Abstract
This literature review aimed to provide a narrative review of evidence on validity of clinical and microbial indicators of infection and to gain insights into the diagnosis of infection in chronic leg ulcers (CLUs). A search was conducted in Cinahl, Medline, the Cochrane Library databases, Embase, Web of Science, ScienceDirect, Pubmed, PsycINFO, ProQuest dissertations, and Google Scholar from January 1990 to July 2017. The inclusion criteria were original studies, systematic reviews, and consensus documents focused on “infection” in CLUs, English language, clinical and community settings, and human. The reviewed studies were inconsistent in criteria for infection between investigated wound types and lack of specificity regarding wound types. There were few studies investigating the criteria for diagnosis of infection in leg ulcers. The identification of leg ulcer infection still remains problematic and relies on out‐of‐date and not uniform evidence. Literature in this area was mostly limited to level III and IV evidence based on The Australian National Health and Medical Research Council Levels of Evidence, or expert opinion. This literature review showed seven clinical signs and symptoms that could be diagnostic for infection in CLUs, including: new, increased, or altered ulcer pain; malodour; increased ulcer area; wound breakdown, delayed or non‐healing; and erythema and increased local temperature, whilst the microbial indicators used to diagnose infected leg ulcers were varied and regarded as less important.
Keywords: diagnosis, identification, indicator, infection, leg ulcer
1. INTRODUCTION
Chronic wounds are defined as wounds that do not heal in a timely and orderly manner, whilst chronic leg ulcers (CLUs) are chronic wounds that are located below the knee.1 CLUs contribute almost 70% of chronic wounds.2 To date, the management of chronic wounds, especially infection, is still a challenging problem because of prolonged healing and reoccurrence. Clinically infected ulcers can result in serious consequences for patients, which can increase the burden to patients, health care systems, and society.3 Whilst many studies focus on management of chronic wound infection, the diagnosis of infection remains problematic and debatable between health professionals. This narrative review aimed to gain insights into the diagnosis of infection in CLUs in the literature from the last three decades.
2. METHODS
2.1. Aims
This literature review aimed to assess the available evidence on diagnosis of infection in CLUs by examining the clinical signs, symptoms, and standards used to diagnose infection in CLUs in the past three decades. The literature review explored the following questions:
How has infection in CLUs been diagnosed?
Which clinical signs and symptoms of infection and microbial indicators have been used to diagnose infected leg ulcers?
What clinical and microbial indicators have been identified as diagnostic of CLU infection?
2.2. Search Strategy
An extensive search for relevant published literature of the online databases CinaHl, Medline, Cochrane Library databases, Embase, Web of Science, ScienceDirect, Pubmed, PsycINFO, ProQuest dissertations, and Google Scholar was undertaken. Because of limited evidence on diagnosing infection in CLUs, this article reviewed literature published from January 1 1990 to July 31 2017. To avoid the accidental exclusion of any relevant studies, broad terms were used. The search terms were: “infect*” AND “leg ulcer*” OR “mixed ulcer*” OR “Venous ulcer*” OR “arterial ulcer*” OR “varicose ulcer*” OR “lower leg ulcer*” OR “lower leg wound*” OR “chronic wound*” with further resources as cited in relevant articles. Publications were restricted to those published in English, with abstracts available and studies conducted on adult humans.
2.3. Inclusion criteria
This literature review focused on studies that examined clinical signs and symptoms of localised and/or spreading infection in CLUs including venous, arterial, and mixed leg ulcers; studies investigating accuracy of using clinical and microbial indicators to diagnose infection; and/or those evaluating the specificity and sensitivity of available suggested criteria for diagnosis of infection. Because of the limited available literature, this review also included studies that investigated treatments for infection in CLUs, but only to examine how infection has been diagnosed and which indicators have been used to diagnose infection. This review included all quantitative studies, such as randomised controlled trials, cohort studies, case–control studies, cross‐sectional studies, and case studies and case series. Systematic review articles were also included if they met the inclusion criteria.
2.4. Exclusion criteria
Those studies that included participants with wound types other than CLUs were excluded. Articles were also excluded if the studies did not clearly describe the criteria used to diagnose wound infection. Studies that used qualitative designs were excluded from this review.
2.5. Levels of evidence
The Australian National Health and Medical Research Council Levels of Evidence were used to rate the findings from reviewed research articles,4 as follows:
| Level I | Evidence from a systematic review of level II studies |
| Level II | Evidence from: a study of test accuracy with an independent, blinded comparison with a valid reference standard, among consecutive persons with a defined clinical presentation in diagnostic accuracy; or a randomised controlled trial study in intervention studies; or a prospective cohort study in prognosis studies |
| Level III‐1 | Evidence from: a study of test accuracy with: an independent, blinded comparison with a valid reference standard, among non‐consecutive persons with a defined clinical presentation in diagnostic accuracy; or a pseudorandomised, controlled trial study in intervention studies |
| Level III‐2 | Evidence from: a comparison with reference standard that does not meet the criteria required for level II and III‐1 evidence in diagnostic study; or a comparative study with concurrent controls (such as non‐randomised, experimental, cohort study, case–control study, interrupted time series with a control group) in intervention studies; or retrospective cohort study |
| Level III‐3 | Evidence from: diagnostic case–control study in diagnostic studies; or a comparative study without concurrent controls, such as historical control and two or more single arm study, interrupted time series without a parallel control group |
| Level IV | Evidence from: study of diagnostic yield (no reference standard) or case series with either post‐test or pretest/post‐test outcomes |
The first reviewer assessed the articles for levels of evidence and sent a narrative summary of the primary results to two other reviewers along with the articles for independent assessment. All disagreements were resolved by discussion and a narrative synthesis of results was undertaken.
3. RESULTS
The search located 8134 articles and 80 articles were included for final full‐text review (Figure 1). There was one systematic review—level I evidence5 which compared the value in identifying pathogens between wound swab and wound biopsy techniques. Six expert opinion or consensus documents, which focused on diagnosis of infection in chronic wounds and/or CLUs were also included.3, 6, 7, 8, 9, 10 Of the remaining 73 articles, eleven focused on clinical signs and symptoms of infection in chronic wounds, 12 focused on diagnosis of infection, and 50 examined the effectiveness of numerous treatment methods for chronic wound infection.
Figure 1.
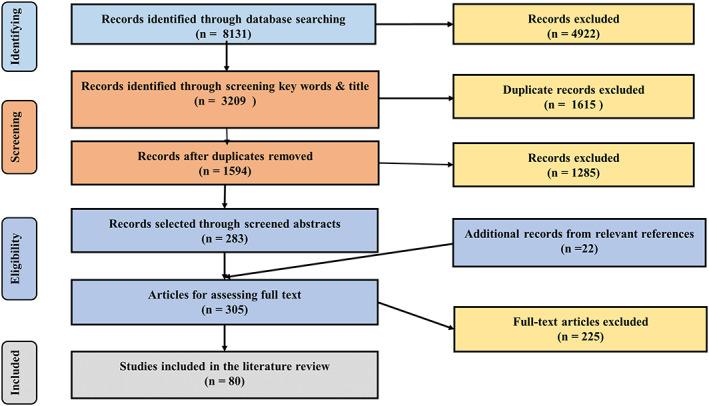
Literature selection process
The level of evidence for most of the studies in relation to diagnosis of infection was low (level II‐IV), with only one study meeting the criteria for level II evidence. The details of level of evidence of these 73 articles are as follows: twelve randomised controlled trials (level II), however, these studies focused on testing the effectiveness of dressings or treatment‐related on patients with infected leg ulcers11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21; one cohort study (level II) examined the effectiveness of a dressing on the bacteria in patients with venous leg ulcers (VLUs)22; 40 level III evidence studies; and nineteen level IV evidence studies, including case series, case studies, and case series with pretest/post‐test outcomes. There was one systematic review that focused on the effectiveness of silver dressing in treatment of chronic wound infection.23 Participant numbers ranged from 1 to 482 with a total of 1274 CLUs (including venous, arterial, and mixed leg ulcers).
Most studies were conducted in Canada and the United Kingdom, however, others were also conducted in United Sates, Germany, and Spain, followed by France and Netherlands. Studies were conducted in different settings, mainly wound clinics, outpatient clinics, and dermatology departments in hospitals or community settings.
This article will first (a) discuss the use of the terms “localised infection,” “critical colonisation,” and the infection continuum in CLUs and/or in chronic wounds; and (b) review the definition of infection in chronic wounds and/or CLUs. The evidence on diagnosis of infection and the clinical and microbial indicators of infection in CLUs used from these studies is then synthesised and discussed under the headings of either (c) existing criteria for diagnosis of infection in CLUs, (d) clinical and microbial indicators that have been used to diagnose infection in CLUs, (e) validation of clinical and microbial indicators, (f) how clinical signs and symptoms of infection have been used to diagnose infection in leg ulcers, (g) other indicators of infection, (h) relationships between clinical judgement and microbiological indicators of infection, and (i) how infection in CLUs has been diagnosed. A summary of literature on diagnosis of infection in CLUs concludes this literature review.
3.1. “Critical colonisation,” “biofilm,” “localised infection,” and the infection continuum in CLUs and/or in chronic wounds
The infection continuum was used to explain the progression of a wound from contamination to infection in the presence of bacteria. Whilst some divided it into contamination, colonisation, critical colonisation, localised infection, spreading infection, and systemic infection,24 many others did not include critical colonisation.3, 10 Kingsley25 defined “critical colonisation” as “host defences unable to maintain healthy balance, either too many microbes or too many species in wound base,” which results in “delay in healing”25 (pp53). Kingsley25 suggested that the clinical signs for critical colonisation included delayed healing despite appropriate treatment, slough, and intransigent in odour. Other researchers diagnosed “critical colonisation” in chronic wounds when the wound presents with at least three out of five clinical signs suggesting “heavy bacterial colonisation”12 or infection/inflammation. Even though the term “critical colonisation” is still used by some,11, 12, 26 an international expert panel has excluded this term from the infection continuum since 2008.3 Recently, the wound infection continuum has included biofilm and its role in wound infection.27 The concept of biofilm has been widely recognised in recent years.28, 29 According to Hurlow and Bowler,30 (pp8) biofilm is defined as “bacteria‐derived living material” that “has a cloudy, translucent and viscous, gel‐like appearance,” “forms above granulation tissue,” attaches “firmly to wound tissue,” and “can be carefully peeled away without causing damage to underlying tissue.” Biofilms may be related to “critical colonisation,” a concept that “compromise wound healing without including clear signs of clinical infection”30 (pp9). Therefore, biofilms were not included in this literature review.
3.2. Definitions of infection in chronic wounds
Infection in chronic wounds was defined in different ways. Some authors defined infection based on microbial standards,19, 31, 32, 33 whilst many defined chronic wound infections based on the clinical pathophysiology in the relationship between bacterial virulence and the host defence24, 34, 35, 36 (Table 1). Despite these differences, authors all agreed that in chronic wounds, infection is present when the host loses its ability to fight against the microbial virulence either from one microbe type or when bacteria interact with each other.3, 10, 38 As a result, the level of toxins and bacterial virulence exceed the host's ability to defend itself.3, 39
Table 1.
Definitions of chronic wound infection
| Author(s), year | Definition of infected chronic wounds |
|---|---|
| Daróczy, 200619 | “infection develops if the number of bacteria colonies is so high (105/cm3) that they can cause local and systemic inflammation and toxic symptoms; the number of bacteria depends on more factors: their species and number, the immunological condition of the host organism, the number of bacterial species present, their virulence and synergic connections” – page 83 |
| East et al, 201533 | “Infection is defined as purulence or two or more other local signs of inflammation in any tissue or part of the lower limb” – page 3 |
| Gardner et al, 200132 | “Infected ulcers were defined as those with 105 or greater organisms per gram of viable, soft wound tissue or wounds containing β‐hemolytic Streptococcus at any level” – page 180 |
| Gardner et al, 200631 | “Infected wounds were defined as those containing 1 x 106 or more organisms per gram of tissue” – page 548 |
| Kingsley, 200324 | “Infection can be defined as the process by which organisms bind to tissue, multiply, and then invade tissue and elicit a marked immune response” – page 3 |
| Enoch & Harding, 200335 | “Wound infection is defined as the presence of replicating microorganisms within a wound with a subsequent host response that leads to a delay in healing.” “The signs and symptoms of local infection are redness (erythema), warmth, swelling, pain and loss of function. Foul odour and pus may accompany this” – page 13/26 |
| Harding et al, 201637 | Clinically infected is “defined as a wound that required the use of systemic antibiotics or topical antimicrobials” or “not clinically infected: exhibiting some signs and symptoms of clinical infection, but not requiring antibiotic or topical antimicrobial treatment” – page 443 |
| Bhat et al, 201434 | “Wound infection is defined as the presence of replicating microorganisms within a wound with a subsequent host response that leads to delayed healing.” “It is important that infection is recognised as early as possible” – page 135 |
| Woo & Sibbald, 200936 |
Superficial critical colonisation or convert infection, or localised infection, or increased bacterial burden: “replicating microbial burden in the wound surface compartment with subtle clinical signs of host injury” – page 41 Deep wound infection: “level of microbial burden or virulence has overwhelmed the host responses and the microorganisms cause clinical injury by invading locally (surrounding or deep skin below the wound base) before potential systemic sepsis” – page 41 |
| Wounds Australia, 201110 | “Wound infection can be defined as multiplication of bacteria that overwhelm host defences, resulting in disruption of healing and damage the wound. Wound infection can result in local and systemic host responses” – page 4 |
Many experts categorised infection in chronic wounds into localised, spreading, and systemic infection; whilst others divided it into superficial and deeper wound infection.14, 40, 41 In fact, this current review showed that the terms “infection in chronic wounds” and/or “infection in CLUs” were the most frequently used compared with “critical colonisation” and “localised infection” in chronic wounds and/or in CLUs. Thus, the term “infection in CLUs” was used throughout this literature review.
3.3. Existing criteria for diagnosis of infection in CLUs
From this review, six sets of criteria for diagnosis of infection in CLUs were found. Of these, five sets were for diagnosis of infection in chronic wounds3, 7, 9, 10, 42 and one set was for diagnosis of infection in granulating wounds.6 However, five out of these six criteria were based on expert opinions3, 6, 7, 9, 10 and one was based on a cross‐sectional study of 41 participants (level III)42 (Table 2).
Table 2.
Clinical signs and symptoms of infection according to suggested criteria
| Clinical signs & symptoms | Cutting & Harding 19946 | Gardner 200142 | Cutting & White 20047 | Sibbald, 20079 | WUWHS, 20083 | Wounds Australia 201110 |
|---|---|---|---|---|---|---|
| 2 experts | Cross‐sectional study | 54 experts | 3 experts | 13 experts—international consensus | National consensus | |
| Ulcer‐related pain | Yes, unexpected pain/tenderness | Yes, increasing pain in the ulcer areaa | Yes, change in the nature of pain | No | Yes, new, increased or altered paina | Yes, increased pain/unexpected pain |
| Malodour | Yes | Yesa | Yes | Yes | Yes | Yes |
| Increase in wound size | No | Yes, 4 weeks period: no change or an increased in the ulcer size | Yes | Yes | No | Yes |
| Purulent exudate | Yes | Yes | No | No | Yes | Yes |
| Wound breakdown | Yes | Yesa | No | Yes | Yesa | Yes |
| Delayed/non‐healing | Yes, delayed healing | Yes | No | Yes | Yesa | No |
| Increased exudate levels | No | Yes | Yes | No | ||
| Erythema | No | Yes | Yes | Yes | Yes | Yes |
| Bridging of the epithelium or soft tissue | Yes | No | No | Yes | Yes | |
| Pocketing at base of a wound | Yes | Yes | No | Yes | Yes | |
| Discolouration of granulation tissue | Yes | Yes | Yes | No | No | No |
| Friable granulation tissue | No | Yesa | No | Yes | Yes | No |
| Increased local temperature | No | Yes, within 4 cm from the ulcer margin | Yes | Yes | Yes | Yes |
| Oedema | No | Yes | No | Yes | Yes, peri‐wound oedema | Yes, localised to peri‐wound tissue |
| Cellulitis | Yes | No | Yes | No | No | No |
| Abscess | Yes | No | No | No | No | No |
| Palpable crepitus from gas in soft tissue | No | No | Yes, for arterial leg ulcers | No | Yes | No |
| Slough or necrotic tissue on the wound surface | No | No | No | Yes | No | No |
| Induration | No | No | No | No | Yes | No |
Highly indicative of infection.
All authors of these six sets of criteria were united in regarding microbial indicators as being less important than clinical indicators when diagnosing infection and agreed that infection should be initially diagnosed based on clinical indicators.3, 6, 7, 9, 10, 42 They also agreed that microbial data usage must be considered in accordance with the individual patient, and bacteria growth alone may be not sufficient to confirm infection.3, 6, 7, 9, 10, 42
Cutting and Harding developed a set of criteria in 1994 to diagnose infection in granulating wounds.6 The criteria included 10 clinical signs: three traditional signs (abscess, cellulitis, and discharge) and seven additional signs (delayed healing, discolouration, friable granulation tissue, unexpected pain, pocketing, bridging at wound base, abnormal smell, and wound breakdown).6 Within the timeframe of this search, this was the first developed criteria for diagnosis of wound infection, however, it was for diagnosis of infection in granulating wounds, therefore may not be absolutely appropriate for use in diagnosis infection in CLUs.6, 43 Despite this limitation, many studies are based on this criteria set to diagnose or to develop new criteria for diagnosis of infection in chronic wounds.7, 42
Gardner's 12 clinical signs and symptoms check list (CSSC) in 200142 was developed based on a cross‐sectional study of 41 participants with chronic wounds (including seven patients with CLUs). This highly cited checklist was underpinned by Cutting and Harding's criteria and included twelve clinical signs and symptoms: increased ulcer pain, erythema, oedema, heat, purulent exudate, serous exudate, delayed healing, discolouration of granulation tissue, friable granulation tissue, pocketing at wound base, foul odour, and wound breakdown. In fact, six signs in this CSSC (delayed healing, discolouration, friable granulation tissue, unexpected pain, pocketing at base of wound, abnormal smell, and wound breakdown) were previously suggested by Cutting and Harding.6 Furthermore, when validating this CSSC in a different study, Gardner et al concluded that only increasing pain and wound breakdown were sufficient indicators of infection with 100% specificity, but none of the twelve clinical signs in the CSSC were considered necessary in identifying infection in chronic wounds.32 The authors also suggested further research to confirm the reliability of this CSSC.32 Cutting and White7 found that the “pocketing at base” was not a valid sign. Dennis et al 44 examined the validity of this CSSC by assessing both clinical signs according to this checklist and bacterial loads from 203 patients with CLUs (with 13.3% infected). The authors found that the CSSC was not well structured and insufficient to represent coherent criteria for diagnosis of chronic wound infection.44
The third reviewed criteria were proposed by Cutting and White7 for diagnosis of infection in different types of chronic wounds, including one criteria set for arterial leg ulcers (ALUs) and one for VLUs.7 The Delphi approach was used with 54 wound experts, to generate criteria in which signs and symptoms of infection were based on levels of importance (high, medium, and low).7 This is the only document that suggested the levels of importance for each clinical indicator. Cellulitis was regarded as one of the most important signs of infection in both types of CLUs. However, cellulitis is characterised by local pain, tenderness, local heat, and erythema.24 Thus, the use of cellulitis as an indicator to diagnose wound infection may not be appropriate. Pus or abscess was also excluded from these criteria.7 The importance of other clinical signs was rated differently between VLUs and ALUs. Dry necrosis turning wet was rated more important than increased pain in ALUs whilst increased exudate was considered less important than increased pain in VLUs.7 Overall, the common signs and symptoms of infection in all CLU types included cellulitis, pain, delayed healing, malodour, and wound breakdown.7 However, the number of signs required to confirm infection was not specified.
Sibbald et al9 divided bacterial damage levels into superficial critical colonisation and deep infection, and suggested assessment models for use to diagnose chronic wound infection, NERDS and STONEES. According to the authors, when bacterial virulence increased and the wound no longer healed as expected the clinicians should assess the wound for clinical signs of critical colonisation based on the NERDS model. The NERDS was a mnemonic term of non‐healing wound, exudative wound, red and bleeding wound, debris in the wound, and smell from the wound.9 When the bacteria were not only present within the wound bed but also multiplying and “spreading to the deeper and surrounding tissue,” clinicians should look for clinical signs in the STONEES model, which is size increasing, temperature, os probes to bone, new breakdown, oedema/erythema, exudate, and smell9 (pp9). These models were created based on a review of literature, including Gardner's criteria,42 and Cutting and White's criteria.7, 9 These suggested criteria have been validated in a cross‐sectional study (level III) of 112 patients with chronic wounds (35 CLUs) and found the criteria's specificity for moderate and heavy bacterial growth was low, 0.80 and 0.69, respectively.36 The authors also determined that no single clinical sign was sufficient to diagnose infection; however, any three of these suggested signs can provide a valid indicator for bacterial damage levels.36
The fifth criteria were proposed by the World Union of Wound Healing Societies (WUWHS).3 In this criteria set, the importance of clinical signs and symptoms was rated, and if two or more of the clinical suggested signs were present chronic wounds were more likely to be infected.3 These criteria highlighted the importance of new, increased or altered pain, and delayed or stalled healing.3 However, no attempts were made to differentiate clinical signs of infection between different types of chronic wounds. When these criteria were used to diagnose infection in 192 patients with 211 chronic wounds from a Dutch nursing home, Rondas et al found that pain, increased exudate, erythema, and delayed healing were the only relevant signs to diagnose infected chronic wounds.45 More importantly, no association between clinical signs of infection and microbiological cultures, taken by a Levine‐technique swab, was found. In addition, these criteria have not been validated and no attempts have been made to differentiate clinical signs of infection between different types of chronic wounds.
The last reviewed criteria were proposed by the Australian Wound Management Association in 2011 and were mainly based on the two previous suggested criteria of Cutting and White in 2004 and the WUWHS criteria in 2008.10 This document highlighted the correlation between levels of bacterial impairment and clinical signs of infection, in which local infection was more likely to present if there were some or all clinical signs (Table 2). Although the document is not a clinical practice guideline, it is a useful document with comprehensive and up‐to‐date knowledge about interaction between microorganisms and the wound. However, the current review did not find any evaluation studies on these criteria.
Overall, seven clinical indicators have been consistently suggested to be diagnostic of infection by these experts: (a) new pain, increasing pain or altered pain in the ulcer area; (b) malodour; (c) increase in ulcer area; (d) wound breakdown; (e) delayed healing; (f) erythema; and (g) increase in local temperature (Table 2).
3.4. Validation of clinical indicators of infection in CLUs
The following section examines how suggested clinical signs and symptoms of infection have been explored and/or validated in clinical research. Ten studies of level II to IV evidence focused on examining and/or validating clinical signs and symptoms of chronic wound infection. These included seven studies used a cross‐sectional design,32, 36, 44, 45, 46, 47, 48 three were case studies49, 50, 51 and one study used the Delphi approach8 (Table 3).
Table 3.
Reviewed studies focused on clinical signs and symptoms of chronic wound infection
| Items | Bowler et al46 | Danielsen et al49 | Gardner, Frantz & Doebbeling32 | Tudor50 | Vowden & Vowden51 | Woo & Sibbald36 | Dennis et al44 | Fierheller & Sibbald47 | Cutting et al8 | Rondas et al48 | Rondas et al45 |
| Study design | Descriptive‐cross‐sectional study | Case study | Cross‐sectional study | Case study | cross‐sectional study | Cross‐sectional study | Cross‐sectional study | Cross‐sectional study | Delphi approach | Cross‐sectional study | multi‐centred cross‐sectional study |
| Sample size/number of CLUs | 71 CLUS | 1 VLU | 36 CWs/7 VLUs | 1 MLU | 482 LUs/269 CLUs | 112 CWs/35 CLUs | 203 CLUs | 40 CLUs | 21 experts | 211 CWs/34 CLUs | 72 CWs/4.8% CLUs |
| Level of evidence | III‐2 | IV | III‐2 | IV | IV | III‐2 | III‐2 | II | III‐1 | III‐3 | |
| Overall comment | malodour resulted from microbial synergy | ulcer enlargement related to infection | could not confirm the reliability of the CSSC | increased wound pain related to infection | erythema, smell, and pus were most frequently reported signs | 4 combined signs had sensitivity of 91.6% heavy bacterial growth | CSSC: no clear structure, not a valid tool to measure infection in leg ulcers | increased peri‐wound skin temperature significantly related to wound infection | a causal relationship between wound infection and the occurrence of pain | no significant relationship between clinical & microbial assessments | increased exudate, erythema, and pain were diagnostic signs of infection |
| Increased ulcer pain | – | N/A | Spec 100% | Yes | – | – | – | – | Yes | – | 56.3% |
| Malodour | Yes | N/A | Spec 88% | Yes | Yes | Spec 86% | Yes | Sens 100% | N/A | – | – |
| Increased wound size | N/A | Yes | N/A | N/A | – | Spec 83% | N/A | N/A | N/A | N/A | N/A |
| Purulent exudate | – | N/A | – | Yes | Yes | N/A | Yes | Sens 87% | N/A | – | – |
| Wound breakdown | N/A | N/A | Spec 100% | Yes | – | Spec 89% | Yes | Sens 100% | N/A | N/A | N/A |
| Delayed/non‐healing | N/A | N/A | Sens 81% | N/A | – | – | Yes | Spec 86% | N/A | – | – |
| Increased exudate levels | N/A | N/A | – | – | – | – | – | Sens 100% | N/A | – | 68.8% |
| Erythema | – | N/A | – | Yes | Yes | Sens 87% | – | Spec 92% | N/A | – | 81.3% |
| Bridging of the epithelium | N/A | N/A | N/A | N/A | – | N/A | N/A | N/A | N/A | – | N/A |
| Pocketing at wound base | N/A | N/A | – | N/A | – | N/A | – | Sens 100% | N/A | – | N/A |
| Discoloured granulation | N/A | N/A | – | Yes | – | N/A | – | Sens 93% | N/A | – | N/A |
| Friable granulation | N/A | N/A | Sens 82% | Yes | – | – | – | – | N/A | N/A | – |
| Increased local temperature | – | N/A | Spec 84% | Yes | – |
Spec 71% Sens 76% |
– | Spec 86%–>2FΔ | N/A | – | – |
| Oedema | – | N/A | – | Yes | – | – | N/A | – | N/A | – | – |
| Cellulitis | – | – | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Abscess | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Slough or necrotic tissue | N/A | N/A | N/A | Yes | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Induration | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | – | – |
CLUs, chronic leg ulcers; CSSC, clinical signs and symptoms check list; CWs, chronic wounds; LUs, leg ulcers; MLUs, mixed leg ulcers; N/A, not applicable; Sens, sensitivity; Spec, specificity; VLUs, venous leg ulcers.
Four studies (two cross‐sectional studies,32, 48 one Delphi study8 and one case study50) concluded increasing pain was diagnostic of chronic wound infection. It is essential to differentiate between pain related to infection and pain related to venous hypertension or other causes. Pain related to wound infection located in the ulcer area can be new pain from a previously non‐painful leg ulcer or increased pain in a patient who had experienced ulcer pain before.7 Gardner et al32 determined increasing pain had a specificity of 100% in indicating infection.
Malodour was determined to be significantly associated with chronic wound infection by two cross‐sectional studies,32, 46 to have a significant relationship with heavy bacterial load36, 44 (cross‐sectional studies), and to be the most frequently presented sign in CLUs.41 Malodour was found to have 100% sensitivity to infection in a cross‐sectional study.47 Malodour is the abnormal and unpleasant smell of the wound and can be an indicator for infection.6, 46 The cross‐sectional study of 71 CLUs (43 infected and 28 non‐infected) by Bowler et al46 compared the severity of malodour between infected and non‐infected CLUs and found malodour was rated as three times higher in infected leg ulcers compared with non‐infected CLUs (18.6% and 6.7%, respectively), (level III). When investigating types of bacteria that produced malodour, the authors concluded that the increase in malodour severity may be a result of synergic interactions between anaerobic and aerobic bacteria.46 Gardner et al32 found foul odour had specificity of 88%. However, the sensitivity of malodour was only 36%.32 Furthermore, in a cross‐sectional study of 203 patients with CLUs, Dennis et al44 found malodour was a significant predictor of bacterial load. Woo and Sibbald36 in a cross‐sectional study of 112 chronic wounds (35 CLUs) also found smell was the clinical sign with the second highest specificity in relation to moderate to heavy bacterial growth, using a wound swab culture with the Levine technique. A descriptive study of 482 patients with chronic wounds (269 CLUs) by Vowden and Vowden51 determined malodour was one of the most frequently presented signs of infection (level IV).
Delayed healing was identified to be a sufficient indicator of wound infection in two cross‐sectional studies32, 36 (level III) and to be significantly associated with high bacterial load44 (level III). Delayed healing is identified if the ulcer size shows no change or even increases despite appropriate treatment.52 Fierheller and Sibbald47 used a cross‐sectional design to study 20 participants without wounds and 40 participants with CLUs (22 infected) and determined delayed healing was one of the three most specific signs of infected leg ulcers with a specificity of 86% (level II).
Wound breakdown was found to be a sufficient indicator of infection in chronic wounds32, 36 (level III) and related to increased bacterial load44 (level II). In a cross‐sectional study of 36 patients with chronic wounds, Gardner et al32 found that all wounds presenting with wound breakdown were diagnosed as infected, based on the positive quantitative culture from wound biopsy tissue (level III). Wound breakdown was also found in another cross‐sectional study to have 100% sensitivity to infection in CLUs.47
Despite not being investigated in many studies, increased wound size was identified as having a specificity of 83% in relation to moderate to heavy bacterial growth,36 and was able to diagnose chronic wound infection. Danielsen et al49 reported ulcer enlargement presented in an adult patient with a CLU infected with Pseudomonas aeruginosa (level IV).
Erythema was found to be significantly associated with chronic wound infection in four studies32, 49, 50, 51 (levels III and IV) and a diagnostic sign of infection in chronic wounds in a multi‐centre cross‐sectional study.45 Erythema was also determined to be the most specific factor related to infected leg ulcers with a specificity of 92% in a study conducted in 20 participants without wounds and 40 participants with CLUs by Fierheller and Sibbald47 (level II). Rondas et al used a multi‐centre cross‐sectional design to investigate signs and symptoms that were used to diagnose infection in 72 chronic wounds (5.6% were CLUs) by physicians in Netherlands.45 The authors found erythema present in 81.3% of infected chronic wounds and was used as a diagnostic sign of infection in chronic wounds.45
In terms of exudate, six studies investigated different exudate‐related indicators in relation to diagnosis of infection in chronic wounds.32, 36, 44, 47, 50, 51 An increased level of exudate was able to diagnose chronic wound infection36 (level III) and identified to have 100% sensitivity to infection47 (level II). Purulent exudate was also identified to be associated with infection in chronic wounds in four studies.32, 47, 50, 51 Fierheller and Sibbald found purulent exudate had a sensitivity of 87% for infection in chronic wounds.47 Purulent exudate was also found significantly associated with higher levels of bacterial load.44
Four studies agreed increased local temperature were associated with infection in chronic wounds.32, 36, 47, 50 Increased temperature in the surrounding skin was found to have high specificity for infected leg ulcers. Fierheller and Sibbald47 in a cross‐sectional study determined increased temperature of the surrounding skin was one of the most specific signs for infected leg ulcers with a specificity of 86%. Gardner et al32 determined the specificity of infection by heat was 84% (level III).
Other clinical signs, such as bridging of the epithelium, abscess, and induration, were not investigated in these studies. However, it is important to acknowledge that the presence of clinical signs and symptoms of infection may differ depending on numerous factors. For instance, Gardner et al52 compared clinical signs of leg ulcer infection in patients with and without diabetes, and the authors highlighted that although no relationships between diabetes and the expression of any clinical signs and symptoms of infection have been found, patients with diabetes were less likely to present with erythema compared with those without diabetes (level III).
Overall, seven clinical signs and symptoms were found to have high specificity for infection, including: (a) new pain, increasing pain, or altered pain in the ulcer area; (b) malodour; (c) increase in ulcer area; (d) wound breakdown; (e) delayed or non‐healing; (f) erythema; and (g) increase in local temperature (Table 3). These clinical indicators were diagnostic for infection in CLUs (level II‐IV). It is interesting to note that these clinical signs and symptoms of infection were also the seven clinical indicators recommended from international experts3, 6, 7, 9, 10, 42 (Table 2).
3.5. How have clinical signs and symptoms of infection been used in practice and research?
This review of the literature showed twenty‐two different clinical indicators were used to identify infection in chronic wounds, and the frequency of clinical signs and symptoms of infection used in the literature are represented in Table 4. These clinical signs and symptoms of localised infection were used differently from study to study. The highest number of clinical indicators used for diagnosis of infection per study was 12 whilst the smallest number was one. The most frequently used clinical signs were malodour, erythema, oedema, increased ulcer‐pain, increased exudate levels or purulent exudate, increased local temperature around the wound, delayed or non‐healing, and friable granulation tissue (Table 4).
Table 4.
Clinical indicators used to diagnose “critically colonised” and/or “locally infected” chronic leg ulcers
| Clinical signs & symptoms | Studies | Total leg ulcers | Total studies |
|---|---|---|---|
| Erythema | Forlee et al53; Beele et al80; Gago et al54; Harding et al37; Lazareth et al12; Murphy55; Rondas et al45; Trial et al56; Walker et al57; Woo et al14; Woo et al58; Woo et al41; Fierheller & Sibbald47; Graham59; Lantis & Gendics22; Lisle60; Rossi & Wertzberger61; Sari et al62; Sibbald et al38; Thai et al63; Tudor50; Vowden & Vowden51; Bhat et al34; Braumann et al64; Bruce et al65 | 967 | 25 |
| Malodour | Forlee et al53; Beele et al80; Harding et al37; Lazareth et al12; Murphy55; Rondas et al45; Trial et al56; Vanscheidt et al13; Walker et al57; Woo et al14; Woo et al41; Fierheller & Sibbald47; Gerry et al92; Graham59; Lantis & Gendics22; Lisle60; Rossi & Wertzberger61; Salavastru et al66; Sibbald et al38; Tudor50; Vowden & Vowden51; Woo & Sibbald36; Bhat et al34; Braumann et al64; Bruce et al65 | 1278 | 25 |
| Increased pain or new/unexpected ulcer pain or pain or continuous pain or persistent pain between two dressing changes | Forlee et al53; Murphy55; Vanscheidt et al13; Fierheller & Sibbald47; Martin et al70; Sibbald et al38; Thai et al63; Tudor50; Woo et al14; Woo et al58; Woo et al41; Eisenstein72; Graham59; Lisle60; and Rondas et al45; Alcaraz et al71; Gago et al54; Bhat et al34; Braumann et al64; Bruce et al65; Beele et al80; Trial et al56; Lantis & Gendics22; Derbyshire69 | 426 | 25 |
| Oedema | Forlee et al53; Gago et al54; Harding et al37; Lazareth et al12; Murphy55; Rondas et al45; Trial et al56; Walker et al57; Woo et al14; Woo et al58; Woo et al41; Eisenstein72; Fierheller & Sibbald47; Graham59; Lantis & Gendics22; Rossi & Wertzberger61; Sibbald et al38; Tudor50; Bhat et al34; Braumann et al64; Bruce et al65; Derbyshire69 | 664 | 22 |
| Increased exudate levels | Forlee et al53; Murphy55; Rondas et al45; Woo et al14; Woo et al58; Woo et al41; Fierheller & Sibbald47; Gerry et al92; Lantis & Gendics22; Martin et al70; Salavastru et al66; Sari et al62; Sibbald et al40; Vowden & Vowden51; Bhat et al34; Dryden et al88 | 936 | 16 |
| Moderate to heavy exudate/heavy exudate | Beele et al80; Harding et al37; Lazareth et al12; Alcaraz et al71; Derbyshire69 | 171 | 5 |
| Purulent exudate/pus discharge | East et al33; Forlee et al53; Gago et al54; Rondas et al45; Trial et al56; Walker et al57; Fierheller & Sibbald47; Graham59; Griffiths et al15; Lantis & Gendics22; Nagoba et al68; Sari et al62; Sibbald et al40; Thai et al63; Tudor50; Vowden & Vowden51; Braumann et al64; Dryden et al88 | 576 | 18 |
| Delayed or non‐healing | Murphy55; Rondas et al45; Woo et al14; Woo et al58; Woo et al41; Gerry et al92; Graham59; Lantis & Gendics22; Nagoba et al68; Sibbald et al40; Sibbald et al38; Alcaraz et al71; Banu et al96; Bhat et al34; Derbyshire69; Dryden et al88; Fierheller & Sibbald47 | 231 | 17 |
| Increased temperature around the wound/warmth/heat/hot to touch | Forlee et al53; Beele et al80; Gago et al54; Rondas et al45; Trial et al56; Woo et al14; Woo et al58; Woo et al41; Graham59; Lantis & Gendics22; Lisle60; Rossi & Wertzberger61; Sibbald et al38; Tudor50; Woo & Sibbald36; Braumann et al64; Bruce et al65 | 244 | 17 |
| Friable granulation tissue bleeds easily | Forlee et al53; Beele et al80; Murphy55; Rondas et al45; Vanscheidt et al13; Walker et al57; Fierheller & Sibbald47; Graham59; Lantis & Gendics22; Sibbald et al40; Sibbald et al38; Tudor50; Woo & Sibbald36; Woo et al14; Woo et al58; Woo et al41 | 665 | 16 |
| Discolouration of granulation tissue | Forlee et al53; Beele et al80; Vanscheidt et al13; Walker et al47; Fierheller & Sibbald47; Graham59; Lantis & Gendics22; Nagoba et al68; Sibbald et al40; Tudor50; Woo et al14; Woo et al58; Woo et al41 | 595 | 13 |
| Yellow/slough | Beele et al80; Nagoba et al68; Rossi & Wertzberger61; Sibbald et al40; Thai et al63; Tudor50; Vowden & Vowden51; Alcaraz et al71; Bhat et al34; Derbyshire69; Dryden et al88 | 390 | 11 |
| Wound breakdown or increase in ulcer area | Vanscheidt et al13; Graham59; Sibbald et al38; Tudor50; Woo & Sibbald36; Woo et al14; Woo et al58; Woo et al41; Salavastru et al66; Woo & Sibbald36 | 676 | 10 |
| Necrotic tissue | Forlee et al53; Beele et al80; Sari et al62; Sibbald et al40; Vowden & Vowden51; Dryden et al88 | 381 | 6 |
| Pocketing | Beele et al80; Murphy55; Vanscheidt et al13; Graham59; | 164 | 4 |
| Cellulitis | Vanscheidt et al13; Isbary et al17; Isbary et al16; Salavastru et al66 | 607 | 4 |
| Induration | Murphy55; Rondas et al45; Gerry et al92 | 8 | 3 |
| Bridging of the epithelium | Vanscheidt et al13; Sibbald et al40 | 132 | 2 |
| Abscess | Vanscheidt et al13 | 126 | 1 |
Malodour was used in 25 studies with a total of 1298 CLUs and erythema was used in 25 studies with 811 CLUs12, 14, 22, 34, 37, 38, 41, 45, 47, 50, 51, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66 (Table 4). Malodour has been used in combination with other clinical signs41, 58, 62 and/or microbial indicators.45, 67, 68, 69 However, in a study that investigated the roles of specific bacteria in wound malodour production between 43 infected and 30 non‐infected leg ulcers, the authors found malodour presented in both infected (18.6%) and non‐infected leg ulcers (6.7%), (level III),.46
Ulcer‐related pain was also used in 22 studies including almost 600 CLUs. In these studies, pain was described differently by authors as either continuous pain,53 persistent or spontaneous pain between two dressing changes,22, 57, 70 extreme pain, and increases or changes in the nature of pain13, 54, 56, 71 or pain.34, 55, 65, 66 New, increasing pain or extreme pain or change in the nature of pain was used by most authors.38, 62, 63, 72 According to Cutting et al,8 the experience of pain should be “best described by the patient from their own subjective stand point.”8 (pp79) Increased pain in a wound can be a result from swelling and increased tension in the wound because of increased tissue fluid.6 A case study reported a patient with mixed leg ulcers50 indicated infection was the cause of increased wound pain (level IV). A Delphi approach study of 21 international health experts concluded the majority of experts regarded new ulcer pain, alteration in ulcer pain, or increasing pain in the ulcer area were indicators of wound infection.8
Increased levels of exudate or change in exudate characteristics, including types and consistency, were also frequently reported signs for early identification of infection. The exudate characteristics were used variously among authors, however, there was consistency in assessing ulcer exudate for any negative changes, such as a significant increase in exudate levels, consistency or odour. Purulent exudate was used in 18 studies with more than 550 CLUs, increased exudate level was used in 16 studies with 926 CLUs, and moderate to heavy exudate was used in 5 other studies with more than 170 CLUs. The exudate level was determined based on the percentage of the dressing stained with exudate.36 However, the evaluation of exudate levels remains problematic, as currently there are no guidelines for this assessment.
Oedema was used in 22 studies with a total of 484 CLUs.12, 14, 22, 37, 38, 41, 47, 48, 50, 54, 55, 56, 57, 58, 59, 60, 62, 73
Seventeen studies used increased temperature around the wound or warmth or hot to touch to diagnose infection in nearly 250 CLUs. Seventeen reviewed studies used delayed healing to diagnose infection in 181 CLUs, five studies used increased ulcer area in almost 500 CLUs, and wound breakdown was also used in five studies with almost 200 CLUs. Friable granulation tissue that bleeds easily was used in 16 studies of 395 CLUs.
Cellulitis was still used as a clinical sign to diagnose infection in CLUs in four studies.16, 17, 67, 74
Altogether, seven clinical indicators, including erythema, malodour, oedema, increased ulcer pain, increased or purulent exudate, delayed healing, and increased local temperature, were found to be used most frequently (Table 4). Except for oedema, other frequently used clinical indicators were also found to have higher specificity for infection in chronic wounds, including CLUs (Table 3).
3.6. Timeframe for assessment
Whilst all authors agreed on assessing for any changes in the ulcer appearance, surrounding ulcer, and general patient health, the recommended timeframe for this assessment varied. Some determined changes by comparing measurements within a 4‐week period.11, 36, 53, 75 Others only compared measurements between two dressing changes,12, 37 or did not clearly describe what timeframe they used to determine a change. For instance, with regard to assessing delayed healing, Gardner32 defined delayed healing if the ulcer area did not change or even increased after four weeks of appropriate treatment. The WUWHS stated if the ulcer surface reduced more than 30% in the first two weeks after commencing treatment, the ulcer is more likely to heal.3 According to Jorgensen et al, delayed healing occurs when the ulcer size does not decrease or even increase over a 4‐week period.11, 75 Sibbald et al9 found that a chronic wound should reduce in size from 20% to 40% over a 4‐week period of commencing appropriate treatment to heal by 12 weeks.9
3.7. Microbial and other indicators of infection
In terms of microbial indicators of infection, this review article focused solely on investigating what microbial standards were suggested to be used or have been used, to diagnose infection in CLUs. The details of bacterial types or bacterial profiles, hence, were out of this article's scope. Traditionally, bacteria in the wound can be identified quantitatively (by culturing) or qualitatively from either wound tissue (obtained by biopsy) or wound exudate (Table 5). However, recently molecular testing or deoxyribonucleric acid (DNA) based methods have gained great attention by both qualitative and quantitative identification of bacteria in chronic wound infection.76, 77, 78, 79 Molecular testing included quantitative polymerase chain reaction (qPCR) and DNA sequencing.79 Four studies compared bacterial characteristics in chronic wounds between culture testing and molecular testing,76, 77, 78, 79 (level III‐IV). The authors agreed that molecular testing cannot only detect the majority of bacteria that were detected by culture methods but also bacteria not detected by culture methods.76, 77, 78, 79 However, further research is needed as molecular testing such as qPCR may not be available to test for some types of bacteria.77
Table 5.
Microbial indicators used to diagnose infected leg ulcers
| Microbial indicators | Authors, year | Total leg ulcers | Total studies |
|---|---|---|---|
| Culture positive | Alcaraz & Kelly71; Danielsen et al49; Flock et al67; Imbernon et al93; Imbernon‐Moya et al91; Isbary et al17; Isbary et al16; Lei et al20; Madhusudhan21; Lisle60; Gerry et al92; Graham59; Nagoba et al68; Martin et al70; Rossi & Wertzberger61; Sari et al62; Thai et al63; Tudor50; Salavastru et al66 | 617 | 20 |
| Bacterial load >105 CPU/g of tissue | Bhat et al34; Gardner et al32; Gardner et al52; Peral et al75; Lantis & Gendics22; Raad et al81 | 127 | 6 |
| Bacterial load >105 CPU/mL fluid or/cm2 fluid | Daróczy19; Kordestani et al18; Sibbald et al38; Woo et al36 | 141 | 4 |
| β‐haemolytic Streptococcus at any levels | Gardner et al32; Gardner et al52 | 14 | 2 |
| Bacterial load >106 CPU/g of tissue | Sibbald et al40 | 20 | 1 |
| If beta‐haemolytic streptococcus present then ≥103 CFU/mL | Kordestani et al18 | 20 | 1 |
CFU, colony‐forming units.
The microbial indicators for infection are still debatable within clinicians and researchers. This literature review found 30 studies used microbial indicators to diagnose infected leg ulcers, however, the microbial indicators used varied widely (Table 5). A positive culture was used to diagnose infection in 20 studies. Four studies used bacterial load >105 CFU/mL fluid or/cm2 fluid,18, 19, 36, 38 6 studies used bacterial load >105 CFU/g of tissue,22, 32, 52, 75, 81, 82 one study used bacterial load >106 CFU/g of tissue,40 one study used bacterial load ≥103 colony‐forming units (CFU)/mL if there was the presence of beta‐haemolytic streptococcus,18 and two studies diagnosed infection in leg ulcers if there was the presence of beta‐haemolytic streptococcus at any levels.42, 80 The samples for microbial analysis were obtained from wound swabs or biopsy with different techniques. Whilst the microbial indicators were used differently to diagnose CLU infection, there was strong evidence on the complexity of bacteria in CLUs. Bowler et al46 examined bacterial profiles between infected and non‐infected leg ulcers (clinical diagnosis), and noted the complexity of the bacterial profile in CLUs and determined the important role of microbial synergy in CLU infection. As bacteria are always present in CLUs, the longer the wound delays in healing, the more likely it will be exposed to and acquire multiple microorganisms.47, 83, 84
Ten studies used a bacterial load >105 CFU of bacteria per gram of tissue22, 32, 34, 52, 75, 81, 82 or per mL of wound fluid18, 19, 36, 38 to diagnose infection in chronic wounds (Table 5). This popular threshold of 105 CFU has been used as a critical criterion for diagnosis of infection in chronic wounds, reasoning on the induction of local tissue damage in the wound because of the increase in the levels of bacterial‐related toxins and inflammatory mediators.14 Historically, the bacterial load at ≥105 CFU per one gram of tissue that was obtained from wound swabbing, biopsy, or ≥ 105 CFU/mL wound fluid can be seen as an indicator for wound infection.85 However, this bacterial load has been shown not to be accurate in diagnosing infection as there may be some bacteria with high virulence levels, especially when they have microbial synergy. In this case, the bacterial load may be lower than ≥105 CFU/g or ≥ 105 CFU/mL; however their pathogenic effects can be greater than each bacterium working independently.84 Gardner et al32, 42 defined infected leg ulcers when the results from biopsy were at ≥105 organisms per gram of viable wound tissue or at any levels if it contained β‐haemolytic Streptococcus. In fact, the prospective study by Cooper et al82 compared the bacterial load using three different techniques to obtain samples (swab, fluid, and biopsy), and found that the bacterial load depended on the wound size and the duration of the wound; as the highest bacterial load was found in the largest and the longest ulcers.82 In a prognostic study with a primary aim of evaluating the diagnostic properties of three enzymes identified from wound fluid of 81 patients with acute or chronic wounds (11 CLUs), Blokhuis‐Arkes et al85 examined the relationship between clinical and microbiological diagnosis of infection and found no relationship between the clinical judgement and microbiological results.
A systematic review compared the Levine or Levine‐like technique for wound swabs to the biopsy technique used to obtain samples for cultures of infected wounds. The authors found that for chronic wounds, including VLUs, both types of techniques were comparable for initial wound monitoring; however, swabs were better when performing quantitative analysis. The swabs were also found to be most valuable for identifying pathogens in infected diabetic foot ulcers that did not involve bone.5 Gardner et al31 in a study of 83 patients with chronic wounds (5 VLUs) defined “true” wound infections if the bacterial load from quantitative cultures was ≥106 organisms per gram of viable wound tissue and compared three techniques to obtain samples, which included wound biopsy, wound swab with the Z technique, and wound swab with the Levine's technique. The authors found that the Levine's technique resulted in the highest accuracy of quantitative cultures compared with the Z‐technique and with biopsy to obtain the specimens.31
3.8. Relationship between clinical judgement and microbiological indicators of infection
This literature review found no studies reporting a significant relationship between clinical signs and symptoms of infection and microbiological results, including the qualitative and quantitative results. Rondas et al48 examined 192 patients with a total of 211 CLUs and found no significant relationships between the clinical diagnosis of infection (using the WUWHS, 2008 criteria) and standardised wound swab results, using the Levine technique. With regard to how microbial results were used in wound infection, a survey of 345 health professionals, with approximately 10‐year experience in wound care across the United States, found that wound infections were mainly diagnosed based on clinical signs and symptoms, and that of those clinically diagnosed as infected, only 60% were cultured.95
3.9. How has infection in CLUs been diagnosed?
The standards for diagnosis of infection in CLUs have been used differently between researchers. This literature review included 50 studies focusing on treatment of critically colonised or localised infected chronic wounds, and one study evaluating the activities of numerous enzymes to diagnose infection.87 Of these studies, 20 studies used clinical indicators only to diagnose infected leg ulcers (Table 6) whilst six studies solely used the microbial standards (Table 8). Twenty‐five studies used both clinical and microbial indicators to diagnose CLU infection (Table 7).
Table 6.
Studies that used clinical indicators only to diagnose infection in chronic leg ulcers
| Author(s), year | Study design | Sample size & Total leg ulcers | Clinical indicators used |
|---|---|---|---|
|
Beele et al, 201080 Belgium and Netherlands |
A randomised, prospective, open label, multi‐centre, multi‐national trial Compared antimicrobial effects of an ionic silver alginate/carboxymethylcellulose dressing with a non‐silver calcium alginate fibre dressing |
36 clinically, critically colonised wounds: 24 CLUs | Continuous pain erythema, warmth, Moderate to serious exudates, >50% yellow/slough, discolouration of granulation tissue, friable granulation tissue that bleeds easily, pocketing at wound base, foul odour, and necrotic |
|
Braumann et a., 201164 Germany |
A cohort study |
52 wounds: 2 CLUs 12 infected wounds |
Pus, malodour, pain, erythema, oedema, and warmth |
|
Bruce et al, 201265 United Kingdom and Ireland |
A multi‐centre pre‐post evaluation study | 14 chronic wounds: 10 CLUs | Erythema, heat, oedema, pain, and odour |
| Derbyshire, 201069 | Case studies | 3 cases: 2 CLUs | Delayed healing, heavy slough, extreme pain between dressing changes, heavy exudate; swelling |
| Dryden et al, 201688 |
Non‐comparative pre‐post evaluation study in a multi‐centre, international setting To explore the clinical effects of Surgihoney RO, a topical wound dressing in bacterial load, biofilm and healing |
114 clinically infected wounds: 33 CLUs | Non‐healing, wound deterioration, green‐tinged or purulent/haemopurulent/seropurulent exudate, heavy or moderate level of exudate, slough or necrotic tissue. |
|
Forlee et al, 201453 South Africa |
A prospective, open, multi‐centre observational study To assess the clinical acceptability of the new gelling fibre dressing containing silver DURAFIBER Ag |
14 VLUs: 12 clinically infected | Wound static or deteriorating, increased exudate/secretion levels, increased pain, increased temperature around the wound, discolouration of granulation tissue, friable granulation, tissue necrosis, local erythema, oedema, purulent drainage, and odour |
|
Gago et al, 200854 Spain |
A prospective, comparative, observational study To compare 3 types of silver dressing: Acticoat Comfeel Ag, hydrocolloid/Biatain Ag polyurethane foam; and Aquacel Ag |
75 patients with infected chronic wounds: 50 leg ulcers | Pain, redness, heat, oedema, and/or purulent exudate |
|
Harding et al, 201637 United Kingdom |
A pre‐market non‐comparative controlled trial study To investigate the safety and performance of a next‐generation antimicrobial dressing AQUACEL Ag+ |
42 patients with clinically infected VLUs | Pain between two dressing changes, peri‐ulcer skin erythema/inflammation, oedema, malodour, and heavy exudate |
|
Jørgensen et al, 200511 15 centres in 7 countries |
A multi‐centre, open, block‐randomised and controlled trial study To compare the effect of a sustained silver‐release foam dressing Contreet Foam with a foam dressing Allevyn Hydrocellular without added silver in critically colonised VLUs with delayed healing |
129 patients with CLUS, critically colonised | Wound healing stalled or delayed compared with the normal expectation for the patient; increased exudate levels within the past 4 weeks; increased pain in the study ulcer area within the past 4 weeks; discolouration of granulation tissue; and foul odour “clinical infection including erysipelas and cellulitis of periulcer skin” |
|
Jorgensen et al, 200874 Denmark |
An open non‐comparative observational study to investigate the effect and safety of Biatain‐Ibu combined with an ionised silver‐releasing wound contact layer‐ Physiotulle Ag | 24 patients with locally infected VLUs | Painful; discolouration of the granulation tissue; exuding, Wound healing stalled or delayed compared with the normal expectation for the patient; and malodour |
|
Lazareth et al, 201212 France |
An open‐labelled, randomised, controlled trial for 4 weeks To assess the ability of a silver lipidocolloid contact layer in comparison with the same wound dressing not impregnated with silver salts to promote the healing process |
102 patients with “heavy bacterial colonisation” VLUs | Pain between 2 dressing changes, peri‐wound erythema, oedema, foul odour, and heavy exudate. Patients presented with at least 3/5 local signs of heavy bacterial colonisation |
| Meaume et al, 200589 |
A randomised open‐label multi‐centre comparative two‐arm parallel‐group trial 13 centres recruited 99 patients with either VLUs or PUs to evaluate the clinical impact of using a silver‐releasing hydro‐alginate dressing |
99 critically colonised chronic wounds: 71 VLUs | Continuous (spontaneous) pain, erythema, oedema, increase local warmth, moderate to high levels of exudate, at least 50% of the wound covered with yellow slough, discoloured or friable granulation tissue, pocketing or undermining at the base of the wound, or foul odour |
|
Murphy, 201655 United Kingdom |
4 case studies To describe the effect of Zorflex |
4 VLUs |
Case 1: painful 7/10, green slough; Case 2 yellow slough ~50%, heavy exudate, the wound failed to progress Case 3 painful non‐healing ulcer, the wound bed was red & inflamed, ~60% slough, wound was static, heavy exudate, excoriation to peri‐wound, failed to respond to treatment Case 4 the wound deteriorated, very painful 7/10, high volumes of exudate caused peri‐wound maceration |
|
Trial et al, 201056 France |
Prospective, open‐label, controlled, and randomised trial To compare the efficacy and tolerability of a new ionic silver alginate matrix Askina Calgitrol Ag with that of a standard silver‐free alginate dressing Algosteril |
42 locally infected chronic wounds: 12 leg ulcers | Clinical infection score: (0–18): fever, local heat, peri‐lesional erythema; persistent pain between 2 dressing changes, oedema, malodour, pus, exudate production |
|
Vanscheidt et al, 200373 Germany |
A multi‐centre, non‐comparative, non‐randomised, pilot trial To evaluate primarily the safety and the initial performance of the ionic silver dressing Aquacel Ag |
15 patients with CLUs: 11 clinically infected | Cellulitis, pain, slough, discharge, erythema, and friable granulation tissue |
|
Vanscheidt et al, 201213 43 centres in the United Kingdom, Germany, France, Denmark and Poland |
International, multi‐centre, double‐blind and randomised controlled clinical trial To evaluate the cytotoxic effect of octenidine dihydrochloride/phenoxyethanol in comparison with Ringer solution |
126 patients with locally infected chronic VLU | Presence of at least 2/9: abscess, cellulites, discharge, discolouration, friable granulation tissue that bleeds easily, unexpected pain/tenderness or change in the nature of pain, pocketing at base of wound or wound breakdown, bridging of the epithelium or soft tissue and abnormal smell |
|
Walker et al, 201557 Canada, United Kingdom, Germany, Denmark, Croatia, Spain, Lithuania, Italy, Czech, Rep Sweden, Bulgaria, Portugal, Slovakia, Netherlands |
An international, multi‐centre, pragmatic, non‐randomised observational study To assess the effectiveness of AQUACEL Ag + dressing in facilitating healing in a variety of hard‐to‐heal wounds that may have been compromised by infection and/or biofilm |
113 patients: 59 CLUs | Purulent exudate, erythema, oedema, malodour, friable granulation tissue, and discoloured granulation tissue |
|
Woo et al, 201214 Canada |
A prospective, open‐label, 4‐week randomised controlled trial To evaluate the effectiveness of a topical silver dressing that consists of silver alginate powder (Arglaes Powder) compared with moisture balance with foam alone‐ Optifoam |
34 critically colonised chronic wounds: 13 CLUs |
A standardised upper—critical colonisation: unhealthy tissue, pain, poor healing, exudate, and reek Lower—deep infection: larger in size, osseous tissue, warmth, oedema, and redness |
|
Woo et al, 201258 Canada |
Case series: 9 patients To evaluate the application of transdermal continuous topical oxygen therapy to promote healing in chronic wounds |
9 patients with CLUs |
Upper: unhealthy tissue, pain, poor healing, exudate, and reek for superficial wound infection Lower: larger size, osseous tissue, warmth, oedema, and redness for deep wound infection |
|
Woo & Heil, 201741 Canada |
Prospective, non‐randomised observational study To evaluate the performance of an antibacterial foam dressing containing methylene blue and gentian violet (Hydrofera Blue Classic dressing) |
29 participants CLUs with localised infection |
Upper: unhealthy tissue, pain, poor healing, exudate, and reek Lower: larger in size & new areas of breakdown, osseous tissue, warmth, oedema, and redness |
CLUs, chronic leg ulcers; PUs, pressure ulcers; VLUs, venous leg ulcers.
Table 8.
Studies that used microbial indicators only to diagnose infection in chronic leg ulcers
| Author(s), year | Study design | Number of CLUs | Microbial indicators used |
|---|---|---|---|
|
Daróczy, 200619 Hungary |
Prospective randomised controlled trial To assess the effectiveness of (a) topical povidone‐iodine with and (b) without compression bandages, (c) to compare the efficacy of systemic antibiotics and topical antimicrobial agents to prevent the progression of superficial skin ulcers. |
63 patients with infected VLUs | Wound swab: the number of bacteria colonies is so high (105 Colonies/cm3) |
|
Dubhashi & Sindwani, 201590 India |
A prospective comparative study To evaluate the use of honey and phenytoin with respect to the process of wound healing, eradication of infection, pain relief and hospital stay |
150 patients: 32 wound infections, 22 VLUs | Culture positive swabs: MRSA was the most common organism isolated in the study (16%) along with other organisms like Pseudomonas and Klebsiella |
|
Imbernon‐Moya et al, 201791 Spain |
3 cases of a chronic venous ulcer infected by multi‐resistant bacteria including Pseudomonas aeruginosa and methicillin‐resistant Staphylococcus aureus | 3 infected VLUs | Culture positive infected by multi‐resistant bacteria including P. aeruginosa and methicillin‐resistant Staphylococcus aureus |
|
Lei et al, 201520 China |
A randomised controlled experiment | 26 patients with CLUs infected with P. aeruginosa | Bacterial culture confirmed P. aeruginosa |
|
Madhusudhan, 201621 India |
A prospective randomised controlled clinical trial over a period of 6 mo | 32 patients with chronic wounds infected with P. aeruginosa | Culture proven to be infected with P. aeruginosa |
|
Peral et al, 201075 Argentina |
A prospective uncontrolled study To investigate the effectiveness of bacterio‐therapy with Lactobacillus plantarum on infected chronic venous ulcers and on interleukin‐8 production |
34 patients with VLUs | A bacterial load at a level > 105 microorganisms per gram of tissue |
CLUs, chronic leg ulcers; MRSA, methicillin‐resistant Staphylococcus aureus; VLUs, venous leg ulcers.
Table 7.
Studies that used both clinical and microbial indicators to diagnose infection in chronic leg ulcers
| Author(s), year | Study design & aims | Total CLUs | Clinical indicators used | Microbial indicators used |
|---|---|---|---|---|
| Alcaraz & Kelly, 200271 |
Case study To describe the effect of honey dressing in management of an infected VLU |
1 infected VLU | Sloughy, painful, and very wet with green exudate | Wound swab, culture: heavy growth of Haemolytic streptococci group G, Proteus spp, and moderate growth of anaerobes |
| Bhat et al,201434 |
Single arm before‐after clinical trial design To test the effectiveness of the Panchavalkala cream on chronic non‐healing wounds that were infected |
50 patients with infected chronic non‐healing wounds | Slough, swelling, redness, discharge, Malodour, pain, and tenderness |
Punch biopsy 105–106 dilutions: mildly infected 107–108 dilutions: moderate infected >108 dilutions: Severe infected |
| Danielsen et al, 199849 | Case study | 1 infected VLU | Ulcer enlargement, no cellulitis | Wound exudate cultured found Pseudomonas aeruginosa exotoxin |
|
Eisenstein, 200872 USA |
Case study | 1 infected VLU | Extreme pain and swelling in the left ankle | MRSA and Enterobacter spp. |
| Flock, Gibbs & Sykes, 200067 | Case study | 1 infected VLU | Ulcer related pain, foul odour, mucopurulent discharge, and oedema | Wound swab & culture: mixed flora and anaerobes |
|
Forlee, Rossington, & Searle, 201453 South Africa |
A prospective, open, multi‐centre observational study | 14 VLUs: 12 clinically infected | Wound static or deteriorating, Increased exudate/secretion levels, Increased pain, Increased temperature around the wound, Discolouration of granulation tissue, Friable granulation, Tissue necrosis, Local erythema, Oedema, Purulent drainage, and Odour (Cutting & Harding, 2004) | Positive tissue biopsy results at the initial assessment ≥104 CFU/g |
|
Gerry et al, 200792 USA |
Case study | 1 infected VLU | Wound failed to heal, extensive induration, foul‐smell, and wept turbid fluid | Culture of the wound identified Stenotrophomonas |
|
Graham, 201459 USA |
A pilot observational study To assess the viability of a MRSA wound healing protocol intended for use in multiple settings |
40 patients with MRSA‐infected lower extremity wounds: 10 VLUs | Erythema, oedema, heat, pain, and purulent exudate, odour, serous exudate, delayed healing, friable granulation tissue, discoloured granulation tissue, pocketing of the wound base, and wound breakdown | Wound swab culture positive for MRSA |
|
Griffiths, Fernandez &Ussia, 200115 Australia |
A double‐blind randomised controlled trial | 35 patients with 49 wounds: 5 VLUs | Using Cutting's criteria: purulent discharge | Wound swab culture: mixed growth of Staphylococcus species and Proteus species |
|
Imbernon et al, 201693 Spain |
Case study | An infected VLU in a patient with diabetes | Disabling and highly painful leg, erythematous edges, seropurulent exudate with haemorrhagic scabs | Culture positive for Methicillin Resistance Staphylococcus aureus |
|
Isbary et al, 201017 Germany |
A prospective randomised controlled phase II trial To examine the safety and efficiency of 5 minutes daily cold atmospheric argon plasma to decrease bacterial load |
38 chronic infected wounds in 36 patients: mostly CLUs |
Did not clearly mention Had at least one chronic infected skin wound large enough for the plasma treatment and a control area of 3 cm2 29/36 patients received systemic antibiotics |
Wound swab Semi‐quantitative assessment Bacterial types were detected from the wounds from culture |
|
Isbary et al, 201216 Germany |
A prospective randomised controlled phase II trial Investigated a 2‐min daily plasma treatment with MicroPlaSter alpha device versus MicroPlaSter beta device |
24 patients with chronic infected wounds: 17 VLUs, 4 ALUs, 2 MLUs |
Did not clearly mention Had at least one chronic infected skin wound large enough for the plasma treatment and a control area of 3 cm2 22 patients received systemic antibiotics |
Wound swab culture to identify bacteria present in the wounds |
|
Kordestani et al, 200818 Iran |
A randomised controlled trial study To compare the wound healing rate and incidence of infection in wounds treated with either a bioactive dressing or the control dressing |
54 patients with either diabetic foot ulcers, pressure ulcers, or leg ulcers | Did not mention/describe but needed to show clinical signs of infection |
Wound swab Infected if the bacterial bioburden >105 CFU/mL, or if beta‐haemolytic streptococcus was present then 103 CFU/mL was the indicator of infection |
|
Lantis & Gendics, 201122 United Kingdom |
A prospective cohort study To determine the in vivo effect of a sustained‐release silver sulphadiazine powder foam dressing—Allevyn Ag Non‐Adhesive on the bacterial burden of VLUs |
24 patients with VLUs | ≥1 clinical signs of infection: oedema, malodour, local/peri‐wound erythema, spontaneous pain between dressing changes, increased exudate, discolouration of granulation tissue, increased temperature at wound, non‐progression of wound closure, and purulent exudate or friable granulation tissue | Had a bioburden of ≥105 CFU/g of tissue |
|
Raad et al, 201081 USA |
A retrospective review of 5 cases To determine the in vivo effect of a sustained‐release silver sulphadiazine powder foam dressing on the bacterial burden of VLUs |
5 patients with VLUs | Ulcers greater than 200 cm2 |
Biopsy, Quantitative cultures: bacterial load ≥105 CFU/g of tissue Two patients had multi‐drug‐resistant pseudomonas, three with MRSA. All five had coliforms present as well |
|
Lisle, 200260 England |
Case study | Hot to the touch, red, painful (pain rated at 8 out of 10 by Mrs R) and with offensive smelling exudate | Swabs cultured positive: MRSA, β‐haemolytic streptococci and mixed enteric flora. | |
|
Nagoba et al, 200868 India |
Two cases |
Case 1: unhealthy granulation tissue and slough, delayed healing despite treatment Case 2: the ulcer had a very bad look with abundant slough and active pus discharge |
The culture of the exudates yielded S. aureus – Case 1 A culture of the exudates yielded S. aureus and Escherichia coli – case 2 |
|
|
Martin et al, 200870 Spain |
Case study | 1 infected leg ulcer |
Painful ulcers in both legs, which carried a chronic lymphoedema background increased pain and exudation |
Mixed flora—consisting of multi‐resistant bacterial organisms—was isolated from both legs. In addition, Vibrio metschnikovii was isolated from the left lower limb |
|
Rossi & Wertzberger, 199661 Italia |
Case study | 1 CLU | The wound: 14 × 7 cm, covered with slough, malodorous, warm to touch, erythematous and oedematous to the knee | Positive culture Swabs |
|
Salavastru et al, 201266 Romania |
A retrospective observational study using the hospital's electronic database | 420 patients with VLUs | Increased exudate production, foul odour, rapid extension of the ulcerated area, hyperpyrexia, and cellulitis | Positive bacteriological swab: S. aureus – present in 55 patients (26.3%), Enterobacter spp. (17.2%), Proteus spp., E. coli and P. aeruginosa, two cases of Enterococcus spp. and one case of Candida albicans |
|
Sari et al, 200962 Turkey |
Prospective pre‐post evaluation study To evaluate the efficacy of a vacuum‐assisted closure ‐V.A.C. Therapy device in the comparative management of clean and infected wounds |
46 patients presented 52 wounds: 35 lower extremity ulcers 31 infected wounds |
The presence of exudation and peri‐lesional erythema were considered signs of inflammation or infection Covered with necrotic tissue, purulent discharge |
Positive wound culture. The most common pathogen isolated in wound cultures was P. aeruginosa followed by S. aureus |
|
Schiffer et al, 201586 Austria |
Prospective cohort study | 95 patients clinically diagnosed with infection: 10 CLUs | Patients were clinically diagnosed with infection by physicians, but did not describe clinical signs | Swab microbiology analysis – did not describe standards used |
|
Sibbald et al, 200140 Canada |
An uncontrolled, open‐label prospective study, single centre, four arm study To evaluate the clinical effect of the ionised nanocrystalline silver dressing on a variety of chronic wounds |
29 patients: 6 VLUs | Non‐healing, devitalised loose yellow debris and necrosis in the base of the ulcer, increased or a bright red granulation tissue that friable and exuberant, bridging of non‐viable epidermis, increased exudate, and exudate becomes purulent | Wound swab – semi‐quantitative ≥106 CFU/g tissue |
|
Sibbald, Coutts & Woo, 201138 Canada |
A multi‐centre, prospective, double‐blind, pilot, randomised controlled clinical trial To evaluate the effectiveness of a PHMB foam dressing compared with a similar non‐antimicrobial foam for the treatment of superficial bacterial burden, wound‐associated pain, and reduction in wound size |
45 subjects with leg (n = 23) and foot (n = 22) ulcers |
Peri‐wound infection: the presence ≥3 criteria from the STONEES: Size, Temperature difference by 3‐ F by infrared thermometry, O—probe/exposed bone, new satellite area breakdown, erythema and oedema, exudate smell, non‐healing, exudate, red friable granulation debris on the surface Smell |
Wound infection was equated to ≥105 colony‐forming units per millilitre |
|
Thai et al, 200263 USA |
Case study describes the effects of ultraviolet light C on wound bioburden and closure in three people with chronic ulcers infected with methicillin‐resistant S. aureus | 3 chronic wounds: 1 mixed led ulcer | Loosely adherent slough, copious amounts of purulent yellow exudate, significant erythema surrounded the wounds, extreme pain limiting patient's mobility and significant sleep disturbances. |
Semi‐quantitative bacterial cultures Presence of three types of bacteria: MRSA, P. aeruginosa and S. aureus |
ALU, arterial leg ulcer; CFU, colony‐forming units; CLUs, chronic leg ulcers; MLU, mixed leg ulcers; MRSA, methicillin‐resistant Staphylococcus aureus; PHMB, polyhexamethylene biguanide; VLUs, venous leg ulcers.
Of 38 studies that used microbial indicators for diagnosis of infection either solely or combined with clinical indicators, 12 studies used quantitative standards to confirm diagnosis of chronic wound infection, 24 studies used positive culture for the confirmation of wound infection, and three studies used both quantitative and qualitative results to confirm the diagnosis of chronic wounds infection (Tables 7 and 8).
This review literature showed twenty‐two different clinical indicators of infection were used (Table 4) to diagnose chronic wound infection. The highest numbers of clinical indicators used for diagnosis of infection per study were 12 whilst the smallest number was one.
Four studies investigated what criteria were used by clinicians to diagnose chronic wound infection.43, 44, 88, 94 The results indicated that clinicians used unreliable and different sets of criteria to diagnose chronic wound infection.88 For example, where the original set of criteria suggested using 11 clinical signs to diagnose infection, the clinicians only used two or five clinical signs. Lorentzen et al87 agreed that the clinical assessment of infection in chronic wounds was a difficult task, with great variability and a low reliability.
4. SUMMARY OF LITERATURE REVIEW ON INFECTION IN CLUS
Early identification of infection in chronic wounds is critical and many studies have attempted to investigate criteria for infection diagnosis from both clinical and microbiological perspectives. Despite significant advances in chronic wound management, the existing evidence for standardised criteria for identifying chronic wound infection is limited. There is a large volume of published studies investigating interventions for wound infections, yet the identification of infection in CLUs still remains problematic and the diagnostic criteria for infection in CLUs are currently not uniform. The reviewed studies were inconsistent and lacked specificity in terms of wound types, clinical characteristics, and indicators used to diagnose infection. Whilst eleven studies identified clinical signs and symptoms of infection in chronic wounds,8, 32, 36, 44, 47, 48, 49, 50, 51 many were conducted more than 10 years ago32, 46, 50 and were of a low level of evidence.8, 32, 35, 44, 45, 46, 49, 50, 51, 52 This literature review could not find strong evidence to describe the optimal criteria for diagnosis of infection in CLUs.
All things considered, this review showed seven frequently used clinical indicators that were suggested by international experts and validated by eleven studies of low‐level evidence, to be diagnostic for chronic wound infection. These include: (a) new pain, increasing pain, or altered pain in the ulcer area; (b) malodour; (c) increase in ulcer area; (d) wound breakdown; (e) delayed or non‐healing; (f) erythema; and (g) increase in local temperature (Tables 2, 3, and 4).
With regard to how these clinical signs and symptoms of infection have been applied to diagnose chronic wound infection, this article showed great variation in practice. Even though most experts agreed on using at least two clinical indicators to diagnose chronic wound infection, the diagnostic standards must be validated in larger samples. Importantly, there has been little research focusing on the clinical signs and symptoms of infection in patients with CLUS, including venous, arterial, and mixed leg ulcers. Early identification of infection can play a vital role in effective management of CLUs, enhancing healing, improving patients' quality of life, and reducing the burden on the health care system. This can only be performed if there is precise guidance available for early identification of chronic wound infection, which is specific on the number of indicators required and how they present for a leg ulcer to be diagnosed as infected.53
5. RECOMMENDATIONS
The following are recommendations for clinicians and researchers when assessing clinical signs and symptoms of infection in patients with CLUs:
When assessing patients with CLUs for clinical signs and symptoms of infection clinicians need to focus on the seven clinical indicators. These include: (a) new pain, increasing pain, or altered pain in the ulcer area; (b) malodour; (c) increase in ulcer area; (d) wound breakdown; (e) delayed or non‐healing; (f) erythema; and (g) increase in local temperature.
Of the seven clinical signs of infection found from this literature review, new pain, increasing pain, or altered pain in the ulcer area was a subjective sign that is best described by patients. Patients' level of pain should be documented accordingly based on the description given by the patient and should not be based on clinicians' assumption.8
Assessing pain as an indicator for infection should be focused on any changes in the nature of pain in the ulcer area. This change can be, for instance, a new ulcer pain or an increase in the patient's existing ulcer pain. Reported pain must be located in the ulcer area and/or surrounding ulcer area and not related to changing dressings.
The assessment of exudate for signs of infection should include the amount of exudate, type of exudate (eg, changing from serous to purulent), and the exudate consistency.
Because of the variation in frequency of dressing changes, the assessment of an increase in ulcer size between two dressing changes may not be consistent. The ulcer area needs to be measured regularly with a consistent approach, with a maximum of 4 weeks between appropriate treatments.11, 36, 53, 75 Any increase ≥20% between two measurements should be considered as an increase in wound size. In addition, if over a 4‐week period of appropriate treatment, the ulcer size does not decrease at least 20%, delayed healing should be noted.9, 36
If a swab is required, the Levine technique should be used to obtain specimens in CLUs as it has been determined to give a better microbial result compared with other techniques.
6. LIMITATIONS AND FURTHER RESEARCH
Because of the scope of the literature review, this article did not investigate the bacterial profile in patients with CLUs, as well as the reliability of current microbial indicators in diagnosis of infection in this specific population.
Further research is required to validate these seven clinical indicators: (a) new pain, increasing pain, or altered pain in the ulcer area; (b) malodour; (c) increase in ulcer area; (d) wound breakdown; (e) delayed or non‐healing; (f) erythema; and (g) increase in local temperature; for their specificity and sensitivity in diagnosis of infection in patients with CLUs; and possibly develop an evidence‐based guideline for diagnosis of infection in CLUs.
CONFLICTS OF INTEREST
The authors have no conflicts of interest to report.
ACKNOWLEDGEMENTS
The first author acknowledges the support of Queensland University of Technology as this literature review article has been undertaken in partial fulfilment of a Doctor of Philosophy. All authors read and approved the final version of this article.
Bui UT, Finlayson K, Edwards H. The diagnosis of infection in chronic leg ulcers: A narrative review on clinical practice. Int Wound J. 2019;16:601–620. 10.1111/iwj.13069
Funding information PhD scholarship
REFERENCES
- 1. Mekkes JR, Loots MAM, Van Der Wal AC, Bos JD. Causes, investigation and treatment of leg ulceration. Br J Dermatol. 2003;148:388‐401. [DOI] [PubMed] [Google Scholar]
- 2. Agale SV. Chronic leg ulcers: epidemiology, aetiopathogenesis, and management. Ulcers. 2013;2013:1‐9. [Google Scholar]
- 3. The World Union of Wound Healing Societies . Principles of Best Practice: Wound Infection in Clinical Practice. An International Consensus. London, UK: Ltd; 2008. [Google Scholar]
- 4. The Australian Government ‐ National Health and Medical Research Council . In: Council NHaMR , ed. NHMRC Levels of Evidence and Grades for Recommendations for Developers of Guidelines. Canberra: National Health and Medical Research Council; 2009. https://www.mja.com.au/sites/default/files/NHMRC.levels.of.evidence.2008-09.pdf. Accessed July 31, 2018. [Google Scholar]
- 5. Copeland‐Halperin L, Kaminsky A, Bluefeld N, Miraliakbari R. Sample procurement for cultures of infected wounds: a systematic review. J Wound Care. 2016;25:S4‐S10. [DOI] [PubMed] [Google Scholar]
- 6. Cutting KF, Harding KG. Criteria for identifying wound infection. J Wound Care. 1994;3:198‐201. [DOI] [PubMed] [Google Scholar]
- 7. Cutting KF, White R. Defined and refined: criteria for identifying wound infection revisited. Br J Community Nurs. 2004;9:S6‐S15. [DOI] [PubMed] [Google Scholar]
- 8. Cutting KF, White RJ, Mahoney P. Wound infection, dressings and pain, is there a relationship in the chronic wound? Int Wound J. 2013;10:79‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sibbald RG, Woo K, Ayello E. Increased bacterial burden and infection: NERDS and STONES. Wounds UK. 2007;3:25‐46. [DOI] [PubMed] [Google Scholar]
- 10. The Australian Wound Management Association . Bacteria Impact on Wound Healing: from Contamination to Infection – Position Document. Perth, Australia: Australian Wound Management Association; 2011. [Google Scholar]
- 11. Jørgensen B, Price P, Andersen KE, et al. The silver‐releasing foam dressing, Contreet foam, promotes faster healing of critically colonised venous leg ulcers: a randomised, controlled trial. Int Wound J. 2005;2:64‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lazareth I, Meaume S, Sigal‐Grinberg ML, Combemale P, Le Guyadec T, Zagnoli A. Efficacy of a silver lipidocolloid dressing on heavily colonised wounds: a republished RCT. J Wound Care. 2012;21:96‐102. [DOI] [PubMed] [Google Scholar]
- 13. Vanscheidt W, Harding K, Téot L, Siebert J. Effectiveness and tissue compatibility of a 12‐week treatment of chronic venous leg ulcers with an octenidine based antiseptic ‐ a randomized, double‐blind controlled study. Int Wound J. 2012;9:316‐323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Woo KY, Coutts PM, Gary Sibbald R. A randomized controlled trial to evaluate an antimicrobial dressing with silver alginate powder for the management of chronic wounds exhibiting signs of critical colonization. Adv Skin Wound Care. 2012;25:503‐508. [DOI] [PubMed] [Google Scholar]
- 15. Griffiths RD, Fernandez RS, Ussia CA. Is tap water a safe alternative to normal saline for wound irrigation in the community setting? J Wound Care. 2001;10:407‐411. [DOI] [PubMed] [Google Scholar]
- 16. Isbary G, Heinlin J, Shimizu T, et al. Successful and safe use of 2 min cold atmospheric argon plasma in chronic wounds: results of a randomized controlled trial. Br J Dermatol. 2012;167:404‐410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Isbary G, Morfill G, Schmidt HU, et al. A first prospective randomized controlled trial to decrease bacterial load using cold atmospheric argon plasma on chronic wounds in patients. Br J Dermatol. 2010;163:78‐82. [DOI] [PubMed] [Google Scholar]
- 18. Kordestani S, Shahrezaee M, Tahmasebi MN, Hajimahmodi H, Ghasemali DH, Abyaneh MS. A randomised controlled trial on the effectiveness of an advanced wound dressing used in Iran. J Wound Care. 2008;17:323‐327. [DOI] [PubMed] [Google Scholar]
- 19. Daróczy J. Quality control in chronic wound management: the role of local povidone‐iodine (Betadine) therapy. Dermatology. 2006;212 (Suppl. 1:82‐87. [DOI] [PubMed] [Google Scholar]
- 20. Lei X, Liu B, Huang Z, Wu J. A clinical study of photodynamic therapy for chronic skin ulcers in lower limbs infected with Pseudomonas aeruginosa . Arch Dermatol Res. 2015;307:49‐55. [DOI] [PubMed] [Google Scholar]
- 21. Madhusudhan VL. Efficacy of 1% acetic acid in the treatment of chronic wounds infected with Pseudomonas aeruginosa: prospective randomised controlled clinical trial. Int Wound J. 2016;13:1129‐1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lantis JC, Gendics C. In vivo effect of sustained‐release silver sulphadiazine foam on bioburden and wound closure in infected venous leg ulcers. J Wound Care. 2011;20:90‐96. [DOI] [PubMed] [Google Scholar]
- 23. Lo SF, Hayter M, Chang CJ, Hu WY, Lee LL. A systematic review of silver‐releasing dressings in the management of infected chronic wounds. J Clin Nurs. 2008;17:1973‐1985. [DOI] [PubMed] [Google Scholar]
- 24. Kingsley A. The wound infection continuum and its application to clinical practice. Ostomy Wound Manage. 2003;49:1‐7. [PubMed] [Google Scholar]
- 25. Kingsley A. A proactive approach to wound infection. Nurs Stand. 2001;15:50‐54, 6, 8. [DOI] [PubMed] [Google Scholar]
- 26. Dissemond J, Gerber V, Kramer A, et al. A practice‐oriented recommendation for treatment of critically colonised and locally infected wounds using polihexanide. J Tissue Viability. 2010;19:106‐115. [DOI] [PubMed] [Google Scholar]
- 27. International Wound Infection Institute . Wound Infection in Clinical Practice: Principles of Best Practice. London, UK: International Wound Infection Institute; 2016. [Google Scholar]
- 28. James GA, Swogger E, Wolcott R, et al. Biofilms in chronic wounds. Wound Repair Regen. 2008;16:37‐44. [DOI] [PubMed] [Google Scholar]
- 29. Malone M, Bjarnsholt T, McBain AJ, et al. The prevalence of biofilms in chronic wounds: a systematic review and meta‐analysis of published data. J Wound Care. 2017;26:20‐25. [DOI] [PubMed] [Google Scholar]
- 30. Hurlow J, Bowler PG. Clinical experience with wound biofilm and management: a case series. Ostomy Wound Manage. 2009;55:38‐49. [PubMed] [Google Scholar]
- 31. Gardner SE, Frantz RA, Saltzman CL, Hillis SL, Park H, Scherubel M. Diagnostic validity of three swab techniques for identifying chronic wound infection. Wound Repair Regen. 2006;14:548‐557. [DOI] [PubMed] [Google Scholar]
- 32. Gardner SE, Frantz RA, Doebbeling BN. The validity of the clinical signs and symptoms used to identify localized chronic wound infection. Wound Repair Regen. 2001;9:178‐186. [DOI] [PubMed] [Google Scholar]
- 33. East JM, Fray DA, Hall DE, Longmore CA. Chronic neuropathic ulcer is not the most common antecedent of lower limb infection or amputation among diabetics admitted to a regional hospital in Jamaica: results from a prospective cohort study. BMC Surg. 2015;15:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bhat KS, Vishwesh BN, Sahu M, Shukla VK. A clinical study on the efficacy of Panchavalkala cream in Vrana Shodhana w.s.r to its action on microbial load and wound infection. Ayu. 2014;35:135‐140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Enoch S, Harding K. Wound bed preparation: the science behind the removal of barriers to healing. Wounds. 2003;15:213‐229. [Google Scholar]
- 36. Woo KY, Sibbald RG. A cross‐sectional validation study of using NERDS and STONEES to assess bacterial burden. Ostomy Wound Manage. 2009;55:40‐48. [PubMed] [Google Scholar]
- 37. Harding KG, Szczepkowski M, Mikosinski J, et al. Safety and performance evaluation of a next‐generation antimicrobial dressing in patients with chronic venous leg ulcers. Int Wound J. 2016;13:442‐448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sibbald RG, Coutts P, Woo KY. Reduction of bacterial burden and pain in chronic wounds using a new polyhexamethylene biguanide antimicrobial foam dressing‐clinical trial results. Adv Skin Wound Care. 2011;24:78‐84. [DOI] [PubMed] [Google Scholar]
- 39. Leaper D, Assadian O, Edmiston CE. Approach to chronic wound infections. Br J Dermatol. 2015;173:351‐358. [DOI] [PubMed] [Google Scholar]
- 40. Sibbald RG, Browne AC, Coutts P, Queen D. Screening evaluation of an ionized nanocrystalline silver dressing in chronic wound care. Ostomy Wound Manage. 2001;47:38‐43. [PubMed] [Google Scholar]
- 41. Woo KY, Heil J. A prospective evaluation of methylene blue and gentian violet dressing for management of chronic wounds with local infection. Int Wound J. 2017;14:1029‐1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gardner SE, Frantz RA, Troia C, et al. A tool to assess clinical signs and symptoms of localized infection in chronic wounds: development and reliability. Ostomy Wound Manage. 2001;47:40‐47. [PubMed] [Google Scholar]
- 43. Cutting KF. Identification of infection in granulating wounds by registered nurses. J Clin Nurs. 1998;7:539‐546. [DOI] [PubMed] [Google Scholar]
- 44. Dennis LA, Dumville JC, Cullum N, Bland JM. Value of a modified clinical signs and symptoms of infection checklist for leg ulcer management. Br J Surg. 2010;97:664‐670. [DOI] [PubMed] [Google Scholar]
- 45. Rondas AA, Schols JM, Stobberingh EE, Halfens RJ. Prevalence of chronic wounds and structural quality indicators of chronic wound care in Dutch nursing homes. Int Wound J. 2015;12:630‐635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bowler PG, Davies BJ, Jones SA. Microbial involvement in chronic wound malodour. J Wound Care. 1999;8:216‐218. [DOI] [PubMed] [Google Scholar]
- 47. Fierheller M, Sibbald RG. A clinical investigation into the relationship between increased periwound skin temperature and local wound infection in patients with chronic leg ulcers. Adv Skin Wound Care. 2010;23(8):369‐381. [DOI] [PubMed] [Google Scholar]
- 48. Rondas AA, Halfens RJ, Schols JM, Thiesen KP, Trienekens TA, Stobberingh EE. Is a wound swab for microbiological analysis supportive in the clinical assessment of infection of a chronic wound? Future Microbiol. 2015;10:1815‐1824. [DOI] [PubMed] [Google Scholar]
- 49. Danielsen L, Balslev E, Doring G, et al. Ulcer bed infection ‐ report of a case of enlarging venous leg ulcer colonized by Pseudomonas aeruginosa . APMIS. 1998;106:721‐726. [PubMed] [Google Scholar]
- 50. Tudor M. Identifying the causes of increased wound pain: the role of the tissue viability nurse. J Wound Care. 2003;12:179‐182. [DOI] [PubMed] [Google Scholar]
- 51. Vowden KR, Vowden P. The prevalence, management and outcome for patients with lower limb ulceration identified in a wound care survey within one English health care district. J Tissue Viability. 2009;18:13‐19. [DOI] [PubMed] [Google Scholar]
- 52. Gardner SE, Frantz RA, Saltzman CL. Diabetes and inflammation in infected chronic wounds. Wounds. 2005;17:203‐205. [Google Scholar]
- 53. Forlee M, Rossington A, Searle R. A prospective, open, multicentre study to evaluate a new gelling fibre dressing containing silver in the management of venous leg ulcers. Int Wound J. 2014;11:438‐445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gago M, Garcia F, Gaztelu V, Verdu J, Lopez P, Nolasco A. A comparison of three silver‐containing dressings in the treatment of infected, chronic wounds. Wounds. 2008;20:273‐278. [PubMed] [Google Scholar]
- 55. Murphy N. Reducing infection in chronic leg ulcers with an activated carbon cloth dressing. Br J Nurs. 2016;25:S38‐S44. [DOI] [PubMed] [Google Scholar]
- 56. Trial C, Darbas H, Lavigne J, et al. Assessment of the antimicrobial effectiveness of a new silver alginate wound dressing: a RCT. J Wound Care. 2010;19:20‐26. [DOI] [PubMed] [Google Scholar]
- 57. Walker M, Metcalf D, Parsons D, Bowler P. A real‐life clinical evaluation of a next‐generation antimicrobial dressing on acute and chronic wounds. J Wound Care. 2015;24:11‐22. [DOI] [PubMed] [Google Scholar]
- 58. Woo KY, Coutts PM, Sibbald RG. Continuous topical oxygen for the treatment of chronic wounds: a pilot study. Adv Skin Wound Care. 2012;25:543‐547. [DOI] [PubMed] [Google Scholar]
- 59. Graham GS. Healing outcomes of MRSA‐infected wounds with a protocol combining Oakin dressing with elements of the de‐escalation theory. J Wound Care. 2014;23:S4‐S11. [DOI] [PubMed] [Google Scholar]
- 60. Lisle J. Use of sugar in the treatment of infected leg ulcers. Br J Community Nurs. 2002;7(suppl 6):40, 42, 44, 46. [DOI] [PubMed] [Google Scholar]
- 61. Rossi A, Wertzberger S. Management problems in the care of an infected leg ulcer. J Wound Care. 1996;5:255‐256. [DOI] [PubMed] [Google Scholar]
- 62. Sari A, Fesli A, Yener T, Basterzi Y, Demirkan F. The efficacy of topical negative pressure in the management of infected and non‐infected wounds. Wounds. 2009;21:95‐101. [PubMed] [Google Scholar]
- 63. Thai TP, Houghton PE, Keast DH, Campbell KE, Woodbury MG. Ultraviolet light C in the treatment of chronic wounds with MRSA: a case study. Ostomy Wound Manage. 2002;48:52‐60. [PubMed] [Google Scholar]
- 64. Braumann C, Guenther N, Menenakos C, et al. Clinical experiences derived from implementation of an easy to use concept for treatment of wound healing by secondary intention and guidance in selection of appropriate dressings. Int Wound J. 2011;8:253‐260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Bruce Z. Using Cutimed® Sorbact® hydroactive on chronic infected wounds. Wounds UK. 2012;8:119‐129. [Google Scholar]
- 66. Salavastru CM, Nedelcu LE, Tiplica GS. Management of leg ulcers in patients with chronic venous insufficiency: the experience of a dermatology clinic in Bucharest, Romania. Dermatol Ther. 2012;25:304‐313. [DOI] [PubMed] [Google Scholar]
- 67. Flock P, Gibbs L, Sykes N. Diamorphine‐metronidazole gel effective for treatment of painful infected leg ulcers. J Pain Symptom Manage. 2000;20:396‐397. [DOI] [PubMed] [Google Scholar]
- 68. Nagoba BS, Gandhi RC, Wadher BJ, Potekar RM, Kolhe SM. Microbiological, histopathological and clinical changes in chronic infected wounds after citric acid treatment. J Med Microbiol. 2008;57:681‐682. [DOI] [PubMed] [Google Scholar]
- 69. Derbyshire A. Innovative solutions to daily challenges: Cutimed Sorbact follow‐up case studies. Br J Community Nurs. 2010;15(Supp1. 2):S24‐S28. [PubMed] [Google Scholar]
- 70. Martin PM, Garaizabal EE, Sanchez LPJ, Sanchez CD. Vibrio metschnikovii from a human infected leg ulcer. Rev Inst Med Trop Sao Paulo. 2008;50:311‐312. [DOI] [PubMed] [Google Scholar]
- 71. Alcaraz A, Kelly J. Treatment of an infected venous leg ulcer with honey dressings. Br J Nurs. 2002;11:859‐860, 62, 64–66. [DOI] [PubMed] [Google Scholar]
- 72. Eisenstein BI. Treatment challenges in the management of complicated skin and soft‐tissue infections. Clin Microbiol Infect. 2008;14 (suppl 2:17‐25. [DOI] [PubMed] [Google Scholar]
- 73. Vanscheidt W, Lazareth I, Routkovsky‐Norval C. Safety evaluation of a new ionic silver dressing in the management of chronic ulcers. Wounds. 2003;15:371‐378. [Google Scholar]
- 74. Jorgensen B, Gottrup F, Karlsmark T, Bech‐Thomsen N, Sibbald RG. Combined use of an ibuprofen‐releasing foam dressing and silver dressing on infected leg ulcers. J Wound Care. 2008;17:210‐214. [DOI] [PubMed] [Google Scholar]
- 75. Peral MC, Rachid MM, Gobbato NM, Huaman Martinez MA, Valdez JC. Interleukin‐8 production by polymorphonuclear leukocytes from patients with chronic infected leg ulcers treated with Lactobacillus plantarum . Clin Microbiol Infect. 2010;16:281‐286. [DOI] [PubMed] [Google Scholar]
- 76. Davies CE, Hill KE, Wilson MJ, et al. Use of 16S ribosomal DNA PCR and denaturing gradient gel electrophoresis for analysis of the microfloras of healing and nonhealing chronic venous leg ulcers. J Clin Microbiol. 2004;42:3549‐3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Melendez JH, Frankel YM, An AT, et al. Real‐time PCR assays compared to culture‐based approaches for identification of aerobic bacteria in chronic wounds. Clin Microbiol Infect. 2010;16:1762‐1769. [DOI] [PubMed] [Google Scholar]
- 78. Rhoads DD, Cox SB, Rees EJ, Sun Y, Wolcott RD. Clinical identification of bacteria in human chronic wound infections: culturing vs. 16S ribosomal DNA sequencing. BMC Infect Dis. 2012;12:321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Sprockett DD, Ammons CG, Tuttle MS. Use of 16S rRNA sequencing and quantitative PCR to correlate venous leg ulcer bacterial bioburden dynamics with wound expansion, antibiotic therapy, and healing. Wound Repair Regen. 2015;23:765‐771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Beele H, Meuleneire F, Nahuys M, Percival SL. A prospective randomised open label study to evaluate the potential of a new silver alginate/carboxymethylcellulose antimicrobial wound dressing to promote wound healing. Int Wound J. 2010;7:262‐270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Raad W, Lantis JC II, Tyrie L, Gendics C, Todd G. Vacuum‐assisted closure instill as a method of sterilizing massive venous stasis wounds prior to split thickness skin graft placement. Int Wound J. 2010;7:81‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Cooper RA, Ameen H, Price P, McCulloch DA, Harding KG. A clinical investigation into the microbiological status of 'locally infected' leg ulcers. Int Wound J. 2009;6:453‐462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Landis SJ. Chronic wound infection and antimicrobial use. Adv Skin Wound Care. 2008;21:531‐540. [DOI] [PubMed] [Google Scholar]
- 84. Bowler PG. Wound pathophysiology, infection and therapeutic options. Ann Med. 2002;34:419‐427. [DOI] [PubMed] [Google Scholar]
- 85. Blokhuis‐Arkes MHE, Haalboom M, van der Palen J, et al. Rapid enzyme analysis as a diagnostic tool for wound infection: comparison between clinical judgment, microbiological analysis, and enzyme analysis. Wound Repair Regen. 2015;23:345‐352. [DOI] [PubMed] [Google Scholar]
- 86. Schiffer D, Blokhuis‐Arkes M, Van Der Palen J, Sigl E, Heinzle A, Guebitz GM. Assessment of infection in chronic wounds based on the activities of elastase, lysozyme and myeloperoxidase. Br J Dermatol. 2015;173:1529‐1531. [DOI] [PubMed] [Google Scholar]
- 87. Lorentzen HF, Gottrup F. Clinical assessment of infection in nonhealing ulcers analyzed by latent class analysis. Wound Repair Regen. 2006;14:350‐353. [DOI] [PubMed] [Google Scholar]
- 88. Dryden M, Dickinson A, Brooks J, Hudgell L, Saeed K, Cutting KF. A multi‐Centre clinical evaluation of reactive oxygen topical wound gel in 114 wounds. J Wound Care. 2016;25(140):2‐6. [DOI] [PubMed] [Google Scholar]
- 89. Meaume S, Vallet D, Morere MN, Teot L. Evaluation of a silver‐releasing hydroalginate dressing in chronic wounds with signs of local infection. J Wound Care. 2005;14:411‐419. [DOI] [PubMed] [Google Scholar]
- 90. Dubhashi SP, Sindwani RD. A comparative study of honey and phenytoin dressings for chronic wounds. Indian J Surg. 2015;77 (suppl 3:1209‐1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Imbernon‐Moya A, Ortiz‐de Frutos FJ, Sanjuan‐Alvarez M, Portero‐Sanchez I, Merinero‐Palomares R, Alcazar V. Topical sevoflurane for chronic venous ulcers infected by multi‐drug‐resistant organisms. Int Wound J. 2017;14:1388‐1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Gerry R, Kwei S, Bayer L, Breuing KH. Silver‐impregnated vacuum‐assisted closure in the treatment of recalcitrant venous stasis ulcers. Ann Plast Surg. 2007;59:58‐62. [DOI] [PubMed] [Google Scholar]
- 93. Imbernón A, Blázquez C, Puebla A, et al. Chronic venous ulcer treatment with topical sevoflurane. Int Wound J. 2016;13:1060‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Rondas A, Schols J, Stobberingh E, Price P. Definition of infection in chronic wounds by Dutch nursing home physicians. Int Wound J. 2009;6:267‐274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Bamberg R, Sullivan PK, Conner‐Kerr T. Diagnosis of wound infections: current culturing practices of US wound care professionals. Wounds. 2002;14:314‐328. [Google Scholar]
- 96. Banu A, Sathyanarayana BC, Chattannavar G. Efficacy of fresh Aloe vera gel against multi‐drug resistant bacteria in infected leg ulcers. Australas Med J. 2012;5:305‐309. [DOI] [PMC free article] [PubMed] [Google Scholar]


