Abstract
Beta antagonist is one of the most effective and the least toxic pharmacological treatments to attenuate the raised catecholamine effects for burned patients. To evaluate the effectiveness and safety of beta blocker compared with placebo or usual care in burned patients, a meta‐analysis of randomised controlled trials (RCTs) was conducted. We searched the database of PubMed, Embase, the Cochrane Library, and Web of Science to 10 April 2020. Two investigators independently assessed articles for inclusion and exclusion criteria and selected studies for the final analysis. We performed the meta‐analysis using a random‐effect model. A total of 12 RCTs were included in the study, including 1887 patients. Propranolol‐treated patients have a decrease in length of hospital stay in adults (weighted mean difference [WMD] = −9.06, 95% CIs = [−12.88, −5.24]) and prepare time of graft (WMD = −7.88, 95% CIs = [−12.27, −3.50]). Similarly, the use of propranolol could significantly decrease heart rate (WMD = −15.16, 95% CIs = [−20.37, −9.94]), rate pressure product (WMD = −1.32, 95% CIs = [−1.67, −0.97]), and mean arterial pressure (WMD = −2.75, 95% CIs = [−4.23, −1.26]). Moreover, there is no significant difference between propranolol and placebo with respect to mortality (risk difference [RD] = 0.00, 95% CIs [−0.03, 0.04]), sepsis (RD = −0.03, 95% CIs [−0.09, 0.03]), and events of post‐traumatic stress disorder (PTSD) and acute stress disorder (RD = −0.01, 95% CIs [−0.07, 0.05]), and also, there is no significant difference in subgroup analysis based on age. The use of beta antagonist in burned patients does reduce length of hospital stay in adults, shorten the preparation time for graft, and reduce heart burden, without increasing mortality, sepsis, or PTSD compared with those who had usual care or placebo. So beta antagonist can be considered as an appropriate treatment strategy in burned patients. More prospective, randomised‐controlled, multi‐centre studies were needed to define their place in therapeutic algorithms.
Keywords: adrenergic antagonist, burns, meta‐analysis, propranolol
1. INTRODUCTION
Burn injury is an important cause of morbidity and mortality worldwide. According to the World Health Organization, in 2004, approximately 180 000 people died of burn injury, and the number of burn patients requiring medical treatment came up to 11 million. 1 Due to significant advances in therapeutic strategies, such as enhancing wound coverage, appropriate infection control, and advanced surgical approaches, the prognosis of severely burned patients has been greatly improved. 1 , 2 However, in an Australia cohort, the all‐cause mortality rate of burned patient was still 1.4 times higher than that of no burned injuries (95% CI: 1.3‐1.5). 3
After being burned, the whole body is shifted into hypermetabolism and catabolism state 2 , 4 due to the release of a large number of catecholamines. 5 This will result in increased resting energy expenditure, rapid muscle loss, and high incidence rates of depression, anxiety, and post‐traumatic stress disorder (PTSD), 6 , 7 thus delaying the recovery process of burn patients, which is one of the main reasons for poor recovery. 4 To our best knowledge, beta antagonist is one of the most effective and least toxic pharmacological treatments to attenuate the raised catecholamine effects. 4 Some experiments have shown that long‐term use of beta antagonists in burn patients might lessen cardiac workload and fat infiltration of the liver, and the latter pathological change also stimulates the process of catabolism condition. 4 , 8 However, Nunez 9 et al synthesised the relevant researches and indicated that the mortality and sepsis of burn patients treated with propranolol were not significantly different from the control group, and there was also insufficient evidence to prove that the use of propranolol could reduce the length of hospital stay among burned patients. More and more randomised controlled trials (RCTs) 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 have been published in recent years. We performed a meta‐analysis with the updated data, hoping to provide more evidence to evaluate the effectiveness and safety of using beta blocker in burned patients.
2. MATERIALS AND METHODS
2.1. Ethics statement
Our systematic review and meta‐analysis were performed in accordance with the Cochrane systematic review handbook (http://handbook.cochrane.org.) to ensure the quality of this study. Literature screening, quality evaluation, and data extraction were performed independently by two reviewers. The PROSPERO registration number is CRD42019123710.
2.2. Search methods
Two reviewers independently conducted a literature search of PubMed, Embase, the Cochrane Library, and Web of Science (last updated to 10 April 2020) on the use of search terms including burn* and beta antagonist* with appropriate synonyms (such as beta block*, atenolol, bisoprolol, carvedilol, metoprolol, propranolol, and other synonyms). There is no language restriction in search process (Table S1).
2.3. Inclusion criteria
Any RCT that fulfilled the following inclusion criteria would be included in our meta‐analysis. (a) Participants (P): the literature that reported the percentage of wounds covering area of the total body surface area (TBSA). (b) Interventions (I) and Comparisons (C): RCTs compared beta antagonist with standard treatment or placebo. (c) Outcomes (O): results of studies included any of the following information: mortality rate, sepsis, length of hospital stay, PTSD, time ready for graft, cardiac function index (heart rate [HR], cardiac index [CI], rate pressure product [RPP], mean arterial pressure [MAP]), fat metabolism, protein metabolism, and resting energy expenditure. Reviews, case reports, conference abstracts, animal experiments, in vitro studies, and studies without randomisation for treatment allocation or studies without usable data were excluded.
The eligibility of each study was evaluated by two reviewers based on the title, abstract and full text. Any disagreement would be resolved through discussion and negotiation. If some differences still existed, the third reviewer would be asked to make the final judgement.
2.4. Data collection
Data and study characteristics were extracted by two reviewers using a standardised collection form (Table S2). The main data included basic study information, population characteristics, interventions in study and control groups, and results of primary outcome indicators as well as study quality. The information was obtained from published data.
2.5. Risk of bias
Two investigators independently evaluated 12 articles based on the Cochrane risk of bias (ROB) tool. 22 The biases included selection bias (sequence generation and allocation concealment), performance bias, detection bias, incomplete data bias, selective reporting, and other biases. According to the information provided in the articles and information obtained by communicating with the authors, we rated each item as “high risk,” “low risk,” or “unclear Risk.” Finally, we computed graphic representations of potential bias by using Review Manager 5.3.
2.6. Data analysis
This meta‐analysis was performed using RevMan 5.3 software provided by the Cochrane Collaboration. The risk difference (RD) and 95% confidence intervals (CIs) were used as the statistical indicators of the enumeration data (eg, mortality rate, sepsis), and the measurement data (eg, length of stay, cardiac function index) used weighted mean difference (WMD) and 95% CIs as the statistical indicators of curative effects. Then, we performed pooled analyses using random effect model to calculate effect sizes and 95% CIs. Moreover, I 2 test was used to evaluate the heterogeneity of each study result. When I 2 > 50%, subgroup analyses based on possible heterogeneity factors (eg, age) were conducted to find the sources of heterogeneity. And we also performed sensitivity analysis to test the stability of the combined results. In our study, P value ≤ .05 was considered a significant difference.
3. RESULTS
3.1. Literature screening
We searched 3104 related records. After duplication, 816 repeated articles were excluded. Then, screening according titles and abstracts, we excluded 2246 irrelevant articles. Finally, 12 studies were included in this meta‐analysis. The literature screening process was shown in Figure 1.
FIGURE 1.
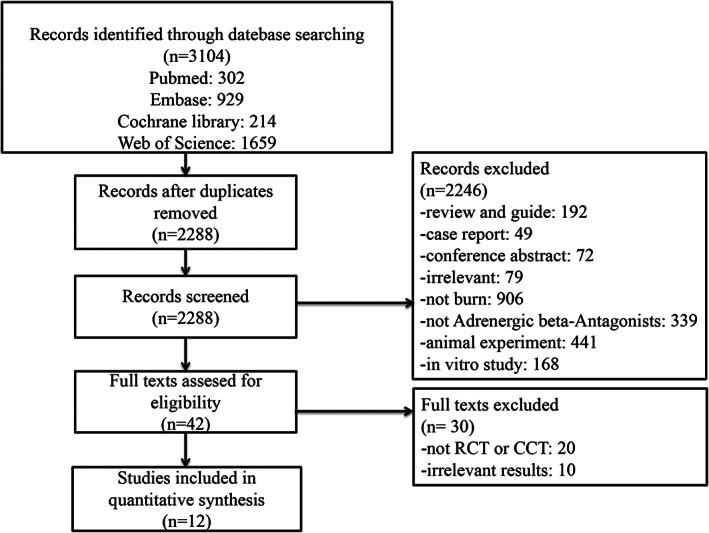
Flowchart according to PRISMA guidelines
3.2. Characteristics of included studies
Our meta‐analysis included 1887 patients from 12 RCTs 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 comparing with or without propranolol treatment. These trials were published from 2001 to 2020. Ten trials were performed in the United States, and the remaining two were in Iran 17 and Pakistanp. 21
Almost all RCTs included patients with burns greater than 20% of TBSA, only one trial 18 limited the TBSA ≤20%. Interventions and comparisons were very similar, with propranolol (titrated to decrease HR by 20% of admission HR) and placebo or standard care being the intervention and comparison. The specific characteristics of the included studies are shown in Table 1.
TABLE 1.
Study and patient population characteristics of included studies
| No. | Study | Country | Study length | Group, n (male) | Age, years | Total body surface area, % | Dose | Administration time |
|---|---|---|---|---|---|---|---|---|
| 1 | Ali, 2015 11 | United States | Not described | Standard care, n = 34 (30) | 38 ± 16 | 59 ± 22 | — | — |
| Propranolol, n = 41 (29) | 41 ± 14 | 49 ± 18 | Average 3.3 ± 3.0 mg/kg/d | Hospitalisation period | ||||
| 2 | Hart, 2002 12 | United States | 3 y | Standard care, n = 19 (13) | 8.4 ± 1.6 | 58 ± 4 | — | — |
| Propranolol, n = 12 (8) | 7.0 ± 1.5 | 56 ± 4 | 0.33 mg/kg q4h | Not described | ||||
| 3 | Herndon, 2001 13 | United States | 1 y | Standard care, n = 12 (9) | 7.8 ± 1.4 | 39 ± 5 | — | — |
| Propranolol, n = 12 (8) | 6.6 ± 1.5 | 57 ± 4 | Decrease the resting heart rate by 20% | 14 d | ||||
| 4 | Herndon, 2012 14 | United States | 10 y | Standard care, n = 89 (56) | 7 ± 5 | 57.5 ± 13.5 | — | — |
| Propranolol, n = 90 (67) | 7 ± 5 | 55.7 ± 16.5 | 4 mg/kg/d | Not described | ||||
| 5 | Herndon, 2016 15 | United States | 18 y | Standard care, n = 248 (146) | 6 ± 0.2 | 52 ± 1 | — | — |
| Propranolol, n = 197 (128) | 5 ± 0.3 | 52 ± 1 | 4.0 ± 0.2 mg/kg/d | Not described | ||||
| 6 | Jeschke, 2007 16 | United States | 11 y | Standard care, n = 143 (83) | 7.8 ± 0.4 | 55 ± 1 | — | — |
| Propranolol, n = 102 (43) | 7.2 ± 0.6 | 54 ± 2 | 0.5‐1.5 mg/kg q6h | 1 mo | ||||
| 7 | Mohammadi, 2009 17 | Iran | 1 y | Standard care, n = 42 (20) | 24.5 ± 12.0 | 33.6 ± 8.7 | — | — |
| Propranolol, n = 37 (22) | 27.7 ± 9.7 | 31.4 ± 7.9 | 1 mg/kg/d | Not described | ||||
| 8 | Orrey, 2014 18 | United States | 2 y | Standard care, n = 23 (19) | 32 ± 10 | ≤20% | — | — |
| Propranolol, n = 20 (15) | 31 ± 9 | ≤20% | 120 mg bid | 20 d | ||||
| 9 | Rosenberg, 2018 10 | United States | 2 y | Standard care, n = 113 (62) | 7.4 ± 4.5 | 56.2 ± 15.4 | — | — |
| Propranolol, n = 89 (73) | 7.2 ± 4.7 | 56.5 ± 14.9 | 4 mg/kg/d | Not described | ||||
| 10 | Sharp, 2010 19 | United States | Not described | Standard care, n = 237 (142) | 7 | 56 | — | — |
| Propranolol, n = 126 (90) | 6 | 55 | Not described | Not described | ||||
| 11 | Wurzer, 2016 20 | United States | 11 y | Standard care, n = 62 (41) | 10 ± 6 | 59 ± 18 | — | — |
| Propranolol, n = 69 (36) | 10 ± 6 | 58 ± 16 | 1 mg/kg/d | Not described | ||||
| 12 | Cheema, 2020 21 | Pakistan | 1 y | Standard care, n = 35 (11) | 18‐60 | 28 ± 3.9 | — | — |
| Propranolol, n = 35 (10) | 28.7 ± 4.4 | 0.5‐3 mg/kg/d | Not described |
4. OUTCOMES
4.1. Time ready for graft (d)
Two studies 17 , 21 showed that using propranolol reduced preparation time before transplant surgery. Based on these results, we made a forest plot for the time ready for graft. The pooled result shows that the preparation time in propranolol is shorter than that in usual care [WMD = −7.88, 95% CI (−12.27, −3.50), P = .03, I 2 = 79%] (Figure 2).
FIGURE 2.

Forest plot for time ready for graft (d) (propranolol versus usual care)
4.2. Length of stay in hospital (d)
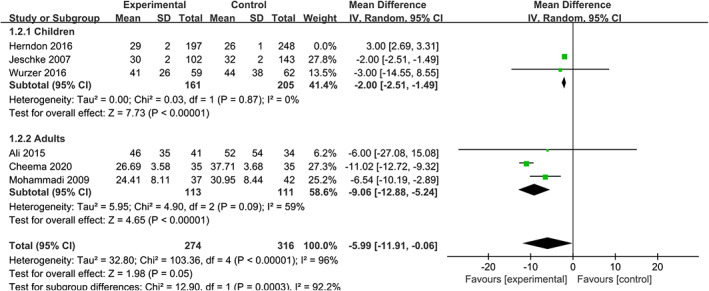
The length of stay in hospital reflects the situation of recovery. According to our results, the use of propranolol does not shorten the time of stay in hospital 11 , 15 , 16 , 17 , 20 , 21 (WMD = −3.97, 95% CIs [−8.21, 0.27], P = .07, I 2 = 99%). Similarly, there is no significant difference in the subgroup of children 15 , 16 , 20 (WMD = 0.10, 95% CIs [−4.50, 4.69], P = .97, I 2 = 99%), but the results in the subgroup of adults are statistically significant 11 , 17 , 21 (WMD = −9.06, 95% CIs [−12.88, −5.24], P = .09, I 2 = 59%) (Figure 3).
FIGURE 3.
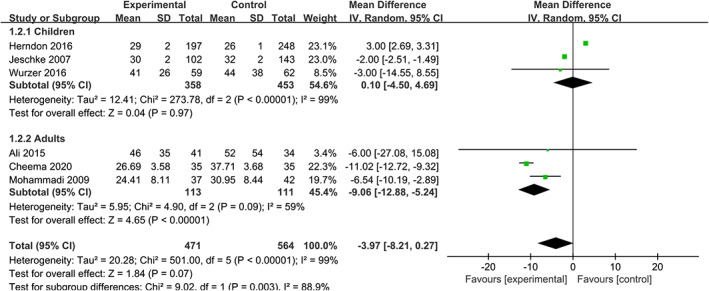
Forest plot for length of stay in hospital (d) (propranolol versus usual care)
4.3. Mortality
Five articles 11 , 15 , 16 , 17 , 20 reported mortality rate for burned patients during hospitalisation, including 965 patients. As shown in Figure 4, there is no significant difference in mortality between the two groups (RD = 0.00, 95% CIs [−0.03, 0.04], P = .78, I 2 = 5%), and also, there is no significant difference in subgroup analysis based on age (adults, 11 , 17 RD = −0.07, 95% CIs (−0.20, 0.07), P = .34, I 2 = 25%; children, 15 , 16 , 20 RD = 0.01, 95% CIs (−0.02, 0.04), P = .49, I 2 = 0%). Overall, the use of propranolol in burned patients does not increase mortality during hospitalisation.
FIGURE 4.
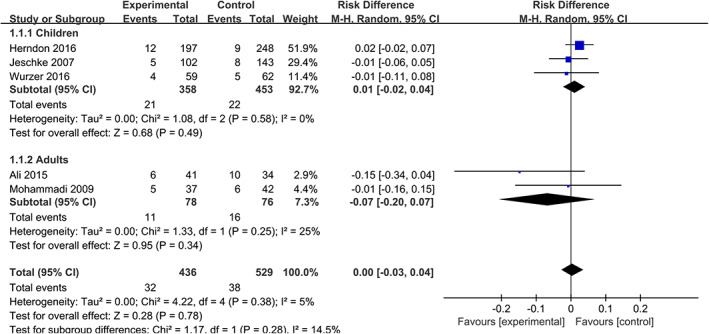
Forest plot for mortality (propranolol versus usual care)
4.4. Sepsis
Similarly, no significant difference is found when we assess the occurrence of sepsis 12 , 13 , 16 , 17 (RD = −0.03, 95% CIs [−0.09, 0.03], P = .37, I 2 = 0%). The analysis in different age groups shows the same conclusion in adults 17 (RD = −0.04, 95% CIs [−0.17, 0.09], P = .57, I 2 = 0%) and children 12 , 13 , 16 (RD = −0.02, 95% CIs [−0.09,0.04], P = .71, I 2 = 0%). To sum it up, treatment with propranolol does not increase infection rate among patients with severe burns (Figure 5).
FIGURE 5.
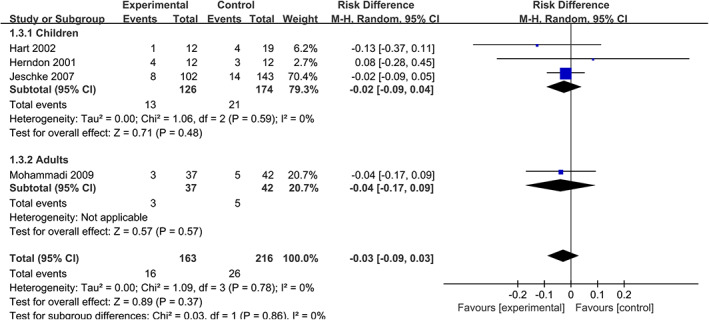
Forest plot for sepsis (propranolol versus usual care)
4.5. HR, RPP, and MAP
We find that the use of propranolol significantly reduces HR 14 , 17 , 20 , 21 (WMD = −15.16, 95% CIs = [−20.37, −9.94], P < .001, I 2 = 90%), RPP 14 , 20 (WMD = −1.32, 95% CIs = [−1.67, −0.97], P < .001, I 2 = 68%), and MAP 14 , 20 (WMD = −2.75, 95% CIs [−4.23, −1.26], P = .003, I 2 = 60%) (Figures 6, 7, 8).
FIGURE 6.
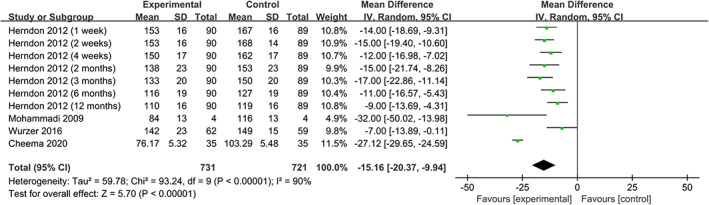
Forest plot for heart rate (propranolol versus usual care)
FIGURE 7.
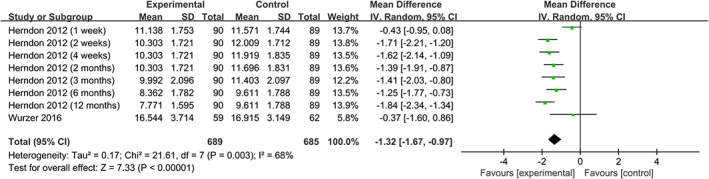
Forest plot for rate pressure product (propranolol versus usual care)
FIGURE 8.
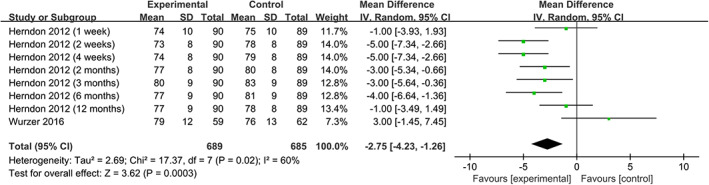
Forest plot for mean arterial pressure (propranolol versus usual care)
4.6. Psychological health
Orrey, Rosenberg, and Sharp used the PSS‐I criteria to determine PTSD, and the “acute stress disorder symptom checklist” for acute stress disorder (ASD). 10 , 18 , 19 The RD of PTSD in propranolol‐treated group is −0.01, but this difference was not statistically significant. (RD = −0.01, 95% CIs [−0.07, 0.05], P = .77, I 2 = 39%) (Figure 9).
FIGURE 9.
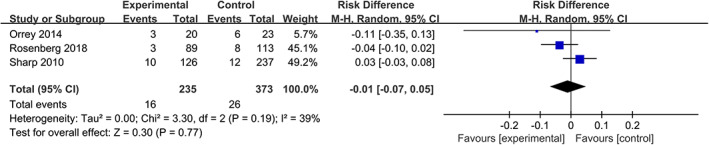
Forest plot for PTSD and ASD (propranolol versus usual care). ASD, acute stress disorder; PTSD, post‐traumatic stress disorder
4.7. Risk of bias
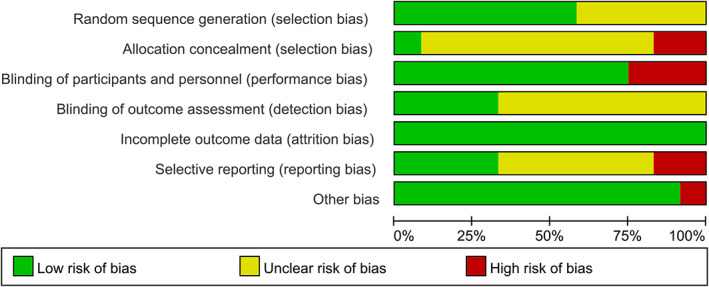
Seven and five trials were rated as low risk and high risk for generating a random sequence, respectively. In addition, in the allocation concealment, the number of studies rated as low risk or high risk was one and two. As for the assessment of blinding implementation, trials rated as low risk of bias in the two parts of implementation and outcome measurement were nine and four, respectively. Then, we evaluated the integrity of the results report, and all the trials were rated as low risk. Finally, we assessed the selective reporting and other bias. To sum it up, articles included in our study could generally be rated as low risk. The overall risk of bias of the included RCTs is best represented in Figures 10 and 11.
FIGURE 10.
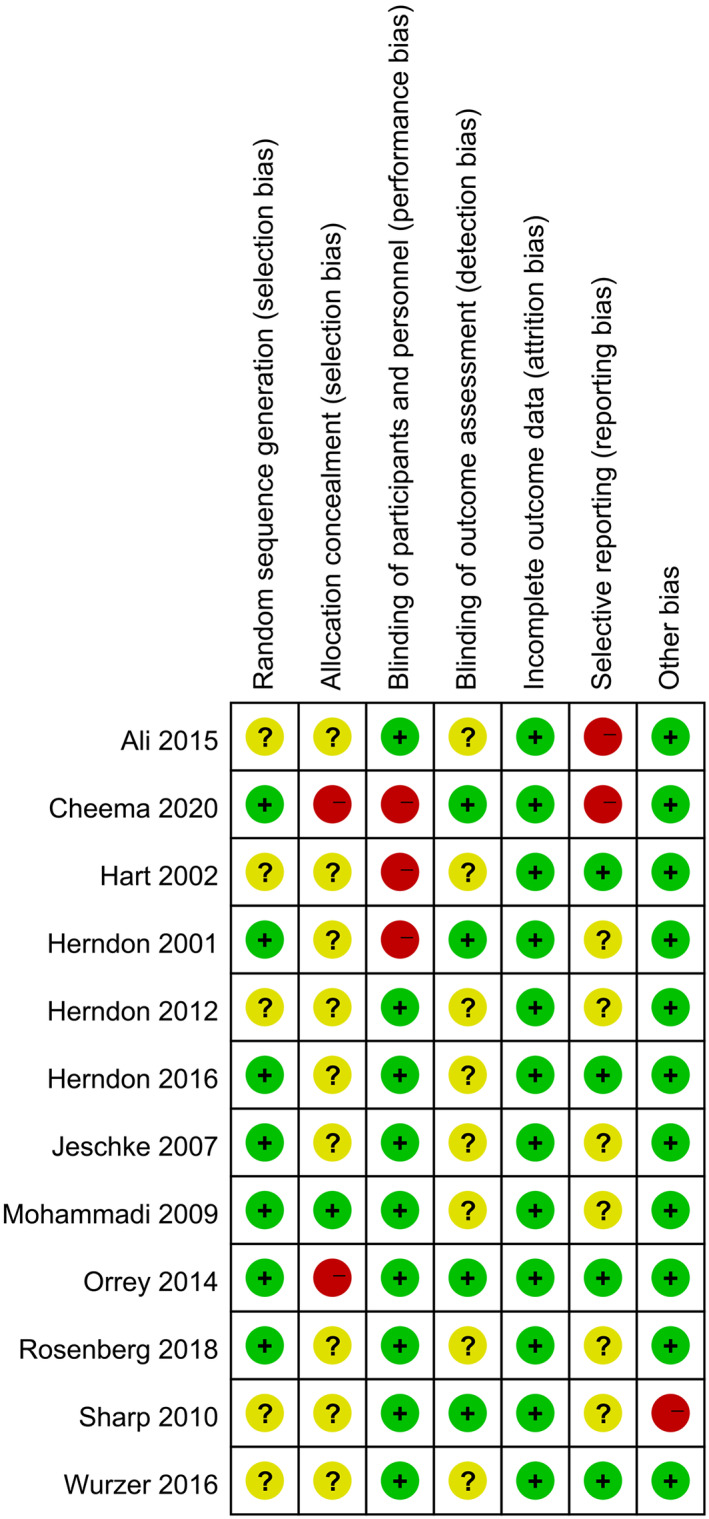
Risk of bias across studies
FIGURE 11.
Risk of bias from individual study
4.8. Sensitivity analysis
When we checked the studies included in the days of hospitalisation one by one, we found that when one study 15 was removed from them, the heterogeneity of the pooled results in the group of children was significantly reduced, and indicated that the use of propranolol could shorten hospital stay. (WMD = −2.00, 95% CIs [−2.51, −1.49], P < .001, I 2 = 0%). We also performed the sensitivity analysis on other combined results, but the final results did not change significantly, indicating that our results were relatively stable. The sensitivity analysis of the meta‐analysis is shown in Figure 12.
FIGURE 12.
Sensitivity analysis
5. DISCUSSION
Some previous clinical trials have suggested that propranolol is one of the most effective and least toxic pharmacological treatments for burns. 4 Moreover, the American Burn Association Consensus in 2013 23 recommended it as a pharmacological method to regulate post‐burn stress response. Several meta‐analyses 8 , 9 , 24 , 25 have also studied it in recent years. However, there was still insufficient evidence to strongly support the previously mentioned conclusions. 9 , 24 , 25 The latest meta‐analysis 9 included eight studies in quantitative synthesis and indicated that there were no differences in mortality or sepsis, while the use of propranolol in burned patients resulted in lower values of HR. As a few new RCTs 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 have been reported recently, we conducted this systematic review and meta‐analysis to evaluate the effectiveness and safety of beta blocker in burned patients. We further confirmed that the use of beta antagonist in burned patients has no significant effect on sepsis and mortality and reduced HR. More importantly, we found that the use of propranolol in burned patients could shorten the time to prepare for graft, reduce the time of stay in hospital for adults and protect the heart function.
Our pooled analysis indicates that the time ready for graft is less in propranolol group than in control group. One possible explanation for this may be that the administration of propranolol improves wound contracture and promotes proper epithelialisation of some superficial parts in the border of deep burn area, and thus improves healing process and decreases the time ready for graft, which is also consistent with animal results. 26 Besides, avoiding wound infection, reducing catabolism, and preserving protein stores as potential benefits of propranolol, which might be other reasons of reducing time ready for graft. 17 In most burns, skin grafting to close wounds is the main treatment method. Transplanting as soon as possible can help effectively reduce residual wounds and the formation of scars on the wound surface. Therefore, reducing the time to prepare for transplantation can improve wound healing process.
Another important finding is that the use of propranolol in burned patients is associated with a reduced length of hospital stay for adults. In other words, it may also reduce medical expenses by shortening hospital stay. Interestingly, this finding is different from the result of previous research. 9 In burned patients, the release of endogenous catecholamines triggers a state of catabolic reactions, 5 , 27 which will lead to depressed immunity, increase infectious complications, impair wound healing, and profound generalised weakness, thereby extending the length of hospital stay. Propranolol can block these effects to some extent. 2 , 4 , 5 In addition, based on our pooled results, the use of propranolol can shorten the preparation time for transplant surgery, which also reduces the total hospital stay. However, the results vary in total people and the subgroup analyses. The length of hospital stay in burned adults shows a significant difference, while no significant difference in burned children. We suppose some other factors including the area and deepness of the burn, the initiation time of the treatment, the dosage of propranolol, and the compliance of patients may have effects on the length of hospital stay. 28 , 29
The release of catecholamines also triggers the systemic inflammatory response, causing protein degradation and catabolism. Consequently, the structure and function of essential organs such as the muscle, skin, heart, immune system, and liver are compromised, contributing to multiple organ failure, sepsis, and mortality. 9 In our results, we found the use of propranolol does not increase the mortality and sepsis, which is consistent with previous studies. 9 , 24 Jeschke et al 16 showed that propranolol was associated with a decrease in serum TNF and IL‐1, but levels only decreased at one time point, indicating that propranolol did not alter the inflammatory reaction compared with controls. What is more, the overall tendency is similar in both total people and the subgroup of children.
And then, we assess the cardiac function. When individuals suffer from trauma, the concentration of catecholamines significantly increases, resulting in an increased burden on the heart. In this case, the patient is prone to develop acute cardiac failure, which is also a common cause of death in adult burned patients. 20 Previous studies have consistently reported that the use of propranolol could significantly reduce HR. 9 And we find similar results with them. The explanation may be that the non‐selective activity of propranolol on β1‐ and β2‐adrenergic receptors theoretically counteracts the increased levels of catecholamines, by binding to adrenergic receptors and blocking the positive inotropic and chronotropic effects of the sympathetic system.
What is more, we also synthesise other cardiac index, like RPP and MAP. Similar to the effect on HR, propranolol also significantly reduces RPP and blood pressure. As RPP is a commonly used measure of myocardial oxygen consumption, 20 , 23 , 30 , 31 we have inferred that propranolol can reduce myocardial oxygen consumption in burned patients. Therefore, combined with the HR, we conclude that propranolol could lessen heart burden and protect the heart function of burned patients. However, there is a large heterogeneity between the studies included in the analysis of HR, RPP, and MAP. And although our sensitivity analysis results indicated that the pooled results were stable, the application of this evidence should still pay attention to the condition of individual patients.
In addition, another superiority of our research is the synthetic analysis of the relevant indicators (PTSD and ASD) for measuring psychological health. According to the current research results, early identification and control of psychological problems such as anxiety or depression helps accelerate the process of wound healing. 32 , 33 Orrey et al 10 , 18 , 19 used the PSS‐I criteria 34 to determine PTSD, while ASD was measured by the “acute stress disorder symptom checklist.” 35 Our results have shown that the occurrence of stress events such as PTSD and ASD in propranolol‐treated group after burns is lower than control (RD = −0.01), but this result is not statistically significant.
Although our research has more comprehensive search strategy (Table S1) and incorporates the latest research compared with previous studies, there are still several shortcomings in our research. Firstly, most of the studies are only for propranolol and we included propranolol to evaluate, so other beta antagonists, such as selective beta blockers, could not be evaluated whether they are effective for burns. In addition, some studies involved in this study are in high risk and high heterogeneity, which might result in inevitable bias. Last but not least, some of the variable and outcome indices were only included by a few studies, which remains to be studied. More prospective, randomised‐controlled, multi‐centre studies were needed to define their place in therapeutic algorithms. Future trials should also assess the impacts of different routes of medication on clinically relevant outcomes and different effects of different areas and depth of the burn.
6. CONCLUSION
Our study indicates that the use of propranolol in burned patients could shorten the time to prepare for graft, reduce the time of stay in hospital in adults, and protect the heart function. Besides, neither does it increase the mortality rate during hospitalisation nor increase the occurrence of sepsis or PTSD. In summary, the use of beta antagonist is an effective and safe choice in burned patients and can be considered as an appropriate treatment strategy. This study is limited by the sample size and quality of the original studies, so further trials on large population with a wider range of outcome measures are warranted to provide more high‐quality evidence.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
Jing Ma, Dingyao Hu, and Zhen Feng were involved in data collection, bias evaluation, analyses of results, and drafting of the article. Jinxing Quan participated in research design and article revising. Jia Tang, Lanlan Guo, and Yali Du participated in data collection. All authors have reviewed the final version of the article and approved to submit to your journal. This article has not been published elsewhere in whole or in part.
Supporting information
Table S1. Search strategies.
Table S2. Data collection.
ACKNOWLEDGEMENTS
This study was supported by National Natural Science Foundation of China (81860091).
Ma J, Hu D, Feng Z, et al. The effectiveness and safety of beta antagonist in burned patients: A systematic review and meta‐analysis. Int Wound J. 2020;17:1881–1892. 10.1111/iwj.13478
Jing Ma, Dingyao Hu and Zhen Feng contributed equally to this work.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available in Rosenberg L's study at https://doi.org/10.1089/cap.2017.0073, reference number 11. The data that support the findings of this study are openly available in Ali A's study at https://doi.org/10.1186/s13054-015-0913-x, reference number 13. The data that support the findings of this study are openly available in David W Hart's study at https://doi.org/10.1097/00000658-200210000-00007, reference number 14. The data that support the findings of this study are openly available in Herndon's, 2001 study at https://doi.org/10.1056/NEJMoa010342, reference number 15. The data that support the findings of this study are openly available in Herndon's, 2012 study at https://doi.org/10.1097/SLA.0b013e318265427e, reference number 16. The data that support the findings of this study are openly available in Herndon's, 2016 study at https://doi.org/10.1097/SLA.0000000000001844, reference number 17. The data that support the findings of this study are openly available in Marc G Jeschke's study at https://doi.org/10.1097/TA.0b013e318031afd3, reference number 18. The data that support the findings of this study are openly available in Ali Akbar Mohammadi's study at https://doi.org/10.1097/BCR.0b013e3181b48600, reference number 19. The data that support the findings of this study are openly available in Danielle C Orrey's study at https://doi.org/10.1097/AJP.0000000000000086, reference number 20. The data that support the findings of this study are openly available in Sherri Sharp's study at https://doi.org/10.1097/TA.0b013e3181a8b326, reference number 21. The data that support the findings of this study are openly available in Paul Wurzer's study at https://doi.org/10.1097/10.1097/SHK.0000000000000671, reference number 22. The data that support the findings of this study are openly available in Saeed Ashraf Cheema's study at https://doi.org/10.1097/10.29271/jcpsp.2020.01.46, reference number 30.
REFERENCES
- 1. Wang Y, Beekman J, Hew J, et al. Burn injury: challenges and advances in burn wound healing, infection, pain and scarring. Adv Drug Deliv Rev. 2018;123:3‐17. [DOI] [PubMed] [Google Scholar]
- 2. Norbury WB, Jeschke MG, Herndon DN. Metabolism modulators in sepsis: propranolol. Crit Care Med. 2007;35(9) Suppl:S616‐S620. [DOI] [PubMed] [Google Scholar]
- 3. Duke JM, Boyd JH, Rea S, Randall SM, Wood FM. Long‐term mortality among older adults with burn injury: a population‐based study in Australia. Bull World Health Organ. 2015;93(6):400‐406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Herndon DN, Tompkins RG. Support of the metabolic response to burn injury. Lancet. 2004;363(9424):1895‐1902. [DOI] [PubMed] [Google Scholar]
- 5. Wilmore DW, Long JM, Mason AD Jr, Skreen RW, Pruitt BA. Catecholamines: mediator of the hypermetabolic response to thermal injury. Ann Surg. 1974;180(4):653‐669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mangano DT, Layug EL, Wallace A, Tateo I. Effect of atenolol on mortality and cardiovascular morbidity after noncardiac surgery. Multicenter study of perioperative ischemia research group. N Engl J Med. 1996;335(23):1713‐1720. [DOI] [PubMed] [Google Scholar]
- 7. Wiechman SA, Patterson DR. Psychosocial aspects of burn injuries. BMJ. 2004;329(7462):391‐393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Baron PW, Barrow RE, Pierre EJ, Herndon DN. Prolonged use of propranolol safely decreases cardiac work in burned children. J Burn Care Rehabil. 1997;18(3):223‐227. [DOI] [PubMed] [Google Scholar]
- 9. Manzano‐Nunez R, Garcia‐Perdomo HA, Ferrada P, Ordonez Delgad CA, Gomez DA, Foianini JE. Safety and effectiveness of propranolol in severely burned patients: systematic review and meta‐analysis. World J Emerg Surg. 2017;12:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rosenberg L, Rosenberg M, Sharp S, et al. Does acute propranolol treatment prevent posttraumatic stress disorder, anxiety, and depression in children with burns? J Child Adolesc Psychopharmacol. 2018;28(2):117‐123. [DOI] [PubMed] [Google Scholar]
- 11. Ali A, Mamachen A, Branski LK, Herndon DN. Propranolol attenuates hemorrhage and accelerates wound healing in severely burned adults. Crit Care. 2015;19:217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hart DW, Wolf SE, Chinkes DL, Lal SO, Ramzy PI. Beta‐blockade and growth hormone after burn. Ann Surg. 2002;236(4):450‐456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Herndon DN, Hart DW, Wolf SE, Chinkes DL, Wolfe RR. Reversal of catabolism by beta‐blockade after severe burns. N Engl J Med. 2001;345(17):1223‐1229. [DOI] [PubMed] [Google Scholar]
- 14. Herndon DN, Rodriguez NA, Diaz EC. Long‐term propranolol use in severely burned pediatric patients: a randomized controlled study. Ann Surg. 2012;256(3):402‐411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Herndon DN, Voigt CD, Capek KD. Reversal of growth arrest with the combined Administration of Oxandrolone and Propranolol in severely burned children. Ann Surg. 2016;264(3):421‐428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jeschke MG, Norbury WB, Finnerty CC, Branski LK. Propranolol does not increase inflammation, sepsis, or infectious episodes in severely burned children. J Trauma. 2007;62(3):676‐681. [DOI] [PubMed] [Google Scholar]
- 17. Mohammadi AA, Bakhshaeekia A, Alibeigi P. Efficacy of propranolol in wound healing for hospitalized burn patients. J Burn Care Res. 2009;30(6):1013‐1017. [DOI] [PubMed] [Google Scholar]
- 18. Orrey DC, Halawa OI, Bortsov AV, et al. Results of a pilot multicenter genotype‐based randomized placebo‐controlled trial of propranolol to reduce pain after major thermal burn injury. Clin J Pain. 2015;31(1):21‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sharp S, Thomas C, Rosenberg L, Rosenberg M, W3rd M. Propranolol does not reduce risk for acute stress disorder in pediatric burn trauma. J Trauma. 2010;68(1):193‐197. [DOI] [PubMed] [Google Scholar]
- 20. Wurzer P, Branski LK, Clayton RP, et al. Propranolol reduces cardiac index but does not adversely affect peripheral perfusion in severely burned children. Shock. 2016;46(5):486‐491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cheema SA, Ahmed UT, Nasir H, Dogar SR, Mustafa Z. Effects of propranolol in accelerating wound healing and attenuation of hypermetabolism in adult burn patients. J Coll Physicians Surgeons Pak. 2020;30(1):46‐50. [DOI] [PubMed] [Google Scholar]
- 22. Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gibran NS, Wiechman S, Meyer W. Summary of the 2012 ABA burn quality consensus conference. J Burn Care Res. 2013;34(4):361‐385. [DOI] [PubMed] [Google Scholar]
- 24. Flores O, Stockton K, Roberts JA, Muller MJ, Paratz JD. The efficacy and safety of adrenergic blockade after burn injury: a systematic review and meta‐analysis. J Trauma Acute Care Surg. 2016;80(1):146‐155. [DOI] [PubMed] [Google Scholar]
- 25. Shan Chew EC, Baier N, Lee JH. Do beta‐blockers decrease the Hypermetabolic state in critically ill children with severe burns? Hosp Pediatr. 2015;5(8):446‐451. [DOI] [PubMed] [Google Scholar]
- 26. Souza BR, Jianine SS, Costa AM. Blockade of B1‐ and B2‐adrenoceptors delays wound contraction and reepithelializationin rats. Clin Exp Pharmacol Physiol. 2006;33(5‐6):421‐430. [DOI] [PubMed] [Google Scholar]
- 27. Chang DW, DeSanti L, Demling RH. Anticatabolic and anabolic strategies in critical illness: a review of current treatment modalities. Shock. 1998;10:155‐160. [DOI] [PubMed] [Google Scholar]
- 28. Berry CC, Patterson TL, Wachtel TL, Frank HA. Behavioural factors in burn mortality and length of stay in hospital. Burns. 1984;10:409‐414. [DOI] [PubMed] [Google Scholar]
- 29. Sánchez J, Perepérez S, Bastida J, Martínez M. Cost‐utility analysis applied to the treatment of burn patients in a specialized center. Arch Surg. 2007;142:50‐57. [DOI] [PubMed] [Google Scholar]
- 30. Kitamura K, Jorgensen CR, Gobel FL, Taylor HL, Wang Y. Hemodynamic correlates of myocardial oxygen consumption during upright exercise. J Appl Physiol. 1972;32(4):516‐522. [DOI] [PubMed] [Google Scholar]
- 31. Katz LN, Feinberg H. The relation of cardiac effort to myocardial oxygen consumption and coronary flow. Circ Res. 1958;6(5):656‐669. [DOI] [PubMed] [Google Scholar]
- 32. Martin L, Byrnes M, Bulsara MK. Quality of life and posttraumatic growth after adult burn: a prospective, longitudinal study. Burns. 2017;43(7):1400‐1410. [DOI] [PubMed] [Google Scholar]
- 33. Haag AC, Landolt MA, Kenardy JA. Preventive intervention for trauma reactions in young injured children: results of a multi‐site randomised controlled trial. J Child Psychol Psychiatry. 2020;10. [DOI] [PubMed] [Google Scholar]
- 34. Foa EB, McLean CP, Zang Y, et al. Psychometric properties of the posttraumatic stress disorder symptom scale interview for DSM‐5 (PSSI‐5). Psychol Assess. 2016;28(10):1159‐1165. [DOI] [PubMed] [Google Scholar]
- 35. Trull TJ, Verges A, Wood PK, Jahng S, Sher KJ. The structure of diagnostic and statistical manual of mental disorders (4th edition, text revision) personality disorder symptoms in a large national sample. Personal Disord. 2012;3(4):355‐369. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Search strategies.
Table S2. Data collection.
Data Availability Statement
The data that support the findings of this study are openly available in Rosenberg L's study at https://doi.org/10.1089/cap.2017.0073, reference number 11. The data that support the findings of this study are openly available in Ali A's study at https://doi.org/10.1186/s13054-015-0913-x, reference number 13. The data that support the findings of this study are openly available in David W Hart's study at https://doi.org/10.1097/00000658-200210000-00007, reference number 14. The data that support the findings of this study are openly available in Herndon's, 2001 study at https://doi.org/10.1056/NEJMoa010342, reference number 15. The data that support the findings of this study are openly available in Herndon's, 2012 study at https://doi.org/10.1097/SLA.0b013e318265427e, reference number 16. The data that support the findings of this study are openly available in Herndon's, 2016 study at https://doi.org/10.1097/SLA.0000000000001844, reference number 17. The data that support the findings of this study are openly available in Marc G Jeschke's study at https://doi.org/10.1097/TA.0b013e318031afd3, reference number 18. The data that support the findings of this study are openly available in Ali Akbar Mohammadi's study at https://doi.org/10.1097/BCR.0b013e3181b48600, reference number 19. The data that support the findings of this study are openly available in Danielle C Orrey's study at https://doi.org/10.1097/AJP.0000000000000086, reference number 20. The data that support the findings of this study are openly available in Sherri Sharp's study at https://doi.org/10.1097/TA.0b013e3181a8b326, reference number 21. The data that support the findings of this study are openly available in Paul Wurzer's study at https://doi.org/10.1097/10.1097/SHK.0000000000000671, reference number 22. The data that support the findings of this study are openly available in Saeed Ashraf Cheema's study at https://doi.org/10.1097/10.29271/jcpsp.2020.01.46, reference number 30.


