ABSTRACT
Background
Dairy foods, particularly yogurt, and plasma biomarkers of dairy fat intake are consistently inversely associated with incident type 2 diabetes. Yet, few trials assessing the impact of dairy on glucose homeostasis include fermented or full-fat dairy foods.
Objectives
We aimed to compare the effects of diets rich in low-fat or full-fat milk, yogurt, and cheese on glucose tolerance and its determinants, with those of a limited dairy diet.
Methods
In this parallel-design randomized controlled trial, 72 participants with metabolic syndrome completed a 4-wk wash-in period, limiting dairy intake to ≤3 servings/wk of nonfat milk. Participants were then randomly assigned to either continue the limited dairy diet, or switch to a diet containing 3.3 servings/d of either low-fat or full-fat dairy for 12 wk. Outcome measures included glucose tolerance (area under the curve glucose during an oral-glucose-tolerance test), insulin sensitivity, pancreatic β-cell function, systemic inflammation, liver-fat content, and body weight and composition.
Results
In the per-protocol analysis (n = 67), we observed no intervention effect on glucose tolerance (P = 0.340). Both the low-fat and full-fat dairy diets decreased the Matsuda insulin sensitivity index (ISI) (means ± SDs −0.47 ± 1.07 and −0.25 ± 0.91, respectively) and as compared with the limited dairy group (0.00 ± 0.92) (P = 0.012 overall). Body weight also changed differentially (P = 0.006 overall), increasing on full-fat dairy (+1.0 kg; −0.2, 1.8 kg) compared with the limited dairy diet (−0.4 kg; −2.5, 0.7 kg), whereas the low-fat dairy diet (+0.3 kg; −1.1, 1.9 kg) was not significantly different from the other interventions. Intervention effects on the Matsuda ISI remained after adjusting for changes in adiposity. No intervention effects were detected for liver fat content or systemic inflammation. Findings in intent-to-treat analyses (n = 72) were consistent.
Conclusions
Contrary to our hypothesis, neither dairy diet improved glucose tolerance in individuals with metabolic syndrome. Both dairy diets decreased insulin sensitivity through mechanisms largely unrelated to changes in key determinants of insulin sensitivity.
This trial was registered at clinicaltrials.gov as NCT02663544.
Keywords: cardiometabolic disease, metabolic syndrome, dairy, glucose tolerance, insulin sensitivity, diabetes, inflammation, liver fat, adiposity, humans
See corresponding editorial on page 495.
Introduction
Type 2 diabetes (T2D) is a major global health issue. In 2017, it was estimated that 451 million people had diabetes worldwide, with the vast majority having T2D (1), costing $850 billion (1). Identifying modifiable determinants of T2D risk is therefore a major public health focus.
Diet is a modifiable lifestyle factor that affects glucose homeostasis and T2D risk (2, 3). Dairy is a food group that is inversely associated with T2D (4–10). This is particularly the case for low-fat dairy and yogurt (9, 11). Although full-fat dairy intake as assessed by questionnaire is mostly not associated with T2D (12, 13), biomarkers of dairy fat intake [i.e., phospholipid pentadecanoic acid (15:0), heptadecanoic acid (17:0), and trans-palmitoleic acid (trans-16:1n–7)] are consistently inversely associated with T2D (14, 15). These latter studies challenge the long-standing view that full-fat dairy foods may promote weight gain and cardiometabolic disease owing to their higher calorie and saturated fat content.
The limited experimental literature is largely inconsistent with the results from observational studies. Randomized controlled trials (RCTs) consistently indicate that increasing dairy intake does not affect glucose tolerance (16–19). Insulin sensitivity, a major determinant of glucose tolerance, also does not change in most RCTs (16–27). Outcomes of existing RCTs may differ from observational findings because they commonly relied on fasting measures of glucose homeostasis only, enrolled participants with normal baseline glucose homeostasis, did not control for changes in weight, and predominantly included low-fat unfermented dairy products (i.e., skim milk). The latter leads to a particular gap in our understanding, because biomarkers of dairy fat intake and yogurt have most consistently been linked to improved metabolic health and a reduced T2D risk (11–13, 28). Only 2 studies have directly compared the impact of low-fat and full-fat dairy on glucose homeostasis, 1 testing cheese (21) and the other milk (29). No RCT that we know of has comprehensively evaluated the impact of a wide variety of low-fat compared with high-fat dairy foods on glucose tolerance or its key determinants. As a result, it remains unclear whether dairy foods are protective against T2D, and whether this effect is dependent on the type of dairy consumed.
To address these gaps, we compared the effect of consuming 3.3 servings/d of low-fat or full-fat dairy foods with a diet limited in dairy on glucose tolerance and its major determinants. In contrast to previous studies, our trial included fermented dairy products in the form of both yogurt and cheese, in addition to milk. Our trial also included dynamic tests of glucose tolerance, insulin sensitivity, and pancreatic β-cell function. Assessment of major determinants of insulin sensitivity included liver fat content, biomarkers of systemic inflammation, and body weight and composition. We hypothesized that regularly consuming milk, yogurt, and cheese, particularly in their full-fat form, would improve glucose tolerance.
Methods
Trial registration
This trial was registered on clinicaltrials.gov on 26 January, 2016 (NCT02663544), before the enrollment of the first study participant. Changes were made after commencement of the study, but before the end of the trial and any laboratory or statistical analyses, to add several outcomes to broaden our ability to interpret trial effects on the primary endpoint. Specifically, we added glucose sensitivity, a measure of pancreatic β-cell function, as an additional secondary outcome measure. We also added fasting insulin, the HOMA-IR, and several measures of adiposity (trunk fat mass, peripheral fat mass, visceral fat mass, waist circumference, hip circumference, and the waist-to-hip ratio) as secondary outcome measures.
Study design
This parallel-design randomized controlled dietary intervention trial was conducted at the University of Washington (UW) and the Fred Hutchinson Cancer Research Center (Fred Hutch) in Seattle, WA. All participants completed a 4-wk wash-in diet period during which dairy food consumption was limited to ≤3 servings of nonfat milk per week (“limited dairy diet”). After completing a baseline clinic visit in the last week of the wash-in period, participants were randomly assigned to either continue the limited dairy diet or switch to a diet containing 3.3 servings/d of either low-fat or full-fat dairy foods in the form of milk, yogurt, and cheese, for 12 wk. Subjects completed a follow-up clinic visit in the final week of the intervention period.
Subjects
We enrolled 18- to 75-y-old, weight-stable participants with the metabolic syndrome (30). Key exclusion criteria included regular recreational drug use; excessive alcohol consumption; recent use of antidiabetic medications or insulin; uncontrolled diabetes [glycated hemoglobin (HbA1c) >8.0%]; history of bariatric surgery; or recent use of medications or diagnosis of any medical condition likely to interfere with study endpoints.
Our primary recruitment strategy was based on automated screens of the UW electronic medical record system. Potentially eligible individuals were contacted by mail, followed by a telephone screening interview, and lastly an in-person screening visit at Fred Hutch. During this screening visit, eligibility was ascertained, the study design and procedures were discussed in detail, and participants sampled the intervention dairy foods. Informed consent was obtained from all participants before study initiation. The Fred Hutch institutional review board approved this study.
Study diets
During the wash-in period, participants were asked to not consume any dairy products other than a maximum of 3 servings (240 mL each) per week of nonfat milk (“limited dairy diet”). At the baseline clinic visit, participants were randomly assigned to 1 of 3 diets: to continue the limited dairy diet, or switch to a diet rich in either low-fat or full-fat dairy foods. The randomization was performed using a random number generator by MK and SH using a block randomization procedure, with a block size of 3, stratified by gender and the screening visit HOMA-IR (<5.0 compared with ≥5.0 or diagnosis of diabetes). Participants were enrolled and assigned to the intervention diets by KAS, GC, MSB, or JNK. Blinding subjects to their randomly assigned diet was not possible because participants could easily discern which diet they had been assigned to based on the texture (i.e., full-fat compared with skim) or the amount (dairy compared with limited dairy) of study dairy provided. Those randomly assigned to stay on the limited dairy diet continued to not consume any dairy foods other than a maximum of 3 servings of nonfat milk per week. In the low-fat dairy diet, participants were asked to consume 3.3 servings/d of dairy in the form of nonfat milk and yogurt, and low-fat cheese. In the full-fat dairy diet arm, participants were asked to consume 3.3 servings/d of dairy in the form of whole milk (3.25% fat), full-fat yogurt (3.1% fat), and full-fat cheese. One serving was defined as 240 mL of milk, 170 g of yogurt, and 42.5 g of cheese. Nonfat and whole milk were produced by Darigold and nonfat and full-fat yogurt by Mountain High (General Mills). The low-fat and full-fat cheeses were chosen to be identical in terms of manufacturer and manufacturing processes, other than fat content, and included low-fat and full-fat cheddar cheese (21.2% and 32.9% fat, respectively; Sargento), gouda (18.0% and 32.2% fat, respectively; Beemster), and mozzarella (10.6% and 21.2% fat, respectively; Frigo/Saputo). The mean total amount of dairy fat in the administered dairy foods was 0 g/d in the limited dairy diet, 8 g/d in the low-fat dairy diet, and 29 g/d in the full-fat dairy diet. The Human Nutrition Laboratory at Fred Hutch provided all study dairy products. During all study diets, participants were instructed to not consume any dairy foods other than those provided by the study, and to otherwise continue to consume their habitual diet ad libitum. They were specifically instructed to incorporate the administered dairy products into their regular meals and snacks, and to consume provided dairy foods daily. On the low-fat and full-fat dairy diets, participants were not required to measure out exactly 3.3 servings of dairy per day, but rather were asked to consume all of the dairy provided before their next dairy food pick-up, such that their dairy consumption would average 3.3 servings/d. They were also asked to record their dairy consumption in a dairy log and to return any leftover dairy foods for weigh-backs.
Clinical procedures and data collection
At both clinic visits, we collected fasting blood; measured body weight and height, waist and hip circumference, and blood pressure; conducted a 3-h frequently sampled oral-glucose-tolerance test (FS-OGTT) to assess glucose tolerance, insulin sensitivity, and pancreatic β-cell function; conducted a whole-body DXA scan on a Lunar iDXA scanner (GE Healthcare) to assess body composition; and conducted an abdominal MRI scan to assess liver fat content.
Participants also completed an unvalidated modified Blair physical activity questionnaire (31) at baseline, clinic visit 1, and monthly during the 12-wk intervention period to assess habitual physical activity throughout the study. Twice during the wash-in diet period and 3 times during the intervention period, participants completed an unannounced 24-h dietary recall interview, administered by a staff member of the Fred Hutch Nutrition Assessment Shared Resource, who was otherwise not associated with the trial. Participants were told that their responses, including any indication of noncompliance, would not be shared with the study team before their completion of or dropout from the study.
Body weight and energy intake are key determinants of glucose tolerance and its determinants. Ad libitum energy intake cannot be measured reliably using subjective assessment methods. Therefore, participants completed a 5-d controlled feeding period during which they were provided with all of their food once during the last 3 wk of the wash-in diet period and again within the first 3 wk of the intervention period. These diets were standardized, based on the average American diet (other than dairy intake), and calibrated to offer 125% of each participant's estimated total energy expenditure. Participants were asked to consume all of the study dairy foods administered to them daily, but to eat the rest of the administered diet ad libitum, and to return all leftover foods to the Human Nutrition Laboratory at Fred Hutch. Returned foods were weighed and subtracted from the weight of the administered foods to calculate total energy intake during these 5-d periods. The purpose of the 5-d feeding periods was to assess whether participants randomly assigned to either the low-fat or full-fat dairy diets would be able to compensate for the energy content of the mandatory dairy foods by reducing their ad libitum consumption of nondairy foods provided by the standardized diet.
Laboratory procedures
High-sensitivity C-reactive protein (CRP), glucose, insulin, C-peptide, and total adiponectin in fasting plasma and HbA1c in fasting RBCs were measured at Northwest Lipid Research Laboratories (NWLRL) in Seattle, WA. CRP was measured by immunonephelometry (Behring Diagnostics), glucose on a Hitachi 917 autoanalyzer (Roche), and insulin and C-peptide on an AIA 600 II autoanalyzer (Tosoh Bioscience). HbA1c analysis was performed using HPLC-based G7 and G8 autoanalyzers (Tosoh Bioscience). Total adiponectin was measured in duplicate by an RIA (EMD Millipore Inc.). The interassay CV at NWLRL for this assay is 8%. High-sensitivity IL-6 was measured in duplicate in the Kratz laboratory by a high-sensitivity ELISA from R&D Systems. The interassay CV was 12%.
As an assessment of compliance with the dietary regimen, we measured the amounts of pentadecanoic acid, heptadecanoic acid, and trans-palmitoleic acid in plasma phospholipids (conducted in the Kraft Lab, Burlington, VT), because these are validated biomarkers of dairy fat intake (32, 33). Plasma phospholipids were extracted according to the method of Folch et al. (34). Plasma phospholipids were isolated from total plasma lipids via solid-phase extraction using aminopropyl cartridges (Thermo Fisher Scientific), and transmethylated with boron trifluoride solution in methanol (Sigma-Aldrich) to FAME (35). FAME were analyzed by GLC (35).
Study outcomes
The a priori–defined primary study outcome was change in glucose tolerance, as assessed by measuring the glucose AUC during a 3-h FS-OGTT. Secondary outcomes included changes in major determinants of oral glucose tolerance (i.e., systemic insulin sensitivity and pancreatic β-cell function) and major determinants of insulin sensitivity (i.e., liver fat content and low-grade chronic systemic inflammation). Systemic insulin sensitivity was assessed using the Matsuda–DeFronzo insulin sensitivity index (ISI) based on data from the FS-OGTT (36). Pancreatic β-cell function was assessed using the insulinogenic index (37), the oral disposition index (oral DI), which is calculated as the product of the Matsuda ISI and the insulinogenic index, and modeled glucose sensitivity (38), all based on the FS-OGTT. Liver fat content was measured by an abdominal MRI scan in the UW Biomolecular Imaging Center on a Philips 3T Ingenia CX whole-body scanner (Philips). Dixon MRI with multiple echo times were obtained, and fat and water MRI images as well as a quantitative liver proton density fat fraction map were produced. The entire liver was segmented on every 10th slice of the fat MRI images to estimate liver fat content. For each slice, the percentage fat was weighted by the contour area as a percentage of the total contoured liver area (sum of the area of all segmented slices), and the weighted fat percentages were summed. Low-grade chronic systemic inflammation was assessed using the concentration of CRP and IL-6 in fasting plasma. Exploratory endpoints reported here to comprehensively assess intervention effects on glucose homeostasis included changes in HbA1c, HOMA-IR (39), and fasting plasma glucose, insulin, and total adiponectin concentrations as well as ad libitum energy intake during the two 5-d controlled feeding periods, body weight, body fat mass, trunk fat mass, peripheral fat mass, visceral fat mass, waist circumference, hip circumference, and the waist-to-hip ratio. We also assessed changes in the overall diet that resulted from the intervention.
Statistical analyses
We aimed to randomly assign 72 participants, with the goal of having ≥60 participants in the primary per-protocol analysis and ≥20 in each intervention group. Sample size was based on assumptions of a baseline 3-h AUC glucose of 24,000 mg/dL × min and an SD of the change of 2300 mg/dL × min, which was estimated to provide 80% power to detect a differential change in AUC glucose between any 2 intervention groups of 10% with a sample size of ≥20/group and an adjusted α-error level of 1.67% (adjusted to account for 3 post hoc tests). The baseline and variability estimates used in the sample size calculation were based on 2 prior pilot intervention trials by our group—1 in prediabetic individuals and the other in obese individuals without diabetes—from which 3-h FS-OGTT data were available.
Statistical analyses were performed using SPSS version 26 (IBM). The level of significance was set to P < 0.05 for all analyses. We conducted both an intent-to-treat (ITT) and a per-protocol analysis, with the latter defined a priori as the primary analysis. For the ITT analysis, we carried the baseline values forward for those time points where data were unavailable. For the per-protocol analysis for each endpoint, subjects were included if they 1) completed the dietary intervention and all clinic visits; 2) were compliant with the dietary regimen (defined as consuming ≥90% of the study dairy foods provided, and consuming ≤10 servings of nonstudy dairy foods during the intervention period); 3) had no changes in medications that might affect the respective study endpoint; and 4) remained free from any illness that might affect the respective study endpoint.
Logarithmic transformations were performed on all outcome variables that were not normally distributed. An unadjusted repeated-measures analysis of variance (RM-ANOVA) with time (clinic visit 1 compared with 2) as the within-subject variable and diet group (limited compared with low-fat compared with full-fat) as the between-subjects variable, with primary emphasis on the time × diet group interaction, was considered model 1. Then, related baseline factors that differed by study arm as defined as a P value < 0.1 were included in the model as covariates, as appropriate (model 2 for glucose homeostasis–related endpoints). This was considered the primary model, because it was thought to more accurately reflect intervention effects (40). We also conducted sensitivity analyses adjusted for changes, defined as the difference between clinic visits 2 and 1, in habitual physical activity (model 2 for energy homeostasis–related endpoints) and those dietary variables that were differentially changed by the intervention diets. In addition, we conducted sensitivity analyses adjusted for our stratification variables: gender and HOMA-IR. For any glucose homeostasis–related variable for which we found differential changes by diet group, we conducted additional secondary analyses adjusting for changes in body fat mass (model 3 for glucose homeostasis–related endpoints) and any body weight or composition measure that changed differentially to determine to which extent any difference observed between the groups may be attributable to a change in measures of adiposity. If the global RM-ANOVA indicated significant time × diet differences between the diet groups for an outcome variable, we conducted post hoc independent-sample t tests comparing the change in that variable in each of the 3 diet groups, or 3 RM-ANOVAs that included only 2 diet groups at a time for post hoc tests. In these analyses, we adjusted for multiple testing according to Bonferroni. Again, post hoc tests were conducted on log-transformed data if the outcome variable failed the test for normality.
Results
Description of participants
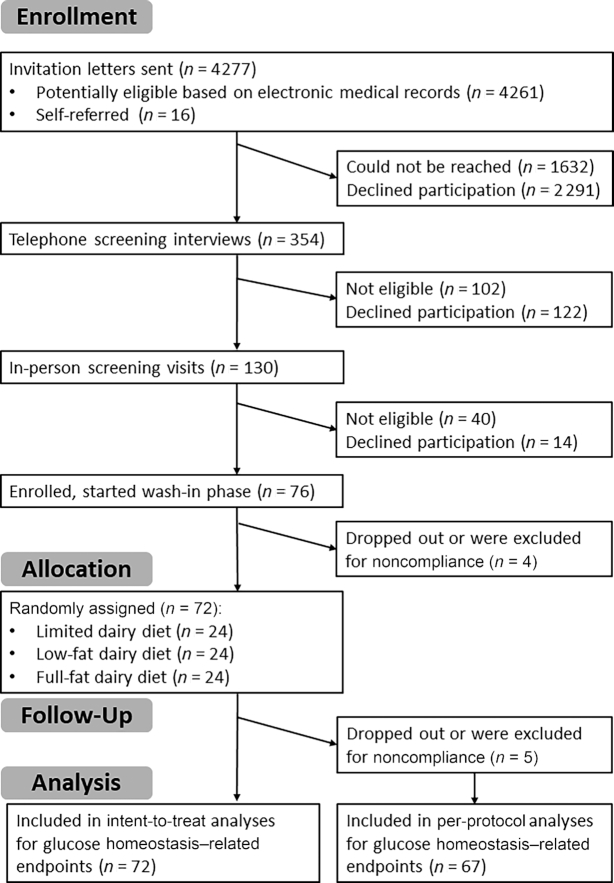
The trial was conducted between January 2016 and October 2018, when the recruitment goal was met. Recruitment letters were sent to 4261 potentially eligible individuals identified by an automated screen and review of the UW electronic medical record system, and to 16 self-referred volunteers (Figure 1). We conducted telephone screening interviews with 354 individuals and invited 130 potentially eligible and interested participants to attend an in-person screening visit, where 90 were deemed eligible for the trial. Excluding individuals unwilling or unable to participate, 76 subjects began the wash-in period. After excluding individuals who either dropped out or were noncompliant with study procedures during the wash-in diet period, a total of 72 adults were randomly assigned to 1 of the 3 diet groups: 24 each to the limited dairy, low-fat dairy, and full-fat dairy diets. All randomly assigned participants were included in the ITT analyses. Five subjects were excluded from the per-protocol analyses of glucose tolerance and related endpoints (n = 67): 3 dropped out before the final clinic visit and 2 were excluded for noncompliance. For body weight– and body composition–related endpoints (n = 66), 1 additional participant was excluded from the per-protocol analyses due to a change in thyroid medication. For the analysis of biomarkers of inflammation (n = 59), an additional 8 participants were excluded for acute illness or a change in medication or supplement intake. For the analysis of liver fat content (n = 61), an additional 6 participants were excluded because they were unable to undergo an MRI scan. Table 1 shows the baseline characteristics, stratified by intervention group, for the per-protocol analysis. Baseline characteristics for those included in the ITT analyses can be found in Supplemental Table 1.
FIGURE 1.
Consolidated Standards of Reporting Trials flow diagram.
TABLE 1.
Baseline characteristics of study participants included in the primary per-protocol analyses for glucose tolerance and related endpoints1
| Variable | Limited dairy (n = 22) | Low-fat dairy (n = 24) | Full-fat dairy (n = 21) | P value |
|---|---|---|---|---|
| Age, y | 56 [46–68] | 64 [58–71] | 65 [58–68] | 0.18 |
| Male sex | 54.5 | 58.3 | 57.1 | 0.97 |
| Caucasian race | 72.7 | 79.2 | 71.4 | 0.87 |
| BMI, kg/m2 | 33.6 ± 5.9 | 32.6 ± 7.3 | 32.8 ± 6.4 | 0.85 |
| Body weight, kg | 101 ± 16 | 96 ± 26 | 95 ± 17 | 0.58 |
| Fat mass, kg | 37.9 [32.2–45.1] | 32.5 [23.6–52.1] | 36.7 [27.4–48.8] | 0.66 |
| Lean mass, kg | 57.7 ± 10.3 | 55.6 ± 14.0 | 53.8 ± 8.8 | 0.54 |
| Visceral adiposity,2 inch3 | 148 [97–202] | 104 [71–202] | 124 [78–177] | 0.35 |
| Physical activity, MET-h/wk | 37.2 [23.4–49.6] | 41.0 [25.7–89.7] | 37.8 [19.6–47.9] | 0.29 |
| 2015 Healthy Eating Index | 71.9 ± 9.2 | 72.3 ± 9.7 | 72.2 ± 8.5 | 0.95 |
| HOMA-IR | 2.5 [1.9–3.5] | 3.3 [1.6–4.4] | 3.0 [1.7–4.4] | 0.98 |
| Fasting glucose, mg/dL | 101 [93–109] | 110 [101–119] | 107 [102–116] | 0.09 |
| Fasting insulin, μU/mL | 9.8 [7.1–14.6] | 12.3 [6.1–15.6] | 11.3 [7.9–14.4] | 0.86 |
| Glycated hemoglobin, % | 5.4 [5.0–5.5] | 5.8 [5.5–6.2] | 5.7 [5.4–5.9] | <0.001 |
| Glucose AUC, mg/dL × min | 25,195 [23,445–30,708] | 29,895 [26,495–32,849] | 27,888 [24,831–29,881] | 0.07 |
| Matsuda insulin sensitivity index | 2.7 [2.0–3.8] | 2.4 [1.8–3.8] | 2.3 [1.9–3.4] | 0.88 |
| Insulinogenic index | 1.0 [0.6–1.5] | 0.7 [0.4–1.4] | 1.2 [0.7–1.9] | 0.51 |
| Glucose sensitivity, pmol × min−1 × m−2 × L × mmol−1 | 92 [64–120] | 74 [41–103] | 86 [68–110] | 0.47 |
| Oral disposition index | 2.3 [1.4–4.5] | 2.3 [1.4–3.2] | 2.8 [1.5–4.8] | 0.59 |
| C-reactive protein, mg/L | 1.2 [0.9–2.5] | 0.9 [0.4–2.1] | 1.5 [0.9–3.0] | 0.52 |
| IL-6, pg/mL | 3.5 [2.5–4.2] | 2.7 [1.8–4.1] | 2.9 [1.6–4.2] | 0.50 |
| Total adiponectin, ng/mL | 5150 [3838–7525] | 6425 [3900–9300] | 5900 [3925–9750] | 0.86 |
| Liver fat content,3 % of total | 5.2 [1.0–8.6] | 4.4 [1.1–10.1] | 3.7 [2.1–10.2] | 0.60 |
n = 67. Values are means ± SDs or medians [IQRs] for nonnormally distributed variables or percentages for categorical variables. P values are based on an ANOVA, except for gender and race, which were based on an independent-samples Kruskal–Wallis test. 1 inch = 2.54 cm. MET, metabolic equivalent of task.
Sample size for visceral adiposity: limited, n = 21; low-fat, n = 22; full-fat, n = 20.
Sample size for percentage liver fat: limited, n = 20; low-fat, n = 22; full-fat, n = 19.
Adverse events
No subject withdrew from the study due to adverse events. Of 5 adverse events reported during the trial, 2 were rated as unrelated to study procedures. Three adverse events were rated as related to study procedures and classified as mild–moderate in severity. One participant in the low-fat dairy group experienced hypoglycemia during both of their FS-OGTTs, with each incident being reported as a separate adverse event. A participant in the limited dairy intervention group experienced vertigo, nausea, and vomiting after the MRI scan.
Adherence to the intervention and dietary intakes
Based on data from the Human Nutrition Laboratory on administered and returned study dairy foods as well as participants’ entries regarding consumption of nonstudy dairy foods on their dairy logs, per-protocol participants (n = 67) consumed (mean ± SD) 98.2% ± 1.8% and 97.9% ± 2.8% of the study dairy foods provided to them during the low-fat and full-fat dairy intervention diet periods, respectively (Table 2). During the limited dairy intervention period, participants consumed a mean ± SD of 74.9% ± 34.9% of the provided (nonmandatory) nonfat milk. Mean ± SD consumption of nonstudy dairy foods was 0.6 ± 1.0, 0.6 ± 0.9, and 1.3 ± 2.3 total servings over the entire 12 wk of the limited, low-fat, and full-fat dairy diet periods, respectively. Consistent data on total consumption of dairy foods were obtained from the mean of 3 unannounced 24-h dietary recall interviews conducted during the intervention period (Table 2). The percentage of 1 key biomarker of dairy fat consumption, pentadecanoic acid in the plasma phospholipid fraction, changed differentially (P = 0.004 for the time × diet interaction in the overall RM-ANOVA), with an increase in the full-fat dairy group compared with the limited dairy group (post hoc, adjusted P = 0.006) and a trend for an increase in the full-fat dairy group compared with the low-fat dairy group (post hoc, adjusted P = 0.075) (Table 2). No statistically significant differential changes were seen for 2 other established biomarkers of dairy fat intake, the plasma phospholipid concentrations of trans-palmitoleic acid and heptadecanoic acid (P = 0.104 and P = 0.203, respectively, for the time × diet interaction in the overall RM-ANOVA).
TABLE 2.
Compliance with the dietary intervention of participants who were included in the per-protocol analysis for glucose tolerance and related endpoints1
| Limited dairy group (n = 22) | Low-fat dairy diet (n = 24) | Full-fat dairy diet (n = 21) | ||||
|---|---|---|---|---|---|---|
| Wash-in diet period | Intervention period | Wash-in diet period | Intervention period | Wash-in diet period | Intervention period | |
| Consumed study dairy foods (administered study dairy foods minus returned study dairy foods) | ||||||
| Reduced-fat milk, servings/d | 0.41 [0.22–0.42] | 0.38 [0.17–0.42] | 0.42 [0.36–0.42] | 1.12 [1.08–1.12] | 0.42 [0.37–0.42] | 0.0 [0.0–0.0] |
| Reduced-fat yogurt, servings/d | 0.0 [0.0–0.0] | 0.0 [0.0–0.0] | 0.0 [0.0–0.0] | 1.00 [0.97–1.01] | 0.0 [0.0–0.0] | 0.0 [0.0–0.0] |
| Reduced-fat cheese, servings/d | 0.0 [0.0–0.0] | 0.0 [0.0–0.0] | 0.0 [0.0–0.0] | 1.14 [1.13–1.14] | 0.0 [0.0–0.0] | 0.0 [0.0–0.0] |
| Whole milk, servings/d | 0.0 [0.0–0.0] | 0.0 [0.0–0.0] | 0.0 [0.0–0.0] | 0.0 [0.0–0.0] | 0.0 [0.0–0.0] | 1.12 [1.10–1.12] |
| Full-fat yogurt, servings/d | 0.0 [0.0–0.0] | 0.0 [0.0–0.0] | 0.0 [0.0–0.0] | 0.0 [0.0–0.0] | 0.0 [0.0–0.0] | 1.01 [0.93–1.01] |
| Full-fat cheese, servings/d | 0.0 [0.0–0.0] | 0.0 [0.0–0.0] | 0.0 [0.0–0.0] | 0.0 [0.0–0.0] | 0.0 [0.0–0.0] | 1.14 [1.13–1.14] |
| 24-h dietary recall data [mean of unannounced 24-h dietary recall interviews in the wash-in (2 d) and intervention (3 d) periods] | ||||||
| Reduced-fat milk, servings/d | 0.59 [0.15–1.06] | 0.53 [0.15–1.29] | 0.74 [0.33–0.81] | 1.11 [0.88–1.75] | 0.50 [0.31–1.09] | 0.0 [0.0–0.42] |
| Reduced-fat yogurt, cups/d | 0.0 [0.0–0.0] | 0.0 [0.0–0.0] | 0.0 [0.0–0.0] | 1.14 [0.59–1.63] | 0.0 [0.0–0.0] | 0.0 [0.0–0.0] |
| Reduced-fat cheese, servings/d | 0.0 [0.0–0.0] | 0.0 [0.0–0.0] | 0.0 [0.0–0.0] | 1.25 [0.67–1.54] | 0.0 [0.0–0.0] | 0.0 [0.0–0.0] |
| Whole milk, servings/d | 0.0 [0.0–0.0] | 0.0 [0.0–0.35] | 0.0 [0.0–0.0] | 0.0 [0.0–0.0] | 0.0 [0.0–0.06] | 1.25 [0.93–1.92] |
| Full-fat yogurt, cups/d | 0.0 [0.0–0.0] | 0.0 [0.0–0.0] | 0.0 [0.0–0.0] | 0.0 [0.0–0.0] | 0.0 [0.0–0.0] | 1.35 [0.84–2.15] |
| Full-fat cheese, servings/d | 0.0 [0.0–0.0] | 0.0 [0.0–0.01] | 0.0 [0.0–0.0] | 0.0 [0.0–0.04] | 0.0 [0.0–0.0] | 1.19 [0.91–1.50] |
| Plasma phospholipid biomarkers of dairy fat intake | ||||||
| 15:0, % of total | 0.19 [0.14–0.21] | 0.16 [0.12–0.20] | 0.16 [0.12–0.17] | 0.14 [0.12–0.17] | 0.15 [0.12–0.19] | 0.19 [0.16–0.21] |
| 17:0, % of total | 0.28 [0.23–0.34] | 0.27 [0.23–0.33] | 0.28 [0.24–0.31] | 0.28 [0.23–0.32] | 0.29 [0.25–0.32] | 0.30 [0.28–0.32] |
| trans-16:1n–7, % of total | 0.017 [0.008–0.027] | 0.013 [0.008–0.022] | 0.016 [0.007–0.024] | 0.015 [0.012–0.019] | 0.012 [0.000–0.018] | 0.024 [0.019–0.027] |
n = 67. Values are medians [IQRs]. One serving was defined as 240 mL for milk, 170 g for yogurt, and 42.5 g for cheese.
The dietary intervention also led to some changes in the participants’ habitual diet, as measured by repeated unannounced 24-h dietary recall interviews (Table 3). The intake of SFAs increased in the full-fat dairy group compared with both the limited and the low-fat dairy groups (P < 0.01 in post hoc testing), and the intake of calcium increased in both dairy groups compared with the limited dairy diet (P < 0.001 in post hoc testing). The intake of MUFAs decreased in the low-fat dairy diet compared with both the limited and full-fat dairy diets (P < 0.05 in post hoc testing), but there was no differential change in PUFA intake. The intake of total sugars (percentage of energy) increased in the low-fat dairy group as compared with the limited dairy diet (P = 0.006, in post hoc testing). Nutrient density–adjusted fiber intake (g/1000 kcal) decreased in the full-fat dairy diet group as compared with the limited dairy diet group (P = 0.024, in post hoc testing), but there was no diet effect on total fiber intake (g/d). Carbohydrate intake (percentage of total energy) increased in the low-fat dairy arm compared with the full-fat dairy arm (P = 0.015 in post hoc testing) and fat intake increased in the full-fat dairy arm compared with the low-fat dairy arm (P < 0.001 in post hoc testing). Protein intake tended to increase in the low-fat dairy arm as compared with the limited dairy arm (P = 0.063 in post hoc testing). Total energy intake increased in the full-fat dairy arm compared with both the limited and low-fat dairy arms (P < 0.05 for both in post hoc testing), with no difference between the latter 2. The 2015 Healthy Eating Index increased in the low-fat dairy group compared with the full-fat dairy groups (P < 0.05 in post hoc testing), whereas the limited dairy group did not differ from either of the full-fat and low-fat dairy groups in post hoc testing.
TABLE 3.
Dietary intakes during wash-in and intervention phases, based on unannounced 24-h dietary recalls, for the participants included in the per-protocol analysis for glucose tolerance and related endpoints1
| Limited dairy group (n = 22) | Low-fat dairy diet (n = 24) | Full-fat dairy diet (n = 21) | RM-ANOVA (time × diet interaction)2 | ||||
|---|---|---|---|---|---|---|---|
| Wash-in diet period | Change during intervention period | Wash-in diet period | Change during intervention period | Wash-in diet period | Change during intervention period | ||
| Energy intake, kcal/d | 1998 [1624–2307] | 81 ± 544a | 2041 [1526–2625] | 224 ± 375a | 1712 [1364–2098] | 554 ± 467b | 0.003 |
| Carbohydrates, %E | 46.1 ± 11.4 | −0.7 [−4.4 to 0.9]a,b | 47.7 ± 8.6 | 1.6 [−4.3 to 6.2]a | 46.3 ± 7.1 | −4.1 [−7.6 to −0.8]b | 0.020 |
| Total sugars, %E | 18.0 ± 7.0 | −1.9 ± 5.6a | 18.5 ± 6.6 | 3.4 ± 5.0b | 17.1 ± 6.2 | 0.9 ± 4.7a,b | 0.004 |
| Added sugars, %E | 9.3 ± 4.5 | −0.5 ± 3.3 | 8.5 ± 4.1 | 0.7 ± 4.3 | 9.1 ± 6.1 | −0.9 ± 4.1 | 0.349 |
| Fiber, g/d | 23.9 ± 8.9 | 1.5 ± 7.9 | 25.7 ± 9.0 | −0.3 ± 6.2 | 21.5 ± 9.0 | 0.2 ± 5.8 | 0.650 |
| Fiber, g/1000 kcal | 12.3 ± 5.0 | 0.3 ± 3.8a | 12.6 ± 3.4 | −1.4 ± 2.5a,b | 12.2 ± 4.4 | −2.8 ± 3.3b | 0.011 |
| Fat, %E | 34.0 ± 8.1 | 1.3 [−2.7 to 5.7]a | 34.2 ± 7.7 | −2.5 [−8.2 to 0.0]b | 35.0 ± 9.0 | 4.4 [0.6–7.3]a | <0.001 |
| SFAs, %E | 8.0 ± 2.0 | 0.7 [−0.3 to 3.8]a | 8.2 ± 2.1 | 0.6 [−1.5 to 2.1]a | 8.7 ± 2.6 | 5.2 [3.4–6.9]b | <0.001 |
| MUFAs, E% | 14.1 ± 4.3 | −0.3 ± 3.8a | 14.0 ± 3.9 | −3.1 ± 2.5b | 13.7 ± 4.1 | −0.2 ± 4.6a | 0.014 |
| PUFAs, %E | 9.2 ± 2.8 | 0.3 ± 2.3 | 9.3 ± 3.1 | −1.8 ± 3.0 | 9.7 ± 3.5 | −1.1 ± 3.4 | 0.058 |
| Protein, %E | 15.4 [12.5–18.1] | 0.0 ± 0.1 | 16.2 [13.7–17.7] | 0.1 ± 0.1 | 15.3 [14.1–18.6] | 0.0 ± 0.1 | 0.024 |
| 2015 Healthy Eating Index | 71.9 ± 9.2 | −2.5 ± 10.0a,b | 72.8 ± 9.7 | 2.9 ± 8.7a | 72.2 ± 8.5 | −5.6 ± 7.6b | 0.008 |
| Calcium, mg/1000 kcal | 307 [222–440] | −9 ± 150a | 298 [252–358] | 401 ± 167b | 338 [276–426] | 277 ± 194b | <0.001 |
n = 67. Values are means ± SDs or medians [IQRs] for nonnormally distributed variables. RM-ANOVA, repeated-measures analysis of variance; %E, percentage of total energy intake.
Reflects an overall comparison of the 3 dietary phases by RM-ANOVA. Values with different superscript letters are statistically significantly different in Bonferroni-adjusted post hoc testing (P < 0.017).
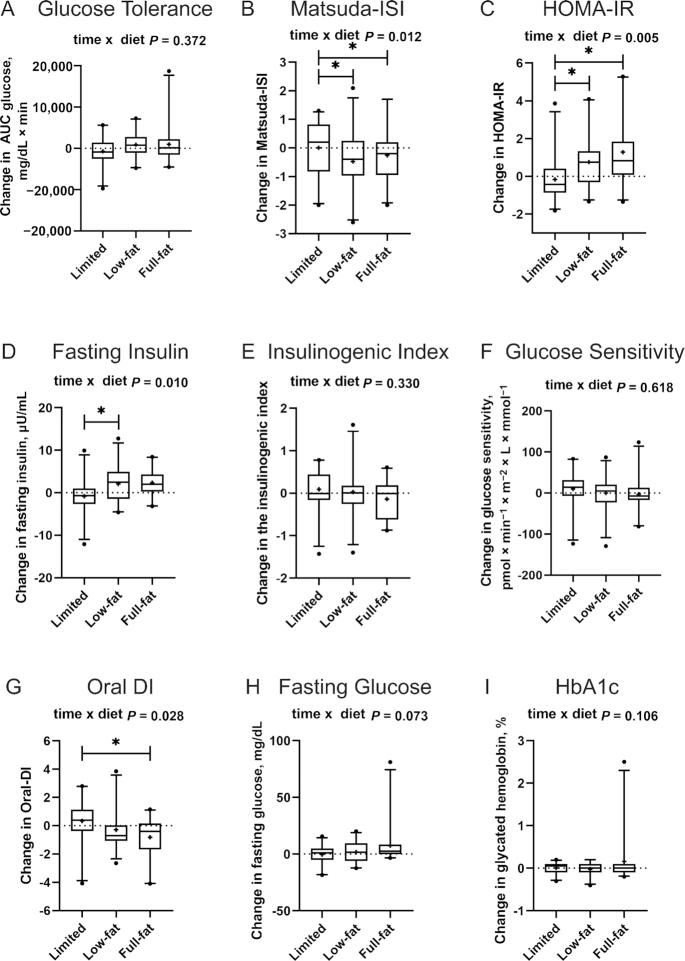
Glucose tolerance and its determinants
Our primary endpoint, glucose tolerance as assessed by AUC glucose, was not differentially affected by the intervention diets (Figures 2, 3). This was the case in the unadjusted analysis (model 1 in Supplemental Table 2), as well as in model 2, which was adjusted for variables that differed or tended to differ across intervention groups at baseline (fasting glucose and HbA1c).
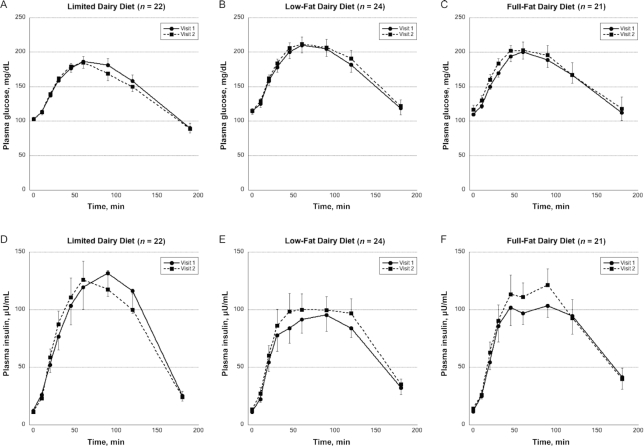
FIGURE 2.
Impact of diets limited in dairy (n = 22, A, D) or rich in low-fat dairy (n = 24, B, E) or full-fat dairy (n = 21, C, F) on plasma glucose (A–C) and insulin (D–F) concentrations in 3-h frequently sampled oral-glucose-tolerance tests. Circles and squares represent means at baseline (visit 1) and after the 12-wk dietary intervention (visit 2), respectively. Error bars represent SEMs.
FIGURE 3.
Changes in measures of glucose homeostasis, insulin sensitivity, and pancreatic β-cell function during the limited dairy diet (n = 22), low-fat dairy diet (n = 24) and full-fat dairy diet (n = 21) (per-protocol analysis, n = 67). Glucose homeostasis and its determinants were assessed by (A) glucose tolerance, i.e., the glucose AUC, (B) the Matsuda ISI, (C) the HOMA-IR index, (D) fasting plasma insulin, (E) the insulinogenic index, (F) glucose sensitivity, (G) the oral DI, (H) fasting plasma glucose, and (I) HbA1c. Outcome variables are represented as the change variable calculated as the value at follow-up minus the value at baseline. Boxes represent IQRs, and whiskers 5th and 95th percentiles, with outliers represented by a solid dot. The medians are represented by horizontal bars across the boxes and the means are represented by crosses. The P values for the time × diet interactions from the overall RM-ANOVA, adjusted for baseline glucose AUC, fasting glucose, and HbA1c, as appropriate, are displayed at the top of each box plot. Bars indicate significant differences between diet groups in post hoc testing (independent-samples t tests or RM-ANOVA with 2 diet groups), again adjusted for baseline AUC glucose, fasting glucose, and HbA1c, as appropriate (*P < 0.05 after adjustment for multiple testing according to Bonferroni). HbA1c, glycated hemoglobin; ISI, insulin sensitivity index; oral DI, oral disposition index; RM-ANOVA, repeated-measures analysis of variance.
Intervention effects were seen for insulin sensitivity, as assessed by the Matsuda ISI (P = 0.012 for the overall time × diet interaction in model 2) (Figure 3, Supplemental Table 2). Specifically, the Matsuda ISI was significantly reduced in both dairy groups compared with the limited dairy group in post hoc tests, after Bonferroni correction (Figure 3B). Consistent with this reduction in insulin sensitivity, we observed a significant intervention effect for HOMA-IR and fasting insulin (overall time × diet interaction P = 0.005 and P = 0.010, respectively) (Figure 3C, Supplemental Table 2). Post hoc t tests showed that HOMA-IR significantly increased in both the low-fat and full-fat dairy groups compared with the limited dairy group (P = 0.030 and P = 0.003, respectively), with no difference between the 2 dairy intervention groups. There was also a significant increase in fasting insulin when comparing the low-fat dairy diet with the limited dairy diet (P = 0.030). The reduction in insulin sensitivity was also evident when assessing the change in plasma insulin concentrations during the 3-h FS-OGTT as compared with AUC glucose (Figure 2).
For measures of pancreatic β-cell function, we detected no overall intervention effect for the insulinogenic index and glucose sensitivity (Figure 3E, F, Supplemental Table 2). However, we detected an intervention effect for the oral DI (overall time × intervention interaction P = 0.028) (Figure 3G), with a statistically significant decrease in the full-fat group compared with the limited dairy group in post hoc testing (P = 0.030).
No intervention effects were seen for any of the major determinants of insulin sensitivity (Figure 4, Supplemental Table 3), including liver fat content (overall time × intervention interaction P = 0.544) and biomarkers of systemic inflammation, including CRP (P = 0.213), IL-6 (P = 0.500), and total adiponectin (P = 0.789).
FIGURE 4.
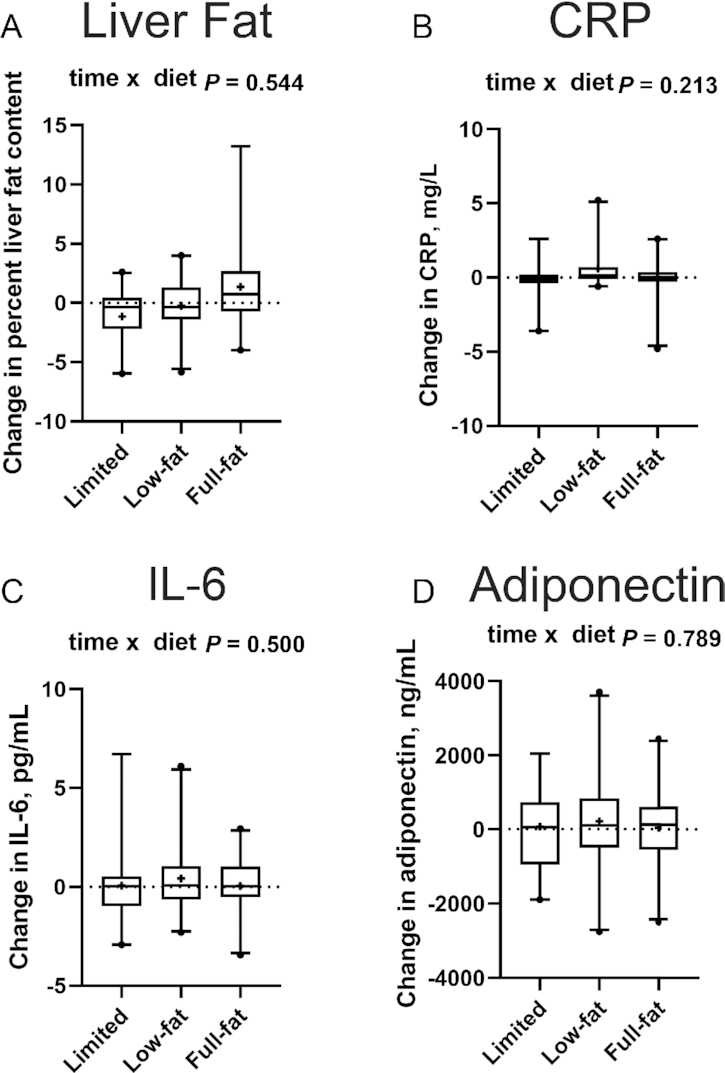
Changes in liver fat content (A) and measures of low-grade chronic systemic inflammation (B–D) in the limited dairy diet (n = 20 and n = 19, respectively), low-fat dairy diet (n = 22 and n = 20, respectively), and the full-fat dairy diet (n = 19 and n = 20, respectively) (per-protocol analysis, n = 61 for liver fat, n = 59 for biomarkers of inflammation). Outcome variables are represented as the change variable calculated as the value at follow-up minus the value at baseline. Boxes represent IQRs, and whiskers 5th and 95th percentiles, with outliers represented by a solid dot. The medians are represented by horizontal bars across the boxes and the means are represented by crosses. The P values for the time × diet interactions from the overall repeated-measures ANOVA are displayed at the top of each box plot. CRP, C-reactive protein.
We also assessed the impact of the intervention diets on other exploratory endpoints related to glucose homeostasis, fasting plasma glucose concentrations, and HbA1c. Whereas no intervention effect was seen for HbA1c (time × intervention interaction P = 0.106) (Figure 3I, Supplemental Table 2), we detected a trend for an intervention effect for fasting glucose (P = 0.073).
In sensitivity analyses, adjustment of models that indicated an intervention effect (Matsuda ISI, fasting insulin, HOMA-IR) for changes in body fat mass, body weight, or waist circumference did not fundamentally change the results, even though in some cases the intervention effects were slightly attenuated (P ≤ 0.070 for all adjusted variables). Sensitivity analyses adjusting for the stratification variables similarly did not fundamentally change any of the results. The sensitivity analyses for the oral DI adjusting for both the changes in waist circumference and weight led to a more substantial attenuation, becoming nonsignificant (P = 0.099 and P = 0.132, respectively; data not shown). We also ran extensive sensitivity analyses adjusting for changes in dietary variables that were differentially changed by the intervention diets (Table 3). The intervention effects for fasting insulin, HOMA-IR, the Matsuda ISI, and the oral DI tended to be very robust and remained significant even after adjustment for changes in percentage energy from carbohydrates, added sugars, total fat, SFAs, MUFAs, and protein; the change in fiber intake (g/1000 kcal); and the change in the 2015 Healthy Eating Index. With the exception of the oral DI, all intervention effects also remained significant after adjustment for change in energy intake. Similarly, sensitivity analyses that excluded 1 outlier in the full-fat dairy group for several of the variables (Figure 3) or adjusted for changes in physical activity did not affect any of the results.
The ITT analysis (n = 72) yielded results consistent with the per-protocol analyses for all endpoints.
Energy intake, body weight, and body composition
During the 5-d controlled feeding period conducted during the intervention period as compared with the 5-d controlled feeding period during the wash-in period, participants received 281 kcal/d and 463 kcal/d more in the form of mandatory dairy foods in the low-fat and full-fat dairy groups, respectively. Comparing intakes from the 5-d controlled periods (intervention compared with wash-in), mean ± SD energy intake remained stable in individuals randomly assigned to stay on the limited dairy diet (mean ± SD: −21 ± 317 kcal/d), increased by +166 ± 267 kcal/d in those who switched to the low-fat dairy diet, and increased by 384 ± 175 kcal/d in participants who switched to the full-fat dairy diet (P < 0.001 in the overall RM-ANOVA; adjusted P < 0.01 in post hoc tests comparing full-fat dairy with both limited dairy and low-fat dairy, with no significant difference between low-fat dairy and limited dairy). Similarly, total energy intake as measured by the repeated 24-h dietary recall interviews increased by (mean ± SD) 224 ± 375 kcal/d and 554 ± 467 kcal/d in the low-fat and full-fat dairy intervention groups, respectively, compared with the wash-in period, whereas it stayed relatively stable in the limited dairy group during the intervention period (P = 0.003 in the overall RM-ANOVA; adjusted P < 0.05 in post hoc tests comparing full-fat dairy with both limited dairy and low-fat dairy, with no significant difference between low-fat dairy and limited dairy) (Table 3). Consistent with these increases in total energy intake, we observed an overall intervention effect for body weight (time × intervention interaction P = 0.005), with a statistically significant increase in the full-fat dairy group compared with the limited dairy group (Figure 5A, Supplemental Table 4), with the low-fat dairy group not significantly different from either after Bonferroni correction. There was a significant overall effect on fat mass (overall time × intervention interaction P = 0.024), but no 2 diets differed from one another after adjustment for multiple comparisons in post hoc testing (Figure 5B, Supplemental Table 4). Further, there was a trend for a difference in lean mass (overall time × intervention interaction P = 0.082) (Figure 5C, Supplemental Table 4). An overall intervention effect was seen for waist circumference (overall time × intervention interaction P = 0.015) (Figure 5D), with a significant increase in both dairy groups compared with the limited dairy group in post hoc testing. No significant intervention effects were seen for hip circumference, the waist-to-hip ratio, or measures of fat distribution including trunk fat, peripheral fat, or visceral fat (Figure 5E–I). As with measures of glucose homeostasis, sensitivity analyses adjusted for changes in physical activity (model 2 in Supplemental Table 4), stratification variables, or key dietary factors, as listed in Table 3, or excluding 1 major outlier in the full-fat dairy group yielded very similar results. The ITT analysis (n = 72) yielded results that were consistent with the per-protocol analyses for all endpoints.
FIGURE 5.
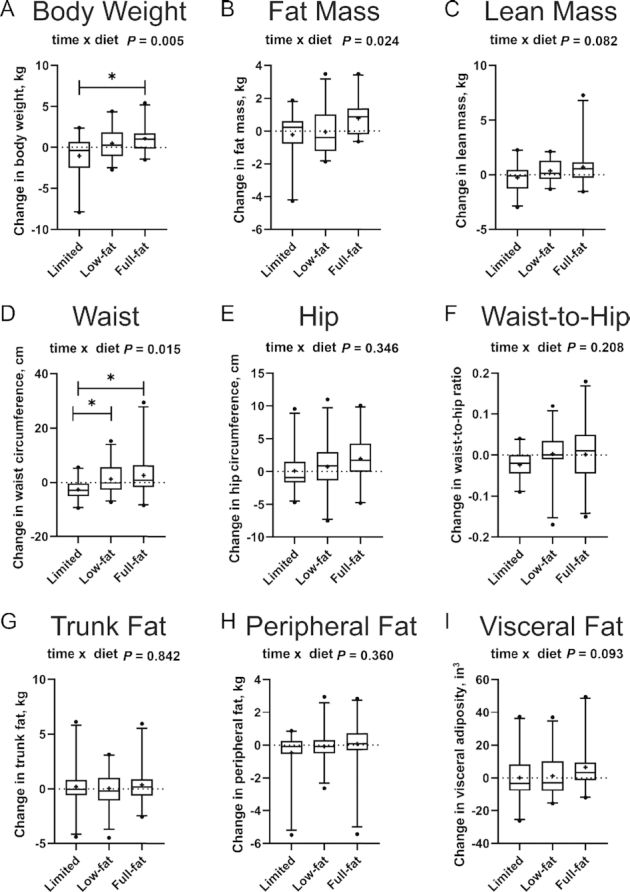
Changes in measures of body weight, anthropometrics, and body composition in the limited dairy diet (n = 21), low-fat dairy diet (n = 24), and the full-fat dairy diet (n = 21) (per-protocol analysis, n = 66). (A) Body weight, (B) total fat mass, (C) lean mass, (D) waist circumference, (E) hip circumference, (F) waist-to-hip ratio, (G) trunk fat mass, (H) peripheral fat mass, and (I) visceral fat area. Outcome variables are represented as the change variable calculated as the value at follow-up minus the value at baseline. Boxes represent IQRs, and whiskers 5th and 95th percentiles, with outliers represented by a solid dot. The medians are represented by horizontal bars across the boxes and the means are represented by crosses. The P values for the time × diet interaction from the overall RM-ANOVA are displayed at the top of each box plot. Bars indicate significant differences between diet groups in post hoc (independent-samples t tests or RM-ANOVA with 2 diet groups) testing (*P < 0.05 after adjustment for multiple testing according to Bonferroni). RM-ANOVA, repeated-measures analysis of variance.
Discussion
In contrast to our hypothesis, consuming 3 servings/d of low-fat or full-fat dairy in the form of milk, yogurt, and cheese did not improve glucose tolerance in men and women with metabolic syndrome. In fact, insulin sensitivity was reduced on both dairy diets compared with the control diet limited in dairy, and this effect was very robust in sensitivity analyses. This reduction in insulin sensitivity was also associated with a reduced oral DI in the full-fat dairy group, suggesting that pancreatic β-cell insulin secretion in response to the standardized glucose load did not increase sufficiently to fully compensate for the decrease in insulin sensitivity. It is possible that this reduction in insulin sensitivity could lead to a reduction in glucose tolerance over time, thereby increasing T2D risk. However, it is curious that the decrease in insulin sensitivity did not seem to be explained by changes in known determinants of insulin sensitivity (i.e., body weight, fat mass, liver fat content, or systemic inflammation), indicating that another mechanism is at play. One potential mechanism that was not explored in this study is that the observed reduction in insulin sensitivity in the dairy groups could plausibly be a physiological response to prevent hypoglycemia after dairy-rich meals. Dairy foods acutely trigger insulin responses far in excess of what would be expected based on their modest glycemic index (41). This insulinotropic effect of dairy may be triggered by branched-chain amino acids found in dairy, which may act directly on the pancreatic β-cell and have also been shown to promote the release of the incretin glucagon-like peptide-1 in intestinal cells in vitro (42–44). Sustained hyperinsulinemia causes insulin resistance (45, 46). Possibly, repeated postprandial hyperinsulinemia triggered by regular dairy consumption could similarly lead to insulin resistance. However, future investigations are needed to assess the impact of the hyperinsulinemia produced by dairy consumption and its potential to affect insulin sensitivity. It therefore remains unclear how the combination of dairy-induced insulin resistance coupled with the acute insulinotropic effect of dairy seen in previous studies affects glucose tolerance and T2D risk in the long term. Another potential mechanism not explored in this study are potential alterations in the gut microbial composition that may have resulted from following our dairy interventions, given that the gut microbiome is an emerging determinant of insulin sensitivity (47).
Another key finding from our trial was that there were no differential effects of our diets on liver fat content or biomarkers of systemic inflammation. The only additional endpoints with intervention effects were body weight, waist circumference, and fat mass. Both body weight and waist circumference were more strongly increased in participants consuming full-fat dairy foods and had intermediate effects in participants consuming low-fat dairy foods. This increase in weight and waist circumference was associated with higher total energy intake, suggesting that participants did not reduce their ad libitum energy intake from nondairy foods enough to fully compensate for the energy consumed in the form of mandatory dairy foods. Energy intake increased in the full-fat dairy group as measured both during the 5-d controlled ad libitum feeding period and through multiple unannounced 24-h dietary recalls, suggesting that the inability to compensate for the caloric content of mandatory dairy foods was sustained throughout the intervention period. This indicates that dairy consumption, and particularly full-fat dairy consumption, in the context of an ad libitum diet, increases energy intake resulting in increased adiposity. This conclusion is in alignment with the result of a recent meta-analysis that showed that dairy intake consistently increased weight in ad libitum studies (48). Our study adds to this literature by providing evidence that full-fat dairy foods increase adiposity to a larger extent than their low-fat counterparts.
Our finding that neither the low-fat nor the full-fat dairy diet had an effect on glucose tolerance is consistent with previous RCTs (16–19). Our data provide additional assurance that null findings in prior trials were not due to the fact that these trials included mostly low-fat unfermented dairy foods. Our finding of decreased insulin sensitivity in participants consuming dairy is also consistent with several prior trials (49–51). At the same time, the majority of previous studies found no effect of dairy consumption on insulin resistance (16–27, 52) and several trials found improvements in insulin sensitivity in participants increasing their dairy intake (52–56). One factor that may explain the differential effects on insulin sensitivity is the duration of the interventions: all studies that showed a reduction in insulin sensitivity were 12 wk in duration or shorter (49–51), whereas almost all studies that showed an improvement in insulin sensitivity were 12 wk in duration or longer (52–54, 56). Another potential factor is that our study was conducted in individuals with the metabolic syndrome, whereas most of the previous studies used comparatively healthier participants, or a population with a less homogeneous metabolic health profile (16–20, 23–25, 27, 49, 50, 52, 53, 55).
The results of our study are greatly at odds with data from observational cohort studies. Five meta-analyses evaluating the effect of dairy foods on T2D concluded that there is a significant inverse relation between dairy consumption and risk of T2D (4–8), with particularly consistent data linking low-fat dairy, yogurt, and plasma biomarkers of dairy fat intake to reduced T2D incidence (11, 14, 15, 57). One possible explanation for the discrepancy between the observational data and findings from trials is that residual confounding may have contributed to the associations in observational studies. It is also possible that effects of increasing dairy consumption on glucose homeostasis were less beneficial in trials given that individuals who are interested and invested in their health are more likely to participate, creating a healthy participant bias. With regards to the data utilizing dairy fat biomarkers, a potential explanation may be that early metabolic changes not commonly measured in observational studies that eventually lead to T2D, such as an increase in liver fat content, affect the concentration of dairy fat biomarkers in plasma phospholipids, thereby confounding the observed association.
This study had multiple strengths that increase our confidence in the results: it is the only study to date to our knowledge that directly compared a variety of low-fat with full-fat dairy foods on glucose tolerance; it assessed glucose homeostasis through dynamic testing; results were basically identical for per-protocol and ITT analyses; we controlled for changes in body weight and fat mass statistically; and participant compliance was excellent. Limitations of this study include a lack of generalizability of the results to populations other than those with metabolic syndrome, and the duration of our dairy intervention, which, even at 12 wk, may have been insufficient to fully capture the long-term implications of habitual dairy consumption.
In conclusion, our study indicates that consuming 3 servings of milk, yogurt, and cheese per day, regardless of fat content, did not affect glucose tolerance in men and women with metabolic syndrome. However, both low-fat and full-fat dairy consumption resulted in a modest decrease in insulin sensitivity. It is unclear whether these changes would persist with prolonged exposure and would affect glucose tolerance and increase the risk of T2D over time. The effect of the dairy diets on insulin sensitivity was not explained by changes in systemic inflammation, liver fat content, body weight, or fat mass. Consuming 3 servings of dairy, particularly full-fat dairy, per day also resulted in an increase in ad libitum energy intake, body weight, and waist circumference. Future studies should investigate potential mechanisms by which dairy consumption may reduce insulin sensitivity and assess whether similar effects are seen in healthy populations. Although results from a single study are insufficient to revise guidelines, the findings from the present study suggest that lower dairy intake may be beneficial in individuals with the metabolic syndrome.
Supplementary Material
ACKNOWLEDGEMENTS
We are deeply indebted to the individuals who volunteered to participate in this trial. We also owe many thanks to the staff of the Fred Hutch Human Nutrition Lab, the Prevention Center, and the Nutrition Assessment Shared Resource, and the staff of the UW Translational Research Unit and the Biomolecular Imaging Center.
The authors’ responsibilities were as follows–––MK, KAS, DKH, JNK, GC, SH, and KMU: designed the research; MK, KAS, JK, DKH, MSB, JNK, and IF: conducted the research; MK, KAS, and MM: analyzed the data; KMU: served as the physician of record; JK: completed the laboratory procedures for the plasma fatty acid analysis; KAS: completed the laboratory procedures for measuring IL-6; MK and KAS: wrote the paper; MK: has primary responsibility for the final content; and all authors: read and approved the final manuscript. This dissertation project of KAS was funded by an international consortium of dairy organizations, including the US National Dairy Council, Dairy Farmers of Canada, the Dutch Dairy Association (Nederlandse Zuivel Organisatie), Dairy Australia, and the French Dairy Interbranch Organization (CNIEL). MK has received honoraria and reimbursements for travel as well as a research grant for this project from several dairy organizations, including the US National Dairy Council, Dairy Farmers of Canada, Nederlandse Zuivel Organisatie, Dairy Australia, and CNIEL. JK has received honoraria and reimbursements for travel as well as research grants from the Vermont Dairy Promotion Council and the National Dairy Council/Dairy Management Inc. MK is a member of the AJCN Editorial Board. The other authors report no conflicts of interest.
Notes
Supported by National Dairy Council/Dairy Management Inc., Dairy Farmers of Canada, Dutch Dairy Association (Nederlandse Zuivel Organisatie), Dairy Australia, and French Dairy Interbranch Organization (CNIEL) contract number 2395 (to MK); NIH grant P30 DK017047 (University of Washington Diabetes Research Center); and NIH grant P30 CA015704 (Fred Hutchinson Cancer Research Center Cancer Center Support Grant). KAS was supported in part by NIH grant T32 CA094880. MSB was supported in part by NIH grants R25CA094880, T32DK007247, and T32HL007028. KMU was supported by the Department of Veterans Affairs.
This study was initiated by the Principal Investigator: MK. The dairy-related funding organizations suggested changes to details of the study design before the conduct of the study, some of which were implemented. Otherwise, the funding organizations had no impact on the design or conduct of the trial, or the analysis and interpretation of study data.
Supplemental Tables 1–4 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Data Availability: An anonymized data set including all data described in the article, code book, and analytic code will be made available upon request to the Principal Investigator (MK).
Abbreviations used: CRP, C-reactive protein; Fred Hutch, Fred Hutchinson Cancer Research Center; FS-OGTT, frequently sampled oral-glucose-tolerance test; HbA1c, glycated hemoglobin; ISI, insulin sensitivity index; ITT, intent-to-treat; NWLRL, Northwest Lipid Research Laboratories; oral DI, oral disposition index; RCT, randomized controlled trial; RM-ANOVA, repeated-measures analysis of variance; T2D, type 2 diabetes; UW, University of Washington.
Contributor Information
Kelsey A Schmidt, Division of Public Health Sciences, Fred Hutchinson Cancer Research Center, Seattle, WA, USA; Nutritional Sciences Program, School of Public Health, University of Washington, Seattle, WA, USA.
Gail Cromer, Division of Public Health Sciences, Fred Hutchinson Cancer Research Center, Seattle, WA, USA.
Maggie S Burhans, Division of Public Health Sciences, Fred Hutchinson Cancer Research Center, Seattle, WA, USA; Department of Epidemiology, University of Washington, Seattle, WA, USA.
Jessica N Kuzma, Division of Public Health Sciences, Fred Hutchinson Cancer Research Center, Seattle, WA, USA.
Derek K Hagman, Division of Public Health Sciences, Fred Hutchinson Cancer Research Center, Seattle, WA, USA.
Imashi Fernando, Division of Public Health Sciences, Fred Hutchinson Cancer Research Center, Seattle, WA, USA; Nutritional Sciences Program, School of Public Health, University of Washington, Seattle, WA, USA.
Merideth Murray, Division of Public Health Sciences, Fred Hutchinson Cancer Research Center, Seattle, WA, USA; Nutritional Sciences Program, School of Public Health, University of Washington, Seattle, WA, USA.
Kristina M Utzschneider, VA Puget Sound Health Care System, Seattle, WA, USA; Department of Medicine, University of Washington, Seattle, WA, USA.
Sarah Holte, Division of Public Health Sciences, Fred Hutchinson Cancer Research Center, Seattle, WA, USA.
Jana Kraft, The College of Agriculture and Life Sciences, The University of Vermont, Burlington, VT, USA.
Mario Kratz, Division of Public Health Sciences, Fred Hutchinson Cancer Research Center, Seattle, WA, USA; Nutritional Sciences Program, School of Public Health, University of Washington, Seattle, WA, USA; Department of Epidemiology, University of Washington, Seattle, WA, USA; Department of Medicine, University of Washington, Seattle, WA, USA.
References
- 1. Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW, Malanda B. IDF Diabetes Atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271–81. [DOI] [PubMed] [Google Scholar]
- 2. Neuenschwander M, Ballon A, Weber KS, Norat T, Aune D, Schwingshackl L, Schlesinger S. Role of diet in type 2 diabetes incidence: umbrella review of meta-analyses of prospective observational studies. BMJ. 2019;366:l2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Palacios OM, Kramer M, Maki KC. Diet and prevention of type 2 diabetes mellitus: beyond weight loss and exercise. Expert Rev Endocrinol Metab. 2019;14(1):1–12. [DOI] [PubMed] [Google Scholar]
- 4. Aune D, Norat T, Romundstad P, Vatten LJ. Dairy products and the risk of type 2 diabetes: a systematic review and dose-response meta-analysis of cohort studies. Am J Clin Nutr. 2013;98(4):1066–83. [DOI] [PubMed] [Google Scholar]
- 5. Gao D, Ning N, Wang C, Wang Y, Li Q, Meng Z, Liu Y. Dairy products consumption and risk of type 2 diabetes: systematic review and dose-response meta-analysis. PLoS One. 2013;8(9):e73965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kalergis M, Leung Yinko SS, Nedelcu R. Dairy products and prevention of type 2 diabetes: implications for research and practice. Front Endocrinol. 2013;4:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Elwood PC, Pickering JE, Givens DI, Gallacher JE. The consumption of milk and dairy foods and the incidence of vascular disease and diabetes: an overview of the evidence. Lipids. 2010;45(10):925–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tong X, Dong J-Y, Wu Z-W, Li W, Qin L-Q. Dairy consumption and risk of type 2 diabetes mellitus: a meta-analysis of cohort studies. Eur J Clin Nutr. 2011;65(9):1027–31. [DOI] [PubMed] [Google Scholar]
- 9. Mitri J, Mohd Yusof BN, Maryniuk M, Schrager C, Hamdy O, Salsberg V. Dairy intake and type 2 diabetes risk factors: a narrative review. Diabetes Metab Syndr. 2019;13(5):2879–87. [DOI] [PubMed] [Google Scholar]
- 10. Gijsbers L, Ding EL, Malik VS, de Goede J, Geleijnse JM, Soedamah-Muthu SS. Consumption of dairy foods and diabetes incidence: a dose-response meta-analysis of observational studies. Am J Clin Nutr. 2016;103(4):1111–24. [DOI] [PubMed] [Google Scholar]
- 11. Guo J, Givens DI, Astrup A, Bakker SJL, Goossens GH, Kratz M, Marette A, Pijl H, Soedamah-Muthu SS. The impact of dairy products in the development of type 2 diabetes: where does the evidence stand in 2019?. Adv Nutr. 2019;10(6):1066–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Soedamah-Muthu SS, de Goede J. Dairy consumption and cardiometabolic diseases: systematic review and updated meta-analyses of prospective cohort studies. Curr Nutr Rep. 2018;7(4):171–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yu E, Hu FB. Dairy products, dairy fatty acids, and the prevention of cardiometabolic disease: a review of recent evidence. Curr Atheroscler Rep. 2018;20(5):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Imamura F, Fretts A, Marklund M, Ardisson Korat AV, Yang W-S, Lankinen M, Qureshi W, Helmer C, Chen T-A, Wong Ket al. Fatty acid biomarkers of dairy fat consumption and incidence of type 2 diabetes: a pooled analysis of prospective cohort studies. PLoS Med. 2018;15(10):e1002670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yakoob MY, Shi P, Willett WC, Rexrode KM, Campos H, Orav EJ, Hu FB, Mozaffarian D. Circulating biomarkers of dairy fat and risk of incident diabetes mellitus among men and women in the United States in two large prospective cohorts. Circulation. 2016;133(17):1645–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gardner CD, Messina M, Kiazand A, Morris JL, Franke AA. Effect of two types of soy milk and dairy milk on plasma lipids in hypercholesterolemic adults: a randomized trial. J Am Coll Nutr. 2007;26(6):669–77. [DOI] [PubMed] [Google Scholar]
- 17. O'Connor S, Julien P, Weisnagel SJ, Gagnon C, Rudkowska I. Impact of a high intake of dairy product on insulin sensitivity in hyperinsulinemic adults: a crossover randomized controlled trial. Curr Dev Nutr. 2019;3(8):nzz083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Engel S, Tholstrup T, Bruun JM, Astrup A, Richelsen B, Raben A. Effect of high milk and sugar-sweetened and non-caloric soft drink intake on insulin sensitivity after 6 months in overweight and obese adults: a randomized controlled trial. Eur J Clin Nutr. 2018;72(3):358–66. [DOI] [PubMed] [Google Scholar]
- 19. Bowen J, Noakes M, Clifton PM. Effect of calcium and dairy foods in high protein, energy-restricted diets on weight loss and metabolic parameters in overweight adults. Int J Obes. 2005;29(8):957–65. [DOI] [PubMed] [Google Scholar]
- 20. Barr SI, McCarron DA, Heaney RP, Dawson-Hughes B, Berga SL, Stern JS, Oparil S. Effects of increased consumption of fluid milk on energy and nutrient intake, body weight, and cardiovascular risk factors in healthy older adults. J Am Diet Assoc. 2000;100(7):810–17. [DOI] [PubMed] [Google Scholar]
- 21. Raziani F, Tholstrup T, Kristensen MD, Svanegaard ML, Ritz C, Astrup A, Raben A. High intake of regular-fat cheese compared with reduced-fat cheese does not affect LDL cholesterol or risk markers of the metabolic syndrome: a randomized controlled trial. Am J Clin Nutr. 2016;104(4):973–81. [DOI] [PubMed] [Google Scholar]
- 22. Dugan CE, Barona J, Fernandez ML. Increased dairy consumption differentially improves metabolic syndrome markers in male and female adults. Metab Syndr Relat Disord. 2014;12(1):62–9. [DOI] [PubMed] [Google Scholar]
- 23. van Meijl LE, Mensink RP. Low-fat dairy consumption reduces systolic blood pressure, but does not improve other metabolic risk parameters in overweight and obese subjects. Nutr Metab Cardiovasc Dis. 2011;21(5):355–61. [DOI] [PubMed] [Google Scholar]
- 24. Thompson WG, Rostad Holdman N, Janzow DJ, Slezak JM, Morris KL, Zemel MB. Effect of energy-reduced diets high in dairy products and fiber on weight loss in obese adults. Obes Res. 2005;13(8):1344–53. [DOI] [PubMed] [Google Scholar]
- 25. Van Loan MD, Keim NL, Adams SH, Souza E, Woodhouse LR, Thomas A, Witbracht M, Gertz ER, Piccolo B, Bremer AAet al. Dairy foods in a moderate energy restricted diet do not enhance central fat, weight, and intra-abdominal adipose tissue losses nor reduce adipocyte size or inflammatory markers in overweight and obese adults: a controlled feeding study. J Obes. 2011:989657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Maki KC, Nieman KM, Schild AL, Kaden VN, Lawless AL, Kelley KM, Rains TM. Sugar-sweetened product consumption alters glucose homeostasis compared with dairy product consumption in men and women at risk of type 2 diabetes mellitus. J Nutr. 2015;145(3):459–66. [DOI] [PubMed] [Google Scholar]
- 27. Benatar JR, Jones E, White H, Stewart RA. A randomized trial evaluating the effects of change in dairy food consumption on cardio-metabolic risk factors. Eur J Prev Cardiol. 2014;21(11):1376–86. [DOI] [PubMed] [Google Scholar]
- 28. Chen Y, Feng R, Yang X, Dai J, Huang M, Ji X, Li Y, Okekunle AP, Gao G, Onwuka JUet al. Yogurt improves insulin resistance and liver fat in obese women with nonalcoholic fatty liver disease and metabolic syndrome: a randomized controlled trial. Am J Clin Nutr. 2019;109(6):1611–19. [DOI] [PubMed] [Google Scholar]
- 29. Engel S, Elhauge M, Tholstrup T. Effect of whole milk compared with skimmed milk on fasting blood lipids in healthy adults: a 3-week randomized crossover study. Eur J Clin Nutr. 2018;72(2):249–54. [DOI] [PubMed] [Google Scholar]
- 30. Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC Jr. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640–5. [DOI] [PubMed] [Google Scholar]
- 31. Blair SN. How to assess exercise habits and physical fitness. New York: Wiley; 1984. [Google Scholar]
- 32. Wolk A, Vessby B, Ljung H, Barrefors P. Evaluation of a biological marker of dairy fat intake. Am J Clin Nutr. 1998;68(2):291–5. [DOI] [PubMed] [Google Scholar]
- 33. Sun Q, Ma J, Campos H, Hu FB. Plasma and erythrocyte biomarkers of dairy fat intake and risk of ischemic heart disease. Am J Clin Nutr. 2007;86(4):929–37. [DOI] [PubMed] [Google Scholar]
- 34. Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226(1):497–509. [PubMed] [Google Scholar]
- 35. Bainbridge ML, Lock AL, Kraft J. Lipid-encapsulated echium oil (Echium plantagineum) increases the content of stearidonic acid in plasma lipid fractions and milk fat of dairy cows. J Agric Food Chem. 2015;63(19):4827–35. [DOI] [PubMed] [Google Scholar]
- 36. Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22(9):1462–70. [DOI] [PubMed] [Google Scholar]
- 37. Herzberg-Schäfer SA, Staiger H, Heni M, Ketterer C, Guthoff M, Kantartzis K, Machicao F, Stefan N, Häring H-U, Fritsche A. Evaluation of fasting state-/oral glucose tolerance test-derived measures of insulin release for the detection of genetically impaired β-cell function. PLoS One. 2010;5(12):e14194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mari A, Schmitz O, Gastaldelli A, Oestergaard T, Nyholm B, Ferrannini E. Meal and oral glucose tests for assessment of β-cell function: modeling analysis in normal subjects. Am J Physiol Endocrinol Metab. 2002;283(6):E1159–66. [DOI] [PubMed] [Google Scholar]
- 39. Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27(6):1487–95. [DOI] [PubMed] [Google Scholar]
- 40. de Souza RJ, Eisen RB, Perera S, Bantoto B, Bawor M, Dennis BB, Samaan Z, Thabane L. Best (but oft-forgotten) practices: sensitivity analyses in randomized controlled trials. Am J Clin Nutr. 2016;103(1):5–17. [DOI] [PubMed] [Google Scholar]
- 41. Östman EM, Liljeberg Elmståhl HGM, Björck IME. Inconsistency between glycemic and insulinemic responses to regular and fermented milk products. Am J Clin Nutr. 2001;74(1):96–100. [DOI] [PubMed] [Google Scholar]
- 42. Chen Q, Reimer RA. Dairy protein and leucine alter GLP-1 release and mRNA of genes involved in intestinal lipid metabolism in vitro. Nutrition. 2009;25(3):340–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nair KS, Short KR. Hormonal and signaling role of branched-chain amino acids. J Nutr. 2005;135(6 Suppl):1547S–52S. [DOI] [PubMed] [Google Scholar]
- 44. Hidayat K, Du X, Shi BM. Milk in the prevention and management of type 2 diabetes: the potential role of milk proteins. Diabetes Metab Res Rev. 2019;35(8):e3187. [DOI] [PubMed] [Google Scholar]
- 45. Del Prato S, Leonetti F, Simonson DC, Sheehan P, Matsuda M, DeFronzo RA. Effect of sustained physiologic hyperinsulinaemia and hyperglycaemia on insulin secretion and insulin sensitivity in man. Diabetologia. 1994;37(10):1025–35. [DOI] [PubMed] [Google Scholar]
- 46. Rizza RA, Mandarino LJ, Genest J, Baker BA, Gerich JE. Production of insulin resistance by hyperinsulinaemia in man. Diabetologia. 1985;28(2):70–5. [DOI] [PubMed] [Google Scholar]
- 47. Lee CJ, Sears CL, Maruthur N. Gut microbiome and its role in obesity and insulin resistance. Ann N Y Acad Sci. 2020;1461(1):37–52. [DOI] [PubMed] [Google Scholar]
- 48. Geng T, Qi L, Huang T. Effects of dairy products consumption on body weight and body composition among adults: an updated meta-analysis of 37 randomized control trials. Mol Nutr Food Res. 2018;62(1):1700410. [DOI] [PubMed] [Google Scholar]
- 49. Hoppe C, Mølgaard C, Vaag A, Barkholt V, Michaelsen KF. High intakes of milk, but not meat, increase s-insulin and insulin resistance in 8-year-old boys. Eur J Clin Nutr. 2005;59(3):393–8. [DOI] [PubMed] [Google Scholar]
- 50. Eelderink C, Rietsema S, van Vliet IMY, Loef LC, Boer T, Koehorst M, Nolte IM, Westerhuis R, Singh-Povel CM, Geurts JMWet al. The effect of high compared with low dairy consumption on glucose metabolism, insulin sensitivity, and metabolic flexibility in overweight adults: a randomized crossover trial. Am J Clin Nutr. 2019;109(6):1555–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Turner KM, Keogh JB, Clifton PM. Red meat, dairy, and insulin sensitivity: a randomized crossover intervention study. Am J Clin Nutr. 2015;101(6):1173–9. [DOI] [PubMed] [Google Scholar]
- 52. St-Onge MP, Goree LL, Gower B. High-milk supplementation with healthy diet counseling does not affect weight loss but ameliorates insulin action compared with low-milk supplementation in overweight children. J Nutr. 2009;139(5):933–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Rideout TC, Marinangeli CP, Martin H, Browne RW, Rempel CB. Consumption of low-fat dairy foods for 6 months improves insulin resistance without adversely affecting lipids or bodyweight in healthy adults: a randomized free-living cross-over study. Nutr J. 2013;12:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wennersberg MH, Smedman A, Turpeinen AM, Retterstøl K, Tengblad S, Lipre E, Aro A, Mutanen P, Seljeflot I, Basu Set al. Dairy products and metabolic effects in overweight men and women: results from a 6-mo intervention study. Am J Clin Nutr. 2009;90(4):960–8. [DOI] [PubMed] [Google Scholar]
- 55. Zemel MB, Richards J, Milstead A, Campbell P. Effects of calcium and dairy on body composition and weight loss in African-American adults. Obes Res. 2005;13(7):1218–25. [DOI] [PubMed] [Google Scholar]
- 56. Stancliffe RA, Thorpe T, Zemel MB. Dairy attentuates oxidative and inflammatory stress in metabolic syndrome. Am J Clin Nutr. 2011;94(2):422–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Drouin-Chartier J-P, Brassard D, Tessier-Grenier M, Côté JA, Labonté ME, Desroches S, Couture P, Lamarche B. Systematic review of the association between dairy product consumption and risk of cardiovascular-related clinical outcomes. Adv Nutr. 2016;7(6):1026–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.