Dear Editors,
1.
Here, we describe a consecutive series of five hidradenitis suppurativa (HS) patients with extensive gluteal and anogenital involvement (FIGURE 1) who were treated with two‐stage surgery preceded by the execution of temporary colostomy or by the positioning of temporary containment devices (TCD).
Figure 1.
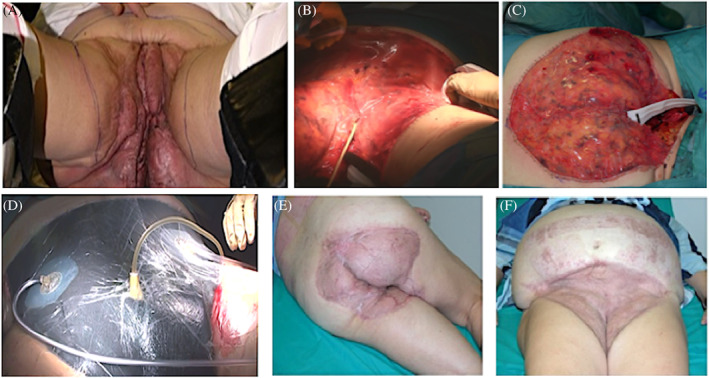
A, Preoperative clinical picture. Hidradenitis suppurativa (HS) anogenital involvement with deep‐seated painful nodules, abscesses, and hypertrophic scars. B, Surgical intervention: wide excision and grafting with Integra; Foley catheter was placed. C, Anal‐gluteal region grafted with a bilayer dermal regenerative template and positioning of TDC. D, Medication with silver‐impregnated dressing secured by a negative pressure wound therapy system. E,F Postoperative clinical picture. The patient presents normal skin without any clinical sign of active HS 9 months after surgery
HS is a recurrent disease characterised by a progression of inflamed nodules to deep‐seated lesions with subsequent scarring. The pathogenesis of the disease is multifactorial and not completely understood, but environmental factors, such as obesity, smoking, traumas, and microbial colonisation, may contribute to disease worsening.1
HS is one of the most disabling dermatological diseases, causing distress and impairment of quality of life. An early diagnosis and regular follow up are required for preventing the development of HS and to improve patient quality of life.
Multiple medical and surgical treatment modalities have been described for HS. As non‐surgical methods rarely result in a lasting cure, surgical treatment is a quite common and accepted therapeutic modality for HS.2
Surgical excision of the affected skin tissue with adequate free margins is the gold standard treatment for the prevention of recurrence. Indeed, radical surgical excision appears to be the most effective solution in terms of patient‐related and disease‐related outcomes.3
Recently, Gonzaga proposed a two‐stage surgical treatment for severe HS.4 According to the authors, the first step consists of an extensive resection down to the fascia of the affected skin, followed by grafting of acellular dermal matrix. After 2 to 3 weeks of revascularisation, a thin split‐thickness skin graft can be placed over the top. HS with anogenital involvement can determine the formation of fistulae to the rectum, vagina, urethra, peritoneum, and/or bladder. Groin location frequently involves the labia, buttocks, and mons pubis. Disruption of lymphatic vessel and lymph nodes by HS inflammation can result in significant increase of risk for the patient of developing chronic lymphoedema. Anogenital HS impairs sexual health and decreases patient quality of life. For these reasons, severe anogenital involvement is still a surgical challenge. Given the increased infectious risk of the anogenital area, temporary colostomy is one of the most common procedures performed in association with extensive surgical practice. Unfortunately, temporary colostomy might cause a decrease in terms of quality of life and carries several surgical risks. In fact, patients need additional surgery to perform colostomy, with subsequent increased time of hospitalisation and risk of infection.
TCD is indicated for immobilised or incontinent patients with liquid or semi‐liquid stool to protect perineal skin and limit the transmission of nosocomial infections.5
To the best of our knowledge, this is the first description of the use of TCD in extensive anogenital HS surgery. Patients presented to the dermatological surgery division after a longstanding course of HS progression (4‐37 years). They had failed previous medical therapy and were at the “severe” stage of the anogenital area according to the HS‐PGA scale. The presence of anal‐rectal involvement, documented through rectoscopy, can be considered a main contraindication for the application of TCD. In our series, only one patient was not eligible for TCD application, and he underwent temporary colostomy.
Wide excision and grafting of Integra dermal regeneration template (Integra Life Sciences Corp., Plainsboro, New Jersey) was performed for all patients who remained hospitalised 4 to 7 days after the procedure. Medications were tailored on patient needs using silver‐impregnated sponge secured by a negative pressure wound therapy system set at 75 mm Hg that was changed every 2 to 3 days for 1 week.
Patients followed a postoperative therapy regimen, comprising a 6‐day course of antibiotic prophylaxis and liquid/semi‐liquid diet. The removal of the silicon membrane and autologous skin grafting was performed on days 16 to 21. Removal of TCD was performed on days 49 to 63. Temporary colostomy was removed on days 98 to 105. Median HS‐PGA decreased from “severe” before the surgical intervention to “clear” in the gluteal and anogenital region for all the six treated patients. Only one major adverse episode was registered in the postoperative period of the sigmoidostomy closure. The patient developed sepsis during hospitalisation and was successfully treated with carbapenem. No other serious adverse event, disease recrudescence, or abnormal scar was reported immediately or 60 months after the surgical procedures.
In conclusion, surgical management of anogenital HS might be extremely complex, but a two‐step surgical procedure could represent the best choice for patients with advanced disease. This approach allows us to performed wide surgical resection, with high rates of subsequent successful grafting. Moreover, in our experience, the use of TCD is safe and less distressing for the patient with respect to colostomy.
REFERENCES
- 1. Bettoli V, Manfredini M, Calamo G, et al. Long‐term adalimumab treatment of hidradenitis suppurativa: results and practical insights from a real‐life experience. Dermatol Ther. 2018;31(6):e12737. [DOI] [PubMed] [Google Scholar]
- 2. Zouboulis CC, Desai N, Emtestam L, et al. European S1 guideline for the treatment of hidradenitis suppurativa/acne inversa. J Eur Acad Dermatol Venereol. 2015;29(4):619‐644. [DOI] [PubMed] [Google Scholar]
- 3. Wormald JCR, Balzano A, Clibbon JJ, Figus A. Surgical treatment of severe hidradenitis suppurativa of the axilla: thoracodorsal artery perforator (TDAP) flap versus split skin graft. J Plast Reconstr Aesthetic Surg. 2014;67(8):1118‐1124. [DOI] [PubMed] [Google Scholar]
- 4. Gonzaga TA, Endorf FW, Mohr WJ, Ahrenholz DH. Novel surgical approach for axillary hidradenitis suppurativa using a bilayer dermal regeneration template: a retrospective case study. J Burn Care Res Off Publ Am Burn Assoc. 2013;34(1):51‐57. [DOI] [PubMed] [Google Scholar]
- 5. Marino F, Manca G. Use of Flexi‐Seal to manage early colostomy complications: letter to the editor. Int Wound J. 2017;14(2):439‐439. [DOI] [PMC free article] [PubMed] [Google Scholar]


