Abstract
The purpose of this study was to use a murine model to determine if melatonin can protect the inner ear from radiation-induced damage. A total of 81 4-week-old Balb/c mice were randomly divided into five groups: control group; 50 mg/kg melatonin group; 5 mg/kg melatonin+radiotherapy group; 50 mg/kg melatonin+radiotherapy group; radiotherapy group. The radiotherapy groups received 16 Gy irradiation and melatonin was administered by intraperitoneal injection 30 min before radiotherapy. On days 3 and 7 after irradiation the function of outer hair cells was determined by auditory brainstem response (ABR) and distortion product otoacoustic emissions (DPOAEs) testing, pathological changes of inner ear cells were observed by light microscopy, and the expression of prestin mRNA was determined. ABR thresholds were increased and wave I latencies were extended after radiotherapy; however, the increases were lower in the groups that received melatonin (P < 0.05). DPOAEs showed radiotherapy-induced hearing loss at 8–12 kHz, and hearing loss was greater on day 7 than day 3. However, hearing loss was less in the melatonin groups (P < 0.05). Histopathological examination showed irradiation resulted in breaks and distortion of the cochlear basement membrane, disruption of the stria vascularis, and swelling of outer hair cells. Melatonin reduced these changes. Radiotherapy upregulated prestin mRNA expression. Radiotherapy-induced upregulation of prestin was decreased in the melatonin groups (P < 0.05), and the decrease was greater in the 50 mg/kg melatonin group (P < 0.05). Melatonin protects against radiation-induced cochlear damage by reducing damage to outer hair cells.
Keywords: melatonin, radiotherapy, cochlear damage, prestin mRNA
INTRODUCTION
The use of ionizing radiation (radiation therapy, radiotherapy) is an important method of radical or palliative treatment for many malignancies [1]. Radiation therapy damages malignant tissue because the ionizing radiation leads to the development of free radicals, which attack various cell components, such as lipids, proteins and DNA, cause lipid peroxidation, and destroyscell membranes. However, ionizing radiation can also damage nearby normal tissues and structures.
The cochlea has a high aerobic metabolism rate [2–4]. Thus, the cochlea is very susceptible to oxidative injury by reactive oxygen species produced by radiotherapy [2, 3]. One of the primary treatments for head and neck cancer is radiotherapy, and damage to the cochlea can result in irreversible sensorineural hearing loss (SNHL) [2, 3]. It has been reported that ~50% of patients who receive radiotherapy for head and neck cancer will eventually develop SNHL [2]. A clinical study reported that a radiation dose of 50–70 Gy can lead to SNHL, and 24–36% of patients who received a dose of 60 Gy sustained permanent hearing loss [5].
High-frequency sounds are localized near the base of the cochlea, and low-frequency sounds near the apex [2–4]. The basal outer hair cells have a significantly lower level of the antioxidant glutathione as compared to the apical outer hair cells [2, 3]. As a result, the basal outer hair cells are more affected by radiotherapy than the apical outer hair cells and high-frequency hearing loss is more severe after radiotherapy than low-frequency hearing loss [2, 3]. Although SNHL is a late complication after radiotherapy, damage and apoptosis of inner ear cells occurs soon after radiotherapy, and the degree of apoptosis is dependent on the dose and time period of irradiation. Prestin, a specific protein in outer hair cells, may be a useful indicator of early damage to the inner ear cause by radiation [6].
The radiation dose and range is often limited when treating head and neck malignancies because of the proximity of vital structures which are susceptible to radiation injury. As a result, a large amount of research has been performed to develop methods to protect vital structures and at the same time provide a sufficient dosage of radiation to the malignancy. To this end, a ‘different concentric circles’ model for administering ionizing radiation, and various agents to protect tissue from the effects of radiation, such as amifostine, melatonin and vitamin E, have been examined [7].
Melatonin is synthesized by the pineal gland and is important for the sleep–wake cycle [8]. However, melatonin is also a free radical scavenger and promotes the activity of antioxidant enzymes such as superoxide dismutase (SOD), glutathione peroxidase, glutathione reductase and catalase [9]. Melatonin functions to stabilize cell membranes by increasing resistance to oxidative damage via reducing the leakage of electrons and the generation of free radicals, and improving the efficiency of electron transport [9].
In addition, lipid peroxidation is inhibited by melatonin [9].
As a potent antioxidant, melatonin plays an important role in the prevention of tissue and organ damage caused by free radicals. A systematic review of animal studies concluded that melatonin can protect tissues from the effects of irradiation [10]. However, few studies have examined whether melatonin can protect the inner ear from radiation damage [11]. Thus, the purpose of this study was to use a murine model to determine if melatonin can protect the inner ear from radiation-induced damage.
MATERIALS AND METHODS
Animals and experimental grouping
The study protocol and animal experiments were approved by the Animal Ethics Committee of Southern Medical University. All animal experiments were conducted in accordance with standard guidelines for the care and use of laboratory animals.
A total of 81, healthy 4-week-old Balb/c mice weighing 12–15 g were obtained from the Southern Medical University Laboratory Animal Center. The tympanic membranes of all mice were examined, and all were observed to have a grossly normal pinna reflex. Melatonin (Sigma-Aldrich, St. Louis, MO, USA) was dissolved in alcoholic saline (0.1 mL, ethanol < 3% v/v).
The 81 mice were randomly divided into five groups: (i) control group (n = 9), mice received 0.9% NaCl intraperitoneally (i.p.); (ii) melatonin group (n = 18), mice received 50 mg/kg melatonin i.p.; (iii) melatonin-5 + radiotherapy group (n = 18), mice received 16 Gy irradiation and 5 mg/kg melatonin i.p. 30 min after irradiation; (iv) melatonin-50 + radiotherapy group (n = 18), mice received 16 Gy irradiation and 50 mg/kg melatonin i.p. 30 min after irradiation; (v) radiotherapy group (n = 18), mice received 16 Gy irradiation and 0.9% NaCl i.p. In each group, the mice were studied 3 and 7 days after irradiation.
Irradiation
Before irradiation the mice were weighed and anesthetized with 0.004 mL per gram body weight chloral hydrate i.p. The mice were fixed in the prone position, and the irradiation area of the inner ear was determined by radiography. The mice were kept in the same fixed position on the linear accelerator bed. A Varian 2100 linear accelerator 6 MeV electron line was used to administer radiation. The dose rate was 400 cGy/min, the distance from the skin was 100 cm and the reference depth was 1.5 cm subcutaneously. Both ears of each mouse received a single dose of 16 Gy irradiation.
Auditory brainstem response
Mice were anesthetized in the same manner used for administration of radiation, and placed in a soundproof room for auditory brainstem response (ABR) recording. Subcutaneous platinum needle electrodes placed in the scalp were used to differentially record ABRs. One electrode was placed at the vertex of the skull, a reference electrode was placed on the same side of the mastoid, and a ground electrode was inserted into the tip of the nose. The stimulus presentation rate was 12.1/s. Responses were amplified using an Intelligent Hearing Systems biological amplifier (USA). Responses were filtered between 300 and 3000 Hz, the observation time was 20 ms, and the number of times of superposition was 800. Beginning at a sound pressure level (SPL) of 90 dB SPL, the SPL of the stimuli was decreased in 10 dB increments until the ABR wave I response was no longer observable.
Distortion product otoacoustic emissions
Mice were anesthetized and placed in a soundproof room as for ABR recording. A special ear-tip probe was used to seal the external auditory canal, then sound stimuli were delivered to the animals via a headset. Detection was begun once the stimulus signal was stable. The distortion product otoacoustic emission (DPOAE) sound stimulus consisted of two simultaneous, permanent pure tones at different frequencies. Stimulus parameters (L1 = L2 = 80 dB SPL) with an f1:f2 ratio of 1.22 were used. The DPOAE amplitude was recorded at 2, 4, 6, 8, 9, 10, 11 and 12 kHz. The DPOAE test results were plotted graphically: 2f1:f2 as a function of the geometric mean of the two primary parameters.
Histopathological studies
On days 3 and 7 after irradiation, six mice in each group were decapitated and the temporal bones were removed. The otic vesicles were dissected in 4% paraformaldehyde (PFA) under a dissecting microscope to reveal the inner ear. The round window membrane was rapidly punctured and the oval window was exposed. A small hole was then drilled in the cupula cochlea, and 4% PFA was injected for perfusion fixation overnight at 4°C. After decalcification, specimens were dehydrated and embedded in paraffin. The paraffin-embedded specimens were cut into 6-mm thick sections, mounted on slides and stained with hematoxylin–eosin. The cochlear sections were examined with an Olympus Bx51 microscope (Japan).
Prestin mRNA extraction and qPCR
On days 3 and 7 after irradiation, the ears of each mouse were sampled. Total RNA of the basilar membrane was eluted with an RNA extraction kit (9108/9109, Takara, DaLian, China). cDNA was synthesized with a RevertAid First Strand cDNA Synthesis Kit (Takara). The reaction components were 2 × SYBR Green Premix EX Taq (10 μL; Takara), forward primer (0.4 μL, 0.2 μM), reverse primer (0.4 μL, 0.2 μM), cDNA (2 μL), and deionized water for a total volume of 20 μL. The PCR parameters were 95°C for 30 s (initial denaturation), followed by 40 cycles at 95°C for 5 s (denaturation), 60°C for 30 s (annealing) and 60°C for 30 s (extension). The two pairs of primers used in real-time PCR were as follows: prestin,forward primer:5’-AGGAGGATATGGAGCCCAATG-3′,reverse primer:5’-CGGTGCACAACCCCATACA-3′; actin, forward primer: 5’-TTCTAGGCGGACTGTTACTGAGCTG-3′, reverse primer: 5’-ACTGCTGTCGCCTTCACCGT-3′. The relative expression of prestin mRNA in the control group and the 2−ΔΔCt values of mice in each group was calculated.
Statistical analysis
Data are presented as mean ± standard deviation. Comparisons of groups was performed with 1-way Kruskal–Wallis analysis of variance (ANOVA), and the post-ANOVA Tukey-B test. A value of P < 0.05 was considered to indicate statistical significance. All statistical analyses were conducted using SPSS version 19.0 software (SPSS Inc., Chicago. IL).
RESULTS
ABR testing
On day 3 after irradiation, the response thresholds were significantly increased after radiotherapy (P < 0.05); however, the increases were less in the two groups that received melatonin than in the radiotherapy group. The ABR wave I latencies were not significantly different between the groups (all, P> 0.05). On day 7 after irradiation, the response thresholds remained higher in the radiotherapy groups: however, the level in the melatonin-50 + radiotherapy group was significantly lower than in the melatonin-5 + radiotherapy group and in the radiotherapy alone group (both, P < 0.05) and the ABR wave I latencies were extended. (Figs 1 and 2; Tables 1 and 2).
Fig. 1.
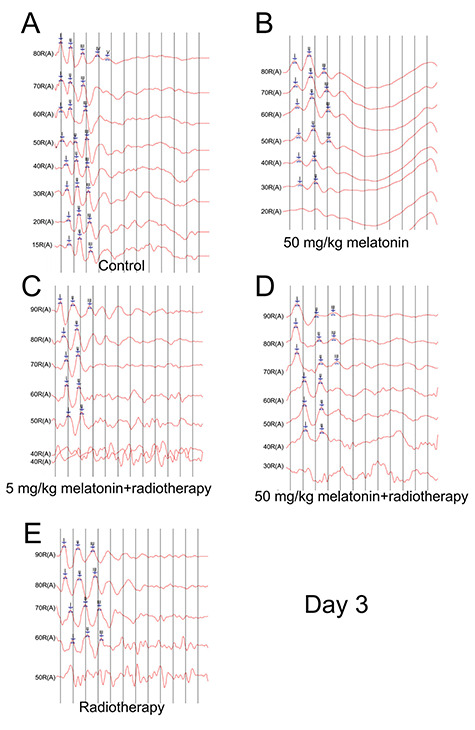
ABR results on day 3 after irradiation. (A) Control group. (B) 50 mg/kg melatonin group. (C) 5 mg/kg melatonin+radiotherapy group. (D) 50 mg/kg melatonin+radiotherapy group. (E) Radiotherapy group.
Fig. 2.
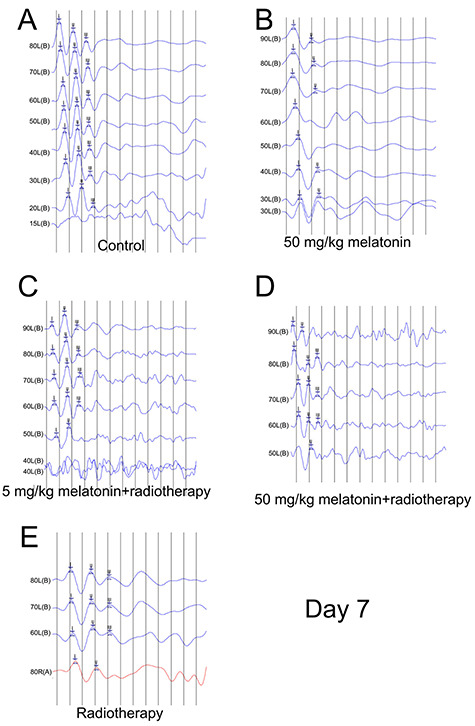
ABR results on day 7 after irradiation. (A) Control group. (B) 50 mg/kg melatonin group. (C) 5 mg/kg melatonin+radiotherapy group. (D) 50 mg/kg melatonin+radiotherapy group. (E) Radiotherapy group.
Table 1.
ABR response thresholds decibel normal Hearing Level (dBnHL) on days 3 and 7 after irradiation; two-way ANOVA with Sidak’s post hoc multiple comparisons test
| Group | Ear | Day 3 | Day 7 | P c |
|---|---|---|---|---|
| Control | 6 | 18.33 ± 2.89 | 18.33 ± 2.89 | 1 |
| Melatonin | 12 | 18.33 ± 2.89b | 21.67 ± 7.64b | 0.52 |
| 5 mg/kg Melatonin+ radiotherapy | 12 | 40.00 ± 0a | 43.33 ± 5.77a,b | 0.42 |
| 50 mg/kg Melatonin+ radiotherapy | 12 | 33.33 ± 5.77ab | 36.67 ± 5.77a,b | 0.52 |
| Radiotherapy | 12 | 50.00 ± 0a | 66.67 ± 5.77a | 0.04* |
a P < 0.05 when compared to the control group at the same time point.
b P < 0.05 when compared to the radiotherapy group at the same time point.
cIndependent samples t-test: *P < 0.05 when with the same group at a different time point.
Table 2.
ABR wave I latencies (ms) on days 3 and 7 after irradiation; two-way ANOVA with Sidak’s multiple comparisons test
| Group | Ear | Day 3 | Day 7 | P c |
|---|---|---|---|---|
| Control | 6 | 1.17 ± 0.06 | 1.17 ± 0.06 | 1 |
| Melatonin | 12 | 1.17 ± 0.06 | 1.20 ± 0.0b | 0.42 |
| 5 mg/kg Melatonin+ radiotherapy | 12 | 1.30 ± 0.0 | 1.27 ± 0.06b | 0.42 |
| 50 mg/kg Melatonin+ radiotherapy | 12 | 1.37 ± 0.06a | 1.30 ± 0.10b | 0.37 |
| Radiotherapy | 12 | 1.30 ± 0.0 | 1.60 ± 0.10a | 0.01* |
a P < 0.05 when compared to the control group at the same time point.
b P < 0.05 when compared to the radiotherapy group at the same time point.
cIndependent samples t-test: *P < 0.05 when with the same group at a different time point.
DPOAEs testing
Stable hearing at all frequencies was observed in the control group and the melatonin group during testing. As expected, animals that were exposed to irradiation exhibited hearing loss, and decreased DPOAE amplitudes were observed from 6–12 kHz. On day 3 after irradiation, the DPOAE responses at each test frequency between the two melatonin+radiotherapy groups were not significantly different (all, P > 0.05). However, on day 7 after irradiation, the DPOAE responses at 10, 11 and 12 kHz in the melatonin-5 + radiotherapy group were significantly lower than in the melatonin-50 + radiotherapy group (P = 0.01, 0.006 and 0.002, respectively) Figs 3–5).
Fig. 3.
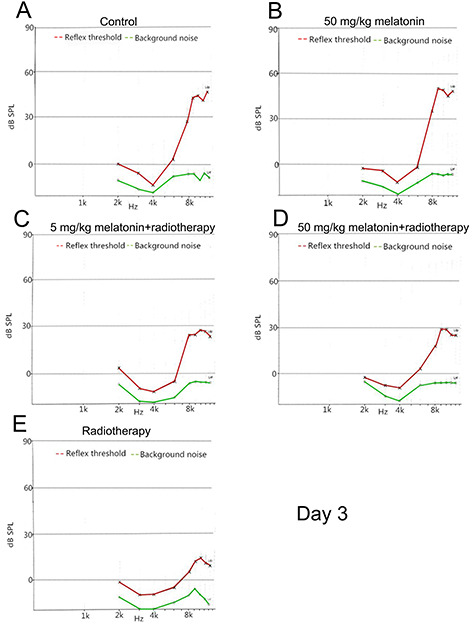
DPOAE amplitude of each frequency on day 3 after irradiation. L1 = L2 = 80 dB SPL. A representative image of a single measurement for each group is shown. (A) Control group. (B) 50 mg/kg melatonin group. (C) 5 mg/kg melatonin+radiotherapy group. (D) 50 mg/kg melatonin+radiotherapy group. (E) Radiotherapy group.
Fig. 5.
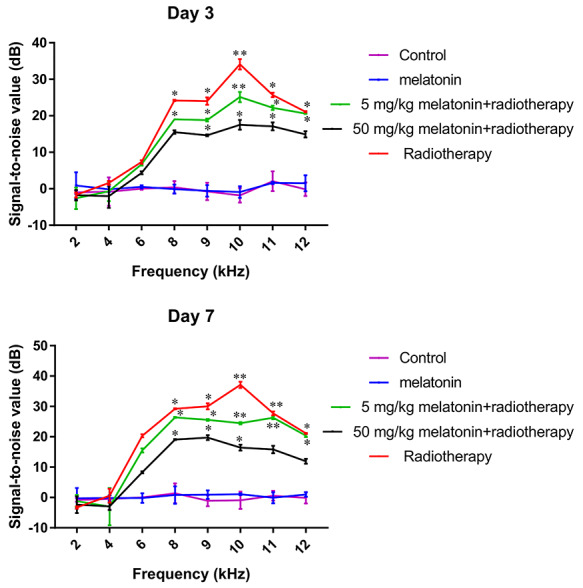
Difference of the average signal-to-noise ratio of DPOAE on day 3 (top) and day 7 (bottom) after irradiation in each group. The results show the mean ± standard deviation of three measurements. Difference = SNR before irradiation − SNR after irradiation. One-way ANOVA: *P < 0.05, **P < 0.01 when compared to the control group at the same time point.
Fig. 4.
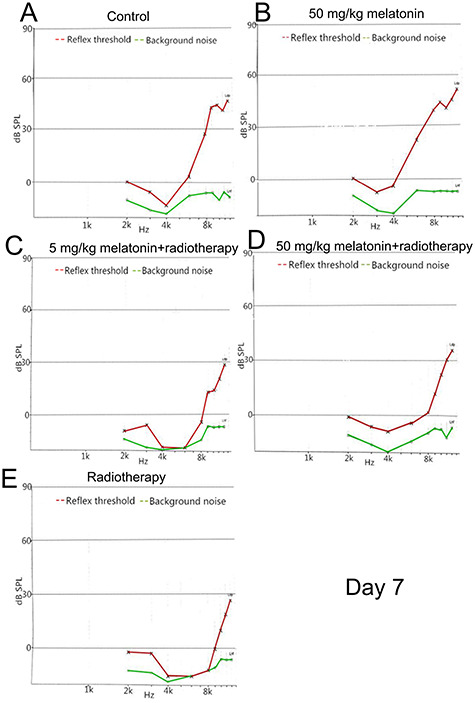
DPOAE amplitude of each frequency on day 7 after irradiation. L1 = L2 = 80 dB SPL. A representative image of a single measurement for each group is shown. (A) Control group. (B) 50 mg/kg melatonin group. (C) 5 mg/kg melatonin+radiotherapy group. (D) 50 mg/kg melatonin+radiotherapy group. (E) Radiotherapy group.
Histopathological studies
On day 3 after irradiation, the inner ear cells appeared essentially normal in each group; however, in the radiotherapy group the cytoplasm of the outer hair cells was slightly edematous and lightly stained. On day 7 after irradiation, the basement membrane of the cochlea was distorted and broken in the radiotherapy group. In the melatonin-5 + radiotherapy group the outer hair cytoplasm was swollen with nuclear condensation or loss, and occasional damage to the vascular stria structure was noted. On the other hand, histopathological evidence indicating inner ear cell damage was markedly reduced in the melatonin-50 + radiotherapy group (Figs 6 and 7).
Fig. 6.
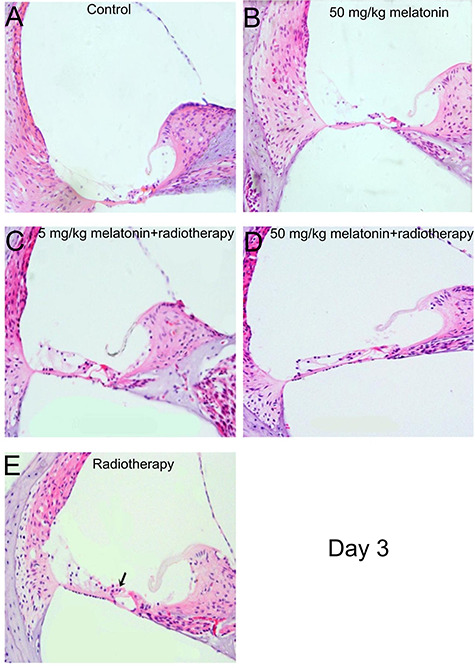
Hematoxylin and eosin staining of the inner ear on day 3 after irradiation. (A) Control group. (B) 50 mg/kg melatonin group. (C) 5 mg/kg melatonin+radiotherapy group. (D) 50 mg/kg melatonin+radiotherapy group. (E) Radiotherapy group. The outer hair cells in the radiotherapy group were slightly swollen and lightly stained (arrow). (Magnification = 40×).
Fig. 7.
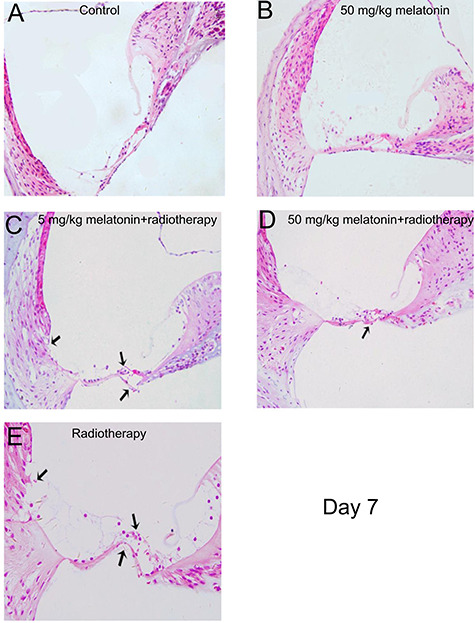
Hematoxylin and eosin staining of the inner ear on day 3 after irradiation. (A) Control group. (B) 50 mg/kg melatonin group. (C) 5 mg/kg melatonin+radiotherapy group. (D) 50 mg/kg melatonin+radiotherapy group. (E) Radiotherapy group. (C, D, E) The cytoplasm of the outer hair cells was swollen and lightly-stained. (C, E) Outer hair cell basement membrane bending deformation and cytoplasmic and nuclear condensation was noted. (E) Stria vascularis destruction was noted (arrows). (Magnification = 40×).
Prestin mRNA expression
We collected the Corti tissue from the mice at 3 and 7 days after the irradiation exposure and examined the mRNA abundance using qPCR. On day 3 after irradiation, prestin mRNA expression was significantly increased in all groups that received irradiation. However, the expression levels were significantly lower in the two groups that received melatonin+radiotherapy than in the radiotherapy alone group (both, P < 0.05), with the expression lower in the melatonin-50 group than in the melatonin-5 group. On day 7 after irradiation, prestin mRNA expression in the radiotherapy group was significantly greater than that on day 3 (P = 0.002). However, prestin mRNA expression levels in the melatonin-50 group were decreased than on day 3 (Table 3; P = 0.001).
Table 3.
Prestin mRNA expression level on days 3 and 7 after irradiation (2-ΔΔCT); two-way ANOVA with Sidak’s multiple comparisons test
| Group | Ear | Day | P c | |
|---|---|---|---|---|
| 3 | 7 | |||
| Control (C) | 6 | 0.054 ± 0.016 | 0.054 ± 0.001 | 2.032 |
| Melatonin (M) | 12 | 0.056 ± 0.009b | 0.043 ± 0.003b | 0.071 |
| 5 mg/kg M + R | 12 | 1.006 ± 0.003a,b | 1.001 ± 0.001a,b | 0.920 |
| 50 mg/kg M + R | 12 | 0.853 ± 0.014a,b | 0.753 ± 0.014a,b | 0.001 |
| Radiotherapy (R) | 12 | 1.742 ± 0.089a | 2.714 ± 0.013a | 0.002 |
a P < 0.05 when compared to the control group at the same time point.
b P < 0.05 when compared to the radiotherapy group at the same time point.
cIndependent samples t-test: *P < 0.05 when with the same group at a different time point.
DISCUSSION
Radiotherapy is a primary treatment for many head and neck cancers; however, it can cause serious side effects involving the ears, such as secretory otitis media, Eustachian tube dysfunction, chronic otitis media, and SNHL, which is often delayed and irreversible [1]. The results of this study showed that pretreatment with melatonin can reduce inner ear damage caused by irradiation.
Injury to the cochlear hair cells and stria vascularis varies with the amount of irradiation given; however, the mechanisms of injury are not clearly understood. Kim et al. [12] have suggested that cellular metabolism is directly affected by radiation, and this leads to cellular dysfunction, degeneration and necrosis. Low et al. [3] suggested that radiation causes stria vascularis microvessel injury, which leads to a disturbance of the microcirculation and thus indirectly reduces the blood supply to the cochlea.
Irrespective of the specific mechanisms, free radicals that are produced by irradiation are considered to be an important causative factor of inner ear damage; thus, reducing excessive free radicals is an important way to protect the cochlea from the effects of radiation. The antioxidant L-N-acetylcysteine (L-NAC) has been shown to reduce the level of reactive oxygen species (ROS) and apoptosis of cochlear cells [13]. As such, the effect of L-NAC provides the basis for the use of antioxidant therapy to reduce radiation-induced cochlear damage.
In the current study, we chose to examine the potential protective effect of melatonin because melatonin is considered a safe drug with few side effects. Melatonin also has a number of properties that make it a good candidate for protection from radiation-induced injury. Melatonin is a strong free radical scavenger, and produces stable oxidation products which prevents the re-formation of free radicals [10]. Melatonin is also strongly lipophilic, and as such can freely penetrate the vast majority of cell membranes and a variety of biological barriers, including mitochondrial membranes, which increases its antioxidant efficiency leading to a reduction in cell apoptosis [7, 10]. Studies have shown that melatonin effectively reduces radiation damage to the spleen [14], liver [15], lungs [16] and ocular lens [17].
A number of studies have suggested that melatonin can protect the inner ear from various types of damage. Ye et al. [18] used guinea pigs and reported that melatonin can protect the cochlea from ototoxicity due to gentamicin. Bas et al. [19] reported that melatonin was more effective at reducing noise-induced injury of the inner ear than tacrolimus or dexamethasone. In a retrospective study, Lasisi et al. [20] showed that a low melatonin plasma concentration was important for the development of high-frequency presbycusis, and melatonin concentration was negatively correlated with age-related hearing loss. These results suggested that melatonin may delay cochlear aging.
Damage to the inner ear due to noise and drugs, and age-related sensorineural deafness are all closely related to excessive production of free radicals that damage the cochlea hair cells. As melatonin has been shown to reduce damage to the inner ear by the aforementioned factors, melatonin may also protect the inner ear from radiation-induced damage. Karaer et al. [11] studied the effect of radiotherapy on the inner ear using a rat model, and found that melatonin treatment attenuates hearing loss and pathological cellular changes (edema and necrosis) in the stria vascularis, hair cells and spiral ganglion cells on the fifth day after irradiation. In the current study, hearing loss occurred after radiation exposure, and high-frequency hearing loss was especially prominent. This indicates that damage to the base of the cochlea was more severe than damage to the apex, and this is likely because metabolism and the development of free radicals is greater in the base than the apex [21].
However, unlike the study by Karaer et al. [11], in our study we administered a single dose of 16 Gy, and we observed inner ear cell pathological changes on days 3 and 7 after irradiation. Early after radiotherapy (day 3), damage was limited and only slight swelling was observed in the cells. During this period, DPOAE testing indicated that the function of outer hair cells was decreased and the cells were damaged; however, it was possible that the cells were in a compensatory state. Pathologic changes of the inner ear cells were, however, observed by light microscopy at day 7 after irradiation. Like the aforementioned study [11], pretreatment with melatonin reduced inner ear cell damage. As such, melatonin appears to have a protective effect against radiation-induced cochlear damage. Therefore, this study further confirmed that melatonin can protect inner ear damage after radiation via different methods (e.g. animal models and radiation modes). A previous study has indicated that the degree of SNHL is closely correlated with the dosage of irradiation, radiotherapy mode, duration of radiotherapy and if radiotherapy is combined with chemotherapy [22]. This may explain why the results of our study are slightly different than those of Karaer et al. [11].
Studies have shown that prestin expression may be involved in the mechanism of inner ear injury caused by factors such as drugs, noise and radiation. Parham [6] reported that outer hair cells were the first to show signs of damage and that prestin could be used as a marker for the early detection of acquired SNHL. We found that the expression of prestin mRNA was upregulated after radiotherapy, but the radiotherapy-induced upregulation of prestin was reduced by pretreatment with melatonin. Prestin mRNA expression may be upregulated because hair cells undergo self-repair to maintain the normal function of the cochlea, and after radiation damage there is an increase in residual hair cells to compensate for damaged cells. Because melatonin decreases the production of free radicals, there is less damage to the hair cells and subsequently there is less upregulation of prestin mRNA.
Melatonin can be administered orally, and by intramuscular and intraperitoneal injection. A study showed that dosages up to 100 mg/kg had no appeared side effects [23]. The results of the current study indicated that melatonin dosages of 5 and 50 mg/kg were both effective at reducing high-frequency hearing loss, the loss and disordered arrangement of outer hair cells, and the upregulation of prestin mRNA; however, the effects were greater at the 50 mg/kg dosage. In this study, no significant side effects were observed, suggesting that the dose range of melatonin was safe. Erdem et al. [24] used a rat model to study the effect of amikacin ototoxicity, and the protective effect of melatonin by measuring DPOAEs at different time points. Interestingly, low-dose melatonin had a protective effect against amikacin-induced ototoxicity; however, at a higher dose melatonin facilitated amikacin-induced ototoxicity. The authors postulated that the vasodilatory effect of melatonin was more prominent than the antioxidant effect at a high dose, which led to an increased accumulation of amikacin in the inner ear. This suggests that studies to determine the optimal dose of melatonin to protect the inner ear from radiation damage are needed.
There are limitations to the study that should be considered. An animal model was used, and thus the results may not be transferable to human patients. Only two dosages of melatonin were examined. As the higher dose provided a greater protective effect, studies using an even higher dose are warranted. In addition, we did not analyze oxidative stress and apoptosis of inner ear cells to further clarify the protective effect of melatonin against radiation. Moreover, control mice received 0.9% NaCl intraperitoneally. However, the control reagent for injection should be alcoholic saline since melatonin was dissolved in alcoholic saline. All these limitations should be addressed in future studies.
CONCLUSIONS
Melatonin can protect the inner ear from radiation damage in a mice model. Further human trials to examine the protective effect of melatonin against radiation damage to the inner ear are warranted. However, due to the large difference in body weight and surface area between mice and human, the dosage range of melatonin used in animal experiments is only used as a reference for human clinical trials.
CONFLICT OF INTEREST
The authors declared there were no conflicts of interest.
FUNDING
Guangdong Provincial Science and Technology Planning Project (No. 2014A020212491).
Contributor Information
Ting Chen, Department of Otolaryngology, GuangDong Women and Children Hospital, Guangzhou, Guangdong, China.
Yuling Luo, Department of Oncology, Nanfang Hospital, Southern Medical University, Guangzhou, Guangdong, China.
Qi Li, Department of Otorhinolaryngology-Head and Neck Surgery, Nanfang Hospital, Southern Medical University, Guangzhou, Guangdong, China.
Chen Yang, Department of Otolaryngology Head and Neck Surgery, Beijing Friendship Hospital, Capital Medical University, Beijing, China.
Yixin Yuan, Department of Otorhinolaryngology-Head and Neck Surgery, Nanfang Hospital, Southern Medical University, Guangzhou, Guangdong, China.
Jinhao Peng, Department of Radiation Oncology, Nanfang Hospital, Southern Medical University, Guangzhou, Guangdong, China.
Molu Ban, Department of Otorhinolaryngology-Head and Neck Surgery, the People's Hospital of Guangxi Zhuang Autonomous Region, Nanning, Guangxi, China.
Yong Liang, Department of Otorhinolaryngology-Head and Neck Surgery, Nanfang Hospital, Southern Medical University, Guangzhou, Guangdong, China.
Wei Zhang, Department of Otorhinolaryngology-Head and Neck Surgery, Nanfang Hospital, Southern Medical University, Guangzhou, Guangdong, China.
References
- 1. Jereczek-Fossa BA, Zarowski A, Milani F et al. Radiotherapy-induced ear toxicity. Cancer Treat Rev 2003;29:417–30. [DOI] [PubMed] [Google Scholar]
- 2. Tan PX, Du SS, Ren C et al. Radiation-induced cochlea hair cell death: Mechanisms and protection. Asian Pac J Cancer Prev 2013;14:5631–5. [DOI] [PubMed] [Google Scholar]
- 3. Low WK, Tan MGK, Chua AWC et al. 12th yahya cohen memorial lecture - the cellular and molecular basis of radiation-induced sensori-neural hearing loss. Ann Acad Med Singapore 2009;38:91–4. [PubMed] [Google Scholar]
- 4. Fischer N, Johnson Chacko L, Glueckert R et al. Age-dependent changes in the cochlea. Gerontology 2020;66:33–9. [DOI] [PubMed] [Google Scholar]
- 5. Merchant TE, Gould CJ, Xiong X et al. Early neuro-otologic effects of three-dimensional irradiation in children with primary brain tumors. Int J Radiat Oncol Biol Phys 2004;58:1194–207. [DOI] [PubMed] [Google Scholar]
- 6. Parham K. Prestin as a biochemical marker for early detection of acquired sensorineural hearing loss. Med Hypotheses 2015;85:130–3. [DOI] [PubMed] [Google Scholar]
- 7. Hall S, Rudrawar S, Zunk M et al. Protection against radiotherapy-induced toxicity. Antioxidants 2016;5:pii: E22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Amaral FGD, Cipolla-Neto J. A brief review about melatonin, a pineal hormone. Arch Endocrinol Metab 2018;62:472–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Poeggeler B, Reiter RJ, Tan D-X et al. Melatonin, hydroxyl radical-mediated oxidative damage, and aging: A hypothesis. J Pineal Res 1993;14:151–68. [DOI] [PubMed] [Google Scholar]
- 10. Zetner D, Andersen LPH, Rosenberg J. Melatonin as protection against radiation injury: A systematic review. Drug Res (Stuttg) 2016;66:281–6. [DOI] [PubMed] [Google Scholar]
- 11. Karaer I, Simsek G, Gul M et al. Melatonin protects inner ear against radiation damage in rats. Laryngoscope 2015;125:E345–9. [DOI] [PubMed] [Google Scholar]
- 12. Kim CS, Shin SO. Ultrastructural changes in the cochlea of the Guinea pig after fast neutron irradiation. Otolaryngol Neck Surg 1994;110:419–27. [DOI] [PubMed] [Google Scholar]
- 13. Low WK, Sun L, Tan MGK et al. L-N-acetylcysteine protects against radiation-induced apoptosis in a cochlear cell line. Acta Otolaryngol 2008;128:440–5. [DOI] [PubMed] [Google Scholar]
- 14. Sharma S, Haldar C, Chaube SK. Effect of exogenous melatonin on X-ray induced cellular toxicity in lymphatic tissue of Indian tropical male squirrel, Funambulus pennanti. Int J Radiat Biol 2008;84:363–74. [DOI] [PubMed] [Google Scholar]
- 15. Shirazi A, Mihandoost E, Ghobadi G et al. Evaluation of radio-protective effect of melatonin on whole body irradiation induced liver tissue damage. Cell J 2013;14:292–7. [PMC free article] [PubMed] [Google Scholar]
- 16. Şener G, Jahovic N, Tosun O et al. Melatonin ameliorates ionizing radiation-induced oxidative organ damage in rats. Life Sci 2003;74:563–72. [DOI] [PubMed] [Google Scholar]
- 17. Shirazi A, Haddadi GH, Asadi-Amoli F et al. Radioprotective effect of melatonin in reducing oxidative stress in rat lenses. Cell J 2011;13:79–82. [PMC free article] [PubMed] [Google Scholar]
- 18. Ye LF, Tao ZZ, Hua QQ et al. Protective effect of melatonin against gentamicin ototoxicity. J Laryngol Otol 2009;123:598–602. [DOI] [PubMed] [Google Scholar]
- 19. Bas E, Martinez-Soriano F, Láinez JM et al. An experimental comparative study of dexamethasone, melatonin and tacrolimus in noise-induced hearing loss. Acta Otolaryngol 2009;129:385–9. [DOI] [PubMed] [Google Scholar]
- 20. Lasisi AO, Fehintola FA. Correlation between plasma levels of radical scavengers and hearing threshold among elderly subjects with age-related hearing loss. Acta Otolaryngol 2011;131:1160–4. [DOI] [PubMed] [Google Scholar]
- 21. Thalmann R, Kusakari J, Miyoshi T. Dysfunctions of energy releasing and consuming processes of the cochlea. Laryngoscope 1973;83:1690–712. [DOI] [PubMed] [Google Scholar]
- 22. Jereczek-Fossa BA, Rondi E, Zarowski A et al. Prospective study on the dose distribution to the acoustic structures during postoperative 3D conformal radiotherapy for parotid tumors: Dosimetric and audiometric aspects. Strahlenther Onkol 2011;187:350–6. [DOI] [PubMed] [Google Scholar]
- 23. Wu Q, Jing Y, Yuan X et al. Melatonin treatment protects against acute spinal cord injury-induced disruption of blood spinal cord barrier in mice. J Mol Neurosci 2014;54:714–22. [DOI] [PubMed] [Google Scholar]
- 24. Erdem T, Ozturan O, Iraz M et al. Dose-dependent dual effect of melatonin on ototoxicity induced by amikacin in adult rats. Eur Arch Oto-Rhino-Laryngology 2005;262:314–21. [DOI] [PubMed] [Google Scholar]


