Abstract
Patients with diabetes mellitus have a lifetime risk of 15% to 25% of developing diabetic foot ulcers (DFUs). DFU is associated with significant morbidity and mortality. Wound imaging systems are useful adjuncts in monitoring of wound progress. Our study aims to review existing literature on the available wound assessment and monitoring systems for DFU. This is a systematic review of articles from PubMed and Embase (1974 – March 2020). All studies related to wound assessment or monitoring systems in DFUs were included. Articles on other types of wounds, review articles, and non‐English texts were excluded. Outcomes include clinical use, wound measurement statistics, hospital system integration, and other advantages and challenges. The search identified 531 articles. Seventeen full‐text studies were eligible for the final analysis. Five modalities were identified: (a) computer applications or hand‐held devices (n = 5), (b) mobile applications (n = 2), (c) optical imaging (n = 2), (d) spectroscopy or hyperspectral imaging (n = 4), and (e) artificial intelligence (n = 4). Most studies (n = 16) reported on wound assessment or monitoring. Only one study reported on data capturing. Two studies on the use of computer applications reported low inter‐observer variability in wound measurement (inter‐rater reliability >0.99, and inter‐observer variability 15.9% respectively). Hand‐held commercial devices demonstrated high accuracy (relative error of 2.1%‐6.8%). Use of spectroscopy or hyperspectral imaging in prediction of wound healing has a sensitivity and specificity of 80% to 90% and 74%to 86%, respectively. Majority of the commercially available wound assessment systems have not been reviewed in the literature on measurement accuracy. In conclusion, most imaging systems are superior to traditional wound assessment. Wound imaging systems should be used as adjuncts in DFU monitoring.
Keywords: artificial intelligence, diabetic foot, mobile applications, wound healing, wounds and injuries
1. INTRODUCTION
Diabetes mellitus (DM) has plagued approximately 425 million people worldwide, with an annual incidence of 6.7 to 7 per 1000 per year in developed countries. 1 Patients with DM are at risk of developing foot ulceration with a lifetime risk of 15% to 25%. 2 Several risk factors have been identified for the development of diabetic foot ulcers (DFUs): vascular disease, neuropathy, and foot deformity; all of which, are long‐term sequelae of poorly controlled DM. 3 DFU is associated with significant morbidity and mortality, with a 2.5 times higher risk of 5‐year mortality as compared to diabetic patients without DFU. 4 Patients with DFU also have to bear the high socioeconomic burden associated with the complications and management of DFUs. 5
Due to the high prevalence and associated morbidity and mortality, it is prudent to ensure proper management of DFUs. Wound care is an important aspect of DFU management. It is a complex process involving a multi‐disciplinary team of doctors, nurses, and allied health professionals, and is a spectrum ranging from 1 wound assessment 2 documentation and monitoring, and 3 intercommunication different various healthcare professionals. 6 , 7 This unmet need for proper wound care gave rise to the innovation of comprehensive wound assessment or monitoring systems.
To date, there are several commercially available wound assessment or monitoring systems available for monitoring of chronic wounds. 8 Wound assessments or monitoring systems range from the use of traditional computer applications and imaging devices to the use of mobile applications for remote assessment; all of which with potential for integration with artificial intelligence to improve measurement outcomes. Features of an ideal imaging system would include high accuracy, portability, convenience, accessibility (integration into existing hospital records and ability for remote assessment) data security, and low costs. However, to our knowledge, only a small proportion of those products are assessed in existing literature. With advancement in technology and rise in commercially available product, a systematic review is ideal to summarise existing evidence on various wound imaging systems available for use in DFU. Our study aims to fill in the gap and summarise various types of wound assessment and monitoring systems in DFUs in existing literature. Our secondary aim is to identify the list of commercially available systems used in DFU monitoring and examine available evidence on their use.
2. METHODS
2.1. Data sources and searches
This is a systematic review on use of wound assessment, imaging, and monitoring systems in DFU. A systematic search of published articles in peer‐reviewed journals was performed on PubMed and Embase (1974‐March 2020). The last search was performed on March 30, 2020. A combination of subject headings and text words on (a) diabetic foot ulcer and (b) multimodal imaging, optical imaging, spectral imaging, computer‐assisted interpretation, artificial intelligence, or mobile applications were used in the search. The complete search strategy is appended in the Appendices (Supplementary Tables S1, S2). Studies selected for the systematic review are in accordance to the quality and standards set by the Preferred Reporting Items for Systematic Reviews and Meta‐analyses (PRISMA). 9
2.2. Study selection
Inclusion criteria were: (a) description, (b) measurement accuracy, or (c) clinical use or challenges of imaging systems, technology, applications, or software in wound assessment of diabetic foot ulcers. Exclusion criteria were: (a) other types of wound such as pressure ulcers, (b) conventional radiological imaging such as plain radiograph, ultrasound, computed tomography or magnetic resonance imaging, (c) review articles, letter to editors or editorials, (d) non‐English articles or (e) articles without full texts.
Two authors (KSC, ZJL) independently screened the studies by title and abstract for potential inclusion in the study. Full‐texts of all eligible articles were subsequently reviewed and assessed for eligibility in the study. Disagreement or conflicts between reviewers were resolved by consensus. The entire study selection process is reflected in the PRISMA flow diagram (Figure 1).
FIGURE 1.
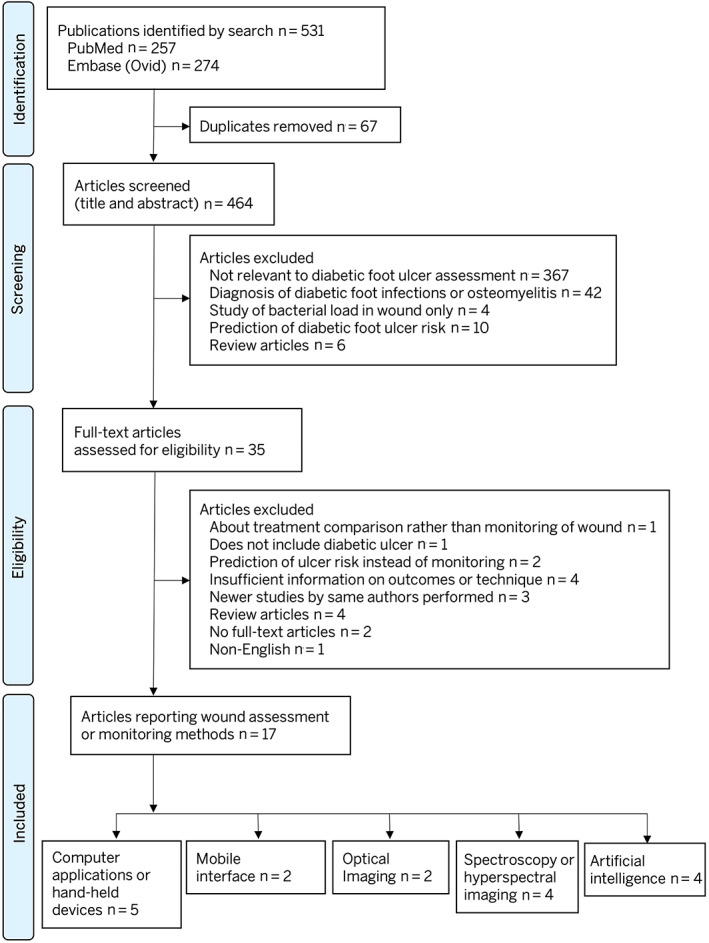
Preferred reporting items for systematic reviews and meta‐analyses (PRISMA) figure showing the study selection process
2.3. Data extraction and quality assessment
Two independent authors (KSC, ZJL) extracted the following information from each study separately: Author, year of study, type of wound assessed, name of system, clinical use, parameters of wound assessed, measurement statistics, ability to integrate into Electronic Health Records (EHRs) or Electronic Medical Records (EMRs) other advantages and challenges. Quality of the included studies were assessed with the revised Methodological Index for Non‐Randomised Studies (MINORS) criteria 10 (Supplementary Table S3). Descriptive studies and technical reports on the use of artificial intelligence were not included in the quality assessment as the use of MINORS criteria is not validated for these types of studies.
2.4. Secondary literature search
A secondary literature search was performed on WoundSource, 8 a reference guide on wound care products, for a list of commercially available wound assessment and monitoring tools available for DFU. The following information was extracted: description, platform, method of measurement, type of measurement, ability for EHR or EMR integration, remote assessment, and other features. The products were subsequently searched on PubMed for available literature on the respective products.
3. RESULTS
Our systematic search yielded 531 articles. Titles and abstracts were screened through 464 articles after removal of duplicates (n = 67). Thirty‐five full text articles were assessed for eligibility for the study and 17 articles were included in the final analysis. 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 The entire process of systematic review is reported in accordance to the PRISMA guidelines and flowchart (Figure 1).
There are five main categories of wound assessment or monitoring techniques or methods: (a) computer applications or hand‐held devices (n = 5), 18 , 19 , 22 , 26 , 27 (b) mobile applications (n = 2), 15 , 17 (c) optical imaging (n = 2), 13 , 21 (d) spectroscopy or hyperspectral imaging (n = 4), 20 , 23 , 24 , 25 and (e) artificial intelligence (n = 4). 11 , 12 , 14 , 16
Uses of wound assessment or monitoring methods were classified into: (a) wound assessment or monitoring and (b) data capturing and storage of information. Sixteen studies reported on wound assessment or monitoring 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 27 ; one study reported on data capturing and storage of information. 26 Of the 16 studies (94.1%) which reported on wound assessment or monitoring, four assessed likelihood of wound healing using spectroscopy or hyperspectral imaging. Table 1 summarises the use of computer applications, hand‐held devices, mobile applications, optical imaging, spectroscopy, or hyperspectral imaging in DFU.
TABLE 1.
Summary of use of various modalities in diabetic foot ulcer (DFU)
| Modality | Author (year) | Name of System | n | Wound Type | Comparator | Clinical Use/Study Objective | Parameters Assessed | Measurement Statistics | EHR or EMR Integration | Advantages | Challenges |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Computer software or hand‐held devices | Jeffcoate et al (2017) 18 | ImageJ (National Institutes of Health) | 31 | Chronic ulcers | No control | Measurement of cross‐sectional area of digital images | 2D: cross‐sectional area |
Inter‐rater reliability: Correlation between observers >0.99 (P = 0.000). No significant overall differenceANOVA F1.358, (P = 0.165) Intra‐rater reliability: Correlation between first and second occasion high: 0.997 [P < 0.001] and 0.999 (P < 0.001 respectively) |
– | Easy training and relatively inexpensive | Unable to measure 3D images |
| Foltynski et al (2013) 19 |
Vistrak device (Smith & Nephew, London, UK) SilhouetteMobile device (ARANZ Medical Ltd., Christchurch, New Zealand) TelaDiaFoS system (Nalecz Institute of Biocybernetics and Biomedical Engineering, Warsaw, Poland) Linear measurement with elliptical method |
16 | DFU |
Visitrak vs TelaDiaFoS vs SilhouetteMobile vs Linear measurement with elliptical method |
Accuracy and precision of various wound measurement devices | 2D: length, width, area |
Relative error (accuracy): Elliptical method: 13.3% Visitrak: 6.8% TelaDiaFoS: 2.1% SilhouetteMobile: 2.3% CV (coefficient of variation) (precision): Elliptical: 6.0% Visitrak: 6.3% TelaDiaFoS: 1.6% SilhouetteMobile: 3.1% |
– | High accuracy of SilhouetteMobile device and TelaDiaFoSsystem permits use in clinical practice | – | |
| Rogers et al (2010) 22 | SilhouetteMobile | 10 | Chronic ulcers | Standard method (length multiplied by width) | Comparative study of accuracy between digital imaging and standard measurements |
3D modelling in SilhouetteMobile Wound area |
Standard manual measurement overestimated wound area by 41% | – | Allows tracking of wound progression with precise measurements | Standard measurement: limited by subjective interpretation and interobserver variability | |
| Laji et al (2001) 26 | FilmMaker Pro (Claris International Inc., Santa Clara, California) | NA | DFU | NA | Creation of digital imaging archive system to track wound progression | NA | NA | Possible integration into hospital server with security features | Relatively inexpensive software and equipment | ‐ | |
| Rajbhandari et al, 1999 27 | DesignCAD (ViaGraÆx Corporation, Pryor, Oklahoma) | 30 | Any ulcer | Traditional calculation with graph paper | Validity of digital measurement in comparison to traditional method | 2D: area |
Inter‐observer variability: Digital imaging mean coefficient of variation 15.9%; traditional tracing method 26.7% (P = 0.05) No significant differences in areas of standard circles measured between methods (P = 0.57) |
– |
Easier to use than traditional method Reproducibility |
Underestimation of ulcer area on curved surfaces which will require 3D imaging | |
| Mobile applications | Yap et al (2018) 15 | FootSnap | 60 | DFU | Non diabetic ulcers (control group) | Software for standardisation of plantar foot images in relation to distance from foot and orientation of camera | 2D: perimeter, surface |
Diabetic foot: Intra‐operator reliability high: JSI 0.89 and 0.91 for each operator Inter‐operator reliability high: JSI 0.89 Normal foot: Intra‐operator reliability high: JSI 0.94 and 0.93 for each operator Inter‐operator reliability high: JSI 0.93 |
– |
Allows easy monitoring and trending of wounds due to standardisation Only marginally lower reliability in diabetic foot compared to normal foot, ensuring suitability for use in diabetic feet |
Training is required and reliability may be lower in new operators Modifications in hardware require alterations in software |
| van Netten et al (2017) 17 | – | 50 | DFU | No control with traditional methods | Diagnostic accuracy of use of mobile phone images to assess DFU | 2D: length, width, area |
Inter‐observer reliability: 0.09 to 0.71 (only decision for peri‐wound debridement reached adequate agreement) Positive likelihood ratio: 1.3 to 4.2 (no strong or convincing evidence) Negative likelihood ratio: 0.13 to 0.88 (only one item has strong diagnostic evidence for negative likelihood ratio) Sensitivity 32% to 97% (4 items with high sensitivity) Specificity 20% to 87% (1 item with high specificity) |
– | – | Poor diagnostic accuracy for DFU and require additional information on wound characteristic on top of the mobile phone images | |
| Optical imaging | Raizman et al (2019) 13 | MolecuLight i:X Wound Imaging device (MolecuLight, Inc., Toronto, Ontario, Canada) | 50 | DFU, venous and arterial ulcer | No control with traditional methods | Use of handheld fluorescence imaging device for wound measurement and detection of bacteria |
2D: length, width, area Extent of fluorescence bacteria detection |
Accuracy: Length and width ≥ 95.75% Area ≥ 94.62% Entire wound fluorescence: Over‐estimation of bacterial load; only 20% of wounds had moderate‐to‐heavy bacterial growth Wound bed fluorescence only: Close relation between extent of fluorescence and bacterial load |
Possible integration | Potential cost savings as fluorescence guidance allows targeted treatment (debridement) | Fluorescence must be performed under dark conditions and visualisation of bacteria in wound does not indicate infection |
| Bowling et al (2011) 21 | NA | 20 | DFU | No control with traditional methods | Reliability and accuracy of remote assessment of 3D images of DFU | 3D: Length, area, surface curvature, depth, volume |
Intra‐operator variability: 3.3% Inter‐operator variability: 11.9% Accuracy: concordance with criterion standard of >50% across all questions on standardised wound assessment form |
Allows for easy storage and transmission of images remotely |
Good quality of presented wound images with interactive ability (such as panning and tilting of image) |
Poor accuracy in determining need for wound debridement due to technical limitations | |
| Spectroscopy or hyperspectral imaging | Weingarten et al (2012) 20 | Near‐infrared spectroscopy | 24 | DFU | NA | Use of near‐infrared spectroscopy to determine wound healing progression |
2D: wound size Oxyhaemoglobin and deoxyhaemoglobin concentration |
At 4 weeks: Sensitivity 0.9 Specificity 0.86 Average slope for healing patients −11.5 (SD 11.8), non‐healing patients +5.6 (SD 12.2), P < 0.002 |
– |
Cost savings of $12 600 per patient if ineffective treatment discontinued after 4 weeks Allows trending of results by graph plotting |
– |
| Neidrauer et al (2010) 23 | Near‐infrared spectroscopy | 16 | DFU | NA | Efficacy of using diffuse near infrared spectroscopy (NIRS) in predicting wound healing in diabetic foot ulcers | Oxyhaemoglobin and deoxyhaemoglobin concentration |
At 10 weeks: 3 μm/week threshold, 5/6 (83%) healing wounds classified correctly, 5/8 (63%) nonhealing wounds classified correctly 6 μm/week threshold: 4/6 (67%) healing wounds classified correctly, 8/8 (100%) nonhealing wounds classified correctly |
– | Allows trending of results by graph plotting | – | |
| Papazoglou et al (2009) 24 | Near‐infrared spectroscopy | 12 | DFU and chronic wounds | NA | Monitoring of chronic wounds using near‐infrared spectroscopy | Oxyhaemoglobin (HbO2) and deoxyhaemoglobin concentration |
Change in (HbO2) – threshold ~3 mM/week: 4/4 (100%) healing wounds correct classified, 4/7 (57%) non healing wounds correctly classified Change in (HbO2) threshold ~6 mM/week; 3/4 (75%) healing wounds correct classified, 7/7 (100% non healing wounds correctly classified |
– | Allows trending of results by graph plotting | – | |
| Nouvong et al (2009) 25 | Hyperspectral imaging | 73 | DFU | NA | The use of hyperspectral imaging to quantify cutaneous tissue haemoglobin oxygenation to assess the healing potential of DFUs | Healing index – derived using oxyhemoglobin concentration [HbO2] and deoxyhemo‐globin concentration [Hhb) |
Sensitivity 80% (n = 43/54) Specificity 74% (n = 14/19) Positive predictive value 90% (n = 43/48) When removing three‐false positive osteomyelitis cases and 4 false negative cases due to measuring of callus: Sensitivity 86% Specificity 88% Positive predictive value 96% |
– | – | – |
Abbreviations: 2D, 2‐dimensional; 3D, 3‐dimensional; EHR, electronic health records; EMR, electronic medical records; NA, not applicable; SD, standard deviation.
3.1. Computer applications
Three studies reported on the use of computer applications on wound assessment or monitoring: Jeffcoate et al and Rajbhandari et al reported low inter‐observer variability in the use of computer applications in digital measurements of wound area (inter‐rater reliability >0.99, and inter‐observer variability 15.9%, respectively). 18 , 27 Laji et al reported the use of an application for digital archiving of wound images to track wound progression. 26 Two studies compared the use of hand‐held commercial devices with traditional method of wound measurement: Foltynski et al reported high accuracy of TelaDiaFoS system (Nalecz Institute of Biocybernetics and Biomedical Engineering, Warsaw, Poland), and SilhouetteMobile device (ARANZ Medical Ltd., Christchurch, New Zealand): relative error 2.1% and 2.3%, respectively, compared to standard method, with relative error of 13.3%. 19 Similarly, Rojers et al reported standard wound measurement resulted in overestimation of wound area by 41%. 22
3.2. Mobile interface
Yap et al described the use of an application (FootSnap) in the standardisation of plantar foot images for easy monitoring of wound, with high intra‐operator reliability (Jaccard similarity index [JSI] 0.89‐0.91) and high inter‐operator reliability (JSI 0.89). 15 Van Netten et al demonstrated poor diagnostic accuracy of remote assessment of DFUs using mobile phone images, with highly variable sensitivity (32%‐97%) and specificity (20%‐87%), and inter‐observer reliability (0.09‐0.71). 17
3.3. Optical imaging
Raizman et al described high accuracy of ≥95.75% and ≥94.62% in the determination of wound length and width, and wound area, respectively, using fluorescence imaging. 13 Bowling et al described the use of optical imaging markers with low intra‐operator variability of 3.3%, 21 but with only >50% concordance across all questions on the standardised wound assessment form on all questions. Table 1 summarises the use of various forms of optical imaging used.
3.4. Spectroscopy or hyperspectral imaging
Three studies reported the use of near‐infrared spectroscopy, 20 , 23 , 24 and one study reported the use of hyperspectral imaging. 25 All of the studies used oxyhaemoglobin and deoxyhaemoglobin concentrations to assess wound healing and progression. The details are summarised in Table 1.
3.5. Artificial intelligence
Table 2 summarises the various forms of artificial intelligence used in wound assessment or monitoring in DFU. Four studies reported the use of artificial intelligence in wound assessment. 11 , 12 , 14 , 16 All of the studies were technical reports on the construction of machine learning methods to assess various aspects of wounds: (a) automated or accurate wound area detection (n = 2), 12 , 16 (b) wound segmentation (n = 1), 14 and (c) recognition of ischemia or infection in wounds (n = 1). 11 Three of the studies reported the need for a large database of wound images to increase the accuracy of the machine learning model (Table 2). 11 , 12 , 16 Ohura et al described limited applicability to other populations due to a limited database of wound images from the Japanese population only. 14
TABLE 2.
Summary of the use of artificial intelligence for wound assessment or monitoring in diabetic foot ulcer (DFU)
| Author (year) | Type of AI | n | Wound Type | Clinical Use | Measurement Accuracy | Remote Assessment Capabilities | Challenges |
|---|---|---|---|---|---|---|---|
| Goyal et al (2020) 11 |
Faster R‐CNN with InceptionResNet V2 BayesNet Random forest InceptionV3 ResNet |
1459 | DFU | Recognition of ischemia and infection in monitoring of DFU |
Average accuracy of all models: Ischemia: 83.3% Infection: 65.8% |
Eventually transit into a remote DFU diagnosis system, but challenges need to be resolved |
Substantial datasets required for good accuracy Images provided for assessment are non‐standardised (type of camera models, devices, poses and illumination) |
| Wang et al (2019) 12 |
Associative hierarchal random field (AHRF) model Conditional random field (CRF) model |
100 | Any foot ulcer | Accurately determining boundaries of ulcer images acquired under various image acquisition conditions |
Wound recognition specificity: AHRF model – 95.5% to 99.2% CRF model 1 (conditional random field) – 89.8% to 92.7% CRF model 2 –91.1% to 98.4% Wound recognition sensitivity AHRF – 76.9% to 84.4% CRF model 1 – 61.8% to 67.4% CRF model 2 – 70.3% to 76.7% |
– | Need to expand diversity of database of real wound images, with possibility of expanding into deep learning in the future |
| Ohura et al (2019) 14 |
Convolutional Neural Networks (CNNs): SegNet LinkNet U‐Net U‐Net_VGG16 |
440 | Chronic wounds | Construct a good wound segmentation model using CNN |
SegNet: AUC 0.994, sensitivity 0.909, Specificity 0.982, accuracy 0.976 LinkNet: AUC 0.987, sensitivity 0.989, specificity 0.989, accuracy 0.972 U‐Net: AUC 0.997, sensitivity 0.993, specificity 0.993, accuracy 0.988 U‐Net_VGG16 AUC 0.998, sensitivity 0.992, specificity 0.992, accuracy 0.989 – highest accuracy |
– | May not be applicable to wound assessment and segmentation of other races (study was conducted in a Japanese population) |
| Wang et al (2017) 16 | Two‐stage support vector machine (SVM) with conditional random field (CRF) image processing | 100 | DFU | Create an automated wound detection method to determine wound area on a smartphone‐based system |
Two‐stage SVM + CRF technique: Sensitivity – 73.3% Specificity – 94.6% Computation time – 20.5 s |
– |
Need to enhance wound image database Need to recruit more clinicians to delineate wound boundaries to minimise variability |
3.6. Wound assessment and monitoring systems available commercially
A total of 18 commercially available wound assessment and monitoring systems were listed on WoundSource. 8 Table 3 summarises the characteristics of these products. Of these 18 products, only 6 of the products were described on their efficacy or accuracy in wound measurements in PubMed indexed peer‐reviewed articles. SilhouetteMobile was the only product which was described in our systematic review, including articles by Foltynski et al and Rogers et al. 19 , 22 The use of the remaining five products described in peer‐reviewed literature was on general wound measurement without the inclusion of diabetic foot ulcers and were hence excluded from this systematic review. The relevant articles on measurement accuracy of the products, are however, referenced and listed in Table 3. 28 , 29 , 30 , 31 , 32 , 33 , 34
TABLE 3.
Overview of existing wound assessment and monitoring systems available commercially
| Product (Company) | Description | Platform | Method of measurement | Type of measurement | EHR or EMR integration | Remote assessment | Other features | Peer‐reviewed article (Author, year) |
|---|---|---|---|---|---|---|---|---|
| CarePICS (CarePICS, LLC, North Carolina) | Application for wound measurement, assessment and monitoring with information sharing between care partners | Mobile application, iOS (Apple Inc., California) and Android (Google LLC, California) | Non‐contact | 3D | Yes, using cloud‐based secure server | Yes | Allows teleconsultation between healthcare providers | NA |
| Digital Wound Management (Healthy.io, Tel Aviv, Israel) | Application for wound assessment, documentation and monitoring | Mobile application | Non‐contact | 3D | – | – | – | NA |
| eKare InSight (eKare Inc., VA) | Application for wound assessment, documentation and monitoring | Mobile application (iOS), powered by Structure Sensor | Non‐contact | 3D | Yes, using cloud‐based secure server | Yes | – | NA |
| EPISCAN I‐200 (Longport, Inc., Pennsylvania) | Use of high frequency ultrasound to detect non‐visible tissue damage | High frequency Ultrasound | Contact | 2D | – | – |
Detect non‐visable tissue damage Progress tracking |
Jasaitiene et al (2015) 28 |
| HealthE*PIX (Healthline Information Systems, Inc., North Carolina) | Digital platform for electronic storage of wound records | Computer software/mobile application | – | – | Yes, also allows integration from existing servers | Yes | – | NA |
| Parable Mobile Wound Management Software (Parable Health, Inc., New York) | Software for wound assessment and electronic documentation | Mobile application | Non‐contact | 3D | – | – | – | NA |
| Scout (WoundVision, Indiana, ) | Imaging and thermal device for wound assessment and documentation | Device | Non‐contact | 3D | Yes | – | Objective and quantitative measurements of skin temperature change |
Langemo et al (2017) 29 Langemo et al (2015) 30 |
| Scout Mobile (WoundVision, Indiana) | Mobile application to standardise wound photography, measurement and assessment | Mobile application (iOS and Android) | Non‐contact | 3D | Yes | – | – | NA |
| Silhouette (ARANZ Medical Ltd., Christchurch, New Zealand) | A group of products which allows wound assessment and electronic documentation of records | Digital platform, mobile application, camera sensor | Non‐contact | 3D | Yes | Yes | – |
Foltynski et al (2013) 19 Rogers et al (2010) 22 |
| SnapshotNIR (Kent Imaging Inc., AB, Canada) | Near‐infrared spectroscopy device to assess tissue oxygenation | Spectroscopy device | Non‐contact | – | – | – | – | NA |
| Swift Skin and Wound (Swift Medical, Ontario, Canada) | Integrated system for wound measurement, documentation and monitoring | Mobile application and computer software | – | 3D | Yes | Yes | Risk calculation for patients with ability to monitor and trend risk levels |
Au et al (2019) 31 Au et al (2018) 32 |
| Tissue Analytics (Tissue Analytics, Inc., Baltimore, Maryland) | Application for wound measurement, documentation and integration into medical records system | Mobile or tablet application | Non‐contact | 3D | Yes | – | – | NA |
| Wound Mapping Ultrasound (Hitachi Aloka Medical America, Inc., Connecticut) | Use of ultrasound to measure the wound, visualise blood flow at wound bed and evaluate soft tissue and cortical surfaces of bones | Ultrasound device | Contact | 2D (but provides information on wound depth) | – | – | – | NA |
| WoundDesk (digitalMedLab, Zürich, Switzerland) | Application for wound assessment, documentation and monitoring | Mobile application (iOS and Android) | Non‐contact | 2D | – | – | Secured exchange with colleagues for multi‐disciplinary management | NA |
| WoundMatrix (WoundMatrix, Inc., Pennsylvania) | Application for wound assessment, documentation and monitoring | Mobile application | Non‐contact | 2D | Yes | Yes | Allows patients to upload wound images to their healthcare provider for immediate review and assessment | Quan et al (2007) 33 |
| WoundRight (WoundRight Technologies, LLC, Georgia) | Application for wound assessment, documentation and monitoring | Mobile application (iOS and Android) | Non‐contact | 2D | – | – |
Track vitals, medical conditions, and report on open or closed wounds Allows prescription of treatment and follow‐up schedules |
NA |
| WoundWiseIQ (Med‐Compliance IQ, Inc., Ohio) | Application for wound assessment, analysis and trending of wound characteristics | Mobile application | Non‐contact | 2D | – | – | – | NA |
| WoundZoom (WoundZoom, Inc., Wisconsin) | Integrated system for wound image capture and assessment and documentation | Device (3D Wound Tablet), mobile application (iOS and Android) and computer software (Windows [Microsoft Corporation, Washington]) | Non‐contact | 3D | Yes | Yes | – | Khong et al (2017) 34 |
Abbreviations: 2D, 2‐dimensional; 3D, 3‐dimensional; EHR, electronic health records; EMR, electronic medical records; NA, not applicable.
3.7. Quality of included studies
The quality of included studies was analysed using the MINORS criteria (Supplementary Table S3). Of the six studies which were non‐comparative, five obtained a score of 12 or less, which reflects high risk of bias. Of the six studies which were comparative, four studies obtained a score of 20 or less, which also reflects a high risk of bias.
4. DISCUSSION
Diabetic foot ulcer is an important complication of diabetes and warrants close monitoring for prevention, promoting wound healing, and preventing recurrence. 35 Several classification systems have been developed to risk stratify and predict the prognosis of DFUs, and its subsequent management. 36 However, it is prudent to perform proper wound care on all patients with DFUs regardless of its severity. Our study analysed the various modalities of wound assessment to aid clinicians and allied health professionals in the management of DFUs.
Wound care begins with wound assessment, which involves the accurate measurement of parameters such as wound area, depth, volume, stage, signs of infection, and healing potential. 37 Majority (94.1%) of the included studies centred around wound assessment and documented the efficacy of various systems in the accuracy and precision of measurements in DFUs. The use of computer software and hand‐held devices has seen optimistic results. Jeffcoate et al and Rajbhandari et al reported low inter‐observer variability in the use of computer applications in digital measurements of wound area (inter‐rater reliability >0.99, and inter‐observer variability 15.9%, respectively). 18 , 27 This is of special significance in the monitoring of DFUs as the management of DFU involves a multidisciplinary team. It is paramount for low inter‐observer variability for precise documentation to track the wound progression or healing and institute proper management.
Spectroscopy or hyperspectral imaging are alternative methods of non‐invasive monitoring which assess the oxyhaemoglobin and deoxyhaemoglobin concentration in the wound bed through the use of diffuse photon wave density (650‐850 nm) to differentiate healing wounds from non‐healing wounds. 38 Of the four studies included in this analysis, Weingarten et al and Nouvong et al demonstrated a sensitivity and specificity of 80% to 90% and 74% to 86%, respectively. 20 , 25 Prediction of wound healing is an important aspect of DFU assessment: Sheehan et al demonstrated that in patients with DFUs who did not achieve a 50% reduction in wound area by 4 weeks were unlikely to heal. 39 Further treatment in this subgroup of patients may be ineffective and may be discontinued. Weingarten et al demonstrated an average cost saving of about $12 600 per patient if ineffective treatment was discontinued for ulcers with low likelihood of healing. 20
In the era of digitalisation, there is a transition from traditional documentation on paper to digital documentation to improve patient care. Digital photographs of DFUs are uploaded to hospital EHRs or EMRs for (a) wound monitoring (b) comanagement in a multidisciplinary team. Laji et al first described the use of a relatively inexpensive and easy to use software for the creation of a digital imaging archive system to track wound progression, with possible integration into EHRs or EMRs (Table 1). 26 To date, there are several commercially available systems have an integrated system of wound assessment, monitoring, and documentation for easy use (Table 3). However, majority of those systems (n = 12/18, 66.7%) have not been reviewed in the literature on its accuracy in wound measurement. It is prudent for future studies to review these systems in terms of (a) measurement accuracy (b) ease of use (c) data integration capabilities (d) remote assessment capabilities (e) costs and (f) other challenges to guide clinicians and allied health professionals on the choice of the better “all‐in‐one” integrated system for wound care in DFU.
The role of artificial intelligence (AI) in medicine is a rapidly growing field, especially in radiology and oncology. 40 However, there are an increasing number of studies on the use of AI in other areas of medicine, such as vascular medicine or surgery. Four articles included in our study examined the use of various AI systems in wound assessment, all of which, were dated less than 5 years ago. 11 , 12 , 14 , 16 Wang et al and Ohura et al demonstrated high specificity and sensitivity in wound recognition and assessment. 12 , 14 Goyal et al further demonstrated the use of AI to recognise ischemia and infection in DFU monitoring, 11 a challenge which has been described in the remote assessment of DFUs. Van Netten et al studied the reliability of remote assessment of DFUs using mobile phone images which failed to demonstrate strong validity or adequate reliability in assessing ischemia and infection in DFUs. 17 AI may be used to increase the reliability of remote assessment of DFU images and will serve as a useful adjunct in comanagement of DFUs.
Last, our study reflects the difficulty in conducting studies to evaluate wound assessments and monitoring systems. Majority of the studies analysed using the MINORS criteria had high risk of bias, where studies did not have a blind or double‐blind evaluation of objective or subjective endpoints, respectively. Sample size calculation was also not performed for majority of the studies, contributing to bias. This observation may be explained by the paucity of existing studies evaluating the efficacy of wound assessment and imaging systems and its emerging role as clinical adjuncts. However, this review provides an extensive overview of the available evidence on the various wound assessment and imaging systems to guide future studies and their methodologies in the evaluation of new commercially available systems. Nevertheless, it is also prudent to note that the use of MINORS criteria has its limitations 10 ; the criteria was originally developed to assess the risk of bias in observational surgical studies and its validation in other types of studies is limited.
Our study has some limitations. Our search was limited to diabetic foot ulcers. We did not expand our search to include other types of wounds such as pressure ulcers, venous ulcers, or burns as the search would be too extensive and would not be the aim of this study. Characteristics of DFUs differ from other types of chronic wounds. Nevertheless, we agree that the type of wound is unlikely to affect the measurement accuracy of wound parameters. Another limitation of this study is the restriction of our literature search to two databases due to limited subscription to research databases. Our literature search on the types of commercially available wound care products is also limited to WoundSource; however, WoundSource has an extensive repository of wound care products reviewed by an editorial board and hence, provides a good overview on the list of commercially available products in wound assessment and monitoring. Our results included a mixture of studies examining on DFUs specifically and chronic wounds in general. Majority of the included articles were also centred around wound assessment and accuracy, with only one article on data capturing and information storage. In addition, we were only able to provide a descriptive analysis of the studies as the studies varied in intervention and outcome measures.
5. CONCLUSION
This review identified the various modalities of wound assessment and monitoring in diabetic foot ulcers including computer software, hand‐held and mobile devices, optical imaging, spectroscopy, and artificial intelligence. Rapid advancement in technology has resulted in the development of various types of wound assessment and monitoring systems which serve as useful adjuncts in improving clinical care, with various products documenting superior accuracy over traditional methods of wound assessments. In line with this, it is prudent to have ongoing studies to evaluate the evidence and outcomes of the extensive list of commercially available wound assessment and monitoring systems.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Supporting information
Supplementary Table S1 Search strategy used in PubMed database
Supplementary Table S2 Search strategy used in Embase database
Supplementary Table S3 Use of the revised MINORS criteria for quality assessment
Chan KS, Lo ZJ. Wound assessment, imaging and monitoring systems in diabetic foot ulcers: A systematic review. Int Wound J. 2020;17:1909–1923. 10.1111/iwj.13481
Funding information Agency for Science, Technology and Research (A*STAR) under its Industry Alignment Fund ‐ Pre‐Positioning Programme (IAF‐PP) as part of Wound Care Innovation for the Tropics (WCIT) Programme, Grant/Award Number: H18/01/a0/ZZ9
DATA AVAILABILITY STATEMENT
The data that supports the findings of this study are available in the supplementary material of this article.
REFERENCES
- 1. Forouhi NG, Wareham NJ. Epidemiology of diabetes. Medicine (Abingdon). 2014;42(12):698‐702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Amin N, Doupis J. Diabetic foot disease: from the evaluation of the “foot at risk” to the novel diabetic ulcer treatment modalities. World J Diabetes. 2016;7(7):153‐164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Crawford F, Cezard G, Chappell FM, et al. A systematic review and individual patient data meta‐analysis of prognostic factors for foot ulceration in people with diabetes: the international research collaboration for the prediction of diabetic foot ulcerations [PODUS]. Health Technol Assess. 2015;19(57):1‐210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Walsh J, Hoffstad O, Sullivan M, et al. Association of diabetic foot ulcer and death in a population‐based cohort from the United Kingdom. Diabet Med. 2016;33(11):1493‐1498. [DOI] [PubMed] [Google Scholar]
- 5. Lo ZJ, Lim X, Eng D, et al. Clinical and economic burden of wound care in the tropics: a 5‐year institutional population health review. Int Wound J. 2020;17:790‐803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Flanagan M. Wound measurement: can it help us to monitor progression to healing? J Wound Care. 2003;12(5):189‐194. [DOI] [PubMed] [Google Scholar]
- 7. Oyibo S, Jude E, Tarawneh I, et al. The effects of ulcer size and site, patient's age, sex and type and duration of diabetes on the outcome of diabetic foot ulcers. Diabet Med. 2001;18(2):133‐138. [DOI] [PubMed] [Google Scholar]
- 8. WoundSource . Wound Assessment & Monitoring Systems WoundSource: Kestrel Health Information; 2020. (cited 2020 07 Apr). Available from: https://www.woundsource.com/product-category/wound-assessment-documentation/wound-assessment-monitoring-systems.
- 9. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264‐269. [DOI] [PubMed] [Google Scholar]
- 10. Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non‐randomized studies [MINORS]: development and validation of a new instrument. ANZ J Surg. 2003;73(9):712‐716. [DOI] [PubMed] [Google Scholar]
- 11. Goyal M, Reeves ND, Rajbhandari S, Ahmad N, Wang C, Yap MH. Recognition of ischaemia and infection in diabetic foot ulcers: dataset and techniques. Comput Biol Med. 2020;117:103616 (no pagination). [DOI] [PubMed] [Google Scholar]
- 12. Wang L, Pedersen PC, Agu E, Strong D, Tulu B. Boundary determination of foot ulcer images by applying the associative hierarchical random field framework. J Med Imaging. 2019;6(2):024002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Raizman R, Dunham D, Lindvere‐Teene L, et al. Use of a bacterial fluorescence imaging device: wound measurement, bacterial detection and targeted debridement. J Wound Care. 2019;28(12):824‐834. [DOI] [PubMed] [Google Scholar]
- 14. Ohura N, Mitsuno R, Sakisaka M, et al. Convolutional neural networks for wound detection: the role of artificial intelligence in wound care. J Wound Care. 2019;28(Supplement10):S13‐S24. [DOI] [PubMed] [Google Scholar]
- 15. Yap MH, Chatwin KE, Ng CC, et al. A new Mobile application for standardizing diabetic foot images. J Diabetes Sci Technol. 2018;12(1):169‐173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang L, Pedersen PC, Agu E, Strong DM, Tulu B. Area determination of diabetic foot ulcer images using a cascaded two‐stage SVM‐based classification. IEEE Trans Biomed Eng. 2017;64(9):2098‐2109. [DOI] [PubMed] [Google Scholar]
- 17. van Netten JJ, Clark D, Lazzarini PA, Janda M, Reed LF. The validity and reliability of remote diabetic foot ulcer assessment using mobile phone images. Sci Rep. 2017;7(1):9480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jeffcoate WJ, Musgrove AJ, Lincoln NB. Using image J to document healing in ulcers of the foot in diabetes. Int Wound J. 2017;14(6):1137‐1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Foltynski P, Ladyzynski P, Sabalinska S, Wojcicki JM. Accuracy and precision of selected wound area measurement methods in diabetic foot ulceration. Diabetes Technol Ther. 2013;15(8):712‐721. [DOI] [PubMed] [Google Scholar]
- 20. Weingarten MS, Samuels JA, Neidrauer M, et al. Diffuse near‐infrared spectroscopy prediction of healing in diabetic foot ulcers: a human study and cost analysis. Wound Repair Regen. 2012;20(6):911‐917. [DOI] [PubMed] [Google Scholar]
- 21. Bowling FL, King L, Paterson JA, et al. Remote assessment of diabetic foot ulcers using a novel wound imaging system. Wound Repair Regen. 2011;19(1):25‐30. [DOI] [PubMed] [Google Scholar]
- 22. Rogers LC, Bevilacqua NJ, Armstrong DG, Andros G. Digital planimetry results in more accurate wound measurements: a comparison to standard ruler measurements. J Diabetes Sci Technol. 2010;4(4):799‐802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Neidrauer M, Zubkov L, Weingarten MS, Pourrezaei K, Papazoglou ES. Near infrared wound monitor helps clinical assessment of diabetic foot ulcers. J Diabetes Sci Technol. 2010;4(4):792‐798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Papazoglou ES, Neidrauer M, Zubkov L, Weingarten MS, Pourrezaei K. Noninvasive assessment of diabetic foot ulcers with diffuse photon density wave methodology: pilot human study. J Biomed Opt. 2009;14(6):064032. [DOI] [PubMed] [Google Scholar]
- 25. Nouvong A, Hoogwerf B, Mohler E, Davis B, Tajaddini A, Medenilla E. Evaluation of diabetic foot ulcer healing with hyperspectral imaging of oxyhemoglobin and deoxyhemoglobin. Diabetes Care. 2009;32(11):2056‐2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Laji K, Kumar J, Bishop J, Page M. Locally developed digital image archive for diabetic foot clinic: a DGH experience. Pract Diabetes Int. 2001;18(7):231‐234. [Google Scholar]
- 27. Rajbhandari SM, Harris ND, Sutton M, et al. Digital imaging: an accurate and easy method of measuring foot ulcers. Diabet Med. 1999;16(4):339‐342. [DOI] [PubMed] [Google Scholar]
- 28. Jasaitiene D, Valiukeviciene S, Linkeviciute G, Raisutis R, Jasiuniene E, Kazys R. Principles of high‐frequency ultrasonography for investigation of skin pathology. J Eur Acad Dermatol Venereol. 2011;25(4):375‐382. [DOI] [PubMed] [Google Scholar]
- 29. Langemo DK, Spahn JG. A reliability study using a long‐wave infrared thermography device to identify relative tissue temperature variations of the body surface and underlying tissue. Adv Skin Wound Care. 2017;30(3):109‐119. [DOI] [PubMed] [Google Scholar]
- 30. Langemo D, Spahn J, Spahn T, Pinnamaneni VC. Comparison of standardized clinical evaluation of wounds using ruler length by width and scout length by width measure and scout perimeter trace. Adv Skin Wound Care. 2015;28(3):116‐121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Au Y, Beland B, Anderson JA, et al. Time‐saving comparison of wound measurement between the ruler method and the swift skin and wound app. J Cutan Med Surg. 2019;23(2):226‐228. [DOI] [PubMed] [Google Scholar]
- 32. Au Y, Laforet M, Talbot K, Wang SC. Skin and wound map from 23,453 nursing home resident records: relative prevalence study. JMIR Dermatol. 2018;1(2):e11875. [Google Scholar]
- 33. Quan SY, Lazarus GS, Kohli AR, et al. Digital imaging of wounds: are measurements reproducible among observers? Int J Low Extrem Wounds. 2007;6(4):245‐248. [DOI] [PubMed] [Google Scholar]
- 34. Khong P, Yeo M, Goh C. Evaluating an iPad app in measuring wound dimension: a pilot study. J Wound Care. 2017;26(12):752‐760. [DOI] [PubMed] [Google Scholar]
- 35. Al‐Rubeaan K, Al Derwish M, Ouizi S, et al. Diabetic foot complications and their risk factors from a large retrospective cohort study. PloS One. 2015;10(5):e0124446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Monteiro‐Soares M, Boyko EJ, Jeffcoate W, et al. Diabetic foot ulcer classifications: a critical review. Diabetes Metab Res Rev. 2020;36:e3272. [DOI] [PubMed] [Google Scholar]
- 37. Wound and Pressure Ulcer Management. Johns Hopkins Medicine; 2020. (cited 2020 07 Apr). Available from: https://www.hopkinsmedicine.org/gec/series/wound_care.html. [Google Scholar]
- 38. Mobley J, Vo‐Dinh T. In: Vo‐Dinh T, ed. Biomedical Photonics Handbook. Boca Raton: CRC Press; 2003. [Google Scholar]
- 39. Sheehan P, Jones P, Caselli A, Giurini JM, Veves A. Percent change in wound area of diabetic foot ulcers over a 4‐week period is a robust predictor of complete healing in a 12‐week prospective trial. Diabetes Care. 2003;26(6):1879‐1882. [DOI] [PubMed] [Google Scholar]
- 40. Chan KS, Zary N. Applications and challenges of implementing artificial intelligence in medical education: integrative review. JMIR Med Educ. 2019;5(1):e13930. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1 Search strategy used in PubMed database
Supplementary Table S2 Search strategy used in Embase database
Supplementary Table S3 Use of the revised MINORS criteria for quality assessment
Data Availability Statement
The data that supports the findings of this study are available in the supplementary material of this article.


