Abstract
The objective of this article is to formulate a new bioengineering theoretical framework for modelling the biomechanical efficacy of cyanoacrylate skin protectants, with specific focus on the Marathon technology (Medline Industries, Inc., Northfield, Illinois) and its modes of action. This work details the bioengineering and mathematical formulations of the theory, which is based on the classic engineering theories of flexural stiffness of coated elements and deformation friction. Based on the relevant skin anatomy and physiology, this paper demonstrates: (a) the contribution of the polymerised cyanoacrylate coating to flexural skin stiffness, which facilitates protection from non‐axial (eg, compressive) localised mechanical forces; and (b) the contribution of the aforementioned coating to reduction in frictional forces and surface shear stresses applied by contacting objects such as medical devices. The present theoretical framework establishes that application of the cyanoacrylate coating provides considerable biomechanical protection to skin and subdermally, by shielding skin from both compressive and frictional (shearing) forces. Moreover, these analyses indicate that the prophylactic effects of the studied cyanoacrylate coating become particularly strong where the skin is thin or fragile (typically less than ~0.7 mm thick), which is characteristic to old age, post‐neural injuries, neuromuscular diseases, and in disuse‐induced tissue atrophy conditions.
Keywords: biomechanical efficacy, medical device‐related pressure ulcers, model, pressure injuries, skin tears
1. INTRODUCTION
In clinical practice, it is often required to protect intact skin from deformation‐inflicted damage which potentially leads to pressure injuries/ulcers. 1 , 2 This occurs, for example, where the skin is fragile or compromised and is subjected to bodyweight forces, or is under skin‐contacting medical devices, or where periwound/stomal skin cannot be completely offloaded. Mechanical forces, which include compressive and frictional components, are applied to skin in each of these scenarios and their structural distortion effects spread to deeper tissues. Fragile skin and subdermal tissues exposed to such forces may develop damage promptly and present dermal erosions, skin tears, or superficial pressure injuries. 3 Prophylactic dressings may be an alternative to consider for protecting vulnerable skin regions; however, in many clinical cases, it is difficult to manage certain anatomical areas with dressings (or dressing cuts), for example, where the regions requiring protection are small or curved or irregularly shaped.
Cyanoacrylates are chemical compounds that include methyl‐2‐cyanoacrylate, ethyl‐2‐cyanoacrylate, n‐butyl‐cyanoacrylate, and 2‐octyl‐cyanoacrylate. These compounds have been available for medical purposes since the 1950s and were first used as tissue adhesives. 4 Cyanoacrylate liquid skin protectants (CLSPs) are commercially available products based on the above‐mentioned compounds. Clinical indications for use of CLSPs are general skin protection (including at pressure points such as the sacrum and heels of supine individuals) and application on perineal, peristomal, perifistula, peritube, and periwound skin, on recently closed wounds, and under negative pressure therapy devices. 5 CLSPs are typically applied by a dedicated wand‐like device to rapidly form a transparent, flexible film that adheres to skin tissues. These CLSP products have high affinity for moisture and, therefore, can bond to surfaces that contain any degree of moisture, including living skin. Specifically, upon topical application to skin, the monomeric substance present in the (wand) applicator instantaneously triggers chain‐reaction polymerisation which, within seconds to a minute, 6 forms a resilient and flexible polymeric barrier to protect the underlying skin. 7 Once polymerised, the formed CLSP coating is structurally stable on adult skin for approximately 3 days, followed by shedding, because of normal skin surface turnover. 7 Published scanning electron microscopy (SEM) studies of porcine skin specimens treated by CLSP (Figure 1) demonstrated intimate and continuous bonding between the polymerised CLSP and skin, as well as formation of a nearly uniform 20‐ to 25‐μm‐thick coating with a relatively smooth external surface. 7
FIGURE 1.
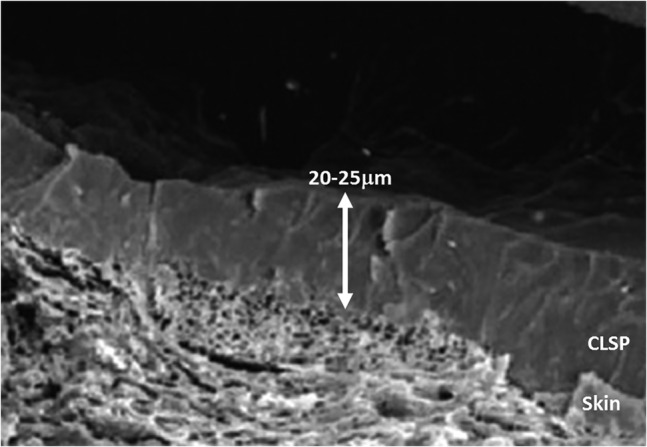
Scanning electron microscopy micrograph of the cyanoacrylate liquid skin protectant (CLSP) bonded to porcine skin. Note the intimate level of bonding of the polymerised CLSP coating to the underlying skin. Reproduced with permission from Vlahovic et al 7
The skin exhibits a stratified structure, consisting of three distinct layers: The lower, fatty connective tissue named the hypodermis supports a relatively thick middle layer called the dermis, and the latter supports the thinner outer layer, the epidermis. The outermost layer of the epidermis, the stratum corneum, consists of flattened corneocytes and is about 10 μm thick. Epidermal stem cells which reside deep in the epidermis constantly proliferate and as the daughter cells move towards the skin surface they are differentiated (keratinized) before reaching the surface, in cycles of approximately 3 to 4 weeks (in young adults) where these cells are sloughed from the surface (in a natural wear process). This turnover process slows down substantially with ageing, which thins the epidermis substantially. 8
The layered structure of skin shapes its mechanical function, which is complex and known to vary by anatomical location, gender (sex steroids modulate epidermal and dermal thickness), age, and health conditions. 9 The mechanical behaviour of skin also depends on the loading mode, magnitudes, and rates of the applied forces (skin is a highly non‐linear and viscoelastic material) as well as the osmolarity of the testing environment. 10 Considering the aforementioned turnover cycles of skin, the mechanical properties of skin depend on the regeneration and repair capacities of the individual, which are, in turn, age‐dependent and may be affected by the function of the inflammatory system, by acute conditions as well as by chronic diseases. 11 , 12 This inherent biological/physiological variability across individuals partially explains the vast variation in reported mechanical properties for skin and skin layers, such as values of elastic moduli that span over several orders of magnitude, 0.04 to 1000 kPa for physiological deformations. 13 Ageing, in particular, increases the stiffness of skin. This primarily occurs because of increased formation and stabilisation of collagen crosslinks in the extracellular matrix, 9 , 14 whereas the overall structure of collagen bundles become disorganised and, thereby, less extensible. 15 In addition, the extracellular protein elastin typically shows a higher degree of calcification in aged skin, with an associated progressive degradation of elastin fibres. 16
Skin is rough by engineering (tribology) standards. The average value of the roughness profile of skin (determined from deviations about the centreline of the epidermis) is between 4 and 40 μm. 13 Roughness is gender‐dependent and increases with age. 17 , 18 Friction of skin with contacting materials and surfaces is of great interest in the context of pressure injury prevention. 19 The coefficient of friction (COF) of skin when in contact with other materials varies with the anatomical site, age, ambient temperature, the level of tissue hydration, the level of wetness at the skin‐material interface, and the amount of hair follicles and hair present on the skin. 20 For untreated, dry skin in contact with common medical textiles, the COF is approximately in the range of 0.4 to 0.6. 13 , 19 , 21 On the fingers and soles of the feet, the COF can be much greater, about 1.2, but at the back, ankle, and upper arms which are relatively smoother, the COF may drop to around 0.2. 13 The aforementioned COFs increase significantly with age 20 corresponding to the reported increase in the surface roughness of skin. 17 , 18
Application of a CLSP adds a thin but firm coating layer on the treated skin (post‐polymerisation) and further influences the surface roughness at that site. The first factor, the addition of a resilient coating layer, increases the local effective stiffness of the treated skin whereas the second factor, smoothing of the external surface, reduces the interfacial COF (Figure 1). Both these factors and their potential interactions thereby change the exposure of skin and subdermal tissues to mechanical loading. The objective of this article is to formulate a new bioengineering theoretical framework for modelling the biomechanical efficacy of CLSPs, in order to show their key modes of action. This work details the specific engineering and mathematical derivations of a novel biomechanical theory that explains how CLSPs function in protecting fragile and compromised skin, based on the classic engineering theories of flexural stiffness of coated elements and deformation friction.
2. METHODS
A new bioengineering theoretical framework is formulated here, for modelling the biomechanical efficacy of CLSPs, with specific focus on the Marathon® CLSP technology (Medline Industries, Inc., Northfield, Illinois) and its modes of action. This new bioengineering theory is based on the theory of flexural stiffness of coated elements adapted from (bio)mechanical engineering (Section 2.1) and the bio‐tribological theory of deformation friction (Section 2.2.), as follows.
2.1. Contribution of the cyanoacrylate skin protectant to flexural skin stiffness
The biomechanical behaviour of a skin site treated with the above‐mentioned CLSP can be approximated based on the composite element (beam) theory, which determines the flexural (bending) stiffness of a layered structure subjected to flexural (non‐axial) forces, such as when a medical device or any other object pushes against the skin at a confined (small) region of contact. For simplicity and to derive closed‐form analytical solutions to the relevant engineering equations describing the modes of action of the CLSP, skin tissue is considered in the following analyses as a homogeneous linearly elastic and isotropic material, having the effective behaviour which incorporates the contributions of the hypodermis, dermis, and epidermis altogether. It should be noted, in this regard, that consideration of the individual contributions of each of these skin layers separately requires use of numerical bioengineering computational methods such as the finite element method, and is not feasible to do analytically.
The total flexural moment applied by such a medical device or an object to the skin, M, around the skin‐object contact site is the sum of the flexural moments generated at the skin tissue (index “s”) and within the polymerised CLSP coating layer (index “c”):
| (1) |
where r is the radius of the local surface curve caused by the compression of the device or object onto the CLSP‐coated skin, E s and E c are the apparent elastic moduli of skin and the polymerised CLSP coating, respectively, and I s and I c are the moments of inertia of the cross‐sections of the skin and the polymerised CLSP coating, respectively. In engineering mechanics theory, the product of the elastic modulus of a material and the cross‐sectional moment of inertia of an element of that material, EI, is defined as the flexural (bending) stiffness. For a given flexural moment that applies, the curvature (deflection) 1/r, which in the present context represents the extent of distortion of the skin (protected by the CLSP coating) under the pushing device/object, grows as the flexural stiffness decreases. Rearranging the terms of the previous equation yields that:
| (2) |
Accordingly, the effective flexural stiffness (EI) of a skin region coated with polymerised CLSP is EI = ESIS + EcIc, or:
| (3) |
The cross‐sectional moment of inertia for a rectangular element is I = wt3/12 where w indicates the width and t is the thickness of the material. The flexural stiffness of a CLSP‐coated skin per unit of width is therefore:
| (4) |
The addition to the flexural stiffness of (untreated) skin per unit of skin width, by application of the CLSP coating, is . The ratio γ of flexural stiffness of CLSP‐coated over untreated skin, which quantifies the improvement in skin protection from flexural (non‐axial) localised forces (such as compressive forces) because of the added flexural stiffness associated with the polymerised CLSP coating, is:
| (5) |
where the greater the γ value is, the more protection that is provided to skin by the polymerised CLSP coating. As could be expected, the above‐mentioned theoretical formulation demonstrates that the added protection depends on both the elastic modulus (Ec) and thickness (tc) of the applied CLSP coating, where, given that the polymerised coating thickness is less than the thickness of the underlying skin, an effective protection theoretically requires that Ec > > Es.
2.2. Reduction of the interfacial friction coefficient and the resulting shear forces through application of the cyanoacrylate skin protectant
A shear force f between the skin and a contacting object is the product of the force perpendicular to the skin‐object interface (called the “normal” force) and the interfacial COF. A reduction in the COF of skin in contact with an object (which is assumed to apply the same normal force) will therefore proportionally reduce the frictional forces and the corresponding surface shear stresses. The relationships between the skin surface structure and its COF with an interacting object are highly complex and depend on environmental parameters, such as temperature and humidity. With that said, the bio‐tribology theory of deformation friction where one material is substantially stiffer than the other is highly useful in approximating the interfacial COF in the present context. This is because many medical devices known to be associated with device‐related pressure injuries, such as tubing and ostomy supplies, are considerably stiffer than skin tissues. 22 The deformation friction theory approximates the sensitivity of the COF to the surface hardness of the less stiff (“softer”) material in the pair of interacting materials, as follows 13 :
| (6) |
where h is the depth of microscopic ploughing of the harder material, that is, of the micro‐topography of a medical device into the other, softer material, which in the present case is skin tissue, and R is the characteristic surface roughness feature (mean asperity radius) of the harder (medical device/object) material micro‐topography.
We now assume that the harder material is indeed a medical device, for example, a silicone nasogastric feeding tube or stoma button. When such a device makes contact with the skin, a ratio δ of the frictional force acting on the CLSP‐coated skin site, over that applied to an untreated skin site (a reference site), can be determined. This ratio δ quantifies the improvement in skin protection post‐treatment, because of the reduced skin COF achieved by the polymerised CLSP coating, and is approximated as follows:
| (7) |
where the lower the δ value is with respect to unity (which is representative of untreated skin), the greater is the protective effect. As a first simple approximation to δ, referred to here as δ1, we consider that the ploughing action of the medical device material surface into the skin, h, is being counteracted elastically according to a Hertz indentation formulation. 23 That is, for a given normal force and characteristic asperity value R, the square root of the third power of ploughing (h3/2) is proportional to the inverse of the elastic modulus 1/E, which yields that:
| (8) |
Given that Ec > > Es as discussed previously, the value δ1 must be lower than unity, which demonstrates a trend of effect in the efficacy of CLSP usage and, specifically, points to the theoretical effectiveness of the CLSP coating in mitigating frictional forces. In fact, the above‐mentioned theoretical result points to a well‐known principle in classic tribology, as follows: The increased effective stiffness of skin because of the contribution of the polymerised CLSP coating (Figure 1) reduces the depth of (microscopic) indention of asperities found on the micro‐topography of any interacting material into the skin tissue. This decreases the deformational component of the COF, which thereby reduces the frictional forces that are proportional to the COF.
Nevertheless, the approximation in Equation 8 is insufficient for the purpose of analysing the prophylactic value of a CLSP, as it does not consider the effect of the thickness of the applied (polymerised) coating on the extent of reduction of the frictional forces post‐treatment (a Hertz approximation inherently assumes a thick skin or a thick coating). Accordingly, a further improved representation of the reduction in frictional (surface shear) forces, in terms of the present modelling framework, is to consider not just the elasticity of the (skin/coating) materials in counteracting the ploughing action of the surface asperities, but also, the contribution of the structural stiffness. Specifically, it is required to evaluate how the interfacial COF is affected by the thickness of the applied CLSP coating.
Accordingly, incorporating the thickness of the polymerised CLSP coating with respect to that of skin yields an improved approximation to δ, referred to here as δ2. Considering the derivation in the previous section (Section 2.1) where we have already determined the ratio of flexural stiffnesses of skin post‐CLSP‐treatment versus the untreated skin condition, one can now use the relationship in Equation 5 to determine the reduction in the frictional force δ2, based on the influence of the polymerised CLSP coating on the flexural stiffness, as follows:
| (9) |
assuming, as before, that tc ≪ ts. The lower the δ2 is, with respect to unity, the more biomechanical protection that the polymerised CLSP coating provides against frictional (shearing) forces. One can verify that the aforementioned Equation 9 results in unity (δ2 = 1) indeed, if the thickness of the polymerised CLSP coating is zero (ie, there is no coating), or if the elastic modulus of the coating Ec approaches that of skin, or is less than that of skin (which makes a coating theoretically ineffective).
3. RESULTS
3.1. Contribution of the cyanoacrylate coating layer to the flexural skin stiffness
Two illustrative and clinically relevant examples can be analysed using Equation 5. The first example is of a healthy adult facial skin at a site that is susceptible to a device‐related pressure injury (eg, the lower lip or nasal dorsum), with “normal” characteristic thickness of 0.7 mm. 24 , 25 , 26 The second case studied here is of a thinner, fragile facial skin of an elderly person, with an abnormally low thickness of 0.35 mm, which is half of the aforementioned normal skin thickness value. 24 , 25 , 26 As indicated previously, skin tissue is inhomogeneous, anisotropic, and viscoelastic, and a wide range of elastic moduli have been reported in the literature, depending on age, anatomical site, and health status, as well as testing protocols and conditions. For the purpose of the present analyses, we assume that the elastic modulus of adult skin falls within the mid‐range of reported empirical data for hydrated human skin, which is 100 kPa for out‐of‐plane (eg, indentation or suction) mechanical loading. 13 , 27 , 28 , 29 , 30 , 31 We further assume, based on reported experimental data, that the elastic modulus of skin increases by 50% (ie, to 150 kPa) with old age. 32 The elastic modulus of CLSP is approximated as that of its main component, cyanoacrylate, which is ~200 MPa depending on the specific chemical formulation33; there are various esters of cyanoacrylic acid in the different homologues. 34 A typical polymerised CLSP coating thickness on skin is ~25 μm 7 (Figure 1).
Substituting the above‐mentioned values for young adult healthy skin in Equation 5 yields that treatment with CLSP will make the adult skin ~1.1‐times stiffer compared with the untreated case. However, and importantly, the thinner, fragile elderly skin will gain substantially more protection by treatment with the CLSP. The CLSP‐treated thinner elderly skin becomes ~1.5‐times stiffer when subjected to flexural distortions with respect to an untreated skin region, thereby allowing the CLSP‐treated skin to more effectively resist and tolerate localised compressive forces and pressures applied by a medical device or other contacting object.
The γ versus skin thickness calculations, plotted in Figure 2, which demonstrate the improvement in skin protection from flexural (non‐axial) forces with application of the CLSP, further depict the conditions of an especially thin and fragile skin and the extents of tissue protection provided by the CLSP in such cases. This theoretical analysis specifically demonstrates that application of CLSP coating is particularly effective where the skin is thin and fragile (less than ~0.7 mm thick), which is typical to ageing, neural injuries, and diseases, as well as to individuals with disuse‐induced tissue atrophies. 35
FIGURE 2.
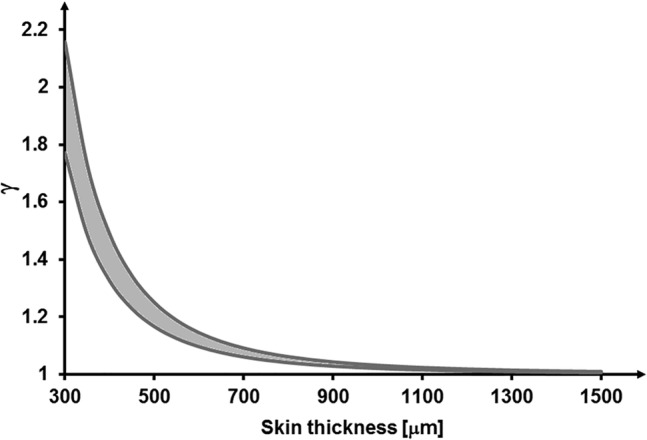
Improvement in skin protection from flexural (non‐axial) forces quantified by the ratio γ for a range of skin elastic moduli (100‐150 kPa) and application of cyanoacrylate liquid skin protectant (CLSP) coating with thickness of 25‐μm post‐polymerisation. The predicted improvement is highly non‐linear and rises substantially for thinner (more fragile) skins such as elderly skin with thickness values lower than ~0.7 mm. An especially thin and fragile (aged) skin region (thinner than ~0.7 mm) which is treated with the CLSP may approximately double its stiffness for resisting flexural distortions with respect to an untreated skin region, thereby allowing treated skin regions to more effectively tolerate localised compressive forces and pressures from a contacting medical device or object. The plotted grey range of γ data resulted from calculations of the γ ratios for the aforementioned range of skin elastic moduli at each skin thickness level
3.2. Reduction of the interfacial friction coefficient and the resulting shear forces
By substituting the representative values for the parameters in Equation 9 as performed in the previous section, the reduction in frictional forces post‐treatment with the CLSP is, in theory, up to ~5% for a healthy adult skin, but up to ~30% for the elderly, thin, and fragile skin. The δ2 versus skin thickness calculations plotted in Figure 3 further depict conditions of an especially thin and fragile skin. Consistent with the results presented in the previous section (Figure 2), this theoretical analysis of reduction in frictional forces demonstrates that application of CLSP coating is particularly effective where skin tissues are thin and fragile (ie, less than ~0.7 mm thick).
FIGURE 3.
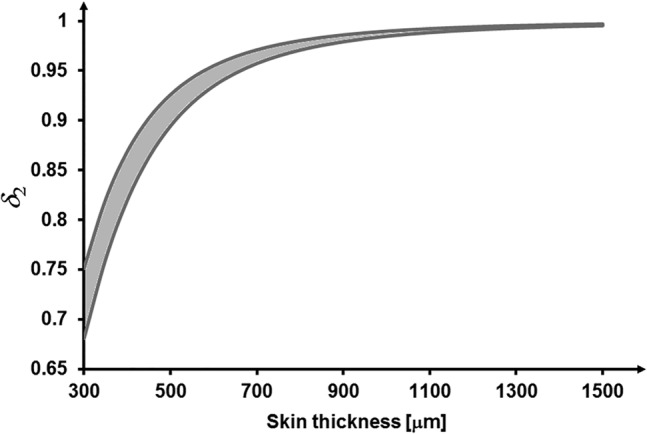
Improvement in skin protection from frictional (shearing) forces quantified by the ratio δ2 for a range of skin elastic moduli (100‐150 kPa) and application of cyanoacrylate liquid skin protectant (CLSP) coating with thickness of 25‐μm post‐polymerisation. The predicted improvement is highly non‐linear and the extent of protection (ie, decrease in δ2) rises substantially for thinner (more fragile) skin tissues such as an elderly skin with thickness values lower than ~0.7 mm. Frictional forces acting on such a thin, fragile (aged) skin region treated with CLSP may be alleviated by up to ~30% with respect to an untreated skin region, thereby allowing the treated skin region to more effectively tolerate localised frictional forces and surface shear stresses induced by a contacting medical device or object. The plotted grey range of δ2 data resulted from calculations of the δ2ratios for the aforementioned range of skin elastic moduli at each skin thickness level
4. DISCUSSION
This article provides, for the first time in the literature, a scientific theoretical description of the bioengineering principles and effects that apply when skin is treated with the commercially available CLSP product studied herein, Medline Marathon®. The present work has specifically analysed the primary biomechanical modes of action of this product, showing its important biomechanical modes of action. The mathematical derivations to describe these key modes of action have been based on established biomechanical engineering and bio‐tribology theories, specifically, the theory of flexural stiffness of coated elements (adapted from (bio)mechanical engineering) and the (tribological) theory of deformation friction. These novel theoretical/mathematical formulations facilitated quantitative analyses of the contribution of the protective layer generated by the CLSP to the flexural stiffness of the treated skin region. Our results demonstrate that the polymerised CLSP coating provides effective protection to fragile, aged skin from flexural (non‐axial, eg, compressive) localised forces. Likewise, the contribution of the studied CLSP coating to reduction in frictional forces and surface shear stresses applied by a contacting object or device (such as a medical device), has also been successfully described here. In addition to the greater stiffness provided by the polymerised CLSP coating, which increases the skin tissue tolerance by reducing the microscopic contact area between skin and objects (which in turn, lowers the deformation friction), the studied CLSP coating also smoothens the natural roughness of skin. This latter effect is again particularly profound for aged, creased, and wrinkled skin tissues. The smoothing of the external surface acts together with the increased surface stiffness to minimise the aggregated microscopic contact areas and thereby, the deformation friction and frictional forces/stresses. This phenomenon is illustrated graphically in Figure 4.
FIGURE 4.
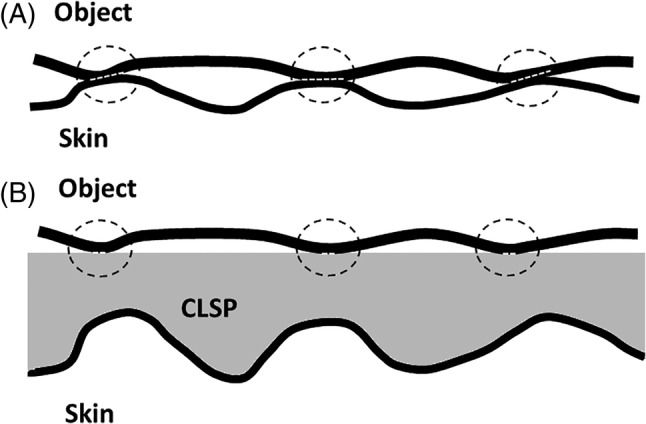
Illustration of how a cyanoacrylate liquid skin protectant (CLSP) which is substantially stiffer than skin tissue reduces the coefficient of friction and thereby, the frictional forces and surface shear stresses applied to skin tissue. Consider a stiff medical device or object with a microscopic surface roughness as seen in, A, which comes into contact with bare skin. The stiff object causes microscopic deformation of the skin and, at locations of asperities, may indent the skin, which then increases the effective contact area of the micro‐topography of the said object with the skin. Frictional forces and shear stresses will therefore apply through these enlarged localised contact areas (marked by dashed white lines at the three circled contact sites). If, however, the same stiff object micro‐topography is encountered by a smoother surface which is also substantially stiffer than skin tissue, that is, the polymerised CLSP coating which does not deform as much when the asperities of the object push against it, then the effective (aggregate) localised contact area is reduced substantially, B. As a result, the overall frictional forces and surface shear stresses, which correlate with the effective contact area, decrease correspondingly
In addition to the well‐documented fragility of elderly skin, because of the slowdown in tissue turnover rates, 8 other chronic or extrinsic conditions may affect skin morphology and contribute to loss of skin thickness. For example, in the chronic phase of central nervous system (CNS) injuries, skin undergoes progressive disuse adaptation, similarly to those reported for musculoskeletal tissues. 35 In persons who experienced a spinal cord injury for example, the skin over the ischial tuberosities and sacrum becomes significantly thinner, which is associated with deficient vascular reactions, decreased fibroblast activity and greater collagen catabolism. 35 As in ageing, the skin of patients with CNS injuries becomes less distensible. 36 The thinner and stiffer skin over weight‐bearing sites is considerably less effective in dissipating mechanical loads through localised deformations, which contributes to the susceptibility of these individuals to pressure injuries, 35 especially at the sacral region where the loss of skin thickness can exceed 30%. 37 Likewise, malnutrition results in significantly decreased skin thickness and lower collagen contents, 38 both of which compromise skin tissue strength. Topical corticosteroids, the most commonly prescribed topical medications for treating rash, eczema, and dermatitis may cause skin atrophy if used for extended periods. 39 In at‐risk patients, ageing skin atrophy and malnutrition often act in combination, each negatively impacting skin thickness, which could induce the skin fragility conditions analysed here (Figures 2 and 3). As the above‐mentioned theoretical results indicate, in such compromised skin conditions, the CLSP becomes increasingly effective in providing protection from dermal erosions, skin tears or superficial pressure injuries.
From a biomechanical perspective, there are fundamental differences between solvent‐based skin protectants and the specific CLSP product analysed here, which contains no solvents. Specifically, Vlahovic et al 7 reported SEM studies of the CLSP investigated here, for which micrographs were obtained post‐application to porcine skin (Figure 1), and compared them with images of solvent‐based protectants. Their SEM work demonstrated continuous tight bonding between the polymerised CLSP coating and the skin underneath, but a discontinuous interface with multiple gaps between the coating and skin for the solvent‐based products. Clearly, any gaps between the coating layer and the underlying skin may result in relative micro‐motion and frictional sliding between the coating and skin if external forces are applied. Such frictional sliding will not decrease tissue loading and may, on the contrary, increase the skin stresses because of exposure to surface friction at the coating‐skin interfaces. Continuous, intimate, and robust bonding between the coating and treated skin is required for the theory presented here (which assumes such ideal bonding) to apply in full. The cyanoacrylate polymers, which do not dissolve in water and polymerise immediately upon exposure to natural skin moisture, provide that gap‐free continuous and resilient bonding to skin, which has been demonstrated in the SEM studies of Vlahovic et al. 7 Importantly, based on the Vlahovic 7 study, the present theory cannot be extrapolated to solvent‐based products.
As with any modelling study, there are some limitations which need to be discussed. First, the analytical approach taken here is not suitable for considering the detailed micro‐topography of skin which is known to depend on the anatomical site, gender, and age and to be influenced by chronic diseases such as diabetes. 39 , 40 , 41 Numerical modelling approaches such as the finite element method are better suited for analyses of the contribution of certain skin micro‐topography features to the damage risk and the potential benefits of application of CLSP on skin of different individuals with varying roughness characteristics. The detailed layered structure of skin tissue (including the variations documented in different populations, eg, the elderly or diabetic) can further be incorporated in such numerical modelling and likewise, the compressive and frictional loading patterns applied by specific medical devices can be simulated. Work underway in our laboratory builds upon the present analytical modelling framework and will add the geometrical level of detail in numerical modelling which will facilitate the above‐mentioned future studies. In addition, although the clinical significance of the present bioengineering framework has not been fully determined and further clinical studies are warranted, the work of Milne et al 43 demonstrated the efficacy of the studied CLSP product in protecting peristomal skin in persons living with an ostomy. They found that application of CLSP under an ostomy skin barrier wafer is a viable option for managing peristomal skin damage resulting in pain and poor wafer adherence. In addition, the Milne 43 study reported that the ability of the CLSP to rapidly create a dry surface for adherence of the skin barrier wafers while alleviating local pain promoted patient self‐care and, hence, better quality of life. Clearly, similar clinical work focusing on other medical device types which produce compressive and frictional forces and associated shearing in skin is merited, to extend the body of literature in this regard.
In conclusion, based on the novel bioengineering theory and analyses detailed here, application of the CLSP product (Medline Marathon®) provides considerable biomechanical protection to skin and subdermal tissues. The theoretical results presented herein demonstrate that the polymerised coating shields the skin from both compressive and frictional forces (and associated shear stresses). Importantly, the prophylactic actions become particularly effective when skin tissues are thin and fragile (less than 0.7 mm thick), which is typical in the elderly and in patients with neural injury or chronic conditions, as well as disuse‐induced tissue atrophy.
ACKNOWLEDGEMENTS
The study was supported by an unrestricted educational grant from Medline Industries, Inc. (Northfield, Illinois).
Gefen A. The bioengineering theory of the key modes of action of a cyanoacrylate liquid skin protectant. Int Wound J. 2020;17:1396–1404. 10.1111/iwj.13401
Funding information Medline Industries, Inc. (Northfield, Illinois)
REFERENCES
- 1. Gefen A. Time to challenge the continued use of the term ‘pressure ulcer’? Br J Nurs. 2017;26(15):S20‐S22. [DOI] [PubMed] [Google Scholar]
- 2. Gefen A, Brienza D, Edsberg L, et al. The etiology of pressure injuries. Prevention and Treatment of Pressure Ulcers/Injuries: Clinical Practice Guideline European Pressure Ulcer Advisory Panel (EPUAP). 3rd ed. Westford, MA: National Pressure Injury Advisory Panel (NPIAP) and the Pan Pacific Pressure Injury Alliance (PPPIA); 2019. [Google Scholar]
- 3. Gefen A. How medical engineering has changed our understanding of chronic wounds and future prospects. Med Eng Phys. 2019;72:13‐18. [DOI] [PubMed] [Google Scholar]
- 4. Walt MJ, Atwood N, Bernatchez SF, Ekholm BP, Asmus R. Skin protectants made of curable polymers: effect of application on local skin temperature. Adv Wound Care. 2017;6(4):109‐114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bryant R, Nix D. Acute and Chronic Wounds: Current Management Concepts. 5th ed. St. Louis, MO: Elsevier; 2016:92. [Google Scholar]
- 6. Burns B. Polycyanoacrylates. Encyclopedia of Polymer Science and Technology. Hoboken, NJ: John Wiley & Sons; 2016:1‐27. [Google Scholar]
- 7. Vlahovic TC, Hinton EA, Chakravarthy D, Fleck CA. A review of cyanoacrylate liquid skin protectant and its efficacy on pedal fissures. J Am Col Certif Wound Spec. 2011;2(4):79‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Farage MA, Miller KW, Elsner P, Maibach HI. Characteristics of the aging skin. Adv Wound Care. 2013;2(1):5‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lynch B, Bonod‐Bidaud C, Ducourthial G, et al. How aging impacts skin biomechanics: a multiscale study in mice. Sci Rep. 2017;7(1):13750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wahlsten A, Pensalfini M, Stracuzzi A, Restivo G, Hopf R, Mazza E. On the compressibility and poroelasticity of human and murine skin. Biomech Model Mechanobiol. 2019. Aug;18(4):1079‐1093. [DOI] [PubMed] [Google Scholar]
- 11. Biggs LC, Kim CS, Miroshnikova YA, Wickström SA. Mechanical forces in the skin: roles in tissue architecture, stability, and function. J Invest Dermatol. 2019;140:284–290. [DOI] [PubMed] [Google Scholar]
- 12. Payne D. Skin integrity in older adults: pressure‐prone, inaccessible areas of the body. Br J Community Nurs. 2020;25(1):22‐26. [DOI] [PubMed] [Google Scholar]
- 13. Gohar R, Rahnejat H. Bio‐tribology (skin). Fundamentals of Tribology. 2nd ed. London, UK: World Scientific Publishing Co.; 2012:365‐366. [Google Scholar]
- 14. Yamauchi M, Woodley DT, Mechanic GL. Aging and cross‐linking of skin collagen. Biochem Biophys Res Commun. 1988;152(2):898‐903. [DOI] [PubMed] [Google Scholar]
- 15. Boss GR, Seegmiller JE. Age‐related physiological changes and their clinical significance. West J Med. 1981;135:434. [PMC free article] [PubMed] [Google Scholar]
- 16. Duncan KO, Leffell DJ. Preoperative assessment of the elderly patient. Dermatol Clin. 1997;15:583‐593. [DOI] [PubMed] [Google Scholar]
- 17. Jacobi U, Chen M, Frankowski G, et al. In vivo determination of skin surface topography using an optical 3D device. Skin Res Technol. 2004. Nov;10(4):207‐214. [DOI] [PubMed] [Google Scholar]
- 18. Lagarde JM, Rouvrais C, Black D. Topography and anisotropy of the skin surface with ageing. Skin Res Technol. 2005;11(2):110‐119. [DOI] [PubMed] [Google Scholar]
- 19. Schwartz D, Magen YK, Levy A, Gefen A. Effects of humidity on skin friction against medical textiles as related to prevention of pressure injuries. Int Wound J. 2018;15(6):866‐874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Veijgen NK, Masen MA, van der Heide E. Variables influencing the frictional behaviour of in vivo human skin. J Mech Behav Biomed Mater. 2013. Dec;28:448‐461. [DOI] [PubMed] [Google Scholar]
- 21. Gefen A. How do microclimate factors affect the risk for superficial pressure ulcers: a mathematical modeling study. J Tissue Viability. 2011. Aug;20(3):81‐88. [DOI] [PubMed] [Google Scholar]
- 22. Bader DL, Worsley PR, Gefen A. Bioengineering considerations in the prevention of medical device‐related pressure ulcers. Clin Biomech. 2019;67:70‐77. [DOI] [PubMed] [Google Scholar]
- 23. Gefen A, Megido‐Ravid M, Azariah M, Itzchak Y, Arcan M. Integration of plantar soft tissue stiffness measurements in routine MRI of the diabetic foot. Clin Biomech. 2001;16(10):921‐925. [DOI] [PubMed] [Google Scholar]
- 24. Fore J. A review of skin and the effects of aging on skin structure and function. Ostomy Wound Manage. 2006. Sep;52(9):24‐35. [PubMed] [Google Scholar]
- 25. Ha RY, Nojima K, Adams WP Jr, Brown SA. Analysis of facial skin thickness: defining the relative thickness index. Plast Reconstr Surg. 2005. May;115(6):1769‐1773. [DOI] [PubMed] [Google Scholar]
- 26. Wilkinson PF, Millington R. Skin (Biological Structure and Function Books). Cambridge, England: Cambridge University Press; 2009:49‐50. [Google Scholar]
- 27. Griffin MF, Leung BC, Premakumar Y, Szarko M, Butler PE. Comparison of the mechanical properties of different skin sites for auricular and nasal reconstruction. J Otolaryngol Head Neck Surg. 2017;46(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liang X, Boppart SA. Biomechanical properties of in vivo human skin from dynamic optical coherence elastography. IEEE Trans Biomed Eng. 2010;57(4):953‐959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pawlaczyk M, Lelonkiewicz M, Wieczorowski M. Age‐dependent biomechanical properties of the skin. Postepy Dermatol Alergol. 2013;30(5):302‐306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zahouani H, Pailler‐Mattei C, Sohm B, Vargiolu R, Cenizo V, Debret R. Characterization of the mechanical properties of a dermal equivalent compared with human skin in vivo by indentation and static friction tests. Skin Res Technol. 2009. Feb;15(1):68‐76. [DOI] [PubMed] [Google Scholar]
- 31. Zheng Y, Mak AF. Effective elastic properties for lower limb soft tissues from manual indentation experiment. IEEE Trans Rehabil Eng. 1999;7(3):257‐267. [DOI] [PubMed] [Google Scholar]
- 32. Agache PG, Monneur C, Leveque JL, De Rigal J. Mechanical properties and Young's modulus of human skin in vivo. Arch Dermatol Res. 1980;269(3):221‐232. [DOI] [PubMed] [Google Scholar]
- 33. MatWeb Material Property Data (MatWeb, LLC) . Overview of materials for cyanoacrylate adhesive. matweb.com
- 34. Mizrahi B, Stefanescu CF, Yang C, et al. Elasticity and safety of alkoxyethyl cyanoacrylate tissue adhesives. Acta Biomater. 2011. Aug;7(8):3150‐3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gefen A. Tissue changes in patients following spinal cord injury and implications for wheelchair cushions and tissue loading: a literature review. Ostomy Wound Manage. 2014;60(2):34‐45. [PubMed] [Google Scholar]
- 36. Park JW, Seo CH, Han SH, Lee YG. Sympathetic influence on biomechanical skin properties after spinal cord injury. Spinal Cord. 2011;49(2):236‐243. [DOI] [PubMed] [Google Scholar]
- 37. Yalcin E, Akyuz M, Onder B, Unalan H, Degirmenci I. Skin thickness on bony prominences measured by ultrasonography in patients with spinal cord injury. J Spinal Cord Med. 2013;36(3):225‐230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Leite SN, Jordão Júnior AA, Andrade TA, Masson Ddos S, Frade MA. Experimental models of malnutrition and its effect on skin trophism. An Bras Dermatol. 2011;86(4):681‐688. [DOI] [PubMed] [Google Scholar]
- 39. Prawer SE, Katz HI. Guidelines for using superpotent topical steroids. Am Fam Physician. 1990;41(5):1531‐1538. [PubMed] [Google Scholar]
- 40. Lechner A, Akdeniz M, Tomova‐Simitchieva T, et al. Comparing skin characteristics and molecular markers of xerotic foot skin between diabetic and non‐diabetic subjects: an exploratory study. J Tissue Viability. 2019. Nov;28(4):200‐209. [DOI] [PubMed] [Google Scholar]
- 41. Sopher R, Gefen A. Effects of skin wrinkles, age and wetness on mechanical loads in the stratum corneum as related to skin lesions. Med Biol Eng Comput. 2011. Jan;49(1):97‐105. [DOI] [PubMed] [Google Scholar]
- 42. Tsukahara K, Hotta M, Osanai O, Kawada H, Kitahara T, Takema Y. Gender‐dependent differences in degree of facial wrinkles. Skin Res Technol. 2013;19(1):e65‐e71. [DOI] [PubMed] [Google Scholar]
- 43. Milne CT, Saucier D, Trevellini C, Smith J. Evaluation of a cyanoacrylate dressing to manage peristomal skin alterations under ostomy skin barrier wafers. J Wound Ostomy Continence Nurs. 2011;38(6):676‐679. [DOI] [PubMed] [Google Scholar]


