Abstract
Abnormal, persistent inflammation after bypass surgery could prevent healing of an ischaemic foot lesion. In 37 patients with peripheral arterial disease (PAD) (Rutherford Grade III Category 5) who underwent infrapopliteal vein graft and midfoot amputation, plasma levels of fibrinogen, C‐reactive protein (CRP), interleukin‐1 (IL‐1), interleukin‐6 (IL‐6), tumour necrosis factor‐α (TNF‐α), and matrix metalloproteinase‐2 and ‐9 (MMP‐2 and MMP‐9) were determined preoperatively and during the follow up. Nine patients without clinical and Doppler evidence of arterial disease, who underwent post‐traumatic midfoot primary amputation, were included in the experiment group, and 15 age‐matched healthy volunteers served as control. In patients who had midfoot amputation for trauma, all wounds healed. Seven (19%) wounds in patients with an occluded graft healed, and five (13%) required major amputation because of a non‐healing wound. Time required for complete healing of the lesion was similar between trauma and PAD patients (8 ± 2 months vs 11 ± 6, respectively, P = NS). Univariate analysis demonstrated that, in PAD patients, the postoperative high levels of TNF‐α, IL‐6, and MMP‐2 and ‐9 were predictive for wound healing failure at 3, 6, and 9 months (P < 0.05), respectively. Furthermore, the subgroup of patients who experienced occlusion of the vein graft during follow up had a significant increase of MMP‐2, ‐9, IL‐6, and TNF‐α at 3, 6, and 9 months (P < 0.05), respectively. Monitoring inflammatory markers allows the determination of patients at risk of healing failure of midfoot amputation after distal revascularisation and might predict the fate of the vein graft.
Keywords: bypass, cytokines, ischaemia, metalloproteinases, trauma
1. INTRODUCTION
Wound healing failure after midfoot amputation, performed simultaneously to distal revascularisation, presents a significant problem,1 potentially leading to major amputation, which significantly increases morbidity and mortality.2 Despite a successful reconstruction, the failure rate of minor amputations is, in fact, approximately 30% to 45%,3, 4 and a major amputation is required in 25% to 30% of these cases.5, 6
The activation of the inflammatory cascade is the primary response to injury. The inflammatory reaction is mediated by inflammatory cytokines such as CRP, IL‐1, IL‐6, and tumour necrosis factor‐α (TNF‐α).7, 8
The healing process is closely related to the balance between extracellular matrix (ECM) synthesis and degradation.9 ECM degradation is mediated by matrix metalloproteinases (MMPs), a family of extracellular endopeptidases. MMP‐2 and ‐9 play a major role in this process because of their affinity for collagen type IV and laminin, the key components of the basement membrane. MMP expression can be induced by cytokines, growth factors, stress, or inflammation. Cytokines such as IL‐6 and TNF‐α have been shown to up‐regulate MMP in several cell types, including fibroblasts, endothelial cells, and vascular smooth muscle cells (VSMCs).10, 11
Conceptually, the levels of circulating inflammatory cytokines could provide an indirect picture of the healing process of a midfoot amputation. In this clinical setting, the analysis of circulating inflammatory cytokines could represent a method to predict and monitor midfoot amputation healing.12
The aim of this study was to evaluate plasma levels of fibrinogen, CRP, IL‐1, IL‐6, TNF‐α, and MMP‐2 and ‐9 as predictors of the wound‐healing process and graft patency in patients undergoing peripheral limb revascularisation followed by a midfoot amputation.
2. MATERIALS AND METHODS
2.1. Study design
2.1.1. Study group
A total of 37 patients of both genders affected with PAD (Rutherford Grade III Category 5)13 and clinical stage 3–4 for risk of limb amputation (Wound Ischemia foot Infection—WIfI classification),14 who underwent an infrapopliteal vein graft and midfoot amputation between January 2012 and December 2015, were enrolled in the present study. This research complies with the principles laid down in the Declaration of Helsinki and was approved by the Institutional Research Committee. Written informed consent for the treatment and the analysis of data for scientific purposes was obtained from all patients.
2.1.2. Control groups
Nine patients, free from peripheral arterial disease, who underwent post‐traumatic midfoot primary amputation at the time of admission were used as controls. Foot injuries occurred as part of an isolated trauma. In these cases, the decision for primary amputation was made by a multidisciplinary team of vascular, orthopaedic, and plastic surgeons. Criteria for primary amputation included ischaemia lasting more than 6 hours and/or bony or soft tissue defects deemed untreatable. The patients were consecutively selected from the Trauma Registry of our institution from January 2012 to December 2015.
Fifteen age‐matched healthy volunteers (mean age, 65 ± 6 years; median age, 66 years; age range, 52–75 years) without atherosclerotic lesions (excluded by carotid and aortic ultrasonography and ankle brachial index measurements) and normal LDL‐cholesterol level were enrolled as a reference for biological parameters.
Exclusion criteria were the presence of chronic venous insufficiency; arterial aneurysms; insulin‐dependent or ‐independent diabetes; acute ischaemia; connective tissue disorders, including rheumatoid arthritis, blood disorders, and use of medications impairing wound healing (ie, cytotoxic antineoplastic, immunosuppressive agents, and corticosteroids); and kidney disease.
2.2. Collection of baseline data
Demographic data, including age, gender, risk factors, and associated diseases, were collected. The risk factors included active tobacco use, cardiac disease (prior myocardial infarction, stable or unstable angina, or ST segment alteration on electrocardiogram), hypertension (diastolic blood pressure, ≥85 mm Hg), renal disease (blood urea nitrogen, >7.1 mmol/L; creatinine level, >266 μmol/L; creatinine clearance, <50 mL/min), pulmonary disease (PO2, <60 mm Hg; PCO2, > 50 mm Hg; pulmonary function tests, <80% of predicted), total cholesterol level ≥5 mmol/L, low‐density lipoprotein level ≥4 mmol/L, high‐density lipoprotein level ≤1 mmol/L, and non‐fasting triglyceride level ≥1.7 mmol/L. Associated diseases were represented by obesity (body mass index, BMI—kg/m2 >20% of ideal) and an alteration in the coagulation assay. Diagnostic investigations consisting of B‐mode ultrasonography and colour imaging and computed tomography angiography (CTA), inflow and run‐off status, surgical procedural details, minor and major complications, length of stay, and early‐ and mid‐term outcomes were recorded.
2.3. Operative procedures and clinical follow up
The Physical Status Classification System proposed by the American Society of Anesthesiologists (ASA)15 was used to determine the surgical risk. All patients belonged to classes II to IV.
2.3.1. Study group
The surgical treatment was planned after evaluating the CTA images. A reversed autogenous vein graft was performed using standard techniques. An autogenous conduit always includes the great saphenous vein. The inflow site included the common femoral artery; distal anastomoses were performed on the tibial vessels. All reconstructions were tunnelled in an anatomical position. Vein graft flow disturbances or intrinsic vein disease were studied at the end of the procedure with angiography. Vein grafts were preferentially performed to revascularise the ischaemic angiosome.16 The necrotic midfoot was amputated (transmetatarsal, Lisfranc, or Chopart amputations) 3 days after revascularisation. No attempts of direct closure were made because it was impossible to safely use the plantar flap to cover the wound. A broad‐spectrum short course (2‐4 days) i.v. antibiotic therapy was started. Negative pressure wound therapy (NPWT) with vacuum‐assisted closure (VAC Therapy System) (KCI Medical S.r.l. via Meucci, 1 Assago—MI, 20090) was used to accelerate wound healing and to prevent infection during in‐hospital stay and was continued on a domiciliary basis. When the NPWT was discontinued, the wounds were medicated until complete healing with standard, comprehensive care, including wound off‐loading, local wound debridement, and wound moisture balance with appropriate dressings.
Postoperatively, acetylsalicylic acid 100 mg/day and statin 10 or 20 mg/day were prescribed to all patients not already on these medications. Follow up consisted of physical examination, ankle brachial index (ABI) measurement, and B‐mode ultrasonography and colour imaging every 4 months. B‐mode ultrasonography and colour imaging and CTA were used to eventually confirm a suspicion of graft occlusion.
2.3.2. Control group
The patients who underwent a post‐traumatic foot amputation had complex tissue defects defined as loss of skin and subcutaneous tissues, resulting in tendon and bones exposure. Wounds were primarily debrided to ensure the removal of all contaminated, devitalised tissue and exposed, not coverable bone segments, sparing the maximum of the viable tissue. A broad‐spectrum short course (2 to 4 days) i.v. antibiotic therapy was started. Once control of the bleeding was achieved, the patients were managed for 2 to 3 days with parenteral antibiotics and gauze dressing, and thereafter, the VAC was applied. Once the NPWT was discontinued, the wounds were medicated until complete healing using the above‐described protocol.
2.4. Laboratory measurements
Plasma and blood samples were collected on the morning of reconstructive arterial surgery and every 3 months thereafter through a direct venipuncture on an antecubital vein of the arm until complete healing of the wound or recurrence of ischaemic symptoms in patients affected with PAD. The samples were collected into tubes containing K2‐EDTA (Terumo Europe NV, Leuven, Belgium) or sodium citrate (BD‐Plymouth, UK) and immediately transported to our laboratory under controlled conditions of temperature and humidity. To evaluate fibrinogen level, the blood sample was centrifuged at 15 000 rpm for 10 minutes to separate plasma. Centrifugations were carried out at room temperature, and samples were analysed immediately using the BCS XP analyser (Siemens Healthcare Diagnostics, Tarrytown NY) with Siemens reagent. After centrifugation of the sampled blood at 3000 rpm for 10 minutes, the serum levels of IL‐1 and IL‐6 were measured with human IL‐1 and IL‐6 Quantikine ELISA kits (R&D Systems, Inc., Minneapolis, MN). The concentration of plasma TNF‐α was determined by human TNF‐α Quantikine ELISA kits (R&D Systems). Measurements of MMP‐2 and ‐9 were obtained using Quantikine ELISA kits (R&D Systems) according to the manufacturer's manual. The mean intra‐ and inter‐assay coefficients of variation for MMP‐2 estimation were <6% and <10% for all cases, respectively. The mean intra‐ and inter‐assay coefficients of variation for MMP‐9 estimation were <3% and <8% for all cases, respectively. The samples collected in tubes containing Lithium Heparin (BD‐Plymouth) were used to evaluate CRP level. The level of high‐sensitivity CRP was analysed by particle‐enhanced immunoturbidimetric methods on COBAS 6000 (Roche Diagnostics, Basel, Switzerland). The limit of detection was 1 mg/L, with a working range extending to 250 mg/L. There was no interference from bilirubin, haemoglobin, triglycerides, and rheumatoid factor up to high concentrations.
2.5. Wound healing
Briefly, wound healing was assessed through direct ulcer tracing onto a clear plastic sheet and subsequent computerised planimetry.17 Healing was calculated by subtracting the final ulcer area (FUA), expressed in centimetres, from the initial ulcer area (IUA), expressed in centimetres, and dividing by the number of follow‐up months to obtain the total area healed per month (cm2/month).
2.6. Statistical analysis
Analysis of our data has been carried out using a computer software program (SPSS Ver. 25.0.0.1; SPSS Chicago, IL for macOS High Sierra ver. 10.13.4, Apple Inc. 1983–2018 Cupertino, CA). All results are expressed as the mean ± SD. Because of sample sizes, non‐parametric tests were applied. The Mann–Whitney U test or the Kruskal‐Wallis H test (one‐way anova), followed by the Bonferroni post‐hoc test calculated by dividing the P value (0.05) by the number of paired comparisons made, was used to analyse continuous variables. The Chi‐square test or Fisher's exact test was used to study categorical variables. Survival, primary patency, and limb salvage rates were assessed using the Kaplan–Meier method. SE of survival, primary patency, and limb salvage rates were estimated at each censored case. Patency and limb salvage rates were calculated based on the number of limbs at risk and survival rates of the number of patients. Differences with an α‐level of <0.05 were considered statistically significant.
3. RESULTS
3.1. Patients overview
Table 1 describes the demographics and preoperative clinical characteristics of patients affected with PAD and those affected with isolated foot trauma. Overall, 32 (69%) patients were considered to be at high surgical risk, and 14 (31%) had a moderate or low operative risk according to ASA classification. Loco regional spinal anaesthesia was used in all patients. No adverse effects related to systemic heparinisation administration when required were recorded.
Table 1.
Patients' demographics, preoperative characteristics, and mechanism of trauma
| PAD patients | Trauma patients | |
|---|---|---|
| Gender (male/female) | 31/6 | 8/1 |
| Age (year ± SD) | 70 ± 7 | 38 ± 12 |
| Active tobacco use | 23 (62%) | 2 (22%) |
| Cardiac disease | 12 (32%) | ‐ |
| Hypertension | 31 (4%) | 1 (11%) |
| Renal disease | 4 (11%) | ‐ |
| Pulmonary disease | 2 (5%) | ‐ |
| Total cholesterol (mmol/L ± SD) | 5 ± 1 | ‐ |
| Low‐density lipoprotein (mmol/L ± SD) | 4 ± 1 | ‐ |
| High‐density lipoprotein (mmol/L ± SD) | 1 ± 2 | ‐ |
| Non‐fasting triglyceride (mmol/L ± SD) | 4 ± 1 | ‐ |
| Obesity | 2 (5%) | |
| Preoperative ABI (mm Hg ± SD) | 0.4 ± 0.1 | 1 ± 0 |
| Vessel run‐off (1/2) | 28/9 | ‐ |
| Clinical stage 3‐4 (WIfI classification) | 16/21 | ‐ |
| ASA II | 7 (19%) | 7 (78%) |
| ASA III | 21 (57%) | 2 (22%) |
| ASA IV | 9 (24%) | ‐ |
| Mechanism of trauma | ‐ | |
| Heavy goods vehicle | NA | 4 (44%) |
| Motor vehicle run over foot | NA | 2 (22%) |
| Crushed with iron plate | NA | 3 (33%) |
Abbreviations: ABI, Ankle Brachial Index; ASA, American Society of Anesthesiologists, NA, not applicable; PAD, peripheral arterial disease.
A reversed autologous saphenous vein graft was constructed, with the proximal anastomosis on the common femoral artery and the distal anastomosis on the anterior tibial artery in 11 (30%) cases, on the posterior tibial artery in 17 (46%) cases, and on the peroneal artery in 9 (24%) cases. The overall length of stay was 10 ± 3 days (median 9 days; range min. 6‐max. 18 days).
3.2. Early outcomes
No early technical failures or major complications were recorded in PAD patients. Minor complications consisted of lymphorrhea at the level of the groin in two cases. These complications spontaneously resolved. No major or minor complications were recorded in the group of patients with isolated foot trauma.
3.3. Midterm outcomes
No patients were lost at follow up (mean 22 ± 13 months; range min. 6‐max. 48 months; median 19.5 months).
During follow up of PAD patients, three (8%) patients died (two myocardial infarctions and one stroke) at 10, 11, and 11 months. One‐year survival rate was 92% (SE = 0.04). One‐year primary patency rate was 65% (SE = 0.08). We observed 12 graft occlusions because of thrombosis (at 7, 8, 8, 9, 9, 10, 11, 11, 11, 11, 12, and 12 months). Five graft occlusions required a major amputation (two above knee and three below knee) because of non‐healing wounds of previous foot amputations. The limb salvage rate at 1 year was 84% (SE = 0.06).
No patient affected with foot trauma died or underwent an above‐ or below‐knee amputation during the follow up.
3.4. Wound healing
Mean wound diameter was 21 ± 5 cm2 (range min. 13‐max. 378 cm2; median 18 cm2). There were no significant differences in wound size between PAD and trauma patients (P = NS), and the type of midfoot amputation had no influence (P = NS) on the healing process.
3.5. Healing process in PAD patients
Twenty‐five (68%) wounds in patients with a patent graft healed in 9 ± 3 months (min. 4‐max. 14 months; median 9 months). Seven (19%) wounds in patients with an occluded graft also healed in 16 ± 10 months (min. 8‐max. 36 months; median 14 months), whereas five (13%) wounds did not heal, and a major amputation was required (P = NS). The wound‐healing process was not influenced by the direct angiosome revascularisation (P = NS).
3.6. Healing process in trauma patients
All wounds healed in 8 ± 2 months (min. 7‐max. 12 months; median 8 months). Table 2 summarises the healing process of PAD and trauma groups.
Table 2.
Healing process after midfoot amputation
| PAD patients | Foot trauma patients | Significance | |
|---|---|---|---|
| 3‐month wounds at risk | 37 | 9 | |
| Wound‐healing process | 1.8 ± 0.6 cm2/month | 2.0 ± 0.5 cm2/month | 0.446 |
| 6‐month wounds at risk | 36 | 9 | |
| Wound‐healing process | 2.1 ± 0.6 cm2/month | 2.4 ± 0.6 cm2/month | 0.191 |
| 9‐month wounds at risk | 23 | 4 | |
| Wound‐healing process | 1.5 ± 0.7 cm2/month | 1.6 ± 0.4 cm2/month | 589 |
| 12‐month wounds at risk | 8 | 1 | |
| Wound‐healing process | 1.4 ± 0.3 cm2/month | 1 ± 0.0 cm2/month | ‐ |
Abbreviation: PAD, peripheral arterial disease.
3.7. Inflammatory markers variation
Briefly, baseline values of IL‐1 (12 ± 3 pg/mL; 12 ± 3 pg/mL), IL‐6 (11 ± 2 pg/mL; 10 ± 2 pg/mL), TNF‐α (9 ± 2 pg/mL; 9 ± 2 pg/mL), CRP (12 186 ± 2610 μg/L; 11 560 ± 1249 μg/L), fibrinogen (9 ± 1 g/L; 10 ± 1 g/L), MMP‐2 (436 ± 92 ng/mL; 440 ± 91 ng/mL), and MMP‐9 (75 ± 10 ng/mL; 75 ± 12 ng/mL) between PAD and trauma patients were similar but significantly higher when compared with controls (1 ± 1 pg/mL; 2 ± 1 pg/mL; 1 ± 1 pg/mL; 1815 ± 484 μg/L; 2 ± 1 g/L; 176 ± 12 ng/mL; and 58 ± 30 ng/mL, respectively) (P < 0.0001, respectively). At 3, 6, and 9 months, we observed a significantly different release of IL‐6 (3 months: 14 ± 5 pg/mL; 10 ± 2 pg/mL; 6 months: 14 ± 5 pg/mL; 9 ± 2 pg/mL, and 9 months: 14 ± 6 pg/mL; 8 ± 1 pg/mL), TNF‐α (3 months: 12 ± 5 pg/mL; 8 ± 2 pg/mL; 6 months: 12 ± 6 pg/mL; 7 ± 2 pg/mL, and 9 months: 13 ± 6 pg/mL; 6 ± 1 pg/mL), MMP‐2 (3 months: 609 ± 270 ng/mL; 387 ± 52 ng/mL; 6 months: 622 ± 310 ng/mL; 368 ± 53 ng/mL, and 9 months: 673 ± 387 ng/mL; 262 ± 70 ng/mL), and MMP‐9 (3 months: 89 ± 23 ng/mL; 73 ± 11 ng/mL; 6 months: 88 ± 22 ng/mL; 72 ± 11 ng/mL, and 9 months: 99 ± 35 ng/mL; 59 ± 16 ng/mL) between PAD and trauma patients (3 months: P < 0.05; P < 0.04; P < 0.02; P < 0.04; 6 months: P < 0.01; P < 0.01 P < 0.02; P < 0.04; 9 months: P < 0.04; P < 0.04; P < 0.05; P < 0.03; respectively). All values are reported in Table 3.
Table 3.
Cytokines, extracellular endopeptidases, and acute phase reactants baseline and 3‐, 6‐, 9‐, and 12‐month values
| PAD patients | Trauma patients | Control subjects | ||
|---|---|---|---|---|
| Baseline (number of patients) | (37) | (9) | (15) | |
| IL‐1 (pg/mL) | 12 ± 3 | 12 ± 3 | 1 ± 1 | |
| IL‐6 (pg/mL) | 11 ± 2 | 10 ± 2 | 2 ± 1 | |
| TNF‐α (pg/mL) | 9 ± 2 | 9 ± 2 | 1 ± 1 | |
| CRP (μg/L) | 12 186 ± 2610 | 11 560 ± 1249 | 1815 ± 484 | |
| Fibrinogen (g/L) | 9 ± 1 | 10 ± 1 | 2 ± 1 | |
| MMP‐2 (ng/mL) | 436 ± 92 | 440 ± 91 | 176 ± 12 | |
| MMP‐9 (ng/mL) | 75 ± 10 | 75 ± 12 | 58 ± 30 | |
| 3 months (number of patients) | (37) | (9) | ||
| IL‐1 (pg/mL) | 11 ± 3 | 12 ± 3 | ||
| IL‐6 (pg/mL) | 14 ± 5 | 10 ± 2 | ||
| TNF‐α (pg/mL) | 12 ± 5 | 8 ± 2 | ||
| CRP (μg/L) | 14 942 ± 16 333 | 11 560 ± 1249 | ||
| Fibrinogen (g/L) | 9 ± 1 | 10 ± 1 | ||
| MMP‐2 (ng/mL) | 609 ± 270 | 387 ± 52 | ||
| MMP‐9 (ng/mL) | 89 ± 23 | 73 ± 11 | ||
| 6 months (number of patients) | (36) | (9) | ||
| IL‐1 (pg/mL) | 11 ± 3 | 10 ± 2 | ||
| IL‐6 (pg/mL) | 14 ± 5 | 9 ± 2 | ||
| TNF‐α (pg/mL) | 12 ± 6 | 7 ± 2 | ||
| CRP (μg/L) | 12 209 ± 2639 | 10 179 ± 1029 | ||
| Fibrinogen (g/L) | 9 ± 1 | 9 ± 1 | ||
| MMP‐2 (ng/mL) | 622 ± 310 | 368 ± 53 | ||
| MMP‐9 (ng/mL) | 88 ± 22 | 72 ± 11 | ||
| 9 months (number of patients) | (23) | (4) | ||
| IL‐1 (pg/mL) | 11 ± 3 | 10 ± 2 | ||
| IL‐6 (pg/mL) | 14 ± 6 | 8 ± 1 | ||
| TNF‐α (pg/mL) | 13 ± 6 | 6 ± 1 | ||
| CRP (μg/L) | 12 318 ± 2520 | 9773 ± 1019 | ||
| Fibrinogen (g/L) | 9 ± 2 | 8 ± 1 | ||
| MMP‐2 (ng/mL) | 673 ± 387 | 262 ± 70 | ||
| MMP‐9 (ng/mL) | 99 ± 35 | 59 ± 16 | ||
| 12 months (number of patients) | (8) | (1) | ||
| IL‐1 (pg/mL) | 10 ± 4 | 7 | ||
| IL‐6 (pg/mL) | 12 ± 6 | 9 | ||
| TNF‐α (pg/mL) | 10 ± 4 | 8 | ||
| CRP (μg/L) | 10 110 ± 1407 | 8800 | ||
| Fibrinogen (g/L) | 9 ± 2 | 7 | ||
| MMP‐2 (ng/mL) | 517 ± 333 | 378 | 3 | |
| MMP‐9 (ng/mL) | 88 ± 36 | 65 |
Abbreviations: CRP, C‐reactive protein; IL‐1, interleukin‐1; IL‐6, interleukin‐6; MMP‐2 and MMP‐9, matrix metalloproteinase‐2 and ‐9; PAD, peripheral arterial disease; TNF‐α, tumour necrosis factor‐α.
In the subgroup of patients affected with PAD, we further analysed the inflammatory markers variation in those who experienced an occluded vein graft. A statistically significant increase of MMP‐2 and ‐9, IL‐6, and TNF‐α in PAD patients with an occluded graft, at 3, 6, and 9 months, respectively, was noted (Figure 1).
Figure 1.
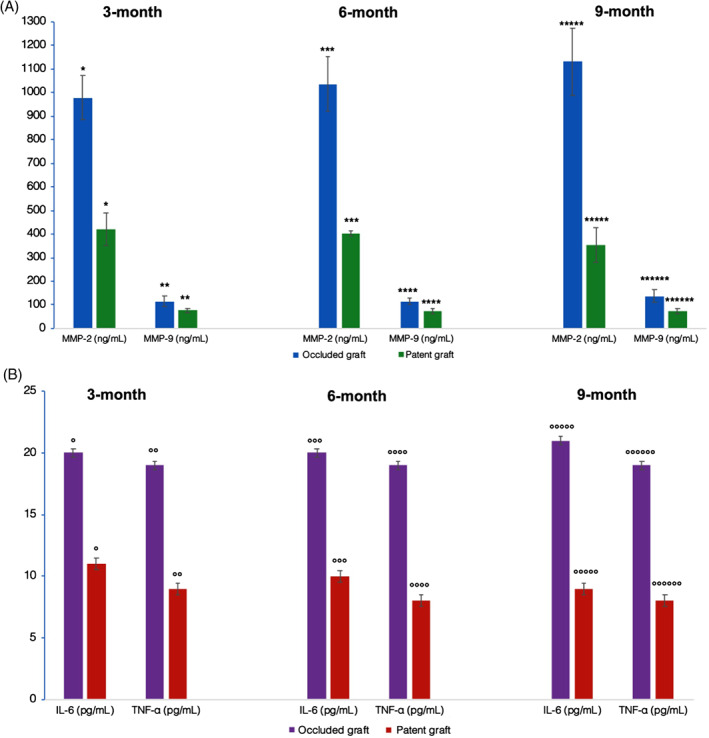
Extracellular endopeptidases (Panel A) and cytokines (Panel B) variations at 3‐, 6‐ and 9‐month in PAD patients with patent or occluded vein grafts (*P < 0.0001; **P < 0.0001; ***P < 0.0001; ****P < 0.0001; *****P < 0.0001; ******P < 0.0001; °P < 0.0001; °°P < 0.0001; °°°P < 0.0001; °°°°P < 0.0001; °°°°°P < 0.0001; °°°°°°P < 0.0001)
4. DISCUSSION
In the present study, we have monitored the changes in the concentration of several inflammatory markers to investigate the process of foot amputation healing in trauma and in PAD patients submitted to peripheral bypass graft treated in accordance with ESVS guidelines.14 The wound‐healing process is accompanied by the release of constitutively available molecules and by the activation of tissue‐resident macrophages, recruitment of neutrophils, and circulating monocytes.18 The inflammatory infiltrate mediates further leucocyte recruitment in a paracrine fashion and mobilises mesenchymal stromal and endothelial cells by synthesis and release of the pro‐inflammatory cytokines, particularly TNF‐α, IL‐1, and IL‐6.19 TNF‐α and IL‐1β up‐regulate MMP expression,20 suggesting that persistent inflammation may have deleterious consequences for the tissues.21 MMPs remodel the ECM and are inflammatory modulators implicated in a wide range of cell signalling processes of both the innate and the adaptive immunity.22 MMPs are known to influence cutaneous wound healing23; MMP‐2 and ‐9 expression is increased in chronic wounds.21, 24
The panel of biochemical tests we chose to study as markers is easily assessable and routinely applicable and, theoretically, might give predictive information on wound healing after midfoot amputation and on the fate of peripheral arterial reconstructions.
Currently, there are some clinical data to support elevated C‐RP as a useful preoperative marker in risk stratification for postoperative amputation in patients undergoing an infrapopliteal revascularisation procedure, whereas fibrinogen is only associated with the severity of PAD at presentation and has no role in predicting the outcome of the reconstructions.25, 26 Our study is the first to assess a variety of inflammatory markers to predict wound healing and, ultimately, the fate of an infrapopliteal reconstruction. In our study, while the preoperative plasma levels of trauma and PAD patients were similar, a different inflammatory response occurred during follow up. Complex foot trauma patients had a significantly lower plasma level of the inflammatory markers because of the different pathology of the amputation. During follow up, PAD patients affected with a non‐healing wound showed a statistically significant stable rise of TNF‐α, IL‐6, MMP‐2, and MMP‐9 when compared with patients with a healing wound. Conversely, the remaining markers steadily decreased in both groups during the follow up; therefore, their utility in predicting wound healing was limited. It is important to consider that even though the risk of ongoing wound infection, the need for tissue debridement, or osteomyelitis are theoretically similar between trauma and PAD patients, their concurrent role in the plasma level elevation of these molecules might mitigate their specificity.
Interestingly, the preoperative plasma level of the inflammatory markers was not predictive of graft patency, but during follow up, a stable increase of TNF‐α, IL‐6, and MMP‐2 and ‐9 also predicted graft occlusion in the absence of clinical or instrumental evidence of impending failure: in all patients with persistent increased levels of TNF‐α, IL‐6, MMP‐2, and MMP‐9, the graft occluded. We should state that the predictable risk factors for vein graft failure, such as the run‐off status, the quality and size of the vein, and the surveillance of restenosis with non‐invasive imaging investigation, are extremely important, and our results do not overtake their role. However, the importance of our molecular markers is based on the lack of a significant association between vein graft failure and the traditional Framingham‐based risk factors. As for the role of inflammatory markers in predicting graft patency, it is difficult to draw meaningful conclusions based on current literature data27, 28 as many studies have short follow up, have measured the markers after graft occlusion, or did not reach significance. Ensuing inflammatory response in the vascular wall following vein grafts as a marker of graft patency is a seductive perspective. Mechanisms that are the basis of this response should be clarified. However, changes in the concentration of MMPs have been previously assessed in patients undergoing revascularisation of arterial districts other than peripheral limb, such as coronary arteries29 or carotid vessels.30 An increase in tissue inflammatory cytokines (TNF‐α and IL‐6) after rabbit common carotid artery injury suggesting their mutual role in promoting the restenotic process has been reported,31 and an increase of IL‐6 in restenotic plaques of patients affected with PAD has been also described.32
We left all foot amputations open because of the extremely complex foot trauma and advanced arterial disease. It is possible that NPWT may have exerted a beneficial effect in the healing process in this clinically difficult setting.33, 34, 35, 36 Plantar flap primary closure is attempted when a rich arterial network from the plantar arteries is present,17 and the wound can be closed without skin tension.37
We are aware that our study has some limitations: the small sample size; the retrospective design of the study; and measuring soluble molecules in peripheral venous samples but not at the level of amputation or reconstruction. Thus, we assumed that the concentration of soluble forms reflects the inflammation process within the amputation site or the graft. Despite the above‐mentioned limitations, present data are encouraging, and their applicability to clinical practice might be extremely helpful.
In conclusion, we recommend monitoring the healing process in PAD patients who underwent peripheral revascularisation and minor amputation with our panel of plasma biomarkers. We are confident that these markers reflect a local inflammatory status able to determine a systemic plasma increase and to predict wound healing after midfoot amputation and the fate of the vein graft.
Sapienza P, Mingoli A, Borrelli V, et al. Inflammatory biomarkers, vascular procedures of lower limbs, and wound healing. Int Wound J. 2019;16:716–723. 10.1111/iwj.13086
REFERENCES
- 1. Matsuzaki K, Hayashi R, Okabe K, Aramaki‐Hattori N, Kishi K. Prognosis of critical limb ischemia: major vs. minor amputation comparison. Wound Repair Regen. 2015;23:759‐764. [DOI] [PubMed] [Google Scholar]
- 2. Serra R, Grande R, Scarcello E, Buffone G, de Franciscis S. Angiosome‐targeted revascularisation in diabetic foot ulcers. Int Wound J. 2015;12:555‐558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vitti MJ, Robinson DV, Hauer‐Jensen M, et al. Wound healing in forefoot amputations: the predictive value of toe pressure. Ann Vasc Surg. 1994;8:99‐106. [DOI] [PubMed] [Google Scholar]
- 4. Caruana L, Formosa C, Cassar K. Prediction of wound healing after minor amputations of the diabetic foot. J Diabetes Complications. 2015;29:834‐837. [DOI] [PubMed] [Google Scholar]
- 5. Becker F, Robert‐Ebadi H, Ricco JB, et al. Chapter I: Definitions, epidemiology, clinical presentation and prognosis. Eur J Vasc Endovasc Surg. 2011;42(Suppl 2):S4‐S12. [DOI] [PubMed] [Google Scholar]
- 6. Criqui MH, Aboyans V. Epidemiology of peripheral artery disease. Circ Res. 2015;116:1509‐1526. [DOI] [PubMed] [Google Scholar]
- 7. Cermak J, Key NS, Bach RR, Balla J, Jacob HS, Vercellotti GM. C‐reactive protein induces human peripheral blood monocytes to synthesize tissue factor. Blood. 1993;82:513‐520. [PubMed] [Google Scholar]
- 8. Ballou SP, Lozanski G. Induction of inflammatory cytokine release from cultured human monocytes by C‐reactive protein. Cytokine. 1992;4:361‐368. [DOI] [PubMed] [Google Scholar]
- 9. Jacob MP. Extracellular matrix remodeling and matrix metalloproteinases in the vascular wall during aging and in pathological conditions. Biomed Pharmacother. 2003;57:195‐202. [DOI] [PubMed] [Google Scholar]
- 10. Sapienza P, di Marzo L, Borrelli V, et al. Basic fibroblast growth factor mediates carotid plaque instability through metalloproteinases‐2 and ‐9 expression. Eur J Vasc Endovasc Surg. 2004;28:89‐97. [DOI] [PubMed] [Google Scholar]
- 11. Borrelli V, di Marzo L, Sapienza P, Colasanti M, Moroni E, Cavallaro A. Role of PDGF and TGF‐b1 in the regulation of metalloproteinases expressions. Surgery. 2006;140:454‐463. [DOI] [PubMed] [Google Scholar]
- 12. De Caridi G, Massara M, Spinelli F, et al. Matrix metalloproteinases and risk stratification in patients undergoing surgical revascularisation for critical limb ischaemia. Int Wound J. 2016;13:493‐499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dayal R, KC. Standardized reporting practices. Rutherford Vascular Surgery. 6th ed. Philadelphia, PA: Elsevier Saunders Inc; 2005:41‐52. [Google Scholar]
- 14. Authors/Task Force Members , Aboyans V, Ricco JB, et al. Editor's choice—2017 ESC guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European Society for Vascular Surgery (ESVS). Eur J Vasc Endovasc Surg. 2018;55:305‐368. [DOI] [PubMed] [Google Scholar]
- 15. American Society of Anesthesiologists . ASA Physical Status Classification System. Last approved by ASA House of Delegates on October 15, 2014. http://www.asahq.org/~/media/sites/asahq/files/public/resources/standards-guidelines/asa-physical-status-classification-system.pdf.
- 16. Klein AJ, Feldman DN, Aronow HD, et al. SCAI expert consensus statement for aorto‐iliac arterial intervention appropriate use. Catheter Cardiovasc Interv. 2014;84:520‐528. [DOI] [PubMed] [Google Scholar]
- 17. Sapienza P, Venturini L, Grande R, et al. Is the endovascular treatment of mild iliac stenoses worthwhile to improve wound healing in patients undergoing femoro‐tibial bypass? Ann Vasc Surg. 2018;47:162‐169. [DOI] [PubMed] [Google Scholar]
- 18. Serra R, Grande R, Buffone G, et al. Extracellular matrix assessment of infected chronic venous leg ulcers: role of metalloproteinases and inflammatory cytokines. Int Wound J. 2016;13:53‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Glass GE, Chan JK, Freidin A, Feldmann M, Horwood NJ, Nanchahal J. TNF‐alpha promotes fracture repair by augmenting the recruitment and differentiation of muscle‐derived stromal cells. Proc Natl Acad Sci U S A. 2011;108:1585‐1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sarén P, Welgus HG, Kovanen PT. TNF‐alpha and IL‐1beta selectively induce expression of 92‐kDa gelatins by human macrophages. J Immunol. 1996;157:4159‐4165. [PubMed] [Google Scholar]
- 21. Amato B, Coretti G, Compagna R, et al. Role of matrix metalloproteinases in non‐healing venous ulcers. Int Wound J. 2015;12:641‐645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Parks WC, Wilson CL, López‐Boado YS. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat Rev Immunol. 2004;4:617‐629. [DOI] [PubMed] [Google Scholar]
- 23. Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999;341:738‐746. [DOI] [PubMed] [Google Scholar]
- 24. Wysocki AB, Staiano‐Coico L, Grinnell F. Wound fluid from chronic leg ulcers contains elevated levels of metalloproteinases MMP‐2 and MMP‐9. J Invest Dermatol. 1993;101:64‐68. [DOI] [PubMed] [Google Scholar]
- 25. Mätzke S, Biancari F, Ihlberg L, et al. Increased preoperative c‐reactive protein level as a prognostic factor for postoperative amputation after femoropopliteal bypass surgery for CLI. Ann Chir Gynaecol. 2001;90:19‐22. [PubMed] [Google Scholar]
- 26. Monahan TS, Owens CD. Risk factors for lower‐extremity vein graft failure. Semin Vasc Surg. 2009;22:216‐226. [DOI] [PubMed] [Google Scholar]
- 27. Owens CD, Ridker PM, Belkin M, et al. Elevated C‐reactive protein levels are associated with postoperative events in patients undergoing lower extremity vein bypass surgery. J Vasc Surg. 2007;45:2‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Blann AD, Adams R, Ashleigh R, Naser S, Kirkpatrick U, McCollum CN. Changes in endothelial, leucocyte and platelet markers following contrast medium injection during angiography in patients with peripheral artery disease. Br J Radiol. 2001;74:811‐817. [DOI] [PubMed] [Google Scholar]
- 29. Kalela A, Limnell V, Aittoniemi J, et al. Serum matrix metalloproteinase‐9 and venous bypass graft occlusion. Scand J Clin Lab Invest. 2006;66:7‐14. [DOI] [PubMed] [Google Scholar]
- 30. Sapienza P, Borrelli V, di Marzo L, Cavallaro A. MMP and TIMP alterations in asymptomatic and symptomatic severe recurrent carotid artery stenosis. Eur J Vasc Endovasc Surg. 2009;37:525‐530. [DOI] [PubMed] [Google Scholar]
- 31. Liang JJ, Xue W, Lou LZ, et al. Correlation of restenosis after rabbit carotid endarterectomy and inflammatory cytokines. Asian Pac J Trop Med. 2014;7:231‐236. [DOI] [PubMed] [Google Scholar]
- 32. Krishnan P, Purushothaman KR, Purushothaman M, et al. Enhanced neointimal fibroblast, myofibroblast content and altered extracellular matrix composition: Implications in the progression of human peripheral artery restenosis. Atherosclerosis. 2016;251:226‐233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. De Caridi G, Massara M, Greco M, et al. VAC therapy to promote wound healing after surgical revascularisation for critical lower limb ischaemia. Int Wound J. 2016;13:336‐342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ubbink DT, Westerbos SJ, Nelson EA, Vermeulen H. A systematic review of topical negative pressure therapy for acute and chronic wounds. Br J Surg. 2008;95:685‐692. [DOI] [PubMed] [Google Scholar]
- 35. Lo Torto F, Ruggiero M, Parisi P, Borab Z, Sergi M, Carlesimo B. The effectiveness of negative pressure therapy on infected wounds: preliminary results. Int Wound J. 2017;14:909‐914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lo Torto F, Monfrecola A, Kaciulyte J, et al. Preliminary result of incisional negative pressure wound therapy and pectorals major muscle flap for median sternotomy wound infection in a high‐risk patient population. Int Wound J. 2017;14:1335‐1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ammendola M, Sacco R, Butrico L, Sammarco G, de Franciscis S, Serra R. The care of transmetatarsal amputation in diabetic foot gangrene. Int Wound J. 2017;14:9‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]


