Abstract
Complications after pressure ulcer reconstruction are common. A complication rate of 21% to 58% and a 27% wound recurrence has been reported. The aim of this study was to decrease postoperative wound‐healing complications with incisional negative pressure wound therapy (iNPWT) postoperatively. This was a prospective non‐randomised trial with a historic control. Surgically treated pressure ulcer patients receiving iNPWT were included in the prospective part of the study (Treatment group) and compared with the historic patient cohort of all consecutive surgically treated pressure ulcer patients during a 2‐year period preceding the initiation of iNPWT (Control). There were 24 patients in the Control and 37 in the Treatment groups. The demographics between groups were similar. There was a 74% reduction in in‐hospital complications in the Treatment group (10.8% vs 41.7%, P = 0.0051), 27% reduction in the length of stay (24.8 vs 33.8 days, P = 0.0103), and a 78% reduction in the number of open wounds at 3 months (5.4 vs 25%, P = −0.0481). Recurrent wounds and history of previous surgery were risk factors for complications. Incisional negative pressure wound therapy shortens hospital stay, number of postoperative complications, and the number of recurrent open wounds at 3 months after reconstructive pressure ulcer surgery, resulting in significant cost savings.
Keywords: cost, flap, negative pressure, pressure ulcer, reconstruction
1. INTRODUCTION
Spinal cord injury (SCI) predisposes patients to a variety of challenges. In addition to several other possible complications after SCI, pressure ulcers are a common problem. Up to 41% of patients with an acute SCI develop a pressure ulcer in their first year after injury,1 37% during their acute care hospitalisation,2 and 73% of patients developed a pressure ulcer in a 20‐year follow‐up study.3 Pressure ulcer development is a serious and distressing adverse event that can represent failure of care. As such, it is of utmost importance to optimise both pressure ulcer prevention and conservative wound care management. Preventative management options include routine skin checks, offloading pressure‐prone areas by positioning, proper seating systems, mattresses, optimising transfers from chair to bed and back, nutrition, continence, spasticity, addictions, smoking, bowel routine, and infections to name a few. When prevention and conservative management fails, surgery is often required.
Surgical management of pressure ulcers is demanding. Complications are common, including wound dehiscence, surgical site infections (SSI), haematomas, seromas, partial or complete flap necrosis, and wound recurrences. A 27% wound recurrence has been reported after flap reconstruction, and the recurrence was related to original wound defect size.4 Overall complications of 21% to 58.7%5, 6, 7, 8 and recurrences of 16% after a mean follow‐up of 55 months have been noted, with more recurrences over the ischial tuberosities.6 Previous reconstruction has been shown to be a risk factor for postoperative dehiscence in ischial tuberosity pressure ulcers as well,9, 10 and wound dehiscence in general is the most common complication.7, 8 Typical risk factors for wound‐healing complications after pressure ulcer reconstruction include BMI < 18, smoking,7, 11 osteomyelitis, diabetes, and ulcers located over the ischial tuberosity.7, 8
Incisional negative pressure wound therapy (iNPWT) creates a negative pressure environment over the sutured or stapled wound that helps hold incision edges together, reduces lateral tension on the wound, stimulates wound edge blood perfusion, removes fluid from the wound and acts as a barrier to external contamination. The dressing is connected to a machine that creates negative pressure. The commonly used mode of therapy is by delivering a continuous negative pressure of 125 mm Hg.
iNPWT has been used to improve wound‐healing outcomes in sutured wounds in various surgical specialties and procedures, including caesarean sections,12, 13 vascular surgery,14, 15 loop ileostomy reversal,16 cardiac surgery,17 open vein harvest in cardiac surgery,18 sternotomy,19 pectoralis major muscle flap for deep sternal infections,20 incisional ventral hernia repairs,21 orthopaedic surgery for acetabular fractures,22 knee arthroplasties,23 hip arthroplasties,24 femoral neck fractures,25 immediate breast reconstructions,26 and post‐bariatric abdominoplasties.27 These studies found that the use of incisional negative pressure therapy immediately after surgery decreased the number of SSIs,12, 13, 16, 17, 19 overall wound complications,13, 15, 17, 27 seroma formation,24, 25 and complications requiring repeat surgery.14, 15, 20 Also noted were reduced length of stay in hospital18, 27 and in intensive care unit,20 reduced wound drainage,21, 24, 25, 26 and time needed for dressing changes.25, 27 Finally, shortened time for drain removal26 and increased ability to self‐care18 have also been identified. However, other studies have reported no difference between conventional and incisional negative pressure wound care.19 Two meta‐analyses showed potential benefit in decreasing SSIs28 or that there is evidence of reducing SSIs, but the evidence is of low quality.29 There is no literature available for the use of iNPWT after pressure ulcer reconstructions.
The purpose of this study was to evaluate the use of iNPWT on surgically treated pressure ulcers in patients with an spinal cord impairment.
2. METHODS
This was a prospective non‐randomised trial with a historic control. All surgically treated pressure ulcer patients receiving postoperative iNPWT since June 2016 were included in the prospective part of the study (Treatment‐group). This group was compared with a historic patient cohort, which consisted of all consecutive surgically treated pressure ulcer patients during a 2‐year period preceding the initiation of iNPWT (Control group).
All patients in both groups were initially assessed by a multidisciplinary team in the spinal cord impairment wound clinic after receiving a referral from the community. Disciplines involved included plastic surgery, physiatry, occupational therapy, physiotherapy, dietitian, and wound care clinician. Attention was paid to wound aetiology, equipment (manual or power chair, seating systems, matrasses, ceiling lift, slings, transfer boards, commodes, and others), transfers from wheelchair to bed and back, spasticity, addictions including smoking, autonomic dysreflexia, wound care, incontinence, bowel routine, infections, sheer forces, and positioning protocols. Management instructions were given to patients, their aids, and the caregivers in the community. For most patients, non‐surgical wound treatment was continued until all the above variables were optimised. They were then placed on the surgical waitlist if appropriate. During the wait time, they were followed in the clinic regularly. Indications for surgical management and surgical treatment itself remained the same across the study period, and all surgeries were performed by the author. Indications for operative management included Gr3‐4 wounds, with full‐thickness skin loss exposing fat or deeper tissues,29 underlying bone exposure, documentation of osteomyelitis, and lack of progression in wound healing in 3 months after optimisation of all patient variables.
Surgical technique included the following: general anaesthesia, appropriate padding, and positioning according to wound location (prone or lateral decubitus position). Wounds were infiltrated with bupivacain 0.25% with epinephrine to control bleeding, and complete sharp wound excision with a 0.5 to 1 cm margin was undertaken. Underlying bony prominences were exposed, removed with an osteotome, and flattened with a file. Meticulous haemostasis, wound irrigation with saline, and layered wound closure with the use of two drains was undertaken: one over the resected bone and one in the flap donor site. Wound closure was performed using one of the following: gluteus maximus muscle advancement inferiorly over the resected bone and layered primary closure for the ischial tuberosity (IT) pressure ulcers with small skin defects; rotational musculocutaneous gluteus maximus or V‐Y posterior thigh flaps for bigger IT ulcers; rotational or V‐Y musculocutaneous gluteus maximus flap for sacral ulcers; or tensor fascial lata flap for greater trochanteric (GT) ulcers. In case of recurrence after having previous surgery with a flap, the old flap was raised and advanced when possible. This was considered “primary closure.”
Three tissue biopsies were taken perioperatively for culture and sensitivities: one from the excised soft tissue (wound), one from the excised bone, and one from the remaining bone after bone excision using sterile technique (“terminal bone”). All patients received antibiotics for 2 weeks unless the terminal bone culture was positive for bacteria, in which case the antibiotics were given for 6 weeks. All antibiotics were targeted to the tissue biopsy culture results.
Postoperatively, the wounds in the Control group were dressed with Steristrip tapes and a Mepore dressing. For the Treatment group, the wound was covered with the Prevena Incision Management System (Prevena Customizable Dressing—90 cm, KCI, an Acelity company, San Antonio, Texas) dressing with the following setting: 125 mm Hg of negative pressure on continuous suction mode (Figure 1). The Prevena dressing was left in place for 7 days, after which it was removed, and a Mepore dressing was applied. All patients were on bed rest for 2 weeks with the head of the bed flat at all times and no hip flexion. Patients were not lying on their flaps and were repositioned every 2 hours by staff. If no wound‐healing complications were noted at 2 weeks, a slow mobilisation progression protocol was initialised. When the patients had progressed to sitting for 2 hours, they typically were transferred to a rehabilitation centre before going home to optimise outcomes. During this period, the following was assessed: home situation in general, seating, cushions, transfers, all other equipment, nutrition, and spasticity. Suture removal was at 3 weeks after surgery.
Figure 1.
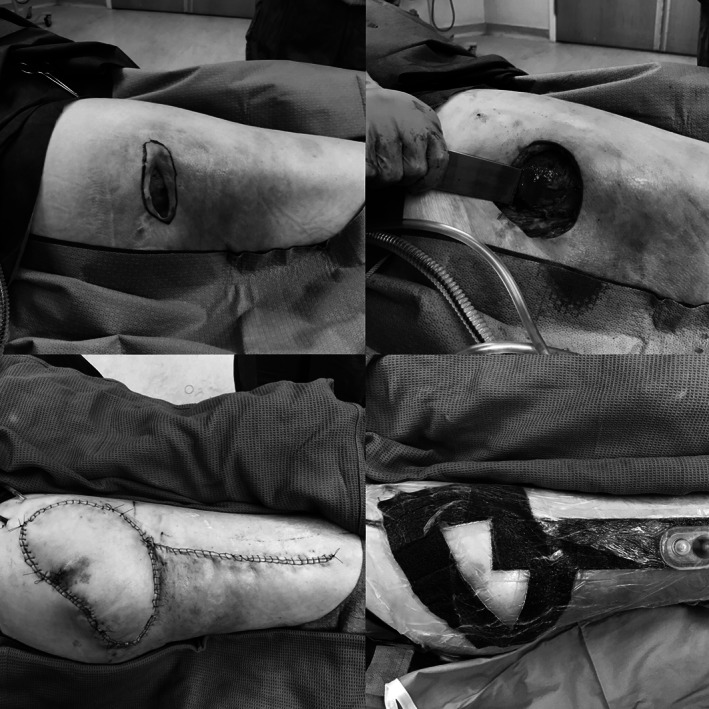
The use of Prevena dressing in the operating room. Clockwise from top left. (A) Greater trochanteric pressure ulcer. (B) Excising the underlying bony prominence after wound excision. (C) Tensor fascial lata flap in place. (D) Prevena dressing in place
The following demographics and data elements were collected: age, height, weight, body mass index (BMI), gender, level of SCI, American Spinal Injury Association (ASIA) classification, smoking status, and comorbidities. Wound parameters included pre‐surgery wound skin defect (width and length), wound depth, wound cavity widest diameter, duration of wound before surgery, anatomic wound location, bone exposure, and previous wound surgeries, excluding debridements only. Surgical and postoperative variables included surgical reconstruction method, in‐hospital all complications, infections, wound dehiscence (anything greater than a 1.5 × 1.5 × 1.5 cm wound at the operative site), haematomas, or seromas requiring intervention, need for acute or delayed repeat surgery, length of stay in hospital, and 90‐day mortality. All patients were assessed 3 months after being discharged from hospital. At that time, any open wound greater than a 1.5 × 1.5 × 1.5 cm at the operative site was considered open.
2.1. Statistical analysis
All comparisons are between the two groups (Treatment vs Control). Continuous measures are summarised as means and range. Comparisons of continuous measures were performed with a Student's t‐test when normally distributed and Wilcoxon two‐sample test for non‐parametric data when the normality assumption was not met. Categorical data are summarised as counts and percent. Chi‐square or Fisher's exact tests were used for categorical data to compare treatment groups. All tests were two‐sided and at a significance level of alpha = 0.05. All analyses were performed with SAS 9.
3. RESULTS
There were 24 patients in the Control group and 37 patients in the Treatment group with a total of 70 wounds treated surgically. The mean (range) age was 56.9 (34‐84) and 55.8 (24‐84) years and BMI 25.1 (16.9‐40.1) and 26.3 (17.6‐34), with male prevalence of 91.7% and 83.7%, respectively. None of these variables were statistically significant between groups. Every patient had an spinal cord impairment: 60 patients with a traumatic SCI and 1 patient with HTLV‐A myelopathy. The percentage of patients who were tetraplegic in the Treatment and Control groups were 43.2% and 25%, respectively (P = 0.1257). The Prevena dressing stayed on a mean of 6.7 days in the Treatment group. Other demographics are presented in Table 1.
Table 1.
Demographics and wound characteristics
| Treatment | Control | P‐value | |||
|---|---|---|---|---|---|
| N | 37 | 24 | |||
| Age, y (ave, range) | 55.8 | 24‐84 | 56.9 | 34‐84 | 0.7101 |
| Men | 83.7% | 91.7% | 0.4621 | ||
| Current smokers | 8.10% | 12.50% | 0.6717 | ||
| Spinal cord impairment | 100% | 100% | |||
| ASI‐A | 78.40% | 79.20% | 0.9415 | ||
| Level of spinal cord impairment | |||||
| Cervical spine (N, %) | 16 | 43.2 | 6 | 25 | 0.1257a |
| Thoracic spine (N, %) | 20 | 54.1 | 18 | 75 | |
| Lumbar spine (N, %) | 1 | 2.7 | 0 | 0 | |
| Wound location (% of group) | |||||
| Ischial tuberosity (IT) | 67.4 | 44.4 | 0.2825b | ||
| Greater trochanteric (GT) | 14.0 | 22.2 | |||
| Sacrum | 18.6 | 25.9 | |||
| Other | 0.0 | 7.4 | |||
| Multiple wounds | 16% | 12.50% | 1.000 | ||
| Wound present (mo) | 24.7 | 2‐164 | 23.5 | 6‐60 | 0.5840 |
| Recurrent ulcer | 24.30% | 45.80% | 0.0804 | ||
| Previous surgeries | 18.90% | 50% | 0.0211 | ||
Comparison between groups of cervical and thoracic (lumbar removed).
Comparison between groups with “Other” wound location removed.
Wound characteristics are also presented in Table 1. Most wounds were over the ischial tuberosities followed by the sacrum and greater trochanters. The number of patients with multiple wounds were similar between groups. There were no differences in wound sizes, including skin defects, wound depths, or maximum wound cavity diameters between the groups. The wound had been present in the Control group for 23.5 and 24.7 months in the Treatment group. The Control group patients had more previous surgeries on their wounds compared with the Treatment group patients (50% vs 18.9%, respectively, P = 0.0211). Most previous surgeries were performed in other hospitals.
The most commonly performed reconstruction was the musculocutaneous gluteus rotation flap (Table 2). There were more primary closures (20.8% vs 2.7%) and gluteus muscle advancements with overlying primary closure (16.7% vs 0%) in the Control group.
Table 2.
Surgical procedures performed
| Surgical procedure (%) | Treatment | Control |
|---|---|---|
| Gluteus rotation flap | 62.2 | 33.3 |
| Gluteus V‐Y flap | 10.8 | 8.3 |
| Tensor fascial lata flap | 10.8 | 12.5 |
| Post thigh flap | 10.8 | 4.2 |
| Primary closure | 2.7 | 20.8 |
| Vastus lateralis muscle flap | 2.7 | 8.3 |
| Gluteus muscle flap with overlying primary closure | 0.0 | 16.7 |
The counts were too small for some categories for a χ 2 test to be valid.
Patients who had recurrent wounds at the time of surgery had more complications (45%) than patients with primary wounds (22.2%) (P = 0.0211). In addition, patients who had had previous surgery were 5.9 times more likely to have a complication compared with patients who had not had surgery before (OR: 0.170; 95% CI (0.047, 0.614). No significant correlation was found between complications and the length of time the wound had been present or presence of multiple wounds.
The number of patients who required repeat surgery during their hospital stay was the same between groups. However, there was a 74% reduction in general in‐hospital complications in the Treatment group (10.8% vs 41.7%, P = 0.0051), 27% reduction in the mean length of stay (mean 24.8 vs 33.8 days, P = 0.0103), 78.4% reduction in surgeries needed for later complications (2.7% vs 12.5%, P = 0.2904), and an 78% reduction in the number of open wounds at 3 months' follow up (5.4% vs 25%, P = −0.0481) (Table 3).
Table 3.
Hospital stay and follow‐up data
| Treatment | Control | P‐value | |
|---|---|---|---|
| Complications during hospital stay (%) | 10.8 | 41.7 | 0.0051 |
| Repeat surgeries, acute (%) | 8.1 | 8.3 | 1.000 |
| Repeat surgery, late (%) | 2.7 | 12.5 | 0.2904 |
| Los (d), mean | 24.8 | 33.8 | 0.0103 |
| Wound open at 3 mo (%) | 5.4 | 25.0 | 0.0481 |
Complications related to wound locations in the whole study group (N = 70 wounds) are presented in Table 4. Overall complications, rates of complications requiring acute surgery, and wounds open at 3 months are compared between treatment groups for each of the anatomical locations. There was a significant difference in the rate of complications during admissions between the three anatomical locations. Of the 12 patients with greater trochanter ulcers, 7 (58.3%) had a complication during admission compared with 17.1% of the ischial tuberosity and 13.3% of the sacral ulcers (P = 0.0104). The biggest reduction in immediate complications were in the ischial tuberosity group where the complications dropped 83.4% in the Treatment group (41.7% vs 6.9%, P = 0.0155). There was no difference, however, in the rate of complications requiring acute surgery nor rate of wounds open at 3 months between anatomical sites.
Table 4.
Postoperative complications and 3‐month follow‐up status by anatomical location
| Sacrum | Ischial tuberosity | Greater trochanter | |
|---|---|---|---|
| Complications during admission (%) | |||
| Control | 28.5 | 41.7 | 83.3 |
| Treatment | 0 | 6.9 | 33.3 |
| P‐value | 0.2000 | 0.0155 | 0.2424 |
| Complications requiring acute surgery (%) | |||
| Control | 0 | 8.33 | 16.7 |
| Treatment | 0 | 10.3 | 16.7 |
| P‐value | N/A | 1.000 | 1.000 |
| Wound open at 3 mo (%) | |||
| Control | 14.3 | 16.7 | 33.3 |
| Treatment | 0 | 3.4 | 16.7 |
| P‐value | 0.4667 | 0.2002 | 1.000 |
Both patients in the Treatment group who required acute repeat surgery had a haematoma and the on of these also had wound edge necrosis. One of these patients was on Rivuroxaban anticoagulation medication during surgery. For the Control group, the acute repeat surgeries were required for a wound dehiscence and for flap tip necrosis. No Prevena dressings were lost because of malfunction, and there were no local side effects or complications related to the dressings. Overall, there were no infectious complications, and the in‐hospital and 3‐month follow‐up mortality was 0% in the whole study population.
4. DISCUSSION
Patients with SCI represent a challenging problems in multiple levels, including patients themselves, caregivers, and nursing and physician staff. It is estimated that 60 000 die from pressure ulcer complications each year in the United States.30 The lack of sensation, inability of self‐adjust positioning, restrictions to obtain proper equipment, and limitations in homecare are contributing factors. A Canadian study showed that the lifetime cost for an SCI patient with a pressure ulcer was about 480 000 CAD, making it a substantial burden to the health care system.31
Incisional negative pressure therapy for closed wounds has shown to be beneficial in several surgical subgroups.12, 13, 14, 15, 17, 18, 19, 20, 22, 23, 24, 25, 26, 27 It has not, however, been reported before in the treatment after pressure ulcer reconstructions. The current study aimed to see whether there was a difference in rates of acute and late wound‐healing complications or other outcome measures when the postoperative dressing was switched from a regular wound dressing to an incisional negative pressure wound dressing in this very specific group of challenging patients.
Most of the wounds in this study were over the ischial tuberosities, which is consistent with previously published literature.4, 7, 8, 9, 10 This relates to the fact that most paralysed patients spend much of their day sitting in their manual or power wheelchairs, and the seating systems are unfortunately not always optimised. Often, relating to their SCI and muscle wasting, there also is a lack of good‐quality soft tissue coverage over the bony prominences in this area. This anatomical location is also very prone to skin breakdown because of excessive moisture from urine and faecal incontinence and sweating, which is often related of autonomic dysreflexia.
This study showed that the use of iNPWT over pressure ulcer reconstruction sites is safe and beneficial, with no dressing‐related complications or side effects. The only difference in the postoperative management between the study groups was the use of the incisional negative pressure dressing as the postoperative dressing in the Treatment group. The surgical treatment and postoperative care were otherwise identical. Negative pressure treatment resulted in several positive outcomes, such as decrease in overall complications, significantly reduced length of stay in hospital, and decrease in the number of open recurrent wounds at 3 months after discharge from hospital, which are all important outcome measures. The reduction in overall complications and hospital stay are directly related to each other and both have been reported before.13, 15, 17, 18, 27 Our results vary from some earlier reports14, 15, 20 where a reduction in the number of complications requiring surgery was reported. Our study did not find this during the initial stay in hospital, but we found a reduction in open wounds at 3 months after surgery, which indirectly resulted in a reduction for the need of later surgical revisions.
Most common known predictive factors for complications in pressure ulcer reconstruction are BMI < 18, smoking,7, 11 osteomyelitis, diabetes, ulcers located over the ischial tuberosity, and a previous reconstruction.9, 10 In the current study, no correlation was found between complications and BMI, diabetes, or the proportion of patients with multiple wounds. Because of the small number of patients with a BMI less than 18 in our study population (one patient with no complications), conclusions regarding the higher risk for complications in this patient croup cannot be drawn. It could be also suspected that patients with a very high BMI would have more complications, but again, this was not evident in our study. The percentage of patients with a previous surgery, however, was higher in the Control group, and this is a detail that might skew our results unfavourably to the Control group.
A multiple logistic regression analysis was performed to compare the complication rates between the treatment groups with previous surgery as a covariate. Both Treatment group and previous surgery were significant predictors of complications. Patients who did not receive an iNPWT were 4.3 time more likely to have a complication compared with the Control group (OR: 0.232; 95%CI (0.060, 0.897). In addition, the patients who had had previous surgery were 5.9 times more likely to develop complications. This aligned with the finding from Jordan et al., who found more dehiscence in ischial tuberosity pressure ulcer reconstructions in patients who had had previous surgeries.9 In addition, in the subgroup of patients that did not have a previous surgery (Treatment group N = 29; Control N = 12), there was a significant reduction in the percentage of patients who developed a complication (Treatment group 3.4% v. Control 33.3%, P = 0.0202). This relates to a 90% drop in complications with the use of iNPWT in the patient group with no previous surgeries.
The reduction in hospital length of stay has been demonstrated before,18 and this is important as it has significant financial implications. Knowing the average cost for 1 day in our hospital, the reduced length of stay in the Treatment group resulted in about 165 000 CAD theoretical savings in total. The cost benefit also shows a cost saving of over $4400 per patient with a $280 investment, a 16‐time saving. This means that, in our institution, the savings from one patient with iNPWT would pay for the iNPWT dressing for 16 patients, for example, for every single pressure ulcer reconstruction for almost 1 year.
There were some limitations to this study. First, the control group was a historic control, and the data were not prospectively collected. Second, the Control group had more previous surgeries, which is a known risk factor for surgical complications after subsequent reconstruction attempts. Third, the Control group had a higher percentage of recurrent wounds. The Treatment group also consisted of 39 consecutive patients with no randomisation. Finally, this study has patients only from a single surgical centre, and the follow‐up time after surgery was only 3 months, and hence, no long term follow‐up data are available.
In summary, iNPWT reduces the number of postoperative complications, shortens hospital length of stay, and reduces the number of recurrent open wounds at 3 months after pressure ulcer reconstruction and subsequent revision surgeries in patients with spinal cord impairment resulting in significant cost savings. As such, this treatment should be considered the postoperative treatment of choice in this challenging patient population.
ACKNOWLEDGEMENTS
The author acknowledges Erin Schmid, RN, WOCN, for her commitment to this project and wound care in general and Dr Peter Lennox for his generous editorial comments.
Papp AA. Incisional negative pressure therapy reduces complications and costs in pressure ulcer reconstruction. Int Wound J. 2019;16:394–400. 10.1111/iwj.13045
REFERENCES
- 1. Stillman MD, Barber J, Burns S, Williams S, Hoffman JM. Complications of spinal cord injury over the first year after discharge from impatient rehabilitation. Arch Phys Med Rehabil. 2017;98(9):1800‐1805. [DOI] [PubMed] [Google Scholar]
- 2. Brienza D, Krishnan S, Karg P, Sowa G, Allegretti A. Predictors of pressure ulcer incidence following traumatic spinal cord injury: a secondary analysis of a prospective longitudinal study. Spinal Cord. 2018;56(1):28‐34. [DOI] [PubMed] [Google Scholar]
- 3. Le Fort M, Espagnacq M, Perrouin‐Verge B, Ravaud J. Risk analyses of pressure ulcer in tetraplegic spinal cord‐injured persons: a French long‐term survey. Arch Phys Med Rehabil. 2017;98(9):1782‐1791. [DOI] [PubMed] [Google Scholar]
- 4. Wurtzer P, Winter R, Stemmer SO, et al. Lomenta DB: risk factors for recurrence of pressure ulcers after defect reconstruction. Wound Repair Regen. 2018;26(1):664‐668. [DOI] [PubMed] [Google Scholar]
- 5. Kwok A, Simpson A, Willcockson J, Donato D, Goodwin I, Agarwal J. Complications and their associations following the surgical repair or pressure ulcers. Am J Surg. 2018;Pii:S0002‐9610(17)31486‐1. [DOI] [PubMed] [Google Scholar]
- 6. Chiu Y, Liao W, Wang T, et al. A retrospective study: multivariate logistic regression analysis of the outcomes after pressure sores reconstruction with fasciocutaneous, myocutaneous and perforator flaps. J Plast Reconstr Aesthet Surg. 2017;70(8):1038‐1043. [DOI] [PubMed] [Google Scholar]
- 7. Bamba R, Madden J, Hoffman A, et al. Flap reconstruction for pressure ulcers. An outcome analysis. Plast Reconstr Surg Glob Open. 2017;5:e1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Biglari B, Buchler A, Reitzel T, et al. A retrospective study on flap complications after pressure ulcer surgery in spinal cord‐related patients. Spinal Cord. 2014;52(1):80‐83. [DOI] [PubMed] [Google Scholar]
- 9. Jordan S, De la Garza M, Lewis V. Two‐stage treatment of ischial pressure ulcers in spinal cord injury patients: technique and outcomes over 8 years. J Plast Reconstr Aesthet Surg. 2017;70(7):959‐966. [DOI] [PubMed] [Google Scholar]
- 10. Tadiparthi S, Hartley A, Alzweri L, Mecci M, Siddiqui H. Improving outcomes following reconstruction of pressure sores in spinal injury patients. A multidisciplinary approach. J Plast Reconstr Aesthet Surg. 2016;69(7):994‐1002. [DOI] [PubMed] [Google Scholar]
- 11. Lane C, Selleck C, Chen U, Tang Y. The impact of smoking and smoking cessation on wound healing in spinal cord‐injured patients with pressure injuries: a retrospective comparison cohort study. J Wound Ostomy Continence Nurs. 2016;43(5):483‐487. [DOI] [PubMed] [Google Scholar]
- 12. Swift S, Zimmerman MB, Hardy‐Fairbanks A. Effect of single‐use negative pressure wound therapy on Postcesarian infections and wound complications for high‐risk patients. J Reprod Med. 2015;60(5–6):211‐218. [PubMed] [Google Scholar]
- 13. Gunatilake R, Swamy G, Brancazio L, et al. Closed‐incision negative‐pressure therapy in obese patients undergoing cesarean delivery: a randomized controlled trial. AJP Rep. 2017;7:e151‐e157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Weir G. The use of a surgical incision management system on vascular surgery incisions: a pilot study. Int Wound J. 2014;11(suppl 1):10‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pledger S, Nink N, Elzien M, Kunole A, Koshty A, Boning A. Reduction of groin wound complications in vascular surgery patients usint closed incision negative pressure therapy (ciNPT): a prospective, randomized, single0institution study. Int Wound J. 2018;15(1):75‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cantero R, Rubio‐Perez I, Leon M, et al. Negative‐pressure therapy to reduce the risk of wound infection following diverting loop ileostomy reversal: an initial study. Adv Skin Wound Care. 2016;29:114‐118. [DOI] [PubMed] [Google Scholar]
- 17. Colli A. First experience with a new negative pressure incision management system on surgical incisions after cardiac surgery for high risk patients. J Cardiothorac Surg. 2011;6:160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lee A, Sheppard C, Kent W, Mewhort H, Sikdar K, Fedak P. Safety and efficacy of prophylactic negative pressure wound therapy following open saphenous vein harvest in cardiac surgery: a feasibility study. Interact Cardiovasc Thorac Surg. 2017;24(3):324‐328. [DOI] [PubMed] [Google Scholar]
- 19. Grauham O, Navasardyan A, Hofmann M, Muller P, Stein J, Hetzer R. Prevention of poststernotomy wound infections in obese patients by negative pressure wound therapy. J Thorac Cardiovasc Surg. 2013;145:1387‐1392. [DOI] [PubMed] [Google Scholar]
- 20. Nickl S, Steindl J, Langthaler D, et al. First experiences with incisional negative pressure wound therapy in a high‐risk poststernotomy patient population treated with pectoralis major muscle flap for deep sternal wound infection. J Reconstr Microsurg. 2018;34(1):1‐7. [DOI] [PubMed] [Google Scholar]
- 21. Olona C, Duque E, Care A, et al. Negative‐pressure therapy in the postoperative treatment of incisional herjnioplasty wounds: a pilot study. Adv Skin Wound Care. 2014;27(2):77‐80. [DOI] [PubMed] [Google Scholar]
- 22. Crist B, Oladeji L, Khazzam M, Della Rocca G, Murthu Y, Stannard J. Role of acute negative pressure wound therapy over primarily closed surgical incisions in acetabular fracture ORIF: a prospective randomized trial. Injury. 2017;48:1518‐1521. [DOI] [PubMed] [Google Scholar]
- 23. Manoharan V, Grant A, Harris A, Hazratwala K, Wilkinson M, McEwen P. Closed incision negative pressure wound therapy vs conventional dry dressing after primary knee arthroplasty: a randomized controlled study. J Arthroplasty. 2016;31:2487‐2494. [DOI] [PubMed] [Google Scholar]
- 24. Pachowsky M, Gusinde J, Klein A, et al. Negative pressure wound therapy to prevent seromas and treat surgical incisions afger total hip athroplasty. Int Orthop. 2012;36(4):719‐722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pauser J, Mordmeyer M, Biber R, et al. Incisional negative pressure wound therapy after hemiarthroplasty for femoral neck fractures—reduction of wound complications. Int Wound J. 2016;13(5):663‐667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gabriel A, Sigalowe S, Maxwell GP. Initial experience using closed incision negative pressure therapy after immediate postmastectomy breast reconstruction. Plast Reconstr Surg Glob Open. 2016;4(7):e819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Abatangelo S, Saporiti E, Giatsidis G. Closed incision negative‐pressure therapy (cNPT) reduces minor local complications in post‐bariatric abdominoplasty body contouring: a retrospective case‐control series. Obes Surg. 2810;28(7):2096‐2104. [DOI] [PubMed] [Google Scholar]
- 28. Semsarzadeh N, Tadisina K, Maddox J, Chopra K, Singh D. Closed incision negative‐pressure therapy is associated with decreased surgical site infections: a meta‐analysis. Plast Reconstr Surg. 2015;136(3):592‐602. [DOI] [PubMed] [Google Scholar]
- 29. De Vries F, Wallert E, Solomkin J, et al. A systematic review and meta‐analysis including GRADE qualification of the risk of surgical site infections after prophylactic negative pressure wound therapy compared with conventional dressings in clean and contaminated surgery. Medicine. 2016;95936:e4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kruger E, Pires M, Ngann Y, Sterling M, Rubay S. Comprehensive management of pressure ulcers in spinal cord injury: current concepts and future trends. J Spinal Cord Med. 2013;36(6):572‐585.24090179 [Google Scholar]
- 31. Chan B, Cadarette S, Wodchis W, Krahn M, Mittmann N. The lifetime cost of spinal cord injury in Ontario, Canada: a population‐based study from the perspective of the public health care payer. J Spinal Cord Med. 2018;20:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]


