Abstract
Chemotherapy for cancer treatment has therapeutic limitations, such as drug resistance, excessive toxic effects and undesirable adverse effects. Therefore, efforts to improve the safety and efficacy of chemotherapeutic agents are essential. Ionizing radiation can improve physiological and pharmacological properties by transforming structural modifications of the drug. In this study, in order to reduce the adverse effects of rotenone and increase anticancer activity, a new radiolytic rotenone derivative called rotenoisin A was generated through radiolytic transformation. Our findings showed that rotenoisin A inhibited the proliferation of breast cancer cells and increased the rate of apoptosis, whereas it had no inhibitory effect on primary epidermal keratinocytes compared with rotenone. Moreover, rotenoisin A-induced DNA damage by increasing reactive oxygen species (ROS) accumulation. It was also confirmed not only to alter the composition ratio of mitochondrial proteins, but also to result in structural and functional changes. The anticancer effect and molecular signalling mechanisms of rotenoisin A were consistent with those of rotenone, as previously reported. Our study suggests that radiolytic transformation of highly toxic compounds may be an alternative strategy for maintaining anticancer effects and reducing the toxicity of the parent compound.
Keywords: rotenone, rotenoisin A, breast cancer, radiolytic transformation, mitogen-activated protein kinase (MAPK)
INTRODUCTION
Breast cancer is the most commonly diagnosed cancer in women worldwide, after non-melanoma skin cancer [1]. Breast cancer is classified into three main subtypes depending on the presence of molecular markers for oestrogen and progesterone receptors and human epidermal growth factor 2 (ERBB2; formerly HER2): hormone receptor-positive/ERBB2-negative (70% of patients), ERBB2-positive (15–20%) and triple-negative (tumour without all three standard molecular markers: 15%) [2]. Breast cancer treatment combines surgery, radiation therapy, chemotherapy and hormonal therapy, depending on the patient’s condition, grade, stage and molecular tumour subtype [3]. Among these, chemotherapy is routinely administered to early-stage breast cancer patients as adjuvant therapy to reduce tumour size or cancer recurrence before and after mastectomy. Representative chemicals currently used to treat early breast cancer include doxorubicin, cyclophosphamide, paclitaxel, trastuzumab, carboplatin fluorouracil and epirubicin [4, 5]. Various combinations of these chemicals are generally recommended for early-stage breast cancer patients and play an important role in the direction of tumour treatment and patient survival. However, the typical drawbacks of these drugs, such as drug resistance and unnecessary adverse effects, remain important challenges to be addressed. In particular, due to adverse effects such as nausea, vomiting, fatigue and joint pain, 28% of breast cancer patients did not continue with their prescribed chemotherapy [6].
Most chemotherapeutic drugs cause DNA damage during replication or destroy rapidly growing cancer cells by other mechanisms [7–9]. However, the drug can damage fast-growing normal cells, causing severe adverse effects. Additionally, in the case of tumours that are resistant to chemotherapeutic drugs, a higher dose is required, which increases cytotoxicity to normal tissues and increases the incidence of multiple drug resistance. Indeed, the severe adverse effects of chemotherapy drugs in healthy tissues and organs are a major cause of increased mortality in cancer patients, limiting chemotherapy. However, limiting the concentration of the drug can minimize adverse effects and damage to normal cells, but the therapeutic efficacy on tumour cells is also significantly reduced. Therefore, it is desirable to develop chemotherapeutic agents with enhanced therapeutic effects and minimal toxicities to normal tissues.
Recently, ionizing radiation has attracted attention as an alternative to improve the bioactivity of drugs and improve their therapeutic efficacy in tumours. Ionizing radiation can enhance pharmacological properties and anticancer activity by inducing structural modifications to the drug [10, 11]. However, research related to radiation conversion is still very limited. We irradiated dexamethasone, a representative anticancer agent in previous studies, to produce a dexamethasone derivative (Dex-IR) with new chemical characteristics [12]. Dex-IR effectively induced apoptosis through caspase–poly(ADP-ribose) polymerase (PARP)-dependent pathways in non-small cell lung cancer (NSCLC). In addition, we confirmed that new compounds are induced by a radiolytic transformation in the isoflavonoid, rotenone. In particular, it has been demonstrated that rotenoisin B, a radiolytic rotenone derivative, effectively inhibits the proliferation of liver cancer cells while reducing adverse effects. Rotenone is an anticancer substance known to induce apoptosis through the activation of reactive oxygen species (ROS) and JNK/p38 mitogen-activated protein kinase (MAPK) in the human breast cancer line MCF-7 [13]. However, low doses of rotenone (<10 nM) cause oxidative damage and death of dopaminergic neurons, and long-term intake has been reported to induce Parkinson’s disease [14]. Recently, we demonstrated the radiolysate, rotenoisin A, using the radioactive transformation method of the isoflavonoid rotenone field by γ-irradiation [15]. After material modification with irradiation in the liquid chromatography analysis, the retention time was reduced from 17.88 min (rotenone) to 9.434 min (rotenoisin A). The secondary product was found to have a different mass (Fig. 1A), and the modified structure was elucidated using spectroscopy (Fig. 1B), adopted from Chul-Hong Park’s study and modified [15]. In this study, we attempted to generate new radiolytic rotenone derivatives through radiolytic transformations to reduce the adverse effects of rotenone and increase its anticancer activity. This study aimed to evaluate the effect of radiolytic rotenone, rotenoisin A, on human breast cancer MCF-7 cells and determine its utility as a novel anticancer target for breast cancer.
Fig. 1.
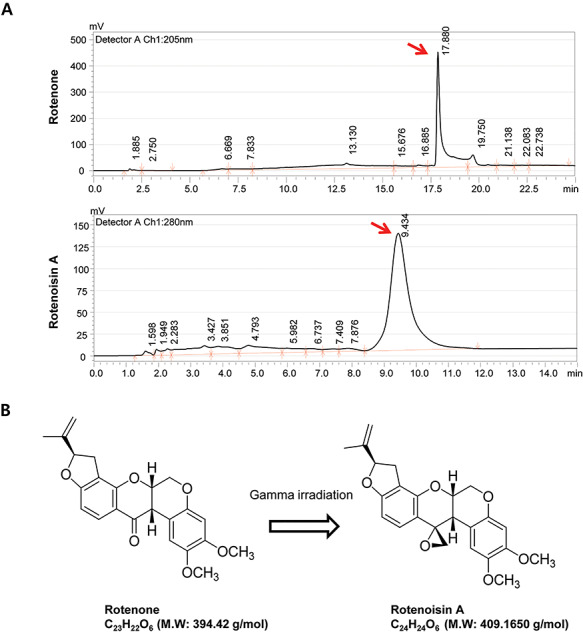
Ionizing radiation induced changes in the molecular properties of rotenone. (A) Chromatograms of rotenone (top) and rotenoisin A (bottom). (B) Structure of rotenone and rotenoisin A. These data we modified from Park et al. 2013 [15].
MATERIALS AND METHODS
Materials and reagents
Rotenone, Cell Counting Kit-8 (CCK-8) and dimethyl sulfoxide (DMSO) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Anti-Bax (#5023), anti-Bcl2 (#2870), anti-caspase 9 (#95020), anti-PARP (#9532), anti-cleaved PARP (#9541), anti-ERK (#4695), anti-p-ERK (#4377), anti-p38 (#9212), anti-p-p38 (#9215), anti-JNK (#9252), anti-p-JNK (#9251), anti-H2AX(#9718) and anti-GAPDH (#2118) were purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA). RPMI 1640, penicillin/streptomycin and foetal bovine serum (FBS) were purchased from Lonza (Walkersville, MD, USA). MitoTracker™ and MitoSOX™ Red were purchased from Thermo Fisher Scientific (Waltham, MA, USA).
γ-Irradiation and high-performance liquid chromatography (HPLC)
Rotenoisin A was prepared using the method described in our previous report [15]. Briefly, a sample solution of rotenone (0.5 g) in MeOH (200 ml) in capped vials was exposed to 50 kGy (absorbed dose) of radiation. Irradiation was carried out at ambient temperature using a cobalt-60 irradiator (point source AECL, IR-79, MDS Nordion International Co. Ltd, Ottawa, ON, Canada) at the Advanced Radiation Technology Institute, Korea Atomic Energy Research Institute (Jeongup, Korea). The source strength was ~320 kCi, with a dose rate of 10 kGy h–1 at the location of the sample. The irradiated methanolic solution was immediately evaporated to remove the solvent and lyophilized. The dried irradiated solution was directly subjected to column chromatography over a silica gel column (2.5 cm i.d. × 32 cm) with CHCl3-MeOH–MeOH to yield pure rotenoisin A (129.1 mg, retention time 4.5 min). HPLC analysis was carried out on a YMC-Pack ODS A-302 column (4.6 mm i.d. × 150 mm; YMC Co., Ltd) and was developed at 40°C with 1% HCOOH/MeCN (1:1, flow rate 1.0 min–1, detection 280 nm).
Cell culture
Human breast cancer MCF-7 cells and primary epidermal keratinocytes (HEKa) were purchased from the American Type Culture Collection (Rockville, MD, USA). MCF-7 cells were cultured in RPMI 1640 medium supplemented with FBS (10%), l-glutamine (4 mM), penicillin (100 U ml–1) and streptomycin (100 μg ml–1). HEKa cells were cultured with Dermal Cell Basal Medium supplemented with a Keratinocyte Growth Kit (ATCC® PCS-200-040™), penicillin (100 U ml–1) and streptomycin (100 μg ml–1). The cells were incubated under sterile conditions at 37°C in a humid environment containing CO2 (5%).
Viability assay and morphological analysis
Cell viability was measured using the CCK-8 assay. The cells were seeded in 96-well plates (1 × 104 cells per well) and incubated overnight. The cells were treated with rotenone or rotenoisin A at the indicated concentrations and incubated for 24 h. A solution of CCK-8 was added to each well, and the plates were incubated for 1 h at 37°C to allow the reaction to take place before removal of the culture medium. Cell viability was determined using a spectrophotometer, and the absorbance was measured at 450 nm (Tecan, Männedorf, Switzerland). The cell viability for each group was expressed as a percentage of the control group. Cell morphology was monitored using an Olympus IX71 fluorescence microscope (Olympus Corporation, Tokyo, Japan).
Oxidative stress assay
MCF-7 cells (2 × 106 cells per well) grown in 12-well plates were incubated with 40 μM rotenone or rotenoisin A for 24 h at 37°C. The oxidative stress assay was conducted by carrying out quantitative measurement of cellular populations undergoing oxidative stress using the Muse™ Cell Analyzer and Muse™ Oxidative Stress Kit (EMD Millipore, Billerica, MA, USA). According to the manufacturer’s protocol, the cells were detached, resuspended to obtain 1 × 106 cells ml–1, and incubated at 37°C for 30 min with the Muse™ Oxidative Stress working solution. The number of oxidized cells was counted using the Muse™ Cell Analyser based on the intensity of red fluorescence. The results were obtained from four independent experiments. Mitochondrial ROS (mROS) levels were measured using the mitochondrion-specific fluorescent hydroethidine-derivative dye, MitoSOX Red (10 μM; Thermo Fisher Scientific), as previously described [16]. Cells were incubated in 96-well black plates with MitoSOX Red for 30 min at 37°C in 5% CO2. The cells were washed twice with phosphate-buffered saline (PBS). MitoSOX Red was measured using a spectrophotometer (Tecan, Männedorf, Switzerland) at excitation and emission wavelengths of 510 and 580 nm, respectively.
Evaluation of apoptosis by annexin-V FITC/propidium iodide (PI)
MCF-7 cells (2 × 106 cells per well) grown in 12-well plates were incubated with 40 μM rotenone or rotenoisin A for 24 h at 37°C. The cells were harvested and washed with Dulbecco’s PBS. The cells were stained with annexin V and the Dead Cell reagent (MCH100105; EMD Millipore) for 20 min, and the flow cytometric assessment was performed using the Muse™ Cell Analyser. The number of apoptotic cells was expressed as the percentage of the live, early/late apoptotic and dead cells, which were determined using the Muse analysis software (Muse 1.1.2; EMD Millipore).
Immunofluorescence staining
Cells (1 × 105 cells per well) were prepared on sterilized glass coverslips (BD Biosciences, Franklin Lakes, NJ, USA) in triplicate. The cells were incubated with rotenone or rotenoisin A for 24 h at 37°C. After incubation, the cells were fixed in 2% paraformaldehyde and 2% glutaraldehyde in PBS for 15 min and permeabilized with 0.25% Triton X-100 in PBS for 10 min. Next, the following procedures were performed according to the experiments. (i) Mitochondria network imaging: cells were stained with 50 nM MitoTracker™ (Thermo Fisher Scientific) for 1 h at 37°C in the dark. (ii) γH2AX staining: cells were incubated with the γH2AX primary antibody for 2 h at room temperature. The cells were washed to remove excess primary antibodies and incubated with the appropriate fluorescently labelled secondary antibodies for 1 h at room temperature. The nuclei were stained with 4,6-diamidino-2-phenylindole (DAPI; D9542, Sigma-Aldrich, St. Louis, MO, USA) and incubated for 5 min. After mounting, fluorescence images were captured using a confocal microscope (LSM 700; Carl Zeiss, Oberkochen, Germany). To quantify the immune-reacted cells, the fluorescence intensity was measured in 10 randomly selected images.
Western blot analysis
MCF-7 cells (1 × 107 cells per well) grown in 6-well plates were incubated with 40 μM rotenone or rotenoisin A for 24 h at 37°C. The cells were washed with PBS and lysed with radioimmunoprecipitation assay (RIPA) buffer. The proteins (30–50 μg) were separated by 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) and transferred to polyvinylidene difluoride (PVDF) membranes. The membranes were blocked with 5% non-fat dry milk for 1 h at room temperature and then incubated overnight with a 1:1000 dilution of primary antibodies at 4°C. The membranes were washed with Tris-buffered saline and incubated with horseradish peroxidase-conjugated secondary antibodies (anti-rabbit IgG, HRP-linked antibody, #7074; Cell Signaling Technology, Inc.) for 2 h at room temperature. The proteins were then visualized using an enhanced chemiluminescence reagent (ECL; EMD Millipore) and exposure to an X-ray film.
Statistical analysis
Statistical differences were evaluated using Student’s t-test. The results were considered statistically significant when the P value was <0.05. All experiments were performed at least three times independently.
RESULTS
Cytotoxicity induced by rotenoisin A compared with rotenone
To explore the inhibitory effect of rotenoisin A on cell growth, MCF-7 cells were treated with various concentrations of rotenoisin A for 24 h. The results from the CCK-8 assay showed that rotenoisin A reduced MCF-7 cell viability in a concentration-dependent manner (Fig. 2A). The reduction reached 34.13 ± 2.68% after 40 μM rotenone treatment, whereas 40 μM rotenoisin A treatment resulted in a reduction in viability to 45.82 ± 2.13% in MCF-7 cells compared with the control. Although the inhibitory effect of rotenoisin A was somewhat weaker than that of rotenone in the MCF-7 cell line, the inhibitory effect of rotenoisin A on HEKa was decreased relative to that of rotenone (Fig. 2B). In previous reports, rotenone used as an organic pesticide has been reported to have neurotoxic effects that could play a role in the development of Parkinson’s disease [17, 18]. Therefore, these results suggest that rotenoisin A may be a new antiproliferative candidate in breast cancer with lower toxicity in normal cells.
Fig. 2.
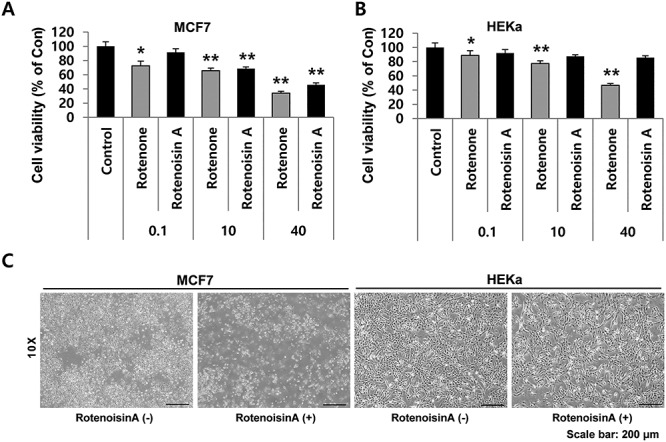
Rotenoisin A inhibited the proliferation of breast cancer (MCF-7) cells but not primary epidermal keratinocytes (HEKa). (A) The MCF-7 cell line was treated with increasing concentrations of rotenone and rotenoisin A for 24 h. The effects of rotenoisin A on the viability of MCF-7 cells at the indicated concentrations were determined using the CCK-8 assay and compared with non-treated cells. (B) HEKa cells were treated with increasing concentrations of rotenone and rotenoisin A for 24 h. The effects of rotenoisin A on the viability of HEKa cells at the indicated concentrations were determined using the CCK-8 assay and compared with those of the rotenone-treated cells. Data are presented as the means ± SEM of three independent experiments (P ≤ 0.05). (C) Representative images of the morphological changes in MCF-7 and HEKa cells were observed at 24 h after rotenone and rotenoisin A treatments, respectively. Scale bar: 200 μm. Con, control.
Rotenoisin A induced accumulation of ROS in MCF-7 cells, resulting in DNA damage
Previous studies have shown the irreversible binding of rotenone to complexes of mitochondrial electron transport chains, followed by the disruption of oxidative phosphorylation by blocking the transfer of electrons from complexes to ubiquinone, resulting in the accumulation of ROS [19, 20]. The Muse™ Cell Analyzer and Mitosox™ assay revealed that rotenoisin A induces the accumulation of cytosolic and mitochondrial ROS in MCF-7 cells. Interestingly, the number of cells with high cytosolic and mitochondrial ROS-positive fractions increased after treatment with 40 μM rotenoisin A, and this trend was maintained over time (Fig. 3A and B).
Fig. 3.
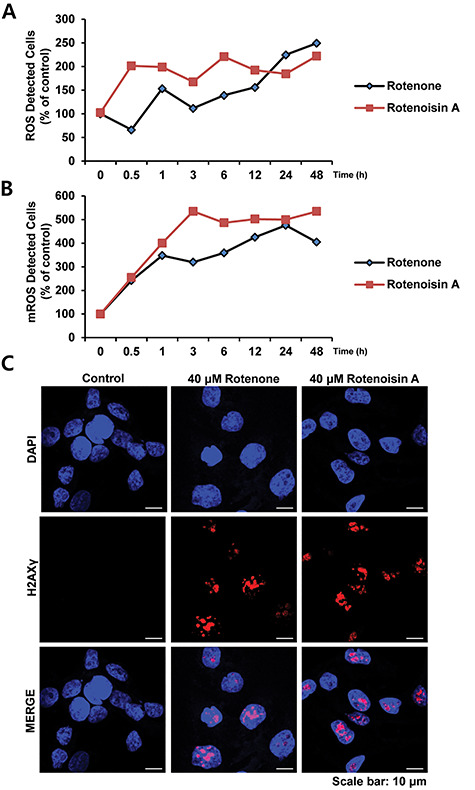
Rotenoisin A increased reactive oxygen species (ROS) accumulation and induced DNA damage. (A) Intracellular ROS detection using the Muse™ Cell Analyser. (B) mROS detection using Mitosox™. (C) γH2AX and MCF-7 cells were cultured on glass coverslips, and the cells were fixed and stained with γH2AX primary antibodies and Alexa Fluor 555-conjugated secondary antibodies. Then, the nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI). Representative images are shown for each control and treatment group, with γH2AX foci indicating double-strand DNA breaks in the nuclei. Scale bar: 10 μm.
Oxidative stress and DNA damage are associated with various human pathological conditions, including ageing and cancer. We monitored γH2AX expression as a measure of DNA damage in MCF-7 cells treated with rotenoisin A for 24 h. Similar to rotenone, an increased γH2AX intensity was observed following treatment with rotenoisin A compared with the control (Fig. 3C). Overall, the results confirmed that increased ROS accumulation due to rotenoisin A treatment results in DNA damage.
Effects of rotenoisin A on disruption of the mitochondrial protein Bcl2/Bax ratio in MCF-7 cells
In many previous studies, rotenone has been identified as an inhibitor of the mitochondrial electron transport chain complex I and is activated by a disrupted electron transport system that results in high ROS accumulation [19, 21, 22]. Mitochondria are not only involved in caspase-dependent apoptosis but also significantly affect the Bcl2 pathway during caspase-independent apoptosis [23, 24]. In this study, we investigated changes in the Bcl2/Bax ratio following rotenone or rotenoisin A treatment. Bcl2 exists in the mitochondrial outer membrane and plays a role in cell survival as well as inhibition of proapoptotic proteins. In addition, Bcl2 also prevents the release of mitochondrial contents, including ROS and cytochrome c, which induces caspase activation. The expression of Bax protein is found in the cytosol, but Bax changes its steric structure when apoptosis signalling begins. During the induction of apoptosis, Bax is associated with cell membranes, specifically mitochondrial membranes [25, 26]. The Bcl2/Bax ratio as a potential molecular marker of the apoptosis pathway is reliable [27, 28]. We found that the expression of Bcl2 in the whole protein decreased 48 h after treatment with 40 μM rotenoisin A. However, Bax protein expression appeared to increase. This tendency is similar to that of natural rotenone. Eventually, it was confirmed that the Bcl2/Bax ratio was decreased by rotenoisin A treatment (Fig. 4A).
Fig. 4.
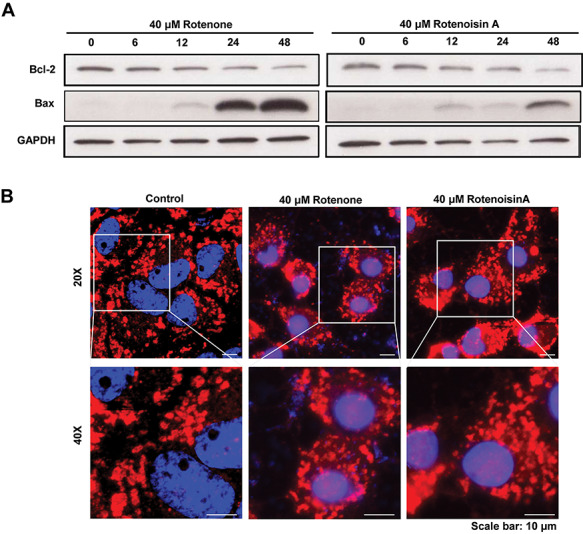
Rotenoisin A decreased Bcl-2/Bax and disrupted the mitochondrial network in MCF-7 cells. (A) Representative images of the western blot analysis. After rotenoisin A treatment, the expressions of Bcl-2 and Bax in MCF-7 cells shifted in a time-dependent manner. (B) Representative images of MitoTracker™ staining. The nuclei were counterstained with DAPI. Scale bar: 10 μm.
Additionally, we observed mitochondria located around the cell nucleus without rotenone or rotenoisin A treatment. After the addition of either substance, dense deposits were observed in the mitochondria, and apoptotic cells displayed shrunken nuclei but increased and swollen mitochondria. The overall distribution also changed irregularly (Fig. 4B). These results suggest that treatment with rotenoisin A not only altered the composition ratio of mitochondrial proteins but also resulted in structural and functional changes.
Rotenoisin A induced phosphorylation of JNK, p38 and ERK MAPKs
To explore the stress index, the levels of the active form of stress-activated MAPKs were measured. Among the MAPKs, JNK and ERK MAPKs act as damaging circuits in the cellular stress environment, inducing excessive oxidative stress [22, 29]. In particular, the activation of p38 MAPK promotes mitochondrial translocation of Bax proteins and plays an important role in intrinsic apoptosis [30, 31]. In this study, rotenoisin A treatment triggered the phosphorylation of JNK and p38, but phosphorylation of ERK was down-regulated in a concentration-dependent manner. These results suggest that damage to the rotenoisin A pathway occurs via MAPKs in MCF-7 cells (Fig. 5A and B).
Fig. 5.
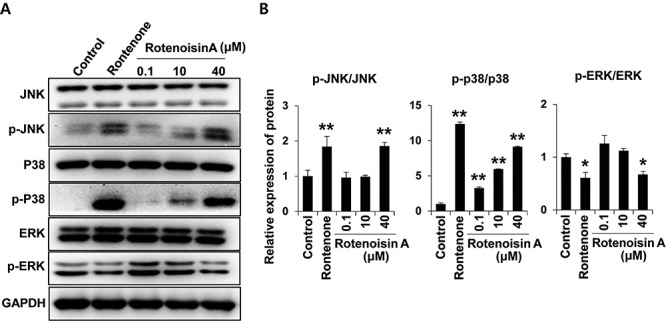
Rotenoisin A stimulated the mitogen-activated protein kinase (MAPK) pathway in MCF-7 cells. (A) Representative images of the western blot analysis for the protein expression of JNK, p-JNK, p38, p-p38, ERK, p-ERK and GAPDH antibodies. (B) Densitometry analysis. The data are presented as the means ± SDs. *P ≤ 0.05 and **P ≤ 0.01 versus control.
Rotenoisin A induced apoptosis in the MCF-7 cell line
In addition to changes in MAPK proteins, the changes in apoptotic proteins were significant. The collapsed mitochondria secrete cytochrome c into the cytoplasm, and this secreted ‘apoptotic cell death factor’ promotes caspase activity [32]. The activity of caspase-9 promoted by apoptosomes eventually leads to DNA repair defects due to PARP dysfunction [33, 34]. In this study, rotenoisin A not only increased cleaved caspase-9 but also increased cleaved PARP expression in a time-dependent manner following treatment (Fig. 6A). Furthermore, apoptosis was confirmed by Annexin/PI staining, which is a commonly employed approach for studying apoptotic cells. The total percentage of apoptosis in vehicle control cells was 12.09 ± 2.24%, which significantly changed to 33.75 ± 3.74% in 40 μM rotenoisin A and 32.74 ± 3.66% in 40 μM rotenone (Fig. 6B). Taken together, it was confirmed that cell damage caused by rotenoisin A resulted in cell death through the apoptosis pathway.
Fig. 6.
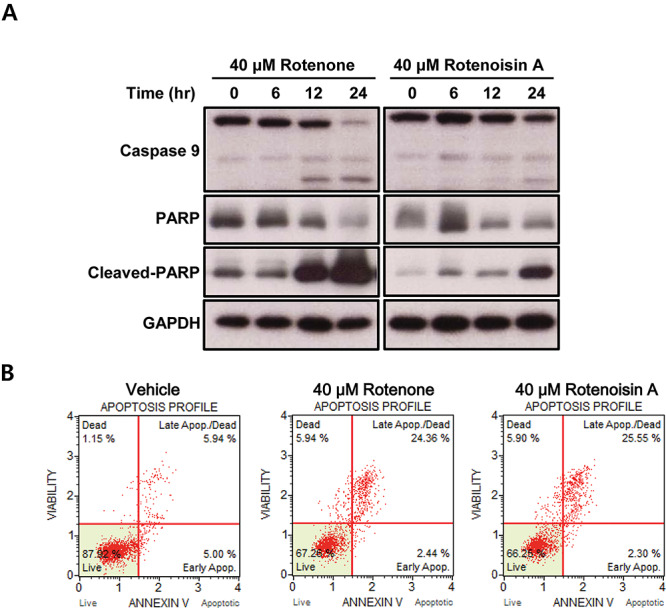
Rotenoisin A induced apoptotic cell death in MCF-7 cells. (A) Representative images of western blot analysis for the protein expression of caspase-9, PARP, cleaved PARP and GAPDH antibodies. (B) Representative images of annexin-V/propidium iodide (PI) measured using the Muse™ Cell Analyser.
DISCUSSION
Rotenone, the isoflavone, naturally protects plants against insects [35, 36]. It is produced from the leaves, seeds or stems of Mexican turnip (Pachyrhizus erosus) plants, or the roots of the Leguminosae family [37]. The insecticidal effect of rotenone has been used since it was observed that insects that ate the plants that produced it died. However, its commercial use has been increasingly discouraged or banned by several reports of concerns about the neurophysiological side effects of rotenone [38, 39].
However, no attempt has been made to utilize the rotenone-induced mitochondrial disruption in mammalian cells and take advantage of this property in human healthcare. In a previous report, we modified rotenone with ionizing radiation and confirmed the changes in chemical properties via nuclear magnetic resonance (NMR) analysis [15]. In this study, we observed the potential of rotenones as antiproliferative candidates for breast cancer. Rotenoisin A, the modified form induced by ionizing radiation, has shown not only similar efficiency in the inhibition of cell growth in MCF-7 cell lines but also decreased toxicity in HEKa cell lines, the normal human keratinocyte, compared with rotenone.
Ionizing radiation allows the introduction of energy into substances to cause favourable chemical changes [40, 41]. Sufficiently irradiated materials can decompose to yield daughter molecules or form new ones. This platform is an advanced technology to transform various materials usefully. Although technology using exposure to ionizing radiation to transform materials is not common, it is physically accurate and has excellent reproducibility. For example, our researchers have confirmed that the reproducibility of rotenoisin A production is consistent when various concentrations and volumes are exposed at a calculated exposure dose. This suggests that γ-irradiation is an economical and innovative way to produce rotenoisin A, a derivative of rotenone.
In this study, although we demonstrated the inhibitory effects of rotenoisin A in a breast cancer cell line, we did not use animal bearing models to identify the systemic efficiency and safety of rotenoisin A. Previous reports have shown that the toxicity of rotenone is less when it is exposed to the skin directly (absorption capacity of <10%) and occurs through ingestion, inhalation or intradermal delivery [42]. We demonstrated the toxicity of rotenone in human keratinocytes, which is counteracted by modification by ionizing radiation. In this context, we believe that rotenoisin A potentially plays a novel role in the inhibition of breast cancer with less toxicity in human organisms. We will continue to systematically explore this advantage using experimental animal studies.
In conclusion, rotenoisin A, a radiolytic rotenone derivative, showed decreased cytotoxicity compared with rotenone in HEKa cells and higher anticancer effects in MCF-7 cells. This modified reagent was able to inhibit cancer cell proliferation via oxidative stress, up-regulation of the MAPK pathway and ultimately apoptotic cell death. In this study, we suggest that the improved efficacy of rotenoisin A establishes it as an anticancer drug with low reversible effects. However, the mechanism underlying rotenoisin A function in complex organs, such as the human body, remains unclear and needs further investigation.
Competing interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
AUTHOR CONTRIBUTIONS
Hyoung-woo Bai: project administration, supervision. Chul-Hong Park and Byung Yeoup Chung: writing—original draft, writing—review and editing. Dong Ho Bak and Seong Hee Kang: methodology.
ACKNOWLEDGEMENTS
This work was supported by the Nuclear R&D Program of the Ministry of Science and ICT, Republic of Korea.
Contributor Information
Dong-ho Bak, Research Division for Biotechnology, Advanced Radiation Technology Institute (ARTI), Korea Atomic Energy Research Institute (KAERI), Jeongeup-si, Jeollabuk-do, Republic of Korea.
Seong Hee Kang, Research Division for Biotechnology, Advanced Radiation Technology Institute (ARTI), Korea Atomic Energy Research Institute (KAERI), Jeongeup-si, Jeollabuk-do, Republic of Korea.
Chul-hong Park, Research Division for Biotechnology, Advanced Radiation Technology Institute (ARTI), Korea Atomic Energy Research Institute (KAERI), Jeongeup-si, Jeollabuk-do, Republic of Korea.
Byung Yeoup Chung, Research Division for Biotechnology, Advanced Radiation Technology Institute (ARTI), Korea Atomic Energy Research Institute (KAERI), Jeongeup-si, Jeollabuk-do, Republic of Korea.
Hyoung-Woo Bai, Research Division for Biotechnology, Advanced Radiation Technology Institute (ARTI), Korea Atomic Energy Research Institute (KAERI), Jeongeup-si, Jeollabuk-do, Republic of Korea; Radiation Biotechnology and Applied Radioisotope Science, University of Science and Technology (UST), Daejeon 34113, Republic of Korea.
References
- 1. da Costa Araldi IC, Bordin FPR, Cadoná FC et al. The in vitro radiosensitizer potential of resveratrol on MCF-7 breast cancer cells. Chem Biol Interact 2018;282:85–92. [DOI] [PubMed] [Google Scholar]
- 2. Waks AG, Winer EP. Breast cancer treatment: a review. JAMA 2019;321:288–300. [DOI] [PubMed] [Google Scholar]
- 3. Kondov B, Milenkovikj Z, Kondov G et al. Presentation of the molecular subtypes of breast cancer detected by immunohistochemistry in surgically treated patients. Open Access Maced J Med Sci 2018;6:961–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fisusi FA, Akala EO. Drug combinations in breast cancer therapy. Pharm Nanotechnol 2019;7:3–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Moreno-Aspitia A, Perez EA. Treatment options for breast cancer resistant to anthracycline and taxane. Mayo Clin Proc 2009;84:533–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Parker PD, Heiney SP, Friedman DB et al. How are health literacy principles incorporated into breast cancer chemotherapy education? A review of the literature. J Nurs Educ Pract 2018;8:77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bagnyukova TV, Serebriiskii IG, Zhou Y et al. Chemotherapy and signaling: how can targeted therapies supercharge cytotoxic agents? Cancer Biol Ther 2010;10:839–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kitao H, Iimori M, Kataoka Y et al. DNA replication stress and cancer chemotherapy. Cancer Sci 2018;109:264–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Annovazzi L, Mellai M, Schiffer DJC. Chemotherapeutic drugs: DNA damage and repair in glioblastoma. Cancers (Basel) 2017;9:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jung HJ, Park HR, Jung U et al. Radiolysis study of genistein in methanolic solution. Radiat Phys Chem 2009;78:386–93. [Google Scholar]
- 11. Huo Z, Wang S, Shao H et al. Radiolytic degradation of anticancer drug capecitabine in aqueous solution: kinetics, reaction mechanism, and toxicity evaluation. Environ Sci Pollut Res 2020;27:20807–16. [DOI] [PubMed] [Google Scholar]
- 12. Lee E-H, Park CH, Choi HJ et al. Dexamethasone modified by gamma-irradiation as a novel anticancer drug in human non-small cell lung cancer. PLoS One 2018;13:e0194341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Deng YT, Huang HC, Lin JK. Rotenone induces apoptosis in MCF-7 human breast cancer cell-mediated ROS through JNK and p38 signaling. Mol Carcinog 2010;49:141–51. [DOI] [PubMed] [Google Scholar]
- 14. Wrangel C, Schwabe K, John N et al. The rotenone-induced rat model of Parkinson's disease: behavioral and electrophysiological findings. Behav Brain Res 2015;279:52–61. [DOI] [PubMed] [Google Scholar]
- 15. Park C-H, Chung BY, Lee SS et al. Radiolytic transformation of rotenone with potential anti-adipogenic activity. Bioorg Med Chem Lett 2013;23:1099–103. [DOI] [PubMed] [Google Scholar]
- 16. Kim J-J, Lee H-M, Shin D-M et al. Host cell autophagy activated by antibiotics is required for their effective antimycobacterial drug action. Cell Host Microbe 2012;11:457–68. [DOI] [PubMed] [Google Scholar]
- 17. Bisbal M, Sanchez M. Neurotoxicity of the pesticide rotenone on neuronal polarization: a mechanistic approach. Neural Regen Res 2019;14:762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tanner CM, Kamel F, Ross GW et al. Rotenone, paraquat, and Parkinson’s disease. Environ Health Perspect 2011;119:866–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Radad K, Rausch W-D, Gille G. Rotenone induces cell death in primary dopaminergic culture by increasing ROS production and inhibiting mitochondrial respiration. Neurochem Int 2006;49:379–86. [DOI] [PubMed] [Google Scholar]
- 20. Li N, Ragheb K, Lawler G et al. Mitochondrial complex I inhibitor rotenone induces apoptosis through enhancing mitochondrial reactive oxygen species production. J Biol Chem 2003;278:8516–25. [DOI] [PubMed] [Google Scholar]
- 21. Moon Y, Lee KH, Park JH et al. Mitochondrial membrane depolarization and the selective death of dopaminergic neurons by rotenone: protective effect of coenzyme Q10. J Neurochem 2005;93:1199–208. [DOI] [PubMed] [Google Scholar]
- 22. Deng YT, Huang HC, Lin JK. Rotenone induces apoptosis in MCF-7 human breast cancer cell-mediated ROS through JNK and p38 signaling. Mol Carcinog 2010;49:141–51. [DOI] [PubMed] [Google Scholar]
- 23. Ola MS, Nawaz M, Ahsan H. Role of Bcl-2 family proteins and caspases in the regulation of apoptosis. Mol Cell Biochem 2011;351:41–58. [DOI] [PubMed] [Google Scholar]
- 24. Finucane DM, Bossy-Wetzel E, Waterhouse NJ et al. Bax-induced caspase activation and apoptosis via cytochromec release from mitochondria is inhibitable by Bcl-xL. J Biol Chem 1999;274:2225–33. [DOI] [PubMed] [Google Scholar]
- 25. Wolter KG, Hsu Y-T, Smith CL et al. Movement of Bax from the cytosol to mitochondria during apoptosis. J Cell Biol 1997;139:1281–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Murphy K, Ranganathan V, Farnsworth M et al. Bcl-2 inhibits Bax translocation from cytosol to mitochondria during drug-induced apoptosis of human tumor cells. Cell Death Differ 2000;7:102–11. [DOI] [PubMed] [Google Scholar]
- 27. Korsmeyer SJ, Shutter JR, Veis DJ, et al. Bcl-2/Bax: a rheostat that regulates an anti-oxidant pathway and cell death. Semin Cancer Biol 1993;4:327–32. [PubMed]
- 28. Leung LK, Wang TT. Differential effects of chemotherapeutic agents on the Bcl-2/Bax apoptosis pathway in human breast cancer cell line MCF-7. Breast Cancer Res Treat 1999;55:73–83. [DOI] [PubMed] [Google Scholar]
- 29. Sui X, Kong N, Ye L et al. p38 and JNK MAPK pathways control the balance of apoptosis and autophagy in response to chemotherapeutic agents. Cancer Lett 2014;344:174–9. [DOI] [PubMed] [Google Scholar]
- 30. Ghatan S, Larner S, Kinoshita Y et al. p38 MAP kinase mediates BAX translocation in nitric oxide-induced apoptosis in neurons. J Cell Biol 2000;150:335–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kang Y-H, Lee S-J. Role of p38 MAPK and JNK in enhanced cervical cancer cell killing by the combination of arsenic trioxide and ionizing radiation. Oncol Rep 2008;20:637–43. [PubMed] [Google Scholar]
- 32. Jiang X, Wang X. Cytochrome c promotes caspase-9 activation by inducing nucleotide binding to Apaf-1. J Biol Chem 2000;275:31199–203. [DOI] [PubMed] [Google Scholar]
- 33. Cui Q, Yu J-h, Wu J-n et al. P53-mediated cell cycle arrest and apoptosis through a caspase-3-independent, but caspase-9-dependent pathway in oridonin-treated MCF-7 human breast cancer cells. Acta Pharmacol Sin 2007;28:1057–66. [DOI] [PubMed] [Google Scholar]
- 34. Tretiakova I, Blaesius D, Maxia L et al. Myrtucommulone from Myrtus communis induces apoptosis in cancer cells via the mitochondrial pathway involving caspase-9. Apoptosis 2008;13:119–31. [DOI] [PubMed] [Google Scholar]
- 35. Haller H, Goodhue L, Jones HA. The constituents of derris and other rotenone-bearing plants. Chem Rev 1942;30:33–48. [Google Scholar]
- 36. Booth A, Moss S, Weyl O Effect of rotenone on gill-respiring and plastron-respiring insects. African Journal of Aquatic Science 2015;40:95–100. [Google Scholar]
- 37. McIndoo NE. A Review of the Insecticidal Uses of the Rotenone-bearing Plants, 1938–1944. United States Department of Agriculture, Agricultural Research Administration, Bureau of Entomology and Plant Quarantine, 1947. [Google Scholar]
- 38. Margabandhu G, Vanisree AJ. Dopamine, a key factor of mitochondrial damage and neuronal toxicity on rotenone exposure and also parkinsonic motor dysfunction—impact of asiaticoside with a probable vesicular involvement. J Chem Neuroanat 2020;106:101788. [DOI] [PubMed] [Google Scholar]
- 39. Subaraja M, Vanisree AJ. Rotenone causing dysfunctional mitochondria and lysosomes in cerebral ganglions of Lumbricus terrestris degenerate giant fibers and neuromuscular junctions. Chemosphere 2016;152:468–80. [DOI] [PubMed] [Google Scholar]
- 40. Kim TH, Kim JK, Ito H et al. Enhancement of pancreatic lipase inhibitory activity of curcumin by radiolytic transformation. Bioorg Med Chem Lett 2011;21:1512–4. [DOI] [PubMed] [Google Scholar]
- 41. Badaboina S, Bai H-W, Na YH et al. Novel radiolytic rotenone derivative, rotenoisin B with potent anti-carcinogenic activity in hepatic cancer cells. Int J Mol Sci 2015;16:16806–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lawana V, Cannon JR. Rotenone neurotoxicity: relevance to Parkinson's disease. Adv Neurotoxicol 2020;4:209–54. [Google Scholar]


