Reid GC, Woods DR, Robb FT. Peptone induction and rifampin‐insensitive collagenase production by vibrio alginolyticus. J Bacteriol. 1980;142:447‐454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Keil‐Dlouha V. Chemical characterization and study of the autodigestion of pure collagenase from Achromobacter iophagus. Biochim Biophys Acta. 1976;429(1):239‐251. [DOI] [PubMed] [Google Scholar]
- 43. Lambert Vidmar S, Lottspeich F, Emod I, Imhoff JM, Keil‐Dlouha V. Collagen‐binding domain of human plasma fibronectin contains a latent type‐IV collagenase. Eur J Biochem. 1991;201(1):79‐84. [DOI] [PubMed] [Google Scholar]
- 44. Steffensen B, Chen Z, Pal S, et al. Fragmentation of fibronectin by inherent autolytic and matrix metalloproteinase activities. Matrix Biol. 2011;30(1):34‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lambert Vidmar S, Lottspeich F, Emod I, Planchenault T, Keil‐Dlouha V. Latent fibronectin‐degrading serine proteinase activity in N‐terminal heparin‐binding domain of human plasma fibronectin. Eur J Biochem. 1991;201(1):71‐77. [DOI] [PubMed] [Google Scholar]
- 46. Chapman HA, Riese RJ, Shi GP. Emerging roles for cysteine proteases in human biology. Annu Rev Physiol. 1997;59:63‐88. [DOI] [PubMed] [Google Scholar]
- 47. Onesti MG, Fino P, Ponzo I, Ruggieri M, Scuderi N. Non‐surgical treatment of deep wounds triggered by harmful physical and chemical agents: a successful combined use of collagenase and hyaluronic acid. Int Wound J. 2014;13:22‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. fidia . Bionect Start, Patient Drug Information Leaflets. 2014. http://pro.fidiapharma.com/lstr_BIONECT_Start.pdf. Accessed January 2004.
- 49. Noruxol . Patient Drug Information Leaflets. 2011. https://farmaci.agenziafarmaco.gov.it/aifa/servlet/PdfDownloadServlet?pdfFileName=footer_007048_028039_FI.pdf&retry=0&sys=m0b1l3. Accessed January 21, 2011.
- 50. Bassetto F, Maschio N, Abatangelo G, Zavan B, Scarpa C, Vindigni V. Collagenase from vibrio alginolyticus cultures: experimental study and clinical perspectives. Surg Innov. 2016;23:557‐562. [DOI] [PubMed] [Google Scholar]
- 51. Sottile J, Hocking DC. Fibronectin polymerization regulates the composition and stability of extracellular matrix fibrils and cell‐matrix adhesions. Mol Biol Cell. 2002;13:3546‐3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Vial C, Gutiérrez J, Santander C, Cabrera D, Brandan E. Decorin interacts with connective tissue growth factor (CTGF)/CCN2 by LRR12 inhibiting its biological activity. J Biol Chem. 2011;286:24242‐24252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sayani K, Dodd CM, Nedelec B, et al. Delayed appearance of decorin in healing burn scars. Histopathology. 2000;36:262‐272. [DOI] [PubMed] [Google Scholar]
- 54. Scott PG, Dodd CM, Tredget EE, Ghahary A, Rahemtulla F. Immunohistochemical localization of the proteoglycans decorin, biglycan and versican and transforming growth factor‐beta in human post‐burn hypertrophic and mature scars. Histopathology. 1995;26:423‐431. [DOI] [PubMed] [Google Scholar]
- 55. Ghosh K, Ren XD, Shu XZ, Prestwich GD, Clark RA. Fibronectin functional domains coupled to hyaluronan stimulate adult human dermal fibroblast responses critical for wound healing. Tissue Eng. 2006;12:601‐613. [DOI] [PubMed] [Google Scholar]
- 56. Keil B. Vibrio alginolyticus (“Achromobacter”) collagenase: biosynthesis, function and application. Matrix Suppl. 1992;1:127‐133. [PubMed] [Google Scholar]
- 57. Van Wart HE, Steinbrink DR. Complementary substrate specificities of class I and class II collagenases from clostridium histolyticum. Biochemistry. 1985;24:6520‐6526. [DOI] [PubMed] [Google Scholar]
- 58. Nomenclature Committee of the International Union of Biochemistry . Units of enzyme activity. Eur J Biochem. 1979;97:319‐320. [Google Scholar]
- 59. Percival SL, McCarty S, Hunt JA, Woods EJ. The effects of pH on wound healing, biofilms, and antimicrobial efficacy. Wound Repair Regen. 2014;22:174‐186. [DOI] [PubMed] [Google Scholar]
- 60. Dereure O, Czubek M, Combemale P. Efficacy and safety of hyaluronic acid in treatment of leg ulcers: a double‐blind RCT. J Wound Care. 2012;21(3):131‐132. 134‐136, 138‐139. [DOI] [PubMed] [Google Scholar]
- 61. Humbert P, Mikosinki J, Benchikhi H, Allaert FA. Efficacy and safety of a gauze pad containing hyaluronic acid in treatment of leg ulcers of venous or mixed origin: a double‐blind, randomised, controlled trial. Int Wound J. 2013;10(2):159‐166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Gravante G, Sorge R, Giordan N, et al. Multicenter clinical trial on the performance and tolerability of the hyaluronic acid‐collagenase ointment for the treatment of chronic venous ulcers: a preliminary pilot study. Eur Rev Med Pharmacol Sci. 2013;17(20):2721‐2727. [PubMed] [Google Scholar]
- 63. Scalise A, Campitiello F, Della Corte A, et al. Enzymatic debridement: is HA‐collagenase the right synergy? Randomized double‐blind controlled clinical trial in venous leg ulcers. Eur Rev Med Pharmacol Sci. 2017;21(6):1421‐1431. [PubMed] [Google Scholar]


