Abstract
The purpose of this study was to analyse the outcome of our established triple treatment strategy in therapy‐resistant deep‐thickness chronic lower leg ulcers. This limb salvage approach consists of ultra‐radical surgical debridement, negative‐pressure wound therapy (NPWT) with or without instillation, and split‐thickness skin grafting. Between March 2003 and December 2019, a total of 16 patients and 24 severe cases of lower leg ulcers were eligible for inclusion in this highly selective population. A total of seven patients received immunosuppressive medication. Complete wound closure was achieved in 25% and almost 90% of included lower leg ulcer cases after 3 and 24 months of our triple treatment strategy, respectively. The overall limb salvage rate was 100%. Bacterial colonisation of these wounds was significantly reduced after multiple surgical debridements and NPWT. Fasciotomy and radical removal of devitalised tissue such as deep fascia, tendons, and muscles combined with NPWT showed promising results in terms of the overall graft take rate. This treatment strategy was considered as last resort for limb salvage in such a critically ill and immunocompromised patient population. Surgeons should be aware of its efficacy and consider the triple treatment strategy especially if no other limb salvage option remains.
Keywords: chronic leg ulcer, negative‐pressure wound therapy, skin transplantation, ultra‐radical debridement
1. INTRODUCTION
Chronic leg ulcers (CLUs) can be defined as wounds that persist longer than 6 weeks and show no healing tendency after 3 months of treatment usually affecting lower legs, ankles, and feet. 1 The most commonly affected population are multimorbid elderly with 0.6% to 3% of people over 60 years and up to 5% of those aged over 80 years. 2 , 3 In order to predict the probability of lower limb amputation, Knighton et al classified chronic wounds according to its severity, which depends on the depth of the defect. 4 Venous insufficiency as the dominating cause accounts for approximately 50% of CLU cases. Wounds of mixed venous‐arterial aetiology account for 18% and wounds related to arterial diseases such as arteriosclerotic occlusion for about 15%. Other diseases such as vasculitis (5%), exogenous factors (4%), and pyoderma gangrenosum (3%) are less common causes. 5 Typical symptoms of CLU are an increase in pain and a foul wound odour. 6 Commonly known adverse sequelae such as a negative body image, social distress, loss of productivity, health care, and personal costs result in an impaired quality of life. 7
The ultimate goal of CLU treatment is to hinder its persistence, attain complete wound closure, and ultimately prevent lower extremity amputation. This does not only reduce socioeconomic burden but also helps to improve patient's quality of life. 8 The complex pathophysiology of CLUs and its underlying cause usually requires a detailed medical history, meticulous physical examination, clinical assessment, and further diagnostic evaluation such as laboratory screenings, venography, and duplex imaging. 6 , 9 , 10 Besides local therapy approaches such as compression therapy, wound dressings, antiseptics, and topical drug application, the establishment of an appropriate systemic therapy based on the underlying disease remains crucial for its treatment success. 11 Several local treatment modalities have evolved and been successfully used in the course of the last few years. 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 A detailed description of emerging treatment strategies, however, lies beyond the scope of this study.
Especially, patients suffering from deep‐thickness CLUs large in size and refractory to conservative treatment have shown to benefit from surgical intervention. 20 This usually involves the debridement of devitalised, necrotic tissue within the wound bed followed by wound coverage using skin grafts or tissue transfer. 20 , 21
Negative‐pressure wound therapy (NPWT), an invention of the late 20th century, has nowadays been broadly used as temporary wound coverage acting as a successful tool for wound bed preparation of many different wound types. This includes but is not limited to acute, chronic, infectious, and burn wounds. 22 , 23 The additional application of intermittent fluid instillation such as normal saline, polyhexanide, povidone‐iodine, or octenidine dihydrochloride to the wound bed for a pre‐defined time period (dwell time) can have a sufficient micro‐debridement effect especially to infectious or contaminated wounds. 24 , 25 , 26 Irrigation of the wound bed and the removal of debris and pathogenic bacteria showed to reduce the microbial film at the wound surface. 27 , 28 NPWT combined with surgical debridement can help to reduce the complexity of surgical wound coverage procedures ultimately acting as salvage procedure for limbs at high risk of amputation. 20 , 29
This retrospective study aims to analyse the success of a surgical treatment approach as limb salvage procedure of chronic lower leg ulcers in critically ill and immunocompromised patients. This includes ultra‐radical surgical debridement of devitalized tissue, NPWT, and split‐thickness skin graft coverage of lower leg defects. The triple treatment concept is believed to improve the wound healing outcome of this highly selected patient collective ultimately preventing the amputation of the affected limb.
2. METHODS
In a retrospective mono‐centre study, data including hospital, operative, general practitioner (GP), and postoperative wound management records were extracted and documented from critically ill and immunocompromised patients suffering from therapy‐resistant CLUs. GPs and wound managers were contacted by telephone if necessary. Patients at high risk of lower limb amputation receiving our triple treatment (ultra‐radical surgical debridement, NPWT with or without instillation [NPWT(i‐d)]), and split‐thickness skin grafting (STSG)) were included. Our hospital database was searched from March 2003 to December 2019. All surgical procedures were performed by experienced plastic surgeons. CLUs persisting at least 3 months before hospital admission with no healing tendency irrespective of its previous treatment attempts were included. Wound severity was determined by its soft tissue defect (grade 3 or higher, according to Knighton et al 4 ) extending over 75% of the lower limb circumference. Ultra‐radical surgical debridement was defined as complete excision of devitalized, infectious tissue with more than 75% of the lower limb circumference. In addition, fasciotomy of affected compartments and fasciectomy of the deep fascial layer of the lower limb was performed until only clean and vital tissue remained. Devitalised tendons and muscles were resected if necessary. Resection was extended into adjacent soft tissue with the aim to remove any calcified and chronically indurated pathological tissue. At least three surgical debridements prior to wound closure attempt were required for study inclusion. The reticulated open‐pore polyurethane foam‐surface negative‐pressure wound dressing (KCI—an Acelity company, San Antonio, Texas) was changed during each surgical debridement. Wounds treated with NPWT with or without instillation and dwell time were included in this study. The nature of this long‐term study and the fact that our high‐volume centre started using NPWT with instillation and dwell time (NPWT(i‐d)) in 2014 made it inevitable to also include immunocompromised patients solely treated with NPWT. Polyhexanide (0.4 mg/1 mL) (Lavasept, B. Braun Medical AG, Germany) was the antiseptic solution used for instillation. All patients received standardised postoperative care during and after hospital admission. NPWT(i‐d) was applied in a standardised fashion and was performed until the fifth day after STSG. The negative pressure was set to −125 mm Hg. However, in cases of pain during NPWT(i‐d), the pressure was reduced to −100 or −75 mm Hg if necessary. The instillation volume was adjusted according to the wound size and depth. Dwell time was set to 20 minutes. Subsequently wound dressings consisting of fat gauze, sterile dressings, and compression bandages were applied and frequently changed until complete wound closure was attained. Low molecular weight heparine was administered subcutaneously during the entire hospital stay preoperatively and postoperatively once a day. Postoperative wound assessments were performed on a regular basis at our outpatient clinic especially during the first 3 months after hospital discharge. The frequency of outpatient visits was usually reduced after the first 3 months according to individual wound conditions. Wounds were assessed by experienced surgeons. Images and notes were taken during each outpatient visit. If necessary, dressing changes were performed by wound managers or GPs on a regular basis after hospital discharge. Cut‐off values determining the wound healing success were set at 3 and 24 months after our triple treatment. Wound healing was defined as epithelialisation of the wound bed after our triple treatment was performed. Additional charts were reviewed for bacterial colonisation of the included lower leg ulcers. Data of the first and the last wound swab before wound coverage with split‐thickness skin graft were collected. This allows an assessment of changes in the bacterial load and flora after NPWT(i‐d) and multiple surgical debridements. Wound swabs were performed by the surgeon in charge in a standardised fashion before wound disinfection and prior to surgical debridement. Wound swabs were analysed for the number of different bacterial species (NDB) and the amount of bacteria under culture (AB) according to the local Institute for Clinical Microbiology. Semi‐quantitative examination of the AB gives a rough estimate of the bacterial colonisation of a wound on an ordinal scale 1 , 2 , 3 , 4 for each bacteria (1: sparse, 2: moderate, 3: several, and 4: plenty). Because of the heterogeneity of the wounds and their bacterial colonisation, the amount of all bacteria found in the wound was calculated through summation of those semi‐quantitative ordinal scaled numbers of each bacteria. 28 All relevant data according to the inclusion criteria were extracted, verified, and documented by two authors on a bespoke pre‐defined Excel sheet (Microsoft, Redmond, Washington).
The following data were collected for comparison:
Patient demographics (gender, age, relevant concomitant diseases, wound location, wound duration, previous local treatments, immunosuppressive medication (Table 1), and American Society of Anaesthesiologists [ASA] score).
Leg ulcer characteristics (length of hospital stay, days from initial ultra‐radical debridement to STSG (Table 2), and number of blood product transfusions).
Leg ulcer outcomes (number of completely healed ulcers after 3 and 24 months (Table 2), time to complete wound healing).
Bacterial load and flora (number of different bacteria, amount of bacteria (Table 3), bacteria shift [gram +/−], and multidrug resistant bacteria).
TABLE 1.
Patient characteristics
| Patient no. | Patient baseline characteristics | Relevant concomitant diseases | Immunosuppressive medication | Previous local treatments | |
|---|---|---|---|---|---|
| Age (in years) | Gender | ||||
| 1 | 62 | M | PAD, sepsis, LV heart failure, HTN, AS, lymphedema lower legs, ARF | Wound antiseptics, antimicrobial wound dressings | |
| 2 | 47 | M | PG, RA, high‐grade left IAD, HTN, nicotine abuse | Infliximab, methotrexate, abatacept, prednisolone | Wound antiseptic, antimicrobial wound dressings |
| 3 | 50 | F | MCTD (sharp syndrome), PF, HTN, AFL | Prednisolone | Antimicrobial wound dressing, wound disinfection, surgical debridement, STSG |
| 4 | 62 | F | CV | Rituximab, mycophenolate mofetil, prednisolone | Steroids |
| 5 | 66 | F | PG, CVI, CHD, IC, HTN, ARF, lymphedema | Mycophenolate mofetil, prednisolone | Wound antiseptics, antimicrobial wound dressing, topical steroids |
| 6 | 57 | M | VTE (DVT), CML, HTN | Mycophenolate mofetil, ciclosporine | Surgical debridement, negative pressure therapy, STSG |
| 7 | 62 | M | CVI, DM, HTN, obesity | Surgical debridement, negative pressure therapy, wound antiseptic, antimicrobial wound dressing, topical steroids, compression therapy, STSG | |
| 8 | 79 | F | CVI, PCV, HTN | Hydroxycarbamide | Surgical and biomechanical debridement, negative pressure therapy, wound antiseptic, silicone wound dressing, zinc |
| 9 | 74 | F | PAD, varicosis, PNP, lymphedema, HTN, CRF | Surgical and biomechanical debridement, wound antiseptics, polyurethane wound dressings, compression therapy | |
| 10 | 77 | M | PAD (Stage I), CHD, HTN | Wound dressings | |
| 11 | 35 | M | VTE (SVT), lipedema (Stage III), lymphedema (Stage III), obesity | Antimicrobial wound dressings, wound desinfection, physiotherapy, lymphatic drainage | |
| 12 | 39 | M | PTS, NF, lower leg phlegmon | Compression therapy, surgical debridement, negative pressure therapy, STSG | |
| 13 | 64 | F | Bullous erysipelas, RA, HTN, CRF, obesity | Methotrexate | Antimicrobial wound dressings, wound desinfection, compression therapy, topical steroids |
| 14 | 64 | M | PAD (Stage II), CHD | Surgical debridement, STSG, antimicrobial wound dressing, compression therapy | |
| 15 | 55 | F | PAD (Stage IV), DM, HTN, CHD, obesity, cardiac valve replacement, left fore foot amputation | Wound antiseptics, topical steroids | |
| 16 | 78 | F | CVI, HTN, heart failure, CRF, obesity | Wound antiseptics, antibiotics, antimicrobial wound dressings, compression therapy, topical steroids | |
Abbreviations: AFL, atrial flutter; ARF, acute renal failure; AS, ankylosing spondylitis; B, both; CHD, chronic heart disease; CV, cryoglobulinemic vasculitis; CVI, chronic venous insufficiency; DM, diabetes mellitus; F, female; HTN, hypertension; IAD, iliac artery disease; IC, ischemic cardiomyopathy; L, left; M, male; MCTD, mixed connective tissue disease; NF, necrotizing fasciitis; PAD, peripheral artery disease; PCV, polycythemia vera; PF, pulmonary fibrosis; PG, pyoderma gangrenosum; PNP, polyneuropathy; R, right; RA, rheumatoid arthritis; STSG, split‐thickness skin graft; SVT, superficial vein thrombosis.
TABLE 2.
Leg ulcer characteristics
| Wound location | Wound duration | Initial ultra‐radical debridement to STSG | Hospital stay | Wound closure after 3 months | Wound closure after 24 months | |||
|---|---|---|---|---|---|---|---|---|
| Patient no. | Lower legs | Categories A‐C | ||||||
| in months | in days | in days | Left leg | Right leg | Left leg | Right leg | ||
| 1 | B | 13 | 15 | 30 | B | B | A | A |
| 2 | L | 25 | 12 | 19 | B | A | ||
| 3 | B | 48 | 11 | 20 | B | B | B | A |
| 4 | B | 192 | 13 | 27 | B | B | A | A |
| 5 | B | 48 | 10 | 32 | B | A | A | A |
| 6 | R | 16 | 43 | 50 | A | A | ||
| 7 | B | 120 | 12 | 19 | B | B | A | A |
| 8 | B | 36 | 12 | 23 | C | A | C | A |
| 9 | R | 3 | 13 | 21 | B | A | ||
| 10 | B | 3 | 9 | 24 | B | A | A | A |
| 11 | B | 10 | 11 | 23 | B | B | A | A |
| 12 | R | 120 | 8 | 20 | B | A | ||
| 13 | L | 5 | 12 | 19 | B | A | ||
| 14 | L | 96 | 10 | 17 | A | A | ||
| 15 | R | 60 | 21 | 29 | C | C | ||
| 16 | L | 36 | 11 | 20 | A | A | ||
Note: B = both, R = right, L = left. Categories (A‐C)—A: complete wound closure; B: wound control (75%‐99% wound closure); C: failed wound closure (<75% wound closure).
Our primary outcome measure was the number of completely healed lower leg ulcers after 3 and 24 months. Wound closure was classified into three different categories (A: complete wound closure, B: wound control [75%‐99% wound closure], and C: leg ulcerations with less than 75% wound closure which were recorded as failures). Secondary outcome measures included time to complete wound healing within category A, change of bacterial load and flora of those chronic wounds during NPWT(i‐d), and surgical debridements. The time to complete wound healing was measured from the day of STSG.
2.1. Statistical analysis
Descriptive analyses were applied to characterise patient demographics. Univariate analyses were performed using Chi‐squared and Fisher's exact tests for categorical variables and paired or unpaired Student's t‐tests for continuous normally distributed variables. Mann‐Whitney‐U and the Kruskal‐Wallis test were used for unpaired non‐normally distributed variables. The Wilcoxon signed rank test was used for paired non‐normally distributed variables. Statistical analyses were performed using GraphPad Prism (GraphPad Software, Inc., California).
3. RESULTS
3.1. Patient demographics
Between March 2003 and December 16, 2019, multimorbid patients (8 female, 8 male) with a total of 24 severe cases of chronic, non‐healing lower leg ulcers were eligible for inclusion. All these wounds were unresponsive to previous non‐surgical and surgical treatments. The age of this highly selected collection of critically ill and immunocompromised patients at the time of ultra‐radical debridement ranged from 35 to 79 years (mean age 60.7 years). CLUs were either located at both lower legs (n = 7), or solely at the right (n = 5) or left (n = 4) lower leg. The median ulcer duration was 36 months (range 3‐192 months) (Figure 1). The included patients suffered from peripheral artery disease (n = 5), chronic venous insufficiency (n = 4), venous thromboembolism (n = 3), autoimmune diseases such as pyoderma gangrenosum (n = 2), mixed connective tissue disease (n = 1), ankylosing spondylitis (n = 1), and cryoglobulinemic vasculitis (n = 1) with several concomitant diseases such as myeloproliferative neoplasm (n = 2), renal failure (n = 5), hypertension (n = 12), diabetes (n = 2), cardiac diseases (n = 7), lymphedema (n = 4), and obesity (n = 5). A total of seven patients received immunosuppressive medication before or during hospital stay. All patients received several local treatments such as wound antiseptics, antimicrobial wound dressings, topical steroids, and antibiotics as well as the application of compression garments prior to hospital admission. A total of five patients received previous unsuccessful wound closure attempts with STSG. All patients received systemic antibiotics during and after their hospital stay in accordance with the wound swab results. A total of 12 patients and 19 leg ulcers were treated with an additional instillation of the wound using an antiseptic solution and only 4 patients received basic NPWT. The median duration of NPWT with or without instillation and dwell time (i‐d) from the initial radical debridement to STSG was 12 days with a range of 8 to 43 days. The median hospital stay was 22 days with a range of 17 to 50 days. However, one specific case with a hospital stay of 50 days and NPWT for 43 days additionally suffered from chronic myeloid leukaemia. Therefore, the patient was transferred to the Department of Haematology and Oncology for bone marrow transplantation between surgical treatments and during NPWT(i‐d). The median ASA score was 3. A total of six patients (37.5%) received between two and six units of red blood cells during their hospital stay.
FIGURE 1.
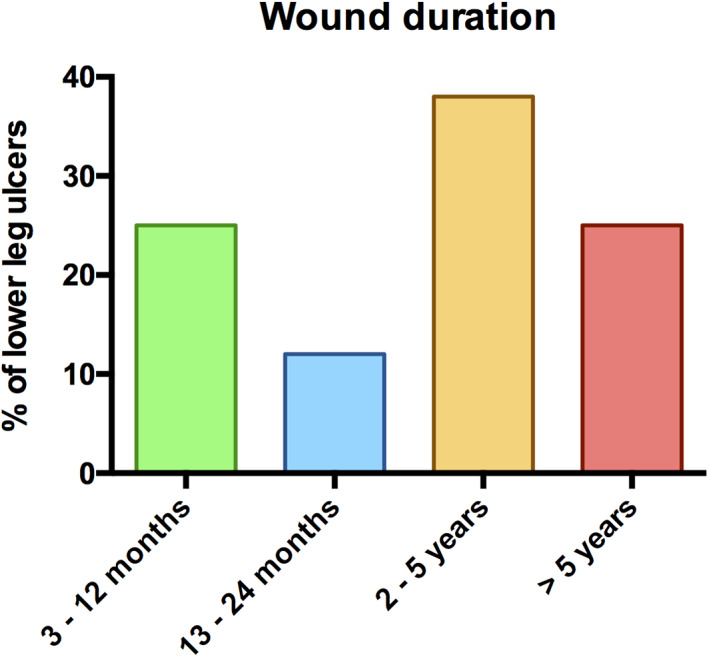
Wound duration of included chronic lower leg ulcers
TABLE 3.
Change of bacterial colonisation of included leg ulcers during ultra‐radical debridement and negative pressure wound therapy
| Patient no. | Wound location | Left leg | Right leg | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Lower legs | First wound swab | Last wound swab | First wound swab | Last wound swab | |||||
| Left/right/both | NDB | AB | NDB | AB | NDB | AB | NDB | AB | |
| 1 | B | 3 | 10 | 3 | 9 | 3 | 10 | 3 | 9 |
| 2 | L | 2 | 7 | 0 | 0 | ||||
| 3 | B | 3 | 12 | 1 | 2 | 3 | 12 | 1 | 3 |
| 4 | B | 2 | 6 | 3 | 7 | 1 | 4 | 2 | 6 |
| 5 | B | 4 | 6 | 6 | 21 | 5 | 17 | 6 | 18 |
| 6 | R | 2 | 6 | 1 | 1 | ||||
| 7 | B | 3 | 12 | 2 | 6 | 5 | 18 | 0 | 0 |
| 8 | R | 3 | 10 | 1 | 4 | 3 | 11 | 0 | 0 |
| 9 | R | 2 | 7 | 4 | 14 | ||||
| 10 | B | 3 | 7 | 4 | 10 | 1 | 2 | ||
| 11 | B | 0 | 0 | 1 | 4 | 3 | 7 | 1 | 2 |
| 12 | R | 2 | 6 | 0 | 0 | ||||
| 13 | L | 1 | 4 | 0 | 0 | ||||
| 14 | L | 2 | 8 | 0 | 0 | ||||
| 15 | R | 3 | 11 | 1 | 3 | ||||
| 16 | L | 1 | 4 | 3 | 9 | ||||
Abbreviations: AB, amount of bacteria; NDB, number of different bacteria.
3.2. Wound healing outcomes
By 3 months, complete wound closure was achieved in six leg ulcers (25%). The number of completely healed wounds (Category A) increased to 21 leg ulcers (87.5%) in 15 patients (94%) 24 months after STSG (Figures 2 and 3). The average time to complete wound healing within Category A was 6.9 months ranging from 2 to 20 months. Wound control (Category B) was achieved in 11 patients (69%) and 16 CLUs (67%) 3 months after STSG. By 24 months, the number of wounds under controlled healing was reduced to one leg ulcer (4%) in one patient (6%) (Category B). Wound closure with ultra‐radical surgical debridement, NPWT(i‐d), and STSG failed in two CLUs (8%) after 3 and 24 months (Category C). Two patients required further surgical revision with surgical debridements, NPWT(i‐d) and STSG 7 and 14 months after initial STSG. However, only one of these two patients could achieve complete wound healing after another triple treatment attempt. The other patient received further local treatment modalities at other departments. The total limb salvage rate of this critically ill patient population was 100%.
FIGURE 2.
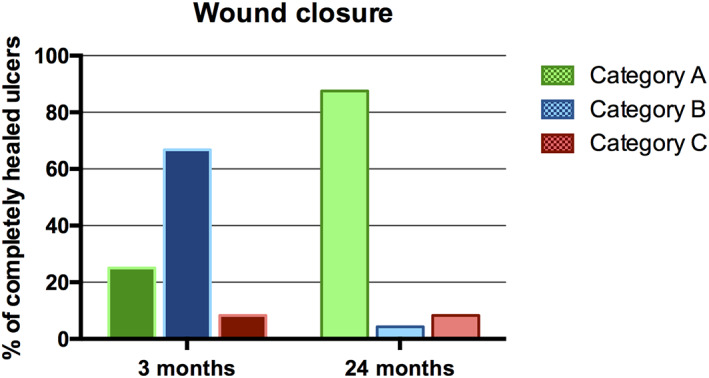
Wound healing outcome of chronic leg ulcers in percentage. Category A: completely healed ulcers; Category B: wound control (75%‐99% wound closure); Category C: failed wound closure
FIGURE 3.
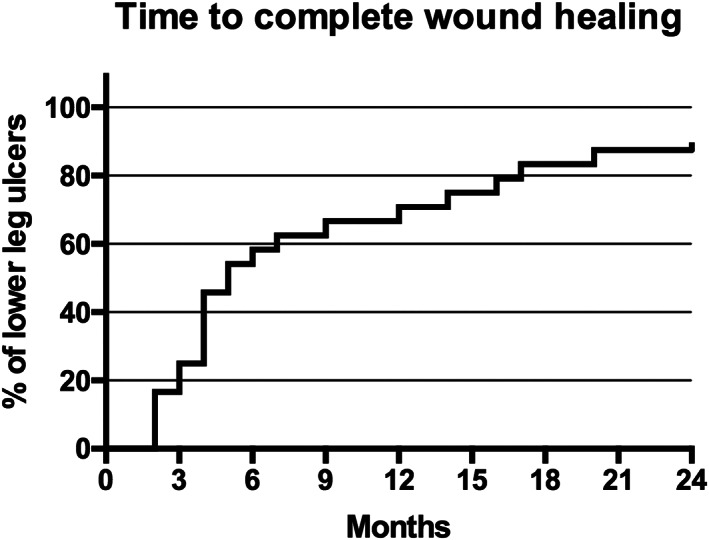
Kaplan‐Meier estimate of time to complete wound healing
3.3. Bacterial load and flora
The average number of different bacterial species during initial ultra‐radical debridement was 2.6 NDB (1.2 SD) ranging from 0 to 5 different bacteria. In 83.3% of the lower leg ulcers, two or more different bacteria were found whereas in 12.5% and in 4.2% of the cases, one and no bacteria was cultivated, respectively. At the time of the last wound swab before STSG, the average number of different bacterial species was reduced to an average of 1.7 NDB (1.8 SD) (P < .036) (Figure 4). The bacterial culture of the last wound swab showed two or more different bacteria in 39.1% of the CLU cases. In 34.8% and 26.1% of the included cases, one and no bacteria was found, respectively. The average amount of bacteria (AB) at the time of the first wound swab was 8.6 (4.2 SD). The last wound swab before STSG showed a significant reduction of 41% (P < .018) with an average bacteria amount of 5.2 (5.9 SD) (Figure 4). The average NDB of the last swab did not show any significant difference between leg ulcers with complete wound closure (category A) and leg ulcers in categories B and C after 3 months. The average amount of bacteria of the last swab did also not significantly differ between leg ulcers classified as category A and leg ulcers classified as categories B and C after 3 months. There was no significant difference in the NDB and AB in leg ulcers classified as category A after 24 months compared with leg ulcers classified as Category B or C after 24 months. A shift of bacteria from Gram‐positive to Gram‐negative or vice versa was not observed. A total of four different patients had wounds colonised with multidrug resistant bacteria during NPWT (i‐d).
FIGURE 4.
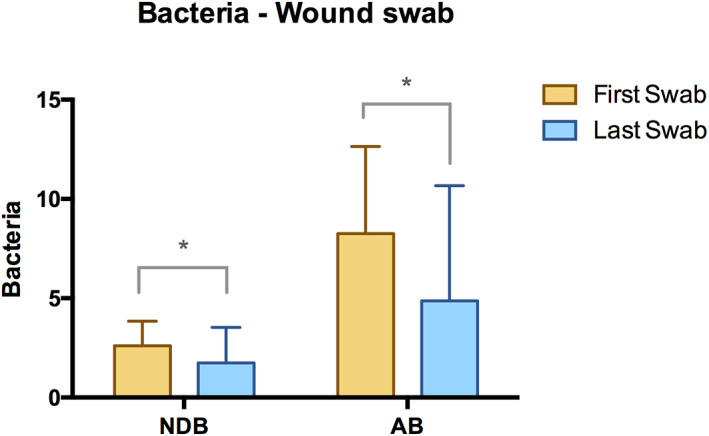
Wound swabs. Average number of different bacteria (NDB) at the time of the first and last wound swabs. Average amount of bacteria (AB) in chronic lower leg ulcers at the time of the first and last wound swab (summation of bacteria load 1 , 2 , 3 , 4 of different bacteria) (* indicates significant difference.)
FIGURE 5.
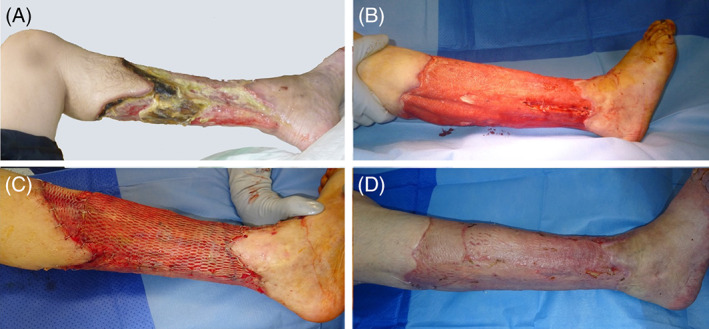
A, Chronic, therapy‐resistant circumferential lower leg ulcer of a 47‐year‐old immunocompromised patient. B, Wound at the lower leg after ultra‐radical debridement and negative pressure wound therapy. C, Split‐thickness skin grafting (STSG) of the lower leg defect. D, Wound healing outcome 3 months after STSG
4. DISCUSSION
CLUs were estimated at 1.51 per 1000 with a lifetime prevalence of 10 per 1000 population. 30 , 31 , 32 Chronic wounds in general are associated with exorbitant costs for health care systems worldwide. 33 , 34 , 35 , 36 Total costs for venous ulcer management including hospitalisation, outpatient and emergency department visits, home health care and prescribed drugs were estimated to be approximately 15 billion USD (United States dollar) in the United States and 1.94 billion GBP (Great Britain pounds) in the United Kingdom annually. 37 , 38 Treatment costs for non‐healing wounds were calculated to be almost 2.5 times higher than those for healed wounds within 1 year. 38 , 39 These numbers demonstrate the global economic burden of CLUs and underlines the importance of adequate wound care and complete wound closure at an early stage. 40
Besides the economic standpoint, CLUs with its adverse sequelae including the amputation of the limb constitute a huge personal burden. The underlying problems causing CLUs are manifold and its treatment usually requires a multidisciplinary approach. The ultimate goal of leg ulcer treatment is to preserve the affected limb. This is known to reduce socioeconomic costs and improve health‐related quality of life. 8 , 41 Despite the common consensus of limb preservation if any possible, about 45 000 limb amputations are inevitably performed as a result of non‐healing chronic wounds in Germany each year. 16 Various reconstructive wound closure methods with different levels of complexity such as skin grafting, local flaps, and free tissue transfer often remain the only way to save the affected limb from an amputation. 42 , 43 Wound size, location, and severity as well as the general health condition determines the choice of the reconstructive surgery procedure for each individual patient. Surgical debridement and NPWT are well‐established tools for wound bed preparation prior to definite wound closure. 22 , 44 NPWT has been successfully applied to various complex non‐healing wounds. NPWT showed to reduce the complexity of the required surgical procedure. 22 , 45 Surgical debridement is a fast and effective way of removing necrotic or infectious tissue in order to restore the wound bed. 44 , 46 The extent of surgical debridement is highly dependent on the severity of the wound condition.
This study represents a clinical series of CLU patients non‐responsive to previous wound treatments, in a critical general health condition and at high risk of limb amputation. Ultra‐radical debridement, NPWT with or without instillation, and STSG were considered as a last resort for limb salvage. Any microvascular free tissue transfer with or without arteriovenous extension was considered unfeasible because of the poor general health condition and vascular status of included patients. 47 CLUs especially those of a certain depth and circular constrictive tissue alterations are at risk of developing a chronic compression syndrome of lower leg muscles. Multifactorial processes, as in calciphylaxis can cause a stiffened and tightened connective tissue of the crural fascia. 48 Crural fasciotomy reduces the pressure within the fascial compartments of the lower leg and has shown to improve wound healing in CLU patients. 49 Therefore, the severity of included wounds 4 made our ultra‐radical surgical debridement strategy inevitable. A total of five patients in our study have a history of STSG. Previous STSG failure in these patients was most likely caused by insufficient surgical debridement. All patients with previous STSG achieved complete wound healing in this study. The ratio of the underlying diseases in this highly selective study population roughly reflects results of previously published epidemiologic studies. 2 , 3 , 6 , 32 Similar to other studies, vascular compromise was the main contributor to CLUs in this case series. All included patients received multiple local and systemic treatment approaches over the course of several months or even years. The fact that seven patients in this study received immunosuppressive medication further demonstrates their critical health condition. The relatively long median wound duration of 36 months corroborates the severity and personal burden of the included cases.
This study illustrates the outcome of our triple treatment approach (ultra‐radical debridement, NPWT with or without instillation, and STSG) in a patient population refractory to multiple treatment approaches with limb amputation as the only other treatment option remaining (Figure 5). Complete wound closure of almost 90%, only two surgical revisions (8%) and no limb amputation highlights its treatment success in such a highly selective study population with no other limb salvage alternative. Similar results were shown in previously published studies. Härmä et al reported on complete wound closure in 78% of the included patients and a wound failure rate of 12% after surgical debridement and split‐thickness skin graft. A total of four patients (10%) had small residual ulcerations (similar to Category B in our study) after a follow‐up of 36 months. Authors claim that an insufficient radical debridement might be responsible for poor wound healing outcomes. Reoperation was required to achieve complete wound healing. 50 A histomorphological examination of recurrent leg ulcers showed increasing signs of dermatoliposclerosis such as extensive fibrosis at the edges of the wound. Authors conclude that an aggressive debridement of the affected tissue benefits CLU healing. 51 Fasciotomy in addition to the removal of infectious, devitalized tissue including the deep fascia, tendons and muscles if necessary, is considered a crucial step in our concept. In contrast, other remedies such as a more superficial “ulcer shaving” approach led to an early relapse of chronic venous leg ulcers and showed a poor healing tendency of skin grafts. 51 A meta‐analysis reported on the outcome of diabetic, non‐infected leg and foot ulcers after a mean period of 2 years. A total of 86% diabetic ulcers achieved complete wound closure. The recurrence rate was 4.2% in this meta‐analysis. 52 Diabetic leg, foot, and ankle wounds had wound healing rates of up to 70% after STSG in previously published case series. 53 , 54 Rose et al, however, reported on a major limb amputation rate of 6% in diabetic wounds. 54 This again underlines the seriousness of the underlying pathology with the ultimate goal of early wound healing and limb salvage. The outcome following previous local non‐surgical and less radical surgical approaches remained suboptimal among these critically ill and partly immunocompromised patients. Recurrent therapy‐resistant complex leg ulcerations of a certain wound size led to the implementation of our triple treatment strategy. Results of our study outline the success of this triple treatment approach as a last resort for limb salvage in such a critical patient population.
Computer‐controlled topical NPWT showed to reduce bacteria levels along with granulation tissue formation, wound size reduction, improvement of local blood circulation, and various improvements at cellular and molecular levels. 22 , 55 , 56 , 57 , 58 This led to its application to various different wound types over the course of the last few decades. 22 , 59 Bacterial colonisation impairs leg ulcer healing ultimately leading to its chronification. 60 Especially the presence of Pseudomonas aeruginosa negatively affects wound healing and split‐thickness skin graft take. 61 The application of various antiseptic fluids to the wound bed for a pre‐defined time period (NPWT(i‐d)) as an adjunct to the basic NPWT device allows an additional removal of debris, exudate and biofilm bacteria ultimately favouring the wound healing process with or without subsequent reconstruction measures. 25 , 62 , 63 Ludolph et al could significantly reduce bacterial colonisation in complex CLUs after NPWT(i‐d). 28 A comparative evaluation of wound colonisation after NPWT versus NPWT(i‐d) was not performed because of the highly selective small number of included cases. In our study, the combination of NPWT irrespective of wound instillation and multiple radical debridements showed a reduced bacterial load in CLUs. The additional administration of systemic antibiotics might also have an impact on the reduced bacterial load and the overall outcome in our study population. The literature, however, still lacks evidence to what extent each of those different interventions individually influence bacterial wound colonisation. This also lies beyond the scope of this study and probably requires a larger sample size in the setting of a prospective study. Our study merely aims to analyse the success of the triple treatment concept in this highly selective immunocompromised patient population. However, wound colonisation in terms of the amount of bacteria and the number of different bacteria at the time of wound closure did not differ between completely healed (Category A) and incompletely healed leg ulcers (Categories B and C). A total of three leg ulcers were colonised with Pseudomonas aeruginosa at the time of the last wound swab prior to STSG. Pseudomonas colonised ulcers could not achieve complete wound closure in 66% of the cases over the course of 2 years after STSG. Additionally, both leg ulcers requiring surgical revision in our study were colonised with Pseudomonas at the time of the last wound swab. This reflects results of previous studies, claiming that the reduction of bacterial load remains of utmost importance for the outcome of CLUs after STSG. In our centre, we routinely apply NPWT for the first 5 days after STSG. This not only protects the wound from external factors but also enables the removal of exudate and stabilises the graft by reducing friction between the graft and the wound bed. A meta‐analysis showed increased graft take and reduced reoperations rates using NPWT after STSG compared with conventional dressings. 64
The authors of this study are aware of its vulnerability to a variety of biases because of its retrospective study design. Robust methods, however, aimed to increase the reliability of those results. Although our department is a high‐volume centre for reconstructive surgery, this study only includes a relatively small number of cases. The low number of included patients introduces the possibility of participation bias. However, this was expected because our criteria aim to include only very specific therapy‐resistant cases in a critically ill and immunocompromised patient population. The authors were aware that wound swabs as used in this study are limited by semi‐quantitative measurements. However, it most likely reflects the entire bacterial flora across the wound bed and is probably the most common clinical way to assess bacterial wound colonisation. 65 Tissue samples by contrast possess the potential of exact volumetric quantitative assessment of the bacterial load. This would require punch biopsies of a pre‐defined tissue size risking to expose or even damage vital structures such as nerves or vessels. Tissue samples rather enable a selective assessment of a small particular wound area. Because of an inhomogeneity of bacteria colonisation across the wound bed, tissue samples usually fail to reflect the bacterial load of the entire wound bed. Despite its limitations, this study shows promising results of the triple treatment approach as a final limb salvage procedure for a highly selective patient population.
5. CONCLUSION
This study demonstrates the efficacy and safety of a surgical approach including ultra‐radical debridement and NPWT with or without instillation followed by split‐thickness skin graft in critically ill leg ulcer patients as an effective limb salvage procedure. This work aims to increase the awareness of its efficacy. Surgeons should consider such a wound closure approach especially if no other options remain and if there is a slight chance of avoiding limb amputation.
CONFLICT OF INTEREST
R. E. H. has received third party funding for scientific research on NPWT from KCI—an Acelity company in the past and has served as a member of a Scientific Advisory Board of KCI‐Acelity in the past. R. E. H. and A. A. served as speakers on scientific symposia of KCI‐Acelity in the past. The authors have no other relevant affiliations or financial involvement with any organisation or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Geierlehner A, Horch RE, Müller‐Seubert W, Arkudas A, Ludolph I. Limb salvage procedure in immunocompromised patients with therapy‐resistant leg ulcers—The value of ultra‐radical debridement and instillation negative‐pressure wound therapy. Int Wound J. 2020;17:1496–1507. 10.1111/iwj.13428
REFERENCES
- 1. Kahle B, Hermanns HJ, Gallenkemper G. Evidence‐based treatment of chronic leg ulcers. Dtsch Arztebl Int. 2011;108(14):231‐237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Briggs M, Closs SJ. The prevalence of leg ulceration: a review of the literature. EWMA J. 2003;3:14‐20. [Google Scholar]
- 3. Rayner RCK, Carville K, Keaton J, Prentice J, Santamaria N. Leg ulcers: atypical presentations and associated comorbidities. Wound Pract Res. 2009;17:168‐184. [Google Scholar]
- 4. Knighton DR, Fylling CP, Fiegel VD, Cerra F. Amputation prevention in an independently reviewed at‐risk diabetic population using a comprehensive wound care protocol. Am J Surg. 1990;160(5):466‐471. (Discussion 71‐72). [DOI] [PubMed] [Google Scholar]
- 5. Körber A, Klode J, Al‐Benna S, et al. Etiology of chronic leg ulcers in 31,619 patients in Germany analyzed by an expert survey. J Ger Soc Dermatol. 2011;9(2):116‐121. [DOI] [PubMed] [Google Scholar]
- 6. Agale S. Chronic leg ulcers: epidemiology, aetiopathogenesis, and management. Ulcers. 2013;2013:9. [Google Scholar]
- 7. Chatterjee SS. Venous ulcers of the lower limb: where do we stand? Indian J Plast Surg. 2012;45(2):266‐274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Luther M. Surgical treatment for chronic critical leg ischaemia: a 5 year follow‐up of socioeconomic outcome. Eur J Vasc Endovasc Surg. 1997;13(5):452‐459. [DOI] [PubMed] [Google Scholar]
- 9. Ghauri AS, Nyamekye IK. Leg ulceration: the importance of treating the underlying pathophysiology. Phlebology. 2010;25(suppl 1):42‐51. [DOI] [PubMed] [Google Scholar]
- 10. Ludolph I, Horch RE, Arkudas A, Schmitz M. Enhancing safety in reconstructive microsurgery using intraoperative indocyanine green angiography. Front Surg. 2019;6:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Naik G, Ivins NM, Harding KG. A prospective pilot study of thigh‐administered intermittent pneumatic compression in the management of hard‐to‐heal lower limb venous and mixed aetiology ulcers. Int Wound J. 2019;16(4):940‐945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Luck J, Rodi T, Geierlehner A, Mosahebi A. Allogeneic skin substitutes versus human placental membrane products in the management of diabetic foot ulcers: a narrative comparative evaluation of the literature. Int J Low Extrem Wounds. 2019;18(1):10‐22. [DOI] [PubMed] [Google Scholar]
- 13. DiDomenico LA, Orgill DP, Galiano RD, et al. Aseptically processed placental membrane improves healing of diabetic foot ulcerations: prospective, randomized clinical trial. Plast Reconstr Surg Glob Open. 2016;4(10):e1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zelen CM, Serena TE, Denoziere G, Fetterolf DE. A prospective randomised comparative parallel study of amniotic membrane wound graft in the management of diabetic foot ulcers. Int Wound J. 2013;10(5):502‐507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Edmonds M, European and Australian Apligraf Diabetic Foot Ulcer Study Group . Apligraf in the treatment of neuropathic diabetic foot ulcers. Int J Low Extrem Wounds. 2009;8(1):11‐18. [DOI] [PubMed] [Google Scholar]
- 16. Ruttermann M, Maier‐Hasselmann A, Nink‐Grebe B, Burckhardt M. Local treatment of chronic wounds: in patients with peripheral vascular disease, chronic venous insufficiency, and diabetes. Dtsch Arztebl Int. 2013;110(3):25‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jeffcoate WJ, Price PE, Phillips CJ, et al. Randomised controlled trial of the use of three dressing preparations in the management of chronic ulceration of the foot in diabetes. Health Technol Assess. 2009;13(54):1‐86. [DOI] [PubMed] [Google Scholar]
- 18. Lalau JD, Bresson R, Charpentier P, et al. Efficacy and tolerance of calcium alginate versus vaseline gauze dressings in the treatment of diabetic foot lesions. Diabetes Metab. 2002;28(3):223‐229. [PubMed] [Google Scholar]
- 19. Blackman E, Moore C, Hyatt J, Railton R, Frye C. Topical wound oxygen therapy in the treatment of severe diabetic foot ulcers: a prospective controlled study. Ostomy Wound Manage. 2010;56(6):24‐31. [PubMed] [Google Scholar]
- 20. Horch RE, Dragu A, Lang W, et al. Coverage of exposed bones and joints in critically ill patients: lower extremity salvage with topical negative pressure therapy. J Cutan Med Surg. 2008;12(5):223‐229. [DOI] [PubMed] [Google Scholar]
- 21. Arkudas A, Horch RE, Regus S, et al. Retrospective cohort study of combined approach for trunk reconstruction using arteriovenous loops and free flaps. J Plast Reconstr Aesthet Surg. 2018;71(3):394‐401. [DOI] [PubMed] [Google Scholar]
- 22. Horch RE, Ludolph I, Muller‐Seubert W, et al. Topical negative‐pressure wound therapy: emerging devices and techniques. Expert Rev Med Devices. 2020;17(2):139‐148. [DOI] [PubMed] [Google Scholar]
- 23. Argenta LC, Morykwas MJ. Vacuum‐assisted closure: a new method for wound control and treatment: clinical experience. Ann Plast Surg. 1997;38(6):563‐576. (Discussion 77). [PubMed] [Google Scholar]
- 24. Wolvos TA. Negative pressure wound therapy with instillation: the current state of the art. Surg Technol Int. 2014;24:53‐62. [PubMed] [Google Scholar]
- 25. Phillips PL, Yang Q, Schultz GS. The effect of negative pressure wound therapy with periodic instillation using antimicrobial solutions on Pseudomonas aeruginosa biofilm on porcine skin explants. Int Wound J. 2013;10(suppl 1):48‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Polykandriotis E, Horch RE, Jost M, Arkudas A, Kees F, Schmitz M. Can systemically administered antibiotics be detected in wound tissues and surfaces under negative pressure wound therapy? Int Wound J. 2019;16(2):503‐510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gabriel A, Shores J, Heinrich C, et al. Negative pressure wound therapy with instillation: a pilot study describing a new method for treating infected wounds. Int Wound J. 2008;5(3):399‐413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ludolph I, Fried FW, Kneppe K, Arkudas A, Schmitz M, Horch RE. Negative pressure wound treatment with computer‐controlled irrigation/instillation decreases bacterial load in contaminated wounds and facilitates wound closure. Int Wound J. 2018;15(6):978‐984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schoni M, Waibel FWA, Bauer D, Gotschi T, Boni T, Berli MC. Long‐term results after internal partial forefoot amputation (resection): a retrospective analysis. Arch Orthop Trauma Surg. 2020. 10.1007/s00402-020-03441-3. [DOI] [PubMed] [Google Scholar]
- 30. Martinengo L, Olsson M, Bajpai R, et al. Prevalence of chronic wounds in the general population: systematic review and meta‐analysis of observational studies. Ann Epidemiol. 2019;29:8‐15. [DOI] [PubMed] [Google Scholar]
- 31. Nelzén O. Prevalence of venous leg ulcer: the importance of the data collection method. Phlebolymphology. 2008;15:143‐150. [Google Scholar]
- 32. Baker SR, Stacey MC. Epidemiology of chronic leg ulcers in Australia. Aust N Z J Surg. 1994;64(4):258‐261. [DOI] [PubMed] [Google Scholar]
- 33. Phillips CJ, Humphreys I, Fletcher J, Harding K, Chamberlain G, Macey S. Estimating the costs associated with the management of patients with chronic wounds using linked routine data. Int Wound J. 2016;13(6):1193‐1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fife CE, Carter MJ. Wound care outcomes and associated cost among patients treated in US outpatient wound centers: data from the US wound registry. Wounds. 2012;24(1):10‐17. [PubMed] [Google Scholar]
- 35. Lo ZJ, Lim X, Eng D, et al. Clinical and economic burden of wound care in the tropics: a 5‐year institutional population health review. Int Wound J. 2020;17:790‐803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Queen D, Harding K. World Union of Wound Healing Societies Meeting, 2020. Int Wound J. 2020;17(2):241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rice JB, Desai U, Cummings AK, Birnbaum HG, Skornicki M, Parsons N. Burden of venous leg ulcers in the United States. J Med Econ. 2014;17(5):347‐356. [DOI] [PubMed] [Google Scholar]
- 38. Guest JF, Ayoub N, McIlwraith T, et al. Health economic burden that different wound types impose on the UK's National Health Service. Int Wound J. 2017;14(2):322‐330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pacella RE, Tulleners R, McCosker L, et al. Reimbursement for the cost of compression therapy for the management of venous leg ulcers in Australia. Int Wound J. 2019;16(4):1069‐1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Harding K, Queen D. Innovation and inertia in wounds. Int Wound J. 2019;16(5):1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kneser U, Arkudas A, Beier JP, et al. Extended skin and soft tissue defects after vascular wounds: plastic surgical concepts. Zentralbl Chir. 2013;138(5):536‐542. [DOI] [PubMed] [Google Scholar]
- 42. Schmauss D, Beier JP, Eisenhardt SU, et al. The "safe" flap—preoperative perforator‐mapping and intraoperative perfusion assessment to reduce flap‐associated morbidity—consensus statement of the German Speaking Working Group for Microsurgery of the peripheral nerves and vessels. Handchir Mikrochir Plast Chir. 2019;51(6):410‐417. [DOI] [PubMed] [Google Scholar]
- 43. Rother U, Muller‐Mohnssen H, Lang W, et al. Wound closure by means of free flap and arteriovenous loop: development of flap autonomy in the long‐term follow‐up. Int Wound J. 2020;17(1):107‐116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Schultz GS, Barillo DJ, Mozingo DW, Chin GA, Wound Bed Advisory Board Members . Wound bed preparation and a brief history of TIME. Int Wound J. 2004;1(1):19‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lang W, Horch RE. Distal extremity reconstruction for limb salvage in diabetic foot ulcers with pedal bypass, flap plasty and vacuum therapy. Zentralbl Chir. 2006;131(suppl 1):S146‐S150. [DOI] [PubMed] [Google Scholar]
- 46. Sibbald RG, Williamson D, Orsted HL, et al. Preparing the wound bed—debridement, bacterial balance, and moisture balance. Ostomy Wound Manage. 2000;46(11):4‐8, 14‐22, and 30‐35. [PubMed] [Google Scholar]
- 47. Illig KA, Moran S, Serletti J, et al. Combined free tissue transfer and infrainguinal bypass graft: an alternative to major amputation in selected patients. J Vasc Surg. 2001;33(1):17‐23. [DOI] [PubMed] [Google Scholar]
- 48. Wollina U, Helm C, Hansel G, et al. Deep ulcer shaving combined with split‐skin transplantation in distal calciphylaxis. Int J Low Extrem Wounds. 2008;7(2):102‐107. [DOI] [PubMed] [Google Scholar]
- 49. Steed DL, Donohoe D, Webster MW, Lindsley L. Effect of extensive debridement and treatment on the healing of diabetic foot ulcers. Diabetic ulcer study group. J Am Coll Surg. 1996;183(1):61‐64. [PubMed] [Google Scholar]
- 50. Härmä M, Asko‐Seljavaara S, Lauharanta J. Surgical treatment of chronic leg ulcers. Acta Derm Venereol. 1994;74(6):484‐485. [DOI] [PubMed] [Google Scholar]
- 51. Reich‐Schupke S, Schmeil I, Kreuter A, et al. Insufficient and incomplete shaving in chronic venous leg ulcers leads to a poor prognosis of the skin graft: a histomorphological analysis. Dermatology. 2011;223(1):87‐92. [DOI] [PubMed] [Google Scholar]
- 52. Yammine K, Assi C. A meta‐analysis of the outcomes of split‐thickness skin graft on diabetic leg and foot ulcers. Int J Low Extrem Wounds. 2019;18(1):23‐30. [DOI] [PubMed] [Google Scholar]
- 53. Ramanujam CL, Stapleton JJ, Kilpadi KL, Rodriguez RH, Jeffries LC, Zgonis T. Split‐thickness skin grafts for closure of diabetic foot and ankle wounds: a retrospective review of 83 patients. Foot Ankle Spec. 2010;3(5):231‐240. [DOI] [PubMed] [Google Scholar]
- 54. Rose JF, Giovinco N, Mills JL, Najafi B, Pappalardo J, Armstrong DG. Split‐thickness skin grafting the high‐risk diabetic foot. J Vasc Surg. 2014;59(6):1657‐1663. [DOI] [PubMed] [Google Scholar]
- 55. Malmsjo M, Ingemansson R, Martin R, Huddleston E. Wound edge microvascular blood flow: effects of negative pressure wound therapy using gauze or polyurethane foam. Ann Plast Surg. 2009;63(6):676‐681. [DOI] [PubMed] [Google Scholar]
- 56. Lu F, Ogawa R, Nguyen DT, et al. Microdeformation of three‐dimensional cultured fibroblasts induces gene expression and morphological changes. Ann Plast Surg. 2011;66(3):296‐300. [DOI] [PubMed] [Google Scholar]
- 57. Egemen O, Ozkaya O, Ozturk MB, Aksan T, Orman C, Akan M. Effective use of negative pressure wound therapy provides quick wound‐bed preparation and complete graft take in the management of chronic venous ulcers. Int Wound J. 2012;9(2):199‐205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Muenchow S, Horch RE, Dragu A. Effects of topical negative pressure therapy on perfusion and microcirculation of human skin. Clin Hemorheol Microcirc. 2019;72(4):365‐374. [DOI] [PubMed] [Google Scholar]
- 59. Renno I, Boos AM, Horch RE, Ludolph I. Changes of perfusion patterns of surgical wounds under application of closed incision negative pressure wound therapy in postbariatric patients1. Clin Hemorheol Microcirc. 2019;72(2):139‐150. [DOI] [PubMed] [Google Scholar]
- 60. Halbert AR, Stacey MC, Rohr JB, Jopp‐McKay A. The effect of bacterial colonization on venous ulcer healing. Australas J Dermatol. 1992;33(2):75‐80. [DOI] [PubMed] [Google Scholar]
- 61. Hogsberg T, Bjarnsholt T, Thomsen JS, Kirketerp‐Moller K. Success rate of split‐thickness skin grafting of chronic venous leg ulcers depends on the presence of Pseudomonas aeruginosa: a retrospective study. PLoS One. 2011;6(5):e20492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Matiasek J, Djedovic G, Mattesich M, et al. The combined use of NPWT and instillation using an octenidine based wound rinsing solution: a case study. J Wound Care. 2014;23(11):590, 2‐590, 6. [DOI] [PubMed] [Google Scholar]
- 63. Matiasek J, Djedovic G, Unger L, et al. Outcomes for split‐thickness skin transplantation in high‐risk patients using octenidine. J Wound Care. 2015;24(suppl 6):S8 and S10–S12. [DOI] [PubMed] [Google Scholar]
- 64. Yin Y, Zhang R, Li S, Guo J, Hou Z, Zhang Y. Negative‐pressure therapy versus conventional therapy on split‐thickness skin graft: a systematic review and meta‐analysis. Int J Surg. 2018;50:43‐48. [DOI] [PubMed] [Google Scholar]
- 65. Gardner SE, Frantz RA, Saltzman CL, Hillis SL, Park H, Scherubel M. Diagnostic validity of three swab techniques for identifying chronic wound infection. Wound Repair Regen. 2006;14(5):548‐557. [DOI] [PubMed] [Google Scholar]


