ABSTRACT
Background
A processed diet, high in fat and low in fiber, is associated with differences in the gut microbiota and adverse health outcomes in humans; however, little is known about the diet–microbiota relation and its impact on pregnancy. Spontaneous preterm birth (SPTB), a pregnancy outcome with serious short- and long-term consequences, occurs more frequently in black and in obese women in the United States.
Objectives
In a prospective, case-control sample matched for race and obesity (cases = 16, controls = 32), we compared the fecal gut microbiota, fecal and plasma metabolites, and diet in the late second trimester. We hypothesized that a Western diet would be associated with reduced microbiota richness and a metabolic signature predicting incidence of SPTB.
Methods
The fecal microbiota was characterized by 16S-tagged sequencing and untargeted metabolomics was used to analyze both plasma and fecal metabolites. Wilcoxon's rank-sum test was used for the comparison of microbiota genera, α-diversity, fecal and plasma metabolites, and dietary variables between term and SPTB. β-Diversity was analyzed using permutational multivariate ANOVA, and metabolite associations were assessed by module analysis.
Results
A decrease in α-diversity was strongly associated with the development of SPTB, especially in the taxonomic class of Betaproteobacteria. Of 824 fecal metabolites, 22 metabolites (mostly lipids) differed between cases and controls (P < 0.01), with greater DHA (22:6n–3) and EPA (20:5n–3) in cases [false discovery rate (FDR) < 0.2]. The most significant fecal metabolite module (FDR-adjusted P = 0.008) was dominated by DHA and EPA. Dietary saturated fat (primarily palmitate) intake was greater in cases (31.38 ± 7.37 compared with 26.08 ± 8.62 g, P = 0.045) and was positively correlated with fecal DHA and EPA (P < 0.05).
Conclusions
Reduced α-diversity of the gut microbiota and higher excretion of omega-3 (n–3) fatty acids in stool may provide a novel biomarker signature predicting SPTB in women with a low-fiber, high-fat diet. Further investigation of these markers in a larger sample is needed for validation.
Keywords: microbiome, metabolome, pregnancy, Western diet, precision nutrition
Introduction
Preterm birth (PTB) occurs before the 37th week of gestation and is one of the leading causes of neonatal morbidity and mortality. Evidence suggests that PTB is a multifactorial syndrome potentially mediated by infection, oxidative stress, inflammation, and maternal perinatal nutrition (1, 2); however, the relation is unclear and the biology of PTB remains poorly understood. Whereas the incidence of infants delivered before 37 weeks of gestation had been on the decline from 2004 to 2013, the percentage of PTB deliveries has recently increased dramatically to 10% as of 2018, with especially high percentages among black women (14%) (3). Obese women are also at high risk of PTB (4). This is particularly concerning because PTB is responsible for nearly one-third of neonatal deaths in the United States (5), and infants born preterm may have serious long-term health conditions due to their immaturity (6), including respiratory distress syndrome, necrotizing enterocolitis, metabolic disease, and the potential for lifelong complications. Shockingly, rates of PTB in the United States are considerably greater than in other similarly developed societies, such as the Nordic countries which have a PTB rate of only 6% (7). Within this context, the identification of biomarkers that predict PTB would be of considerable utility, especially if they provide new insights into pathogenesis, which could lead to novel approaches to reduce risk.
There has been increasing interest in the gut microbiota and its association with health and disease in human populations. Studies in animal models suggest that there may be a cause-and-effect relation between the bacteria within the gastrointestinal tract and human health (8–11). Many studies demonstrate that an alteration in the composition of the human gut microbiota, its genome (referred to as the “metagenome”), and its metabolites (the “metabolome”) are associated with disease states; however, there are fewer studies suggesting that these features can be used as a biomarker to predict the future development of disease. Analyses of samples matched to clinical outcomes have suggested that the gut microbiome might have predictive value in the treatment of inflammatory bowel disease (11) or in the prevention of cardiovascular disease (9), type 2 diabetes (8, 10), and metabolic syndrome (10). However, prospective studies are needed to validate the predictive value of these biomarkers. In metabolic syndrome, the most consistent feature associated with a disease state was decreased gut microbial richness; less microbial richness is associated with diet—specifically lower fiber and higher fat intake—and residence in an industrialized nation (10). There has been much interest in the impact of diet on the gut microbiota where effect sizes seem to be personalized and relatively modest (12, 13). Nevertheless, the high-dimensional nature of microbiota features may be helpful in the development of personalized dietary interventions to prevent and/or treat disease (14).
Using a case-control sample from a prospective cohort of pregnant women, we sought to determine whether, in the context of a high-fat, low-fiber diet, maternal fecal microbial diversity and metabolome during the second trimester contained features associated with future development of spontaneous PTB (SPTB). We hypothesized that a processed, high-fat, low-fiber diet would be associated with alterations in the gut microbiota, fecal metabolome, and plasma metabolome that could provide a metabolomic signature of eventual SPTB.
Methods
Sample
In accordance with an Institutional Review Board–approved protocol, informed consent was obtained from 301 primiparous women who were enrolled into the study at 20–26 weeks of gestation. The study participants received prenatal care from 1 of 2 hospital-affiliated obstetric practices, delivered at the same hospital, and remained enrolled in this observational study until after the delivery of their infant. The key exclusion criteria were women with multiple gestation, fetal chromosomal abnormality or major fetal anomaly, intrauterine fetal demise, maternal HIV, chronic kidney disease, transplantation, history of weight loss surgery, prior completed pregnancy, vegan diet, and chronic use of immunosuppressive medications or steroids.
The study design included a cross-sectional survey of diet, the fecal microbiome, and the fecal and plasma metabolome at 20–26 weeks of gestation, followed by prospective observation for the primary clinical outcome of spontaneous preterm compared with term delivery. The diagnosis of SPTB was obtained from medical record review, using American College of Obstetricians and Gynecologists definitions (15) and confirmed by a perinatologist. Gestational age at delivery was determined by best obstetric dating based on last menstrual period and confirmed by prenatal ultrasound.
An embedded case-control study (Supplemental Figure 1) was constructed by matching all women who underwent SPTB (cases, n = 16) to 2 women who delivered at term without complications such as gestational diabetes, gestational hypertension, or pre-eclampsia (controls, n = 32). Because of the recognized greater risk of PTB in black and obese women, cases were matched to controls by race (black/nonblack) and prepregnancy BMI group (obese/nonobese). Racial-ethnic identity was determined by self-report. Owing to the small number of women who identified as Asian or “other,” they were included with Caucasians to form a nonblack category for comparison with women who self-identified as black. Prepregnancy BMI was calculated from height and measured weight at the initial prenatal visit (in kg/m2). According to CDC guidelines (16), BMI ≥ 30 was used to categorize obesity. Gestational weight gain was calculated as the maternal weight at the last prenatal visit before delivery minus the weight at the first prenatal visit.
Three 24-h dietary recalls were obtained during weeks 20–26 of gestation via unannounced telephone calls within a 10-d interval before collection of the stool sample. A trained research nutritionist administered the recalls using the validated multipass method supported by Nutrition Data System for Research software (University of Minnesota Nutrition Coordinating Center) (17). Oral prenatal vitamin content was not included in the food recalls, but all patients reported taking these supplements. Food recall data were cleaned for implausible energy intake when energy intake was <600 or >3500 kcal/d (18). This excluded diet data in 1 case, where the energy intake was reported as 4801 kcal/d, 76 kcal · kg body weight−1 · d−1.
After the third dietary recall was obtained, the study parti-cipants collected their next stool sample and shipped it to the laboratory. During the following obstetric clinic visit, a sample of ≤50 mL blood was drawn and processed to obtain plasma. Plasma and fecal samples were stored at −80°C until further analysis. The study protocol did not allow missing data. Study participants were then monitored prospectively for the timing of their delivery. Of the original 301 enrolled subjects, 4 delivered their infants in a different hospital that did not permit exact tracking of birth timing, and these subjects were excluded from the analyses.
The stool samples were assayed for gut microbial identity using 16S tagged gene sequencing processed by Quantitative Insights Into Microbial Ecology (QIIME) 2, including de-multiplexing, quality control, amplicon sequence variant identification, and taxonomic assignment. The maternal plasma and fecal water samples were assayed for untargeted metabolomics by Metabolon (Metabolon, Inc.).
Statistical and bioinformatics analysis
16S tagged sequencing data analysis
The relative abundance per sample was summarized at the genus level. Richness, Shannon indexes, and Menhinick indexes were calculated to represent the microbiome α-diversity. The weighted (proportional abundance) and unweighted (presence-absence) Unifrac distances were calculated to represent the microbiome β-diversity. R package “vegan” was used for α- and β-diversity calculation. Two control samples had very low read counts and were removed in the analyses that were related to microbiome data. The mean number of reads per sample (± SD) that could be assigned to the genus level was 30,409.71 ± 9733.2.
Differential abundance analysis
Wilcoxon's rank-sum test was used for the comparison of microbiome genera, α-diversity, and stool and plasma metabolites between term and SPTB. Differences in dietary intake variables were compared between term and SPTB cases using a 2-tailed t test with unequal variance. Permutational multivariate analysis of variance (PERMANOVA) was applied for the comparison of β-diversity between term and SPTB. A false discovery rate (FDR) of 0.20 was used to select the bacterial genera or metabolites that were associated with SPTB. All analyses were performed using R version 3.5.1 (R Foundation). For our given sample size, we had a power of 80% to detect an effect size (as measured by Cohen's d) of 0.88 at an α level of 0.05 for the primary outcome of microbial diversity. For other secondary outcomes, owing to multiple comparison adjustments, only associations with a very large effect size could be detected.
Weighted Gene Co-expression Network Analysis metabolite module
For the stool metabolites, Weighted Gene Co-expression Network Analysis (WGCNA) (19) was applied to 1) identify functional modules of stool metabolites based on pairwise correlations, 2) correlate the modules with SPTB based on principal component analysis, and 3) find the most important metabolites within the module of interest. Such a network analysis accounts for high correlations among certain metabolites, provides effective dimension reduction, and leads to more biologically interpretable results. By using WGCNA, the correlations raised to a certain power (adjacencies) were calculated between all pairs of metabolites, so that the degree distribution would fit a scale-free model. The adjacencies were then transformed into topological overlap measures (TOMs) and TOM-based distance matrices. Finally, a dynamic branch-cutting method was applied to detect clusters (modules) of metabolites depending on the shape of the clustering tree. Pearson's correlation was calculated between each module (represented by the first principal component) and SPTB. The important metabolites in each module were defined as metabolites with high metabolite significance (represented by absolute Pearson's correlation with SPTB > 0.4) and high intramodular connectivity (represented by absolute Pearson's correlation with the first principal component of the module > 0.8). R package WGCNA was used for the analysis.
Results
Baseline characteristics
From a prospective cohort observation of 301 pregnant women, 16 women delivered spontaneously earlier than 37 weeks of gestation (Supplemental Figure 1). Four women were considered to have early SPTB (24–30 wk) and 12 were categorized as late SPTB (31–36 wk). All cases, including 8 black and 8 nonblack women (6 white and 2 Asian), were matched with 2 controls of the same self-reported race and obesity status (18 obese and 30 nonobese women). As expected, owing to the preterm delivery, gestational weight gain and gestational age at delivery were lower in cases, but gestational weight gain per week was similar for both groups (Table 1).
TABLE 1.
Baseline demographic and delivery characteristics of cases who developed spontaneous preterm birth and controls1
| Variable | Cases (n = 16) | Controls (n = 32) | P value |
|---|---|---|---|
| Baseline demographic characteristics | |||
| Age, y | 28.88 ± 4.92 | 28.38 ± 5.77 | 0.756 |
| Black | 9 (56.25) | 18 (56.25) | 1.00 |
| White | 5 (31.25) | 10 (31.25) | |
| Asian | 2 (12.5) | 4 (12.5) | |
| Private insurance | 10 (62.5) | 23 (71.9) | 0.527 |
| Medicaid | 6 (37.5) | 9 (28.1) | |
| First clinic BMI, kg/m2 | 27.93 ± 6.32 | 27.82 ± 5.76 | 0.957 |
| Normal or underweight | 4 (25.00) | 17 (53.13) | 0.149 |
| Overweight | 6 (37.51) | 3 (9.38) | |
| Obese | 6 (37.51) | 12 (37.50) | |
| Antibiotics within 8 wk before enrollment | 2 (12.5) | 3 (9.4) | 0.738 |
| Smoking within 8 wk before enrollment | 1 (6.3) | 0 (0) | 0.153 |
| Assisted reproduction therapy | 1 (6.3) | 3 (9.4) | 0.712 |
| First-trimester vaginal bleeding | 2 (12.5) | 4 (12.5) | 1.00 |
| Second-trimester vaginal bleeding | 1 (6.3) | 0 (0) | 0.153 |
| Delivery characteristics | |||
| Gestational weight gain, kg | 8.89 ± 5.44 | 13.29 ± 6.19 | 0.017 |
| Gestational weight gain, kg/gestational age | 0.265 ± 0.147 | 0.339 ± 0.157 | 0.122 |
| Gestational age at delivery, wk | 33.13 ± 3.77 | 39.44 ± 1.22 | <0.0001 |
1Values are mean ± SD for continuous variables and the P value is based on the t test; values are n (%) for categorical variables and the P value is based on the chi-square test.
α-Diversity of fecal microbiota was reduced in women with SPTB
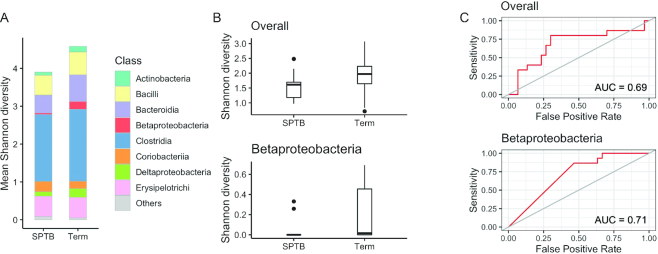
In order to determine the difference of diversity at the genus level in the fecal microbiota, 3 measures of α-diversity were evaluated: 1) richness, the total number of different genera in a sample; 2) Shannon diversity, which accounts for the proportion (evenness) of each genus in a sample; and 3) Menhinick's index, the number of genera divided by the square root of the total number of individuals. The 3 α-diversity indexes were all significantly reduced in fecal microbiota collected between 20 and 26 weeks of gestation from women who ultimately had SPTB (Figure 1A, Shannon diversity P = 0.043; Figure 1B, richness P = 0.009; Figure 1C, Menhinick's index P = 0.014). To determine the taxonomic features responsible for this difference, Shannon diversity was calculated for each bacterial class (Figure 2A). The bacterial class of Betaproteobacteria was identified as showing a significant reduction in Shannon diversity in SPTB cases (Wilcoxon's rank-sum test, FDR < 0.2) (Figure 2B), where the AUC on logistic regression analysis was 0.71 (Figure 2C). No specific genera in Betaproteobacteria showed a statistically significant difference in relative abundance in cases compared with controls (Wilcoxon's rank-sum at FDR = 0.2) (Supplemental Figure 2).
FIGURE 1.
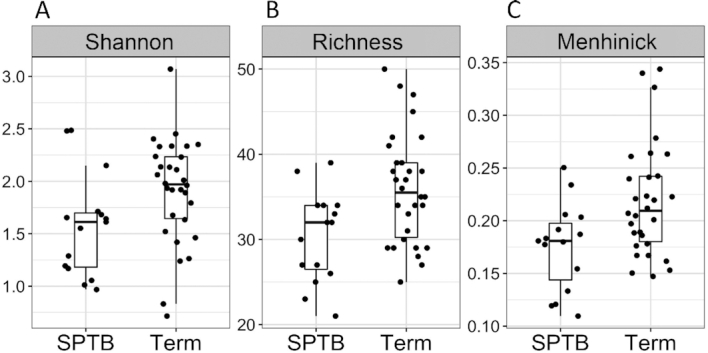
Shannon diversity and richness of the gut microbiota at 20–26 weeks of gestation in women who spontaneously delivered preterm (n = 16) relative to those with term deliveries (n = 32). (A) Shannon diversity (Wilcoxon's rank-sum test, P = 0.043). (B) Microbiota richness (Wilcoxon's rank-sum test, P = 0.009). (C) Menhinick's index (Wilcoxon's rank-sum test, P = 0.014). In each plot the bar is the median value, and the whiskers indicate the 25% and 75% distribution. SPTB, spontaneous preterm birth.
FIGURE 2.
Shannon diversity of bacterial classes at 20–26 weeks of gestation in women who spontaneously delivered preterm (n = 16) relative to those with term deliveries (n = 32). (A) The composition of mean Shannon diversity by bacterial class. (B) Shannon diversity of Betaproteobacteria in SPTB compared with term (Wilcoxon's rank-sum test, false discovery rate < 0.2). In each plot the bar is the median value, and the whiskers indicate the 25% and 75% distribution. (C) AUC in a receiver operating characteristic analysis using a logistic regression model to predict SPTB with the contribution of Shannon diversity of Betaproteobacteria (AUC = 0.71) and of the overall microbiota (AUC = 0.69). SPTB, spontaneous preterm birth.
Owing to the small sample size, to test for predictive performance using within-class diversity, we performed leave-one-out cross-validation analysis. The resulting AUC of Betaproteobacteria was 0.60 (Supplemental Figure 3).
β-Diversity of the fecal microbiome differed in mothers who had SPTB compared with full-term birth
Both weighted and unweighted Unifrac distance were significantly different between cases and controls (PERMANOVA, p=0.034 for weighted analysis, Figure 3A; p=0.013 for unweighted analysis, Figure 3B). We were unable to identify any statistically significant discriminatory taxa based on the relative abundances at the genus level (106 genera tested) at an FDR of 0.20.
FIGURE 3.
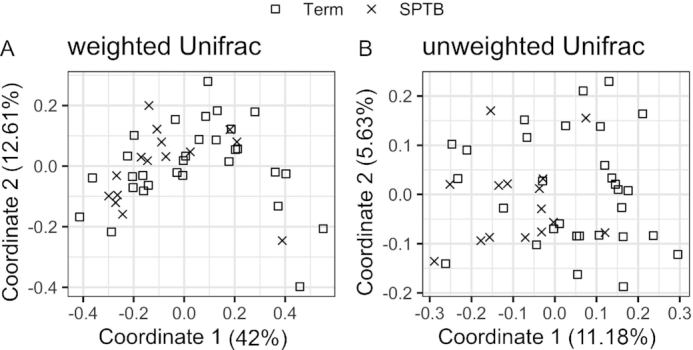
Principal components analysis plot of β-diversity between women who later experienced SPTB (n = 16) and term controls (n = 32). (A) The phylogenetic distance of proportional abundance (weighted Unifrac, P = 0.067) and (B) presence-absence (unweighted Unifrac, P = 0.013), analyzed by permutational multivariate ANOVA in SPTB and term. SPTB, spontaneous preterm birth.
SPTB was associated with an increase in 2 lipid metabolites in the fecal metabolome
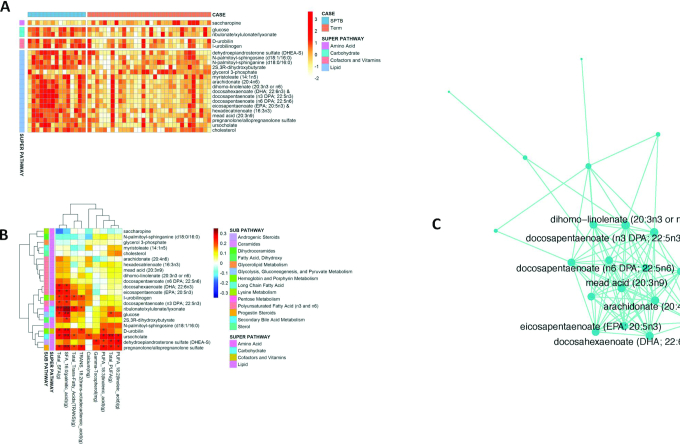
To identify additional analytic features associated with SPTB, we performed untargeted metabolomics analysis on maternal fecal and plasma samples collected at the prenatal visit. Out of 824 fecal metabolites, 22 were different in abundance between cases and controls (P < 0.01, Wilcoxon's rank-sum test). The majority of fecal metabolite differences were found among fatty acids and cholesterol hormone metabolites (Figure 4A). After correction for multiple comparisons (FDR < 0.20), both DHA (22:6n–3) and EPA (20:5n–3) were increased in the fecal metabolites of women with SPTB (Figure 4A).
FIGURE 4.
Untargeted fecal metabolites in cases and controls. (A) Of 824 fecal metabolites, 22 were differentially abundant in women with SPTB compared with term deliveries (P < 0.01 level and & = FDR < 0.2). (B) Heatmap comparing partial Kendall correlations between differentially abundant dietary micronutrients in columns and fecal metabolites in rows. *,**Significant correlation: *P < 0.05; **P < 0.01. (C) The most significantly associated module, built around ω-3 fatty acid metabolites, identified by Weighted Gene Co-expression Network Analysis of fecal metabolites (FDR = 0.008). Individual metabolites are represented by nodes, and are connected by edges whose topological overlap is above the threshold of 0.05. The centrality of a node (the number of adjacent edges) is represented by the node size. FDR, false discovery rate; SPTB, spontaneous preterm birth.
Similarly, out of 825 plasma metabolites, 4 [β-citrylglutamate, propionylglycine, caproic acid (6:0), and 14-hydroxydocosahexanoic acid (HDoHE)/17-HDoHE] were different in abundance (P ≤ 0.01), although none were significant after correction for multiple comparisons (Supplemental Table 1).
Cases with SPTB had greater intake of dietary fat—both saturated and polyunsaturated—than women who delivered at term
Given the previously described associations between dietary intake and the gut microbiota (12, 13, 20), we used the mean of three 24-h dietary recalls administered before collection of the stool sample to characterize the average diet of cases and controls (Table 2). Women with SPTB had greater intake of dietary fat (90.2 g compared with 75.2 g, P = 0.028) with a trend toward a higher percentage of calories from fat (37.4% compared with 34.6%, P = 0.075); however, both groups were close to the upper limit of the reference range for dietary fat intake based on the American Heart Association recommendations (20%–35% of calories from fat). The intake of saturated fat was higher in cases (31.4 g compared with 26.1 g, P = 0.045), predominantly palmitic acid (16:0) (16.4 g compared with 13.7 g, P = 0.044). There was no difference in intake of monounsaturated fat. PUFA intake was greater in women with SPTB (22.2 g compared with 16.5 g, P = 0.018; 9% compared with 8% kcal, P = 0.047). Both omega (ω)-3 fatty acids (2.7 g compared with 1.9 g, P = 0.014) and ω-6 fatty acids (21.7 g compared with 16.3 g, P = 0.05) were higher in mothers with SPTB. Both groups had a ratio of ω-6 to ω-3 higher than the recommended ratio of <4:1, which is consistent with the overall poor quality of diet in this cohort of women (8.3:1 for cases compared with 9.1:1 for controls, P = 0.39). All women reported taking prenatal vitamins that included 200 mg DHA. However, both groups had <0.2 g/d of the beneficial long-chain ω-3 fatty acids, DHA and EPA, from their diet. In contrast, both cases and controls met the minimum DRI (1.4 g/d during pregnancy) of the precursor ω-3 fatty acid found in seed oils, α-linolenic acid (18:3n–3), with cases ingesting significantly more α-linolenic acid than controls (2.3 g compared with 1.7 g, P = 0.009). Intakes of ω-6 linoleic acid (18:2n–6) (19.1 g compared with 14.3 g, P = 0.024) and trans-octadecadienoic acid (18:2n–6) (0.45 g compared with 0.33 g, P = 0.015) were also higher in cases; these fatty acids are predominantly found in processed vegetable and seed oils. Consistent with higher intake of vegetable and seed oils, cases also had higher amounts of γ-tocopherol (17.0 mg compared with 12.0 mg, P = 0.018) and a trend toward higher amounts of Δ-tocopherol (4.2 mg compared with 2.9 mg, P = 0.052) (21).
TABLE 2.
Mean daily dietary intake of selected nutrients in cases and controls1
| Variable | Cases (n = 15) | Controls (n = 32) | P value |
|---|---|---|---|
| Energy, kcal | 2163.02 ± 322.68 | 1943.84 ± 413.35 | 0.077 |
| Energy, kcal/kg weight | 30.16 ± 8.78 | 27.97 ± 9.54 | 0.456 |
| Protein, g | 87.52 ± 19.11 | 84.58 ± 26.16 | 0.699 |
| Protein, % kcal | 16.33 ± 3.56 | 17.41 ± 4.07 | 0.384 |
| Protein, g/kg weight | 1.19 ± 0.43 | 1.17 ± 0.41 | 0.849 |
| Carbohydrate, g | 258.07 ± 53.96 | 239.97 ± 58.46 | 0.317 |
| Carbohydrate, % kcal | 47.64 ± 5.83 | 49.65 ± 7.84 | 0.382 |
| Total fiber, g | 18.43 ± 6.27 | 18.64 ± 8.76 | 0.934 |
| Soluble fiber, g | 5.45 ± 1.58 | 5.33 ± 1.65 | 0.814 |
| Insoluble fiber, g | 12.89 ± 5.04 | 13.23 ± 7.47 | 0.875 |
| Fat, g | 90.17 ± 19.52 | 75.19 ± 21.68 | 0.028 |
| Fat, % kcal | 37.43 ± 5.32 | 34.55 ± 4.92 | 0.075 |
| Saturated fat, g | 31.38 ± 7.37 | 26.08 ± 8.62 | 0.045 |
| Saturated fat, % kcal | 13.01 ± 1.97 | 12.0 ± 2.82 | 0.218 |
| Cholesterol, mg | 339.26 ± 166.25 | 344.05 ± 162.99 | 0.926 |
| Trans fat, g | 2.57 ± 0.91 | 1.99 ± 1.05 | 0.071 |
| Trans fat, % kcal | 1.07 ± 0.34 | 0.893 ± 0.34 | 0.144 |
| Monounsaturated fats, g | 29.42 ± 7.17 | 26.26 ± 7.57 | 0.183 |
| Monounsaturated fats, % kcal | 12.24 ± 2.52 | 12.07 ± 1.94 | 0.800 |
| Polyunsaturated fats, g | 22.15 ± 9.17 | 16.52 ± 6.35 | 0.018 |
| Polyunsaturated fats, % kcal | 9.16 ± 3.38 | 7.56 ± 1.96 | 0.047 |
| ω-3 Fatty acids, g | 2.65 ± 1.05 | 1.89 ± 0.89 | 0.014 |
| ω-6 Fatty acids, g | 21.72 ± 9.16 | 16.25 ± 6.18 | 0.050 |
| ω-6:ω-3 | 8.25 ± 2.06 | 9.14 ± 2.44 | 0.389 |
| β-Carotene, retinol equivalents | 3631.33 ± 3103.16 | 4058.27 ± 4551.32 | 0.744 |
| Retinol, μg | 509.76 ± 237.79 | 525.90 ± 324.09 | 0.864 |
| Vitamin D, μg | 7.46 ± 8.44 | 6.02 ± 4.68 | 0.457 |
| β-Tocopherol, mg | 0.48 ± 0.22 | 0.39 ± 0.16 | 0.116 |
| γ-Tocopherol, mg | 16.95 ± 7.87 | 11.97 ± 5.74 | 0.018 |
| Δ-Tocopherol, mg | 4.16 ± 2.45 | 2.88 ± 1.85 | 0.052 |
| Vitamin K, μg | 193.17 ± 145.54 | 143.52 ± 94.12 | 0.166 |
| Solid fats, g | 40.60 ± 11.69 | 33.37 ± 12.92 | 0.072 |
| Sodium, mg | 3320.89 ± 699.61 | 3047.50 ± 811.09 | 0.257 |
| Calcium, mg | 1197.62 ± 211.19 | 1001.51 ± 346.85 | 0.049 |
| Magnesium, mg | 314.36 ± 96.39 | 295.34 ± 95.12 | 0.528 |
| Iron, mg | 15.43 ± 3.27 | 16.44 ± 6.39 | 0.569 |
| SFA 4:0 butyric acid, g | 0.95 ± 0.44 | 0.74 ± 0.34 | 0.083 |
| SFA 6:0 caproic acid, g | 0.52 ± 0.27 | 0.42 ± 0.21 | 0.170 |
| SFA 8:0 caprylic acid, g | 0.42 ± 0.22 | 0.32 ± 0.29 | 0.062 |
| SFA 10:0 capric acid, g | 0.78 ± 0.34 | 0.65 ± 0.29 | 0.182 |
| SFA 12:0 lauric acid, g | 1.13 ± 0.59 | 1.05 ± 0.67 | 0.698 |
| SFA 14:0 myristic acid, g | 3.21 ± 1.19 | 2.58 ± 1.04 | 0.075 |
| SFA 16:0 palmitic acid, g | 16.38 ± 3.51 | 13.72 ± 4.32 | 0.044 |
| SFA 17:0 margaric acid, g | 0.16 ± 0.08 | 0.15 ± 0.13 | 0.781 |
| SFA 18:0 stearic acid, g | 6.87 ± 0.08 | 5.73 ± 1.99 | 0.157 |
| SFA 20:0 arachidic acid, g | 0.18 ± 0.07 | 0.14 ± 0.07 | 0.055 |
| SFA 22:0 behenic acid, g | 0.17 ± 0.12 | 0.12 ± 0.13 | 0.189 |
| MUFA 14:1 myristoleic acid, g | 0.11 ± 0.07 | 0.10 ± 0.09 | 0.627 |
| MUFA 16:1 palmitoleic acid, g | 1.32 ± 0.35 | 0.20 ± 0.48 | 0.411 |
| MUFA 18:1 oleic acid, g | 27.39 ± 6.83 | 24.26 ± 6.99 | 0.157 |
| MUFA 20:1 gadoleic acid, g | 0.36 ± 0.31 | 0.34 ± 0.34 | 0.805 |
| MUFA 22:1 erucic acid, g | 0.02 ± 0.03 | 0.01 ± 0.02 | 0.685 |
| PUFA 18:2 linoleic acid, g | 19.12 ± 8.42 | 14.30 ± 5.52 | 0.024 |
| PUFA 18:3 linolenic acid, g | 2.34 ± 0.87 | 1.73 ± 0.65 | 0.009 |
| PUFA 18:4 parinaric acid, g | 0.02 ± 0.04 | 0.01 ± 0.03 | 0.426 |
| PUFA 20:4 arachidonic acid, g | 0.25 ± 0.31 | 0.21 ± 0.23 | 0.634 |
| PUFA 20:5 EPA, g | 0.10 ± 0.17 | 0.04 ± 0.12 | 0.173 |
| PUFA 22:5 docosapentaenoic acid, g | 0.06 ± 0.10 | 0.04 ± 0.07 | 0.472 |
| PUFA 22:6 DHA, g | 0.18 ± 0.34 | 0.11 ± 0.25 | 0.370 |
| Trans 18:1 Trans octadecenoic acid, g | 2.01 ± 0.77 | 1.57 ± 0.91 | 0.113 |
| Trans 18:2 Trans octadecadienoic acid, g | 0.45 ± 0.16 | 0.33 ± 0.15 | 0.015 |
| Trans 16:1 Trans hexadecenoic acid, g | 0.06 ± 0.03 | 0.05 ± 0.03 | 0.277 |
1Values are mean ± SD or unpaired t test P values.
We used partial Kendall correlation to examine the associations between the statistically significant dietary intake variables and fecal metabolites, which can be visualized in a heatmap (Figure 4B). Total saturated fat intake, the majority of which was palmitic acid, was positively associated with fecal metabolite concentrations of DHA, EPA, and docosapentaenoic acid (DPA; 22:5n–3) (each P < 0.05), as well as sugars (glucose, ribulonate/xylulonate/lyxonate), cholesterol breakdown (d-urobilin, ursocholate), and steroid hormone metabolites (pregnanolone/allopregnanolone sulfate) (each P < 0.01). Total PUFA intake, including both linolenic and linoleic acid, was positively associated with fecal concentrations of ursocholate, dehydroepiandrosterone sulfate (DHEA-S), and pregnanolone/allopregnanolone sulfate (all P < 0.05). These correlations provide objective analytic measures that parallel the dietary information collected by self-reported recall.
Four modules in the fecal metabolome were associated with SPTB
After individual tests on single metabolites, we used WGCNA to identify and evaluate whether specific metabolic modules or pathways were correlated with SPTB. A total of 20 modules were recovered from stool metabolomics data, and the module-level correlation analysis identified 4 modules highly correlated with SPTB (Supplemental Figure 4, Pearson correlation value > 0.3; P value < 0.05). The modules represented as connected graphs of metabolites are shown in Figure 4C and Supplemental Figures 5–7, where 2 metabolites are connected if the edge weight was >0.05. To summarize the metabolite measures within a module, the first principal component was calculated and was associated with SPTB. Members of the 4 most strongly associated modules included most of the differentially abundant metabolites identified from the individual variable tests (Wilcoxon's rank-sum, P value < 0.01). The 4 most associated modules included closely related metabolites that are similar in chemical structure and/or closely related in biological function. The module most strongly associated with SPTB included 26 metabolites and was enriched in ω-3 fats (Figure 4C, Supplemental Table 2). Three other modules with P value < 0.05 are in Supplemental Figures 5–7 and Supplemental Table 2. One module (Supplemental Figure 5, P value = 0.01, FDR-adjusted P = 0.070) contained a high representation of steroid hormones, including bile acids and DHEA-S for which elevated concentrations have recently been associated with preterm labor (22). Another module (Supplemental Figure 6, P value = 0.03, FDR-adjusted P = 0.158) was most strongly represented by ceramide and sphingosine signaling molecules that are recognized as proinflammatory. Further, sphingosine-1-phosphate has been identified for its role in myometrial contraction in response to LPS-induced infection (23), thus highlighting the potential role these modules may play in the etiology of SPTB. The module including sugars, fats, and alcohol was associated with SPTB overall (Supplemental Figure 7, P value = 0.009, FDR-adjusted P = 0.07), although no single member of the module was associated with SPTB with a Pearson's correlation value > 0.4.
Discussion
In this prospective, case-control study of pregnant women matched by nationally recognized variables associated with SPTB, we identified features of the fecal microbiota and metabolome that discriminate between women who eventually develop SPTB and those who carry their pregnancy to term. A decrease in α-diversity was strongly associated with the development of SPTB where a reduction in the taxonomic class of Betaproteobacteria performed moderately well in discriminating SPTB. Whereas there were no features in the maternal plasma metabolome associated with the development of SPTB after adjusting significance levels for FDR, the fecal metabolome had distinct increases in excretion of lipid and steroid hormone metabolites—specifically DHA, EPA, DHEA-S, and allopregnanolone—that were associated with greater dietary fat intake (especially SFAs, PUFAs, and trans fatty acids). These results not only suggest that the composition of the gut microbiota may ultimately have utility in predicting SPTB, but, together with the fecal metabolome, may also be congruent with previous epidemiologic studies showing that the consumption of a high-fat, low-fiber diet is associated with an increased risk of SPTB (5, 7, 24–26).
Few studies have examined the association between diet and the composition of the gut microbiota during pregnancy. In 2 studies of the same cohort of Norwegian pregnant women, higher dietary fiber and moderate fat intake were associated with increased microbial richness and fewer Bacteroidaceae (27), whereas higher intake of monounsaturated fat, cholesterol, and fat-soluble vitamins was associated with increased potentially proinflammatory bacteria of the genus Proteobacteria (28). Another study reported reduced Shannon diversity in postpartum fecal samples from women delivering preterm but did not report dietary intake or metabolomics data (29). Our study is the first that we know of to describe maternal gut microbiota and fecal metabolomics-based features that are associated with SPTB in a prospective cohort and to identify reduced microbial diversity in Betaproteobacteria. Although most Proteobacterial bacterial taxa associated with disease states belong to the class of Gammaproteobacteria, Betaproteobacteria taxa have also been associated with disease. For example, Alcaligenes spp. have been associated with inflammatory diseases in mice and humans (30) and both Sutterella and Parasutterella have been associated with both diet and disease (31, 32). We also noted weaker associations with plasma metabolites with potential relevance during pregnancy. 14-HDoHE is a lipoxygenase metabolite of DHA recognized for low activation of platelets (33). 17-HDoHE is a DHA-derived oxylipin signaling molecule that has been linked with reduced oxidative stress and oxidative damage in hepatocytes, and suggested as a possible novel biomarker of surgical systemic inflammatory stress (34). It has also been suggested to be a proresolving mediator and peroxisome proliferator–activated receptor-α/γ agonist in cell culture (35). In pregnancy, the concentration of plasma oxylipins and precursor fatty acids has been suggested as a potential predictor of SPTB (36). In the current study, the ratio of 14-HDoHE to 17-HDoHE was increased in women with SPTB; however, the meaning of this difference during pregnancy is not yet clear. Importantly, these analytic features were consistent with the subtle differences in diet between cases and controls based on dietary recall data.
Numerous researchers have shown that diet has significant influence on the diversity and richness of the gut microbiota (12, 20, 37–43). Greater intake of processed foods and proinflammatory fatty acids (reflected by higher SFAs, ω-6 PUFAs, and trans fatty acids) has been linked to obesity, diabetes, and cardiovascular disease, yet less is known about the impact of greater intake of processed and inflammatory fats during pregnancy. Large international cohort observations have linked a high-fat, low-fiber diet with increased risk of SPTB and a lower-fat, higher-fiber diet with reduced risk (7, 24–26). Similarly, highly processed, high-fat diets that are low in fiber are linked with decreased microbiota diversity, whereas those rich in fiber but low in fat are associated with increased diversity (12, 20). The diet in this study might be described as a comparison of a high-fat, low-fiber diet in controls with a yet worse quality diet in cases. Both were poor in quality but the diet in cases was worse and associated with SPTB in our cohort. Although fiber intake was low in both cases and controls, the predominant dietary difference was greater fat intake (especially SFAs, PUFAs, and trans fatty acids) in women with SPTB. The analytic data on the composition of the gut microbiota, together with 2 features in the fecal metabolome, were congruent with consumption of a poor-quality diet that includes higher amounts of proinflammatory dietary fat in mothers at risk of SPTB.
The dietary recall data in our study were suggestive of higher intake of plant and seed oils, including significantly greater intake of PUFAs—predominantly ω-6—as well as higher γ-tocopherol in processed foods. This signature was associated with higher maternal fecal concentrations of the steroid hormone metabolite DHEA-S. Both DHEA-S and allopregnanolone are fetal steroid molecules derived from breakdown of cholesterol that may signal intrauterine fetal stress. Intriguingly, maternal serum DHEA-S concentrations have been identified as one of the signals for cervical ripening (44) and indicators of successful induction of labor in term pregnancies (45). Similarly, the higher concentrations of fecal EPA and DHA paired with a decrease in α-diversity of the gut microbiota seen in women with SPTB were congruent with the dietary recall data showing that these women were consuming a higher-fat diet than controls. In addition, several bile acid metabolites (d-urobilin, l-urobilinogen, and ursocholate) cosegregated in the fecal metabolomics analysis, a finding that would be expected with greater dietary fat intake and a subsequent increase in biliary secretions (Figure 4A, B).
Trans fatty acids are associated with cardiovascular disease and proinflammatory cytokine production by toll like receptor-4 activation, with the potential for deleterious effects on the fetus (46). Trans fatty acids have also been suggested as a risk factor for inadequate placental delivery of ω-3 DHA to the fetus. Trans fatty acids are inversely related to DHA and arachidonic acid (20:4n–6) concentrations in maternal plasma during pregnancy and at delivery and in fetal cord blood (47, 48), suggesting possible displacement by trans fatty acids of essential fatty acids in the case of placental transfer of this essential fatty acid for brain development to the fetus. In contrast to women in this cohort who delivered at term, trans fat intake in cases (2.7 g/d) exceeded the recommended intake amounts of <2 g/d and was also considerably greater than current Danish intake amounts (<1 g/d) where the rates of SPTB are lower than in the United States (48).
This study is limited by its small sample size, with only 16 cases of SPTB (5%). By contrast, national data include cases of medically indicated PTB due to pregnancy complications and women with a history of SPTB in prior pregnancies, both excluded from this study. However, this sample is an unusually rich representation of black women in a northeastern urban setting, a segment with limited previous description of diet and the gut microbiome during pregnancy. Even though black women have been recognized as at higher risk of SPTB (3), studies identifying novel predictive variables are limited, thus the current study is important. The limited generalizability of these findings to other samples with different racial and ethnic diversity or rural settings will require further study.
Nonetheless, the ability to validate self-reported dietary recall data by using independently determined quantifiable analytic features adds to the strength of our findings. It is also important to consider that both cases and controls had poor-quality, highly processed high-fat, low-fiber diets, so our ability to detect a dietary effect should have been modest. Yet, we observed differences in gut microbiota diversity and fecal metabolites. Future studies are needed comparing pregnant women eating a minimally processed healthy diet with those consuming a highly processed unhealthy diet to more adequately determine the potential impact of diet quality on the gut microbiota and birth timing during pregnancy.
In conclusion, data obtained several weeks before delivery suggest that reduced α-diversity of the fecal microbiota and increased fecal concentration of lipid (DHA, EPA, DPA) and steroid (DHEA-S and allopregnanolone) metabolites might provide a biomarker signature associated with future SPTB in women consuming a high-fat, low-fiber diet. Further investigation of these parameters in a larger sample is needed to evaluate the reproducibility of the findings. Translational studies in a mouse model of SPTB also might enlighten and enable full assessment of the absorption dynamics and any role that tissue concentrations of ω-3 fatty acids might play.
Supplementary Material
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—CWC, GDW, and ME: designed the research; CWC and ME: conducted the research; CWC, HL, YL, and GDW: analyzed the data or performed statistical analysis; CWC, GDW, YL, and VG: wrote the manuscript; CWC and GDW: had primary responsibility for the final content; and all authors: read and approved the final manuscript. The authors report no conflicts of interest.
Notes
Supported by the University of Pennsylvania March of Dimes Prematurity Research Center (to ME). The project was also supported in part by National Center for Research Resources and National Center for Advancing Translational Sciences, NIH grant UL1TR000003, the PennCHOP Microbiome Program, and Center for Molecular Studies in Digestive and Liver Disease grant NIH P30 DK050306. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Neither funding source influenced the study design or findings.
Supplemental Figures 1–7 and Supplemental Tables 1 and 2 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
VG and YL contributed equally to this work as first authors.
GDW and CC contributed equally to this work as senior authors.
Abbreviations used: DHEA-S, dehydroepiandrosterone sulfate; DPA, docosapentaenoic acid; FDR, false discovery rate; HDoHE, hydroxydocosahexanoic acid; PERMANOVA, permutational multivariate analysis of variance; PTB, preterm birth; SPTB, spontaneous preterm birth; TOM, topological overlap measure; WGCNA, Weighted Gene Co-expression Network Analysis.
Contributor Information
Victoria Gershuni, Department of Surgery, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, USA.
Yun Li, Department of Biostatistics, Epidemiology, and Informatics, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, USA.
Michal Elovitz, Department of Maternal and Fetal Medicine, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, USA.
Hongzhe Li, Department of Biostatistics, Epidemiology, and Informatics, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, USA.
Gary D Wu, Department of Gastroenterology, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, USA.
Charlene W Compher, School of Nursing, University of Pennsylvania, Philadelphia, PA, USA.
Data Availability
Data described in the article, code book, and analytic code will be made publicly and freely available without restriction at https://github.com/yli0131/MGM.
References
- 1. Esplin MS. Overview of spontaneous preterm birth: a complex and multifactorial phenotype. Clin Obstet Gynecol. 2014;57(3):518–30. [DOI] [PubMed] [Google Scholar]
- 2. Voltolini C, Torricelli M, Conti N, Vellucci FL, Severi FM, Petraglia F. Understanding spontaneous preterm birth: from underlying mechanisms to predictive and preventive interventions. Reprod Sci. 2013;20(11):1274–92. [DOI] [PubMed] [Google Scholar]
- 3. Martin JA, Hamilton BE, Osterman MJK, Driscoll AK, Drake P. Births: final data for 2017. Natl Vital Stat Rep. 2018;67(8):1–50. [PubMed] [Google Scholar]
- 4. de Jongh BE, Paul DA, Hoffman M, Locke R. Effects of pre-pregnancy obesity, race/ethnicity and prematurity. Matern Child Health J. 2014;18(3):511–17. [DOI] [PubMed] [Google Scholar]
- 5. Martin CL, Sotres-Alvarez D, Siega-Riz AM. Maternal dietary patterns during the second trimester are associated with preterm birth. J Nutr. 2015;145(8):1857–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. The American College of Obstetricians and Gynecologists (ACOG) . Preterm labor and birth FAQ. [Internet]. Washington (DC): ACOG; 2019; [cited 13 May, 2019]. Available from: https://www.acog.org/-/media/For-Patients/faq087.pdf?dmc=1&ts=20190513T1547129735. [Google Scholar]
- 7. Englund-Ögge L, Brantsæter AL, Sengpiel V, Haugen M, Birgisdottir BE, Myhre R, Meltzer HM, Jacobsson B. Maternal dietary patterns and preterm delivery: results from large prospective cohort study. BMJ. 2014;348:g1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Forslund K, Hildebrand F, Nielsen T, Falony G, Le Chatelier E, Sunagawa S, Prifti E, Vieira-Silva S, Gudmundsdottir V, Pedersen HKet al. Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature. 2015;528:262–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhu W, Gregory JC, Org E, Buffa JA, Gupta N, Wang Z, Li L, Fu X, Wu Y, Mehrabian Met al. Gut microbial metabolite TMAO enhances platelet hyperreactivity and thrombosis risk. Cell. 2016;165(1):111–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pedersen HK, Gudmundsdottir V, Nielsen HB, Hyotylainen T, Nielsen T, Jensen BAH, Forslund K, Hildebrand F, Prifti E, Falony Get al. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature. 2016;535(7612):376–81. [DOI] [PubMed] [Google Scholar]
- 11. Ananthakrishnan AN, Luo C, Yajnik V, Khalili H, Garber JJ, Stevens BW, Cleland T, Xavier RJ. Gut microbiome function predicts response to anti-integrin biologic therapy in inflammatory bowel diseases. Cell Host Microbe. 2017;21(5):603–10.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wu GD, Chen J, Hoffmann C, Bittinger K, Chen Y-Y, Keilbaugh SA, Bewtra M, Knights D, Walters WA, Knight Ret al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334(6052):105–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Johnson AJ, Vangay P, Al-Ghalith GA, Hillmann BM, Ward TL, Shields-Cutler RR, Kim AD, Shmagel AK, Syed AN, Personalized Microbiome Class Students et al. Daily sampling reveals personalized diet-microbiome associations in humans. Cell Host Microbe. 2019;25(6):789–802.e5. [DOI] [PubMed] [Google Scholar]
- 14. Zmora N, Zilberman-Schapira G, Suez J, Mor U, Dori-Bachash M, Bashiardes S, Kotler E, Zur M, Regev-Lehavi D, Brik RB-Zet al. Personalized gut mucosal colonization resistance to empiric probiotics is associated with unique host and microbiome features. Cell. 2018;174(6):1388–405.e21. [DOI] [PubMed] [Google Scholar]
- 15. Manuck TA, Esplin MS, Biggio J, Bukowski R, Parry S, Zhang H, Huang H, Varner MW, Andrews W, Saade Get al. The phenotype of spontaneous preterm birth: application of a clinical phenotyping tool. Am J Obstet Gynecol. 2015;212(4):487.e1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. CDC . About adult BMI. [Internet]. Atlanta, GA: Division of Nutrition, Physical Activity, and Obesity, National Center for Chronic Disease Prevention and Health Promotion; 2020; [accessed 23 December, 2020]. Available from: https://www.cdc.gov/healthyweight/assessing/bmi/adult_bmi/index.html. [Google Scholar]
- 17. University of Minnesota Nutrition Coordinating Center (UoMNCC) . Nutrition Data System for Research software and methodology version 2009. [Internet]. Minneapolis, MN: UoMNCC; 2009; [cited 19 April, 2016]. Available from: http://www.ncc.umn.edu/products/. [Google Scholar]
- 18. Tabung FK, Satija A, Fung TT, Clinton SK, Giovannucci EL. Long-term change in both dietary insulinemic and inflammatory potential is associated with weight gain in adult women and men. J Nutr. 2019;149(5):804–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Langfelder P, Horvath S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics. 2008;9:559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wu GD, Compher C, Chen EZ, Smith SA, Shah RD, Bittinger K, Chehoud C, Albenberg LG, Nessel L, Gilroy Eet al. Comparative metabolomics in vegans and omnivores reveal constraints on diet-dependent gut microbiota metabolite production. Gut. 2016;65(1):63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jiang Q, Christen S, Shigenaga MK, Ames BN. γ-Tocopherol, the major form of vitamin E in the US diet, deserves more attention. Am J Clin Nutr. 2001;74(6):714–22. [DOI] [PubMed] [Google Scholar]
- 22. El Sayed MA, El Kelani OA, Rezk MA, Solyman Attalah AE, Rawash MSA. Maternal serum dehydroepiandrosterone sulfate as a predictor of labor inhibition in preterm labor. Menoufia Med J. 2018;31:57–62. [Google Scholar]
- 23. Vyas V, Ashby CR Jr, Reznik SE. Sphingosine kinase: a novel putative target for the prevention of infection-triggered preterm birth. Obstet Gynecol Int. 2013;2013:302952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Grieger JA, Grzeskowiak LE, Clifton VL. Preconception dietary patterns in human pregnancies are associated with preterm delivery. J Nutr. 2014;144(7):1075–80. [DOI] [PubMed] [Google Scholar]
- 25. Rasmussen MA, Maslova E, Halldorsson TI, Olsen SF. Characterization of dietary patterns in the Danish National Birth Cohort in relation to preterm birth. PLoS One. 2014;9(4):e93644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hillesund ER, Øverby NC, Engel SM, Klungsøyr K, Harmon QE, Haugen M, Bere E. Associations of adherence to the New Nordic Diet with risk of preeclampsia and preterm delivery in the Norwegian Mother and Child Cohort Study (MoBa). Eur J Epidemiol. 2014;29(10):753–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Röytiö H, Mokkala K, Vahlberg T, Laitinen K. Dietary intake of fat and fibre according to reference values relates to higher gut microbiota richness in overweight pregnant women. Br J Nutr. 2017;118(5):343–52. [DOI] [PubMed] [Google Scholar]
- 28. Mandal S, Godfrey KM, McDonald D, Treuren WV, Bjørnholt JV, Midtvedt T, Moen B, Rudi K, Knight R, Brantsæter ALet al. Fat and vitamin intakes during pregnancy have stronger relations with a pro-inflammatory maternal microbiota than does carbohydrate intake. Microbiome. 2016;4(1):55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dahl C, Stanislawski M, Iszatt N, Mandal S, Lozupone C, Clemente JC, Knight R, Stigum H, Eggesbø M. Gut microbiome of mothers delivering prematurely shows reduced diversity and lower relative abundance of Bifidobacterium and Streptococcus. PLoS One. 2017;12(10):e0184336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sonnenberg GF, Monticelli LA, Alenghat T, Fung TC, Hutnick NA, Kunisawa J, Shibata N, Grunberg S, Sinha R, Zahm AMet al. Innate lymphoid cells promote anatomical containment of lymphoid-resident commensal bacteria. Science. 2012;336(6086):1321–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Laitinen K, Mokkala K. Overall dietary quality relates to gut microbiota diversity and abundance. Int J Mol Sci. 2019;20(8):1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Carding S, Verbeke K, Vipond DT, Corfe BM, Owen LJ. Dysbiosis of the gut microbiota in disease. Microb Ecol Health Dis. 2015;26:26191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lagarde M, Guichardant M, Bernoud-Hubac N, Calzada C, Véricel E. Oxygenation of polyunsaturated fatty acids and oxidative stress within blood platelets. Biochim Biophys Acta Mol Cell Biol Lipids. 2018;1863(6):651–6. [DOI] [PubMed] [Google Scholar]
- 34. Wolfer AM, Scott AJ, Rueb C, Gaudin M, Darzi A, Nicholson JK, Holmes E, Kinross JM. Longitudinal analysis of serum oxylipin profile as a novel descriptor of the inflammatory response to surgery. J Transl Med. 2017;15(1):83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Egawa D, Itoh T, Akiyama Y, Saito T, Yamamoto K. 17-OxoDHA is a PPARα/γ dual covalent modifier and agonist. ACS Chem Biol. 2016;11(9):2447–55. [DOI] [PubMed] [Google Scholar]
- 36. Ramsden CE, Makrides M, Yuan Z-X, Horowitz MS, Zamora D, Yelland LN, Best K, Jensen J, Taha AY, Gibson RA. Plasma oxylipins and unesterified precursor fatty acids are altered by DHA supplementation in pregnancy: can they help predict risk of preterm birth?. Prostaglandins Leukot Essent Fatty Acids. 2020;153:102041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. De Filippis F, Pellegrini N, Vannini L, Jeffery IB, La Storia A, Laghi L, Serrazanetti DI, Di Cagno R, Ferrocino I, Lazzi Cet al. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut. 2016;65(11):1812–21. [DOI] [PubMed] [Google Scholar]
- 38. Segata N. Gut microbiome: westernization and the disappearance of intestinal diversity. Curr Biol. 2015;25(14):R611–13. [DOI] [PubMed] [Google Scholar]
- 39. Makki K, Deehan EC, Walter J, Bäckhed F. The impact of dietary fiber on gut microbiota in host health and disease. Cell Host Microbe. 2018;23(6):705–15. [DOI] [PubMed] [Google Scholar]
- 40. Flint HJ, Duncan SH, Louis P. The impact of nutrition on intestinal bacterial communities. Curr Opin Microbiol. 2017;38:59–65. [DOI] [PubMed] [Google Scholar]
- 41. Flint HJ, Duncan SH, Scott KP, Louis P. Links between diet, gut microbiota composition and gut metabolism. Proc Nutr Soc. 2015;74(1):13–22. [DOI] [PubMed] [Google Scholar]
- 42. Graf D, Di Cagno R, Fåk F, Flint HJ, Nyman M, Saarela M, Watzl B. Contribution of diet to the composition of the human gut microbiota. Microb Ecol Health Dis. 2015;26:26164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kovatcheva-Datchary P, Nilsson A, Akrami R, Lee YS, De Vadder F, Arora T, Hallen A, Martens E, Björck I, Bäckhed F. Dietary fiber-induced improvement in glucose metabolism is associated with increased abundance of Prevotella. Cell Metab. 2015;22(6):971–82. [DOI] [PubMed] [Google Scholar]
- 44. Rechberger T, Abramson SR, Woessner JF Jr. Onapristone and prostaglandin E2 induction of delivery in the rat in late pregnancy: a model for the analysis of cervical softening. Am J Obstet Gynecol. 1996;175(3 Pt 1):719–23. [DOI] [PubMed] [Google Scholar]
- 45. Maciulla J, Goolsby L, Racowsky C, Reed K. Maternal serum dehydroepiandrosterone sulfate levels and successful labor induction. Obstet Gynecol. 1998;91(5 Pt 1):771–3. [DOI] [PubMed] [Google Scholar]
- 46. Mennitti LV, Oliveira JL, Morais CA, Estadella D, Oyama LM, do Nascimento CMO, Pisani LP. Type of fatty acids in maternal diets during pregnancy and/or lactation and metabolic consequences of the offspring. J Nutr Biochem. 2015;26(2):99–111. [DOI] [PubMed] [Google Scholar]
- 47. Decsi T, Campoy C, Demmelmair H, Szabó E, Marosvölgyi T, Escolano M, Marchal G, Krauss-Etschmann S, Cruz M, Koletzko B. Inverse association between trans isomeric and long-chain polyunsaturated fatty acids in pregnant women and their newborns: data from three European countries. Ann Nutr Metab. 2011;59(2–4):107–16. [DOI] [PubMed] [Google Scholar]
- 48. Decsi T, Boehm G. trans Isomeric fatty acids are inversely related to the availability of long-chain PUFAs in the perinatal period. Am J Clin Nutr. 2013;98(2):543S–8S. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the article, code book, and analytic code will be made publicly and freely available without restriction at https://github.com/yli0131/MGM.