Abstract
Tendinopathy is a very common disease in the general population as well as in athletes. The aim of the present study was to examine the tendon thickness and cross‐sectional area (CSA) in subjects with chronic mid‐portion Achilles tendinopathy (AT) who engaged in either an eccentric exercise (EE) programme with vibration training or an EE programme combined with cryotherapy. A sample of 61 patients with chronic mid‐portion AT were recruited and divided into two groups: EE programme vibration training (n = 30) and EE programme combined with cryotherapy (n = 31). Three ultrasound assessments were performed: pre‐intervention and at 4, and at 12 weeks. The comparison of thickness and CSA measures at baseline, 4, and 12 weeks showed a significant (P < 0.05) increase at 0, 2, 4, and 6 cm in maximal isometric contraction and at rest in subjects with chronic mid‐portion AT. The EE vibration training resulted in a statistically significant CSA increase compared with the cryotherapy group in patients with chronic mid‐portion AT.
Keywords: exercise therapy, tendinopathy, ultrasonography
1. INTRODUCTION
Tendinopathy is a very common disease in the general population as well as among athletes.1 Because of the Achilles tendon's structure, as one of the strongest and largest tendons in the human body, it is highly susceptible to suffer from tendinopathy.2 Of individuals who suffer from Achilles tendinopathy (AT), 75% are between 30 and 49 years old, and they are usually injured while performing sports.2 Kujala et al3 estimated that the incidence of tendon injuries is between 30% and 50% of all sports injuries and 6% in sedentary people. In addition, AT is diagnosed in 55% to 65% of all Achilles tendon examinations.4
The aetiology of tendinopathy remains unclear. Histopathological examination of the tendon tissue clearly shows no evidence of prostaglandin‐mediated inflammation.5, 6 Pingel et al7 reported that degenerative changes in tendon structure, poor neovascularisation, and core tendon growth are caused by a failed healing process. Moreover, a lack of flexibility in the lower limb; biomechanical factors such as hyper‐pronation, disturbances in blow flow, and a high body mass index (BMI); and a sedentary lifestyle are considered risk factors.8, 9, 10 Ohberg and Alfredson11 reported that the appearance of new vessels in the tendon pain areas is related to pain. A nerve ingrowth in the mid‐Achilles portion could be another source of pain.1, 12
Several studies have shown the efficacy of loading interventions, such as eccentric exercise (EE) training, as one of the main treatment options for AT.13, 14 Likewise, Beyer et al4 compared effectiveness of eccentric training (EEC) to heavy slow resistance training in patients with AT and found benefits to both interventions. Mafi et al15 reported better short‐term clinical results for EEC compared with concentric exercise in subjects with AT.
Hilgers et al16 presented whole‐body vibration training as an alternative to conventional interventions for motor control disorders in the lower limbs, showing benefits in endurance and muscle strength in individuals with multiple sclerosis. Therefore, Horstmann et al17 suggested that improving triceps surae muscle strength, modulating the neuromuscular system, increasing lower limb muscle flexibility, and relieving pain symptoms are likely good approaches for patients with AT. Following those suggestions, they carried out a study in 58 patients with AT, performing a 12‐week whole‐body training intervention that demonstrated benefits in pain, sonographic, and muscle strength parameters. Because of this, whole‐body vibration has proven to be a good therapeutic choice in AT subjects.
Cryotherapy treatment has also shown benefits in subjects with AT. Knobloch and Hufner18 found that cryotherapy improves pain and normalises blow flow in subjects with AT. In addition, it has been observed that cryotherapy and compression were effective at increasing tendon oxygen saturation in patients with AT.19 Therefore, it has been observed that cryotherapy could have positive effects combined with other therapies and alone in patients with AT.
Rehabilitative ultrasound imaging (RUSI) has been used to evaluate musculoskeletal features that could influence a physical therapy evaluation, such as thickness and cross‐sectional area (CSA).20 RUSI has been used to examine motor control.21 Considering the lower limbs, Lobo et al22 reported that peroneus longus CSA was reduced in subjects with lateral ankle sprains. CSA and thickness of the abductor hallucis and flexor halluces were reduced in subjects with hallux valgus.23 Angin et al24 found thinner plantar fascia in subjects with pes planus. In addition, other novel evaluation systems, such as infrared thermography, are being studied to assess the Achilles tendons,25, 26 but further research is needed in this emerging line. Therefore, RUSI is considered a relatively inexpensive, non‐invasive, and portable technique that provides an examination of the thickness and CSA of multiples tissues.25 Moreover, it has been used to measure thickness27, 28 and CSA29, 30 in subjects with AT.
The aim of the present study was to examine the tendon thickness and CSA in subjects with chronic mid‐portion AT who performed an EE programme vibration training compared with an EE programme combined with cryotherapy. It was hypothesised that an EE programme vibration training is more effective than an EE programme combined with cryotherapy in patients with mid‐portion AT.
2. METHODS
2.1. Design
A prospective, single‐blinded, randomised, controlled clinical trial (NCT03029910) was performed from January to December 2017 following the Consolidated Standards of Reporting Trials (CONSORT) guidelines.
2.2. Ethical considerations
The study was approved by the ethics committee of Hospital Universitario de la Princesa (2828A), Madrid, Spain. All the participants in the study signed the informed consent form. The study also adhered to the ethical standards of the Declaration of Helsinki for human experimentation.29
2.3. Participants
For this study, 61 patients with chronic mid‐portion AT (age: 41.2 ± 10 years) were recruited and divided into two groups: EE programme vibration training (n = 30) and EE programme combined with cryotherapy (n = 31) (Figure 1). Participants’ inclusion criteria were as follows: aged between 18 and 65 years, having symptoms for at least 3 months,31 and having mid‐portion Achilles pain (2–7 cm proximal to insertion) on palpation.13 Exclusion criteria were a lower limb disease within the last 12 months, previous fracture or surgical intervention,31 any systemic disease,13 and past negative experiences with EE32 vibration and cryotherapy interventions.
Figure 1.
CONSORT flow algorithm outlining participant enrolment, allocation, follow up, and attrition numbers for this study
2.4. Eccentric exercise programme
Both groups performed a 12‐week EE programme based on the guidelines given by Alfredson et al13. The patients performed 90 repetitions by completing three sets of 15 repetitions in two training positions (knee fully extended and knee slightly flexed) once a day. Throughout the intervention, the EE programme was performed in self‐loading. The subjects were advised that they might feel slight pain.13
2.5. Eccentric exercise vibration training
The vibration training was performed on a Power Plate My3 (Performance Health Systems, Northbrook, Illinois). Following the guidelines in Hazell et al33, patients were placed in a standing position on the vibration platform that was set to a vibration frequency of 35 Hz at an amplitude of 4 mm for 5 minutes. The patients performed the EE programme on the platform during the vibration.
2.6. Cryotherapy intervention
Prior to the EE programme, a cryotherapy intervention was carried out. Patients were dressed in shorts without shoes and socks and were seated on a chair. Patients immersed the affected lower limb in a 70‐L bucket and 55‐cm deep at 8°C± 2°C water for 17 minutes.34 Immediately after the intervention with cryotherapy, the EE programme was carried out.
2.7. Sonographic assessment
A sonographic assessment was performed with a LogiQ P7 (GE Healthcare; UK) with a 4 to 13 MHz‐range linear transducer (L6–12‐ RS type; 38‐mm footprint). According to Rompe et al,35 ultrasound images were obtained in a prone position with both feet free from the examination table. Achilles tendon thickness and CSA measures were recorded at 0, 2, 4, and 6 cm from the calcaneal insertion at rest and in maximal isometric contraction. During the study investigation, three assessments were carried out: pre‐intervention and at 4 and at 12 weeks. The mean of three repeated values was calculated for each measure. ImageJ software (version 2.0; US National Institutes of Health, Bethesda, Maryland) was used to measure all of the images offline.36
2.8. Statistical analysis
SPSS 23.0 software (IBM SPSS Statistics for Windows; IBM Corp., NY, New York) was used for data analysis. The Shapiro‐Wilks test was used for the normality assumption. For the baseline comparison, the Student t test was used considering the homogeneity of variance using Levene's test. A two‐way analysis of variance (anova) for repeated measures was used to examine the effects of intra‐subjects (pre‐ and post‐) and inter‐subject (treatment group) aspects on the dependent variables. The post hoc analyses were carried out by means of Bonferroni's correction. An α error of 0.05 (95% confidence interval) and a desired power of 80% (β error of 0.2) were used. The level of significance was set at P < 0.05.
3. RESULTS
Regarding Table 1, socio‐demographic data did not show statistically significant differences (P > 0.05). As shown in Table 2, the comparison of thickness measures at baseline, 4, and 12 weeks showed a significant (P < 0.05) increase at 0, 2, 4, and 6 cm in maximal isometric contraction and at rest. No statistically significant differences were found between intervention groups. Regarding Table 3, the comparison of CSA measures at baseline, 4, and 12 weeks showed a significant (P < 0.05) increase at 0, 2, 4, and 6 cm in maximal contraction and at rest in favour of the EE vibration training group. (Figure 2A‐D, respectively). At last, the comparison of CSA measures at baseline, 4 and 12 weeks showed a significant (P < 0.05) increase at 2, 4, and 6 cm, being positive between contraction and at rest.
Table 1.
Socio‐demographic data
| Data | Cryotherapy | EE vibration training | P‐value | |
|---|---|---|---|---|
| Gender | 0.645 | |||
| Men (n, %) | 5 ± 16.13 | 4 ± 13.33 | ||
| Women (n, %) | 26 ± 83.87 | 26 ± 86.66 | ||
| Age (years) | 42.1 ± 9.2 | 41.1 ± 8.2 | 0.645 | |
| BMI (kg/m2) | 24.8 ± 2.4 | 25.2 ± 2.5 | 0.442 | |
| Injury time, mean (SD) | 4.4 ± 2.6 | 4.1 ± 4.4 | 0.145 | |
Abbreviations: BMI, body mass index; EE, eccentric exercise.
Table 2.
Thickness measures
| Measurement (cm) | Cryotherapy intervention (n = 31) | Eccentric exercise vibration training (n = 30) | Intra‐subject effects | |
|---|---|---|---|---|
| Time P‐ value | Treatment X Time P‐value | |||
| Thickness at rest 0 cm | 0.001* | 0.359 | ||
| Baseline | 4.65 ± 0.8 | 4.77 ± 0.9 | ||
| 4 weeks | 4.79 ± 0.7 | 4.82 ± 0.7 | ||
| 12 weeks | 5.09 ± 0.7 | 5.3 ± 0.7 | ||
| Thickness contraction 0 cm | 0.001* | 0.121 | ||
| Baseline | 4.85 ± 0.8 | 5.08 ± 0.8 | ||
| 4 weeks | 5.10 ± 0.7 | 5.17 ± 0.7 | ||
| 12 weeks | 5.29 ± 0.8 | 5.67 ± 0.8 | ||
| Thickness at rest 2 cm | 0.001* | 0.333 | ||
| Baseline | 5.70 ± 1.1 | 5.97 ± 1,2 | ||
| 4 weeks | 5.98 ± 1.1 | 6.07 ± 1,0 | ||
| 12 weeks | 6.20 ± 1.1 | 6.43 ± 1,0 | ||
| Thickness contraction 2 cm | 0.001* | 0.830 | ||
| Baseline | 5.94 ± 1.3 | 6.11 ± 1.3 | ||
| 4 weeks | 6.15 ± 1.2 | 6.32 ± 1.1 | ||
| 12 weeks | 6.50 ± 1.3 | 6.60 ± 1.0 | ||
| Thickness at rest 4 cm | 0.001* | 0.874 | ||
| Baseline | 6.41 ± 2.0 | 6.92 ± 1.8 | ||
| 4 weeks | 6.64 ± 1.9 | 7.20 ± 1.8 | ||
| 12 weeks | 7.02 ± 2.1 | 7.55 ± 1.8 | ||
| Thickness contraction 4 cm | 0.001* | 0.660 | ||
| Baseline | 6.70 ± 2.3 | 7.25 ± 2,2 | ||
| 4 weeks | 6.97 ± 2.1 | 7.40 ± 2,1 | ||
| 12 weeks | 7.26 ± 2.3 | 7.84 ± 2.2 | ||
| Thickness at rest 6 cm | 0.001* | 0.354 | ||
| Baseline | 7.64 ± 2.1 | 8.94 ± 2.7 | ||
| 4 weeks | 7.91 ± 2.2 | 9.22 ± 2.8 | ||
| 12 weeks | 8.01 ± 2.2 | 9.49 ± 2.8 | ||
| Thickness contraction 6 cm | 0.001* | 0.428 | ||
| Baseline | 8.01 ± 2.6 | 9.36 ± 3.2 | ||
| 4 weeks | 8.24 ± (2.5) | 9.69 ± 3.3 | ||
| 12 weeks | 8.39 ± (2.6) | 9.96 ± 3.4 | ||
Values are mean ± SD unless otherwise indicated.
P‐value statistically significant differences.
Table 3.
Cross‐sectional area measurements
| Measurement (cm2) | Cryotherapy intervention (n = 31) | Eccentric exercise vibration training (n = 30) | Intra‐subject effects | |
|---|---|---|---|---|
| Time P‐value | Treatment × time P‐value | |||
| CSA at rest 0 cm | 0.001* | 0.013* | ||
| Baseline | 77.82 ± 29.4 | 80.77 ± 23.3 | ||
| 4 weeks | 83.38 ± 28.9 | 85.53 ± 24.0 | ||
| 12 weeks | 90.35 ± 34.7 | 100.88 ± 32.9 | ||
| CSA contraction 0 cm | 0.001* | 0.002* | ||
| Baseline | 86.07 ± 34.4 | 85.47 ± 25.9 | ||
| 4 weeks | 91.59 ± 33.7 | 93.27 ± 27.9 | ||
| 12 weeks | 97.87 ± 38.4 | 109.30 ± 36.5 | ||
| CSA at rest 2 cm | 0.001* | 0.003* | ||
| Baseline | 171.32 ± 69.6 | 173.78 ± 58.4 | ||
| 4 weeks | 178.88 ± 70.6 | 183.91 ± 60.4 | ||
| 12 weeks | 187.84 ± 80.2 | 213.25 ± 80.8 | ||
| CSA contraction 2 cm | 0.001* | 0.006* | ||
| Baseline | 172.88 ± 65.9 | 174.74 ± 52.9 | ||
| 4 weeks | 184.71 ± 71.8 | 189.23 ± 54.8 | ||
| 12 weeks | 195.90 ± 80.8 | 223.58 ± 81.6 | ||
| CSA at rest 4 cm | 0.001* | 0.001* | ||
| Baseline | 193.51 ± 79.5 | 192.62 ± 59.8 | ||
| 4 weeks | 202.52 ± 77.7 | 206.04 ± 65.6 | ||
| 12 weeks | 210.83 ± 83.6 | 233.35 ± 81.2 | ||
| CSA contraction 4 cm | 0.001* | 0.005* | ||
| Baseline | 192.21 ± 70.0 | 190.54 ± 48.8 | ||
| 4 weeks | 207.13 ± 75.0 | 210.22 ± 60.4 | ||
| 12 weeks | 218.65 ± 84.9 | 241.62 ± 78.0 | ||
| CSA at rest 6 cm | 0.001* | 0.015* | ||
| Baseline | 216.37 ± 86.0 | 224.75 ± 72.7 | ||
| 4 weeks | 225.00 ± 87.0 | 237.06 ± 79.3 | ||
| 12 weeks | 236.19 ± 93.0 | 263.92 ± 88.5 | ||
| CSA contraction 6 cm | 0.001* | 0.019* | ||
| Baseline | 212.94 ± 79.6 | 212.79 ± 61.4 | ||
| 4 weeks | 225.71 ± 84.0 | 231.64 ± 66.6 | ||
| 12 weeks | 238.75 ± 91.3 | 263.19 ± 80.1 | ||
| CSA difference contraction: at rest 0 cm | 0.144 | 0.143 | ||
| Baseline | 8.25 ± 8.1 | 4.70 ± 8.4 | ||
| 4 weeks | 8.21 ± 6.6 | 7.73 ± 7.1 | ||
| 12 weeks | 7.52 ± 5.1 | 8.42 ± 7.0 | ||
| CSA difference contraction: at rest 2 cm | 0.001* | 0.585 | ||
| Baseline | 1.56 ± 17.5 | 0.96 ± 24.2 | ||
| 4 weeks | 5.83 ± 15.6 | 5.32 ± 9.7 | ||
| 12 weeks | 8.06 ± 12.3 | 10.33 ± 18.5 | ||
| CSA difference contraction: at rest 4 cm | 0.002* | 0.930 | ||
| Baseline | −1.30 ± 26.7 | −2.08 ± 22.9 | ||
| 4 weeks | 4.61 ± 17.2 | 4.19 ± 16.8 | ||
| 12 weeks | 7.83 ± 11.0 | 8.27 ± 20.1 | ||
| CSA difference contraction: at rest 6 cm | 0.021* | 0.543 | ||
| Baseline | −3.43 ± 27.4 | −11.96 ± 29.5 | ||
| 4 weeks | 0.72 ± 17.7 | −5.41 ± 20.5 | ||
| 12 weeks | 2.56 ± 13.1 | −0.73 ± 20.4 | ||
Abbreviation: CSA, cross‐sectional area.
Values are mean (SD) unless otherwise indicated.
P‐value statistically significant differences.
Figure 2.
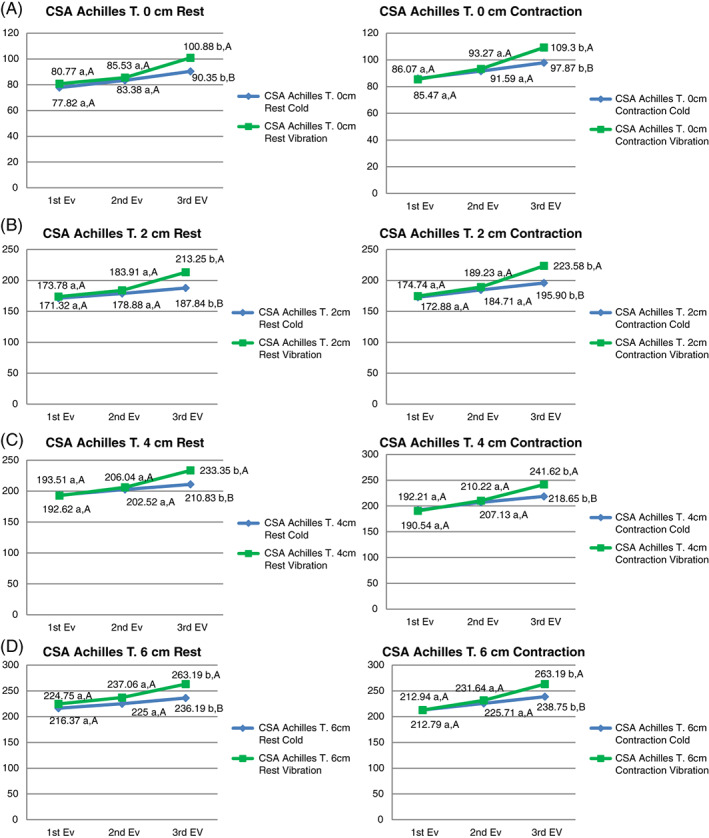
Cross‐sectional area values at 0, 2, 4, and 6 cm. A, B, Significant difference between follow up and baseline values (P < 0.05). A‐B, significant difference between groups (P < 0.05). EV, evaluation
4. DISCUSSION
The goal of the study was to examine whether the EE vibration training programme and cryotherapy with EE led to benefits for thickness and CSA in patients with mid‐portion AT.
4.1. Tendon thickness
Both groups in our study showed an increase in tendon thickness at 0, 2, 4, and 6 cm in maximal isometric contraction and at rest. According to Docking and Cook,37 a pathological tendon may compensate for areas of disorganisation by increasing in tendon thickness. In addition, the increase of a tendon thickness could be related to the mechanical stimuli induced by high‐intensity exercise, such an EE, which can be the primary mechanism for muscle and tissue hypertrophy.36 Cook et al.38 reported that only 30% of subjects with AT could return to values prior to the injury. Although AT tendons can improve their function, the ultrasound values did not improve.39
4.2. Cross‐sectional area
The results of the present study showed a CSA increase at 0, 2, 4, and 6 cm in both intervention groups in maximal isometric contraction and at rest. Between groups, statistically significant differences were found (P < 0.05) in favour of the EE vibration training group in all measures. Similar results were found in a study by Rosenberg et al.,40 reporting a CSA increase in subjects who performed resistance vibration training. In addition, the intensity of the contraction force produced by the vibratory training depends on the previous stretching of the tissue, being maximal during EE.17 Therefore, a more intense muscular contraction is produced with EE vibration training, causing adaptations such as CSA tendon increase. In line with Arya and Kulig,41 the results of our study show an increase of CSA in subjects with mid‐portion AT. Cook and Purdam42 reported that the CSA increase is produced to compensate the compression forces that occur in the lower limb.
For the difference between CSA in muscle contraction and at rest, statistically significant differences (P < 0.05) were found at 2, 4, and 6 cm from the insertion of the calcaneus at 12 weeks in both groups. The difference in CSA was increased at the end of the intervention with respect to the baseline. According to Reeves and Cooper,43 a transverse tendon deformation only occurred in selected regions of the distal Achilles tendon at 20% and 30% of maximal voluntary contraction, unlike the proximal portion of the Achilles. Therefore, the contraction tendon surface is slightly lower in the proximal tendon areas at low intensity. Nuri et al31 reported a decrease in CSA with a 50% maximal voluntary contraction in subjects with AT. Our study showed a CSA increase being positive the difference between contraction and at rest, supporting EE and hypertrophy theories.38 Likewise, no statistically significant differences were observed between EE vibration training and cryotherapy, which indicates that this improvement is due exclusively to the performance of the EE.
4.3. Limitations
Several limitations should be considered in the present study. First, the vibration and cryotherapy interventions contained an eccentric exercise; therefore, it is difficult to conclude whether the increases in measures were caused by the eccentric exercise or the vibration and cryotherapy in a reliable manner. Second, other ultrasonography modes, such as M‐mode, and software analysis, such as sonoelastography, were not used but may be useful for the study of muscle tissue characteristics.44 Short‐term follow up was performed, but long‐term follow up must be performed in future studies for to know if the effects in CSA are maintained.
5. CONCLUSIONS
EE vibration training programme and cryotherapy with EE produced a thickness and CSA increase at 0, 2, 4, and 6 cm in maximal isometric contraction and at rest in subjects with chronic mid‐portion AT. No statistically significant differences were found between intervention groups for thickness measures. EE vibration training has shown a statistical CSA increase than the cryotherapy group in patients with chronic mid‐portion AT.
CONFLICTS OF INTEREST
The authors have no conflicts of interest to report.
Romero‐Morales C, Javier Martín‐Llantino P, Calvo‐Lobo C, et al. Ultrasonography effectiveness of the vibration vs cryotherapy added to an eccentric exercise protocol in patients with chronic mid‐portion Achilles tendinopathy: A randomised clinical trial. Int Wound J. 2019;16:542–549. 10.1111/iwj.13074
This paper has been reviewed by anti‐plagiarism Turnitin programme that guarantees the originality of the manuscript.
REFERENCES
- 1. Van Sterkenburg MN, van Dijk CN. Mid‐portion Achilles tendinopathy: why painful? An evidence‐based philosophy. Knee Surg Sports Traumatol Arthrosc. 2011;19(8):1367‐1375. 10.1007/s00167-011-1535-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Järvinen TAH, Kannus P, Paavola M, Jarvinen TLN, Józsa L, Järvinen M. Achilles tendon injuries. Curr Opin Rheumatol; 2001. Mar;13(2):150–5. PMID: 11224740 [DOI] [PubMed] [Google Scholar]
- 3. Kujala UM, Sarna S, Kaprio J. Cumulative incidence of achilles tendon rupture and tendinopathy in male former elite athletes. Clin J Sport Med. 2005;15(3):133‐135. [DOI] [PubMed] [Google Scholar]
- 4. Beyer R, Kongsgaard M, Hougs Kjaer B, Ohlenschlaeger T, Kjaer M, Magnusson SP. Heavy slow resistance versus eccentric training as treatment for achilles tendinopathy: a randomized controlled trial. Am J Sports Med. 2015. Jul;43(7):1704‐1711. 10.1177/0363546515584760. [DOI] [PubMed] [Google Scholar]
- 5. Alfredson H, Thorsen K, Lorentzon R. In situ microdialysis in tendon tissue: high levels of glutamate, but not prostaglandin E2 in chronic Achilles tendon pain. Knee Surg Sports Traumatol Arthrosc. 1999;7(6):378‐381. 10.1007/s001670050184. [DOI] [PubMed] [Google Scholar]
- 6. Enwemeka CS. Inflammation, cellularity, and fibrillogenesis in regenerating tendon: implications for tendon rehabilitation. Phys Ther. 1989;69(10):816‐825. [DOI] [PubMed] [Google Scholar]
- 7. Pingel J, Fredberg U, Mikkelsen LR, et al. No inflammatory gene‐expression response to acute exercise in human Achilles tendinopathy. Eur J Appl Physiol. 2013;113(8):2101‐2109. 10.1007/s00421-013-2638-3. [DOI] [PubMed] [Google Scholar]
- 8. Lake JE, Ishikawa SN. Conservative treatment of Achilles tendinopathy: emerging techniques. Foot Ankle Clin. 2009;14(4):663‐674. [DOI] [PubMed] [Google Scholar]
- 9. Magnan B, Bondi M, Pierantoni S, Samaila E. The pathogenesis of Achilles tendinopathy: a systematic review. Foot Ankle Surg. 2014;20(3):154‐159. 10.1016/j.fcl.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 10. Frey C, Zamora J. The effects of obesity on orthopaedic foot and ankle pathology. Foot Ankle Int. 2007;28(9):996‐999. 10.3113/FAI.2007.0996. [DOI] [PubMed] [Google Scholar]
- 11. Ohberg L, Alfredson H. Effects on neovascularisation behind the good results with eccentric training in chronic mid‐portion Achilles tendinosis? Knee Surg Sports Traumatol Arthrosc. 2004. Sep;12(5):465‐470. 10.1007/s00167-004-0494-8. [DOI] [PubMed] [Google Scholar]
- 12. Andersson G, Danielson P, Alfredson H, Forsgren S. Nerve‐related characteristics of ventral paratendinous tissue in chronic Achilles tendinosis. Knee Surg Sports Traumatol Arthrosc. 2007. Oct;15(10):1272‐1279. 10.1007/s00167-007-0364-2. [DOI] [PubMed] [Google Scholar]
- 13. Alfredson H, Pietila T, Jonsson P, Lorentzon R. Heavy‐load eccentric calf muscle training for the treatment of chronic Achilles tendinosis. Am J Sports Med. 1998;26(3):360‐366. 10.1177/03635465980260030301. [DOI] [PubMed] [Google Scholar]
- 14. Stanish WD, Rubinovich RM, Curwin S. Eccentric exercise in chronic tendinitis. Clin Orthop Relat Res. 1986;208:65‐68. [PubMed] [Google Scholar]
- 15. Mafi N, Lorentzon R, Alfredson H. Superior short‐term results with eccentric calf muscle training compared to concentric training in a randomized prospective multicenter study on patients with chronic Achilles tendinosis. Knee Surg Sports Traumatol Arthrosc. 2001;9(1):42‐47. 10.1007/s001670000148. [DOI] [PubMed] [Google Scholar]
- 16. Hilgers C, Mundermann A, Riehle H, Dettmers C. Effects of whole‐body vibration training on physical function in patients with multiple sclerosis. Neuro Rehabilitation. 2013;32(3):655‐663. 10.3233/NRE-130888. [DOI] [PubMed] [Google Scholar]
- 17. Horstmann T, Jud HM, Frohlich V, Mundermann A, Grau S. Whole‐body vibration versus eccentric training or a wait‐and‐see approach for chronic Achilles tendinopathy: a randomized clinical trial. J Orthop Sports Phys Ther. 2013;43(11):794‐803. 10.2519/jospt.2013.4762. [DOI] [PubMed] [Google Scholar]
- 18. Knobloch K, Hufner T. Conservative treatment of Achilles tendinopathy. Unfallchirurg. 2010;113(9):705‐711. 10.1007/s00113-010-1808-6. [DOI] [PubMed] [Google Scholar]
- 19. Knobloch K, Grasemann R, Spies M, Vogt PM. Midportion achilles tendon microcirculation after intermittent combined cryotherapy and compression compared with cryotherapy alone: a randomized trial. Am J Sports Med. 2008;36(11):2128‐2138. 10.1177/0363546508319313. [DOI] [PubMed] [Google Scholar]
- 20. Potter CL, Cairns MC, Stokes M. Use of ultrasound imaging by physiotherapists: a pilot study to survey use, skills and training. Man Ther. 2012. Feb;17(1):39‐46. 10.1016/j.math.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 21. Teyhen DS, Gill NW, Whittaker JL, Henry SM, Hides JA, Hodges P. Rehabilitative ultrasound imaging of the abdominal muscles. J Orthop Sports Phys Ther. 2007;37(8):450‐466. 10.2519/jospt.2007.2558. [DOI] [PubMed] [Google Scholar]
- 22. Lobo CC, Morales CR, Sanz DR, Corbalan IS, Marin AG, Lopez DL. Ultrasonography comparison of peroneus muscle cross‐sectional area in subjects with or without lateral ankle sprains. J Manipulative Physiol Ther. 2016;39(9):635‐644. 10.1016/j.jmpt.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 23. Lobo CC, Marin AG, Sanz DR, et al. Ultrasound evaluation of intrinsic plantar muscles and fascia in hallux valgus: a case‐control study. Medicine (Baltimore). 2016;95(45):e5243. 10.1097/MD.0000000000005243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Angin S, Crofts G, Mickle KJ, Nester CJ. Ultrasound evaluation of foot muscles and plantar fascia in pes planus. Gait Posture. 2014;40(1):48‐52. 10.1016/j.gaitpost.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 25. Rodriguez‐Sanz D, Losa‐Iglesias ME, Becerro de Bengoa‐Vallejo R, et al. Skin temperature in youth soccer players with functional equinus and non‐equinus condition after running. J Eur Acad Dermatol Venereol. 2018;32(11):2020‐2024. 10.1111/jdv.14966. [DOI] [PubMed] [Google Scholar]
- 26. Rodríguez‐Sanz D, Becerro‐de‐Bengoa‐Vallejo R, Losa‐Iglesias ME, et al. Effects of compressive stockings and standard stockings in skin temperature and pressure pain threshold in runners with functional ankle equinus condition. J Clin Med. 2018;7(11). 10.3390/jcm7110454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Whittaker JL, Warner MB, Stokes M. Comparison of the sonographic features of the abdominal wall muscles and connective tissues in individuals with and without lumbopelvic pain. J Orthop Sports Phys Ther. 2013;43(1):11‐19. 10.2519/jospt.2013.4450. [DOI] [PubMed] [Google Scholar]
- 28. Shaikh Z, Perry M, Morrissey D, Ahmad M, Del Buono A, Maffulli N. Achilles tendinopathy in club runners. Int J Sports Med. 2012. May;33(05):390‐394. 10.1055/s-0031-1299701. [DOI] [PubMed] [Google Scholar]
- 29. Scott A, Huisman E, Khan K. Conservative treatment of chronic Achilles tendinopathy. CMAJ. 2011;183:1159‐1165. 10.1503/cmaj.101680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Magnusson SP, Beyer N, Abrahamsen H, Aagaard P, Neergaard K, Kjaer M. Increased cross‐sectional area and reduced tensile stress of the Achilles tendon in elderly compared with young women. J Gerontol A Biol Sci Med Sci. 2003;58(2):123‐127. [DOI] [PubMed] [Google Scholar]
- 31. Nuri L, Obst SJ, Newsham‐West R, Barrett RS. Three‐dimensional morphology and volume of the free Achilles tendon at rest and under load in people with unilateral mid‐portion Achilles tendinopathy. Exp Physiol. 2018;103:358‐369. 10.1113/EP086673. [DOI] [PubMed] [Google Scholar]
- 32. Holt GR. Declaration of Helsinki‐the world's document of conscience and responsibility. South Med J. 2014;107(7):407. 10.14423/SMJ.0000000000000131. [DOI] [PubMed] [Google Scholar]
- 33. Hazell TJ, Jakobi JM, Kenno KA. The effects of whole‐body vibration on upper‐ and lower‐body EMG during static and dynamic contractions. Appl Physiol Nutr Metab. 2007;32(6):1156‐1163. 10.1139/H07-116. [DOI] [PubMed] [Google Scholar]
- 34. Astrom M, Gentz CF, Nilsson P, Rausing A, Sjoberg S, Westlin N. Imaging in chronic achilles tendinopathy: a comparison of ultrasonography, magnetic resonance imaging and surgical findings in 27 histologically verified cases. Skeletal Radiol. 1996;25(7):615‐620. [DOI] [PubMed] [Google Scholar]
- 35. Rompe JD, Nafe B, Furia JP, Maffulli N. Eccentric loading, shock‐wave treatment, or a wait‐ and‐see policy for tendinopathy of the main body of tendo achillis: a randomized controlled trial. Am J Sports Med. 2007;35(3):374‐383. 10.1177/0363546506295940. [DOI] [PubMed] [Google Scholar]
- 36. Khanmohammadi R, Someh M, Ghafarinejad F. The effect of cryotherapy on the normal ankle joint position sense. Asian J Sports Med. 2011;2:91‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Docking SI, Cook J. Pathological tendons maintain sufficient aligned fibrillar structure on ultrasound tissue characterization (UTC). Scand J Med Sci Sports. 2016;26(6):675‐683. 10.1111/sms.12491. [DOI] [PubMed] [Google Scholar]
- 38. Cook JL, Khan KM, Kiss ZS, Purdam CR, Griffiths L. Prospective imaging study of asymptomatic patellar tendinopathy in elite junior basketball players. J Ultrasound Med. 2000. Jul;19(7):473‐479. [DOI] [PubMed] [Google Scholar]
- 39. Sidhu G, Beyene J, Rosenblum ND. Outcome of isolated antenatal hydronephrosis: a systematic review and meta‐analysis. Pediat Nephrol. 2006;21:218‐224. 10.1007/s00467-005-2100-9. [DOI] [PubMed] [Google Scholar]
- 40. Rosenberger A, Beijer Å, Johannes B, Schoenau E, Mester J, Rittweger J, Zange J Changes in muscle cross‐sectional area, muscle force, and jump performance during 6 weeks of progressive whole‐body vibration combined with progressive, high intensity resistance training. J Musculoskelet Neuronal Interact; 2017; 17(2): 38–49. PMID: 28574410 [PMC free article] [PubMed] [Google Scholar]
- 41. Arya S, Kulig K. Tendinopathy alters mechanical and material properties of the Achilles tendon. J Appl Physiol. 2010;108(3):670‐675. 10.1152/japplphysiol.00259.2009. [DOI] [PubMed] [Google Scholar]
- 42. Cook JL, Purdam CR. Is tendon pathology a continuum? A pathology model to explain the clinical presentation of load‐induced tendinopathy. Br J Sports Med. 2009. Jun;43(6):409‐416. 10.1136/bjsm.2008.051193. [DOI] [PubMed] [Google Scholar]
- 43. Reeves ND, Cooper G. Is human Achilles tendon deformation greater in regions where cross‐sectional area is smaller? J Exp Biol. 2017;220(9):1634‐1642. 10.1242/jeb.157289. [DOI] [PubMed] [Google Scholar]
- 44. Karjalainen PT, Aronen HJ, Pihlajamäki HK, Soila K, Paavonen T, Bostman OM. Magnetic resonance imaging during healing of surgically repaired Achilles tendon ruptures. Am J Sports Med. 1997;25(2):164‐171. 10.1177/036354659702500204. [DOI] [PubMed] [Google Scholar]


