Abstract
Exact data regarding the clinical role of maggot debridement therapy (MDT) for wound care in a specific country are not available. Thus, we analysed the use of MDT in hospitalised patients in Germany. Detailed lists of all hospitalised cases treated with MDT in Germany for the years 2011 to 2016 were provided by the Federal Statistical Office as well as the lists of the 15 most frequent principal and additional diagnoses, respectively, and the 10 most frequent procedures documented with MDT in 2016. Within the 6‐year time period of the study, the number of cases treated with MDT increased by 11% from 4513 in 2011 to 5.017 in 2016. Lower leg and foot were the most frequent anatomic sides of treatment counting up to 83.9% of all cases. In addition, MDT procedures for temporary soft tissue coverage including negative pressure wound therapy were often performed: for treatment of large areas in 36.7% and small areas in 6.2%. 41.3% of all cases treated with MDT had infection with Escherichia coli and 35.9% of all cases with Bacillus fragilis. Our analysis shows a limited use of MDT with a small increase only in the last 6 years in German hospitals. MDT is predominately used to treat foot or leg ulcers.
Keywords: debridement, foot ulcer, infection, maggots
1. INTRODUCTION
An important issue in wound management is the process called debridement,1 which is defined as the removal of foreign debris and devitalized or contaminated tissues from a wound bed so that the surrounding healthy tissues are exposed. Maggot debridement therapy (MDT) was first introduced in the United States in 1931. Probably, it is a much older and its roots reach back to antiquity. It was routinely used in the United States until the mid‐1940s in over 300 hospitals.2 With the advent of antibacterials, maggot therapy became rare until the early 1990s, when it was reintroduced first in the United States, and later in Israel, the United Kingdom, Germany, Sweden, Switzerland, Ukraine, and Thailand. Sterile maggots of the green bottle fly, Lucilia sericata, are used for MDT.
One of the major advantages of MDT is that the maggots separate the necrotic tissue from the living tissue, making a surgical debridement easier.3 In 80% to 95% of the cases, a complete or significant debridement of the wound is achieved. A systematic meta‐analysis published in 2014 reported that MDT had a significantly increased positive effect on wound healing compared with conventional therapies including mechanical debridement, surgical debridement, or hydrogel, with a pooled relative risk (RR) of 1.80 (95% CI 1.24–2.60).4 The subgroup analysis revealed that the combined RRs were 1.79 (95% CI 0.95–3.38) for patients with diabetic foot ulcers (DFU) and 1.70 (95% CI 1.28–2.27) for patients with other types of ulcers. The time to healing of the ulcers was significantly shorter among patients treated with MDT, with a pooled standardised mean difference (SMD) of −0.95 (95% CI –1.24, −0.65). For patients with DFU, the SMD was −0.79 (95% CI –1.18, −0.41), and for patients with other types of ulcers, the SMD was −1.16 (95% CI –1.63, −0.69). Contraindications mentioned by the companies providing maggots are known hypersensitivity, wounds close to large vessels, as the danger of life‐threatening vascular injury exists, wounds with insufficient blood flow or acute, and rapidly progressive or life‐threatening infections. It should not be used in sterile body cavities and if surgical debridement is required.
Despite many studies addressing specific questions, exact data regarding the general role of MDT for wound care in a specific country are not available. Thus, we analysed the use of MDT in hospitalised patients in Germany.
2. PATIENTS AND METHODS
Roughly 90% of the population in Germany are statutorily insured and entitled to receive health promotion and disease prevention benefits to maintain and restore their health or to improve their state of health. The statutory health insurance is funded by the statutory health insurance funds, which, being public law corporations, are financially and organizationally independent. They carry out the tasks assigned to them by the State independently from the interprofessional team model.
The national statistics published by the Federal Statistical Office includes data from all hospitals in Germany that use the Diagnosis Related Group (DRG) system, representing more than 99% of hospitals. These hospitals are legally obliged to deliver extensive data on hospital treatment, including demographic data, diagnoses, comorbidities, complications, and procedures to the “Institute for the Hospital Remuneration System” (InEK), which transfers the data for a yearly summary to the Federal Statistical Office. Since 2005 all diagnoses were coded with the International Statistical Classification of Diseases and Related Health Problems 10th Revision (ICD‐10) with the German Modification (ICD‐10‐GM) in the corresponding annual version.
MDT is coded as 8‐192.3* since 2011 and additional information is given regarding the anatomical site of the body that is affected (Table 1). MDT is reimbursed only in hospitalised patients in Germany currently.
Table 1.
Absolute numbers of documented OPS codes covering MDT in hospitalised cases in Germany in the years 2011 up to 2016
| Years | |||||||
|---|---|---|---|---|---|---|---|
| OPS‐code | Anatomic side of treatment | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 |
| 8‐192.30 | Lip | 10 | 14 | 8 | 9 | 9 | 9 |
| 8‐192.34 | Other parts of the head | 21 | 21 | 21 | 16 | 21 | 20 |
| 8‐192.35 | Neck | 11 | 16 | 13 | 11 | 7 | 14 |
| 8‐192.36 | Shoulder and axilla | 5 | 8 | 8 | 6 | 14 | 7 |
| 8‐192.37 | Upper and elbow | 28 | 27 | 12 | 14 | 13 | 30 |
| 8‐192.38 | Forearm | 10 | 20 | 3 | 14 | 13 | 23 |
| 8‐192.39 | Hand | 21 | 39 | 35 | 34 | 29 | 34 |
| 8‐192.3a | Chest wall and back | 58 | 49 | 49 | 40 | 49 | 61 |
| 8‐192.3b | Abdominal region | 80 | 102 | 110 | 93 | 77 | 98 |
| 8‐192.3c | Inguinal and genital region | 70 | 84 | 85 | 77 | 74 | 126 |
| 8‐192.3d | Buttocks | 204 | 210 | 169 | 165 | 193 | 196 |
| 8‐192.3e | Thighs and knees | 166 | 180 | 169 | 140 | 128 | 171 |
| 8‐192.3f | Lower leg | 1419 | 1450 | 1516 | 1547 | 1378 | 1393 |
| 8‐192.3g | Foot | 2359 | 2594 | 2913 | 2799 | 2701 | 2814 |
| 8‐192.3x | Others | 51 | 28 | 45 | 34 | 31 | 21 |
| Total | 4513 | 4842 | 5156 | 4999 | 4737 | 5017 | |
3. STATISTICAL ANALYSES
Detailed lists of all cases treated with MDT in Germany for the years 2011 to 2016 were provided from the Federal Statistical Office. These data count the cases with MDT and not the number of single procedures.
In addition, the Federal Statistical Office provided lists of the 15 most frequent principal and additional diagnoses associated with the NPWT for the year 2016 as well as the 10 most frequent procedures documented in these cases. According to its definition, each principal diagnosis represents a single case that has been treated with MDT with one application at least. A single inpatient case is counted in the statistics on principal and additional diagnoses in parallel and could have multiple additional diagnoses or procedures.
Calculations were done using Microsoft® Excel 2003 and Microsoft® Access 2003.
4. RESULTS
Within the 6‐year time period of the study, the number of cases treated with MDT increased by 11% from 4513 in 2011 to 5017 in 2016 (Table 1). In general, males were treated more frequently than females (Figure 1). Lower leg and foot were the most frequent anatomical sides of treatment counting up to 83.9% of all cases, followed by inguinal region, buttocks, and thighs and knees with 9.8%. Treatment of the upper limb or even the head was performed rarely.
Figure 1.
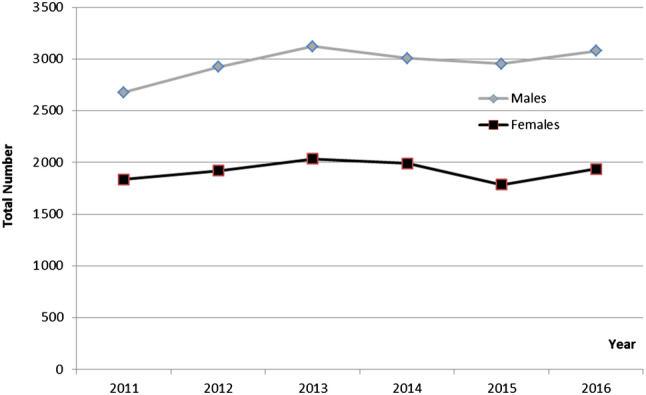
Absolute numbers of documented OPS‐codes covering MDT in hospitalised male and female cases in Germany in the years 2011 up to 2016
To explore the role of MDT for different clinical problems, we analysed the most frequent principal diagnoses in cases treated with MDT (Table 2). In 2016, the six most frequent principal diagnoses represent 67% of all cases. The principal diagnosis of peripheral arterial disease (PAD) and DFU counted up to one quarter of all cases each. Other diagnoses were much rarer.
Table 2.
Most frequent principal diagnosis in hospitalised cases with documented MDT
| Range | Code | Principal diagnosis | Absolute number | Rate % |
|---|---|---|---|---|
| 1 |
I70.24 I70.25 |
Atherosclerosis of arteries of extremities with ulcer or gangrene | 1345 | 26.8 |
| 2 |
E11.50 E11.74 E11.75 E10.75 |
Diabetes mellitus, type 1 or type 2 with multiple complication: with diabetic foot syndrome | 1342 | 26.7 |
| 3 | I83.2 I83.0 | Varicose veins of lower extremities with ulcer | 313 | 6.2 |
| 4 | L97 | Ulcer of lower limb, not elsewhere classified | 183 | 3.6 |
| 5 |
T81.4 T87.4 |
Infection following a procedure, not elsewhere classified and infection of amputation stump | 127 | 2.5 |
| 6 | L89.34 | Stage IV decubitus ulcer: sacral | 50 | 1.0 |
| Total | 3360 | 67.0 |
To explore comorbidities that may influence the clinical course of the cases and the need of MDT, we analysed the most frequent additional diagnosis in cases treated with MDT. It shows that infections had an important impact. 41.3% of all cases treated with MDT had infection with Escherichia coli, 35.9% of all cases with Bacillus fragilis, 22.4 with Pseudomonas aeruginosa, and 22.0% with Streptococcus group D and Enterococcus (Table 3).
Table 3.
Most frequent secondary diagnosis indicating contamination or infection in hospitalised cases with documented MDT
| Range | Code | Secondary diagnose | Total number | Rate (%) |
|---|---|---|---|---|
| 1 | B96.2 | Escherichia coli as the cause of diseases classified to other chapters | 2071 | 41.3 |
| 2 | B95.6 | Bacillus fragilis as the cause of diseases classified to other chapters | 1803 | 35.9 |
| 3 | B96.5 | Pseudomonas aeruginosa as the cause of diseases classified to other chapters | 1126 | 22.4 |
| 4 | B95.2 | Streptococcus group D and Enterococcus as the cause of diseases classified to other chapters | 1106 | 22.0 |
To explore the treatment strategies that were performed in addition to MDT, we analysed the most frequent procedures in cases treated with MDT (Table 4). It shows that MDT was often performed in addition to NPWT. The procedure of temporary soft tissue coverage including NPWT for treatment of large areas was documented in 36.7% and of small areas in 6.2% of all cases with MDT. Surgical debridement was performed in 33.1% and 31.3% of wounds associated with minor amputation and 6.7% with revision of amputation at the foot.
Table 4.
Most frequent procedures in hospitalised cases with documented MDT
| Range | Code | Procedures without double coding | Total number | Rate (%) |
|---|---|---|---|---|
| 1 |
5‐916.a0 5‐916.a1 |
Temporary soft tissue coverage—large area: vacuum therapy—system: on the skin and subcutaneous tissue or deep, subfascial or on the bones and joints of the extremities | 1839 | 36.7 |
| 2 |
5‐896.1g 5‐896.1f |
Surgical debridement with removal of diseased tissue on skin and subcutaneous tissue: large area: foot | 1658 | 33.1 |
| 3 |
5‐865.6 5‐865.7 5‐865.8 |
Amputation and disarticulation foot: • transmetatarsal • toe amputation • toe beam resection |
1568 | 31.3 |
| 4 | 5‐866.5 | Revision of an amputation area: foot region | 338 | 6.7 |
| 5 | 5‐869.1 | Other operations on bones and joints of the extremities: soft tissue debridement | 318 | 6.4 |
| 6 | 5‐896.0g | Temporary soft tissue coverage—small area: vacuum therapy system: on the skin and subcutaneous tissue: foot | 313 | 6.2 |
5. DISCUSSION
Our analysis gives an insight of the general use of MDT in hospitalised patients in Germany. In detail, it shows the following:
In hospitalised cases, MDT is used in a limited number of cases.
MDT is primarily used at the lower limb and predominately in cases with ulcers associated with PAD or diabetes mellitus.
Infection is a frequent clinical finding in patients treated with MDT.
Over the last decade, MDT has been recognised by many clinicians as a potential adjunct to conventional therapy, and many patients with non‐healing, chronic ulcers have been treated.5 Numerous case reports and case series have described the successful use of MDT in a variety of ulcers. However, comparative clinical trials and in particular randomised controlled trials investigating the efficacy of MDT are sparse. When evaluating maggots as debriding agents, some studies report MDT as being significantly more effective than hydrogel or a mixture of conventional therapy modalities, including hydrocolloid, hydrogel, and saline moistened gauze.1, 5, 6, 7 A multicentre, randomised, controlled, open, observer blind, parallel group study including 88 patients provided good evidence to show that MDT, in the form of a BioFOAM dressing, is considerably more quickly than a hydrogel in debrided venous and mixed leg ulcer, although the possibility of resloughing should be closely monitored.8 Although the clinical benefit of MDT in most of the clinical settings has not been proved by randomised controlled studies, so far the use in specific wounds at the foot may be based on good clinical experiences. The quality of the studies makes it difficult to conclude that MDT shortens healing time. On the other hand, some studies reported specific effects of maggots on wound healing in addition to the debridement. MDT shall induce neogranulation and angiogenesis in diabetic foot wounds after MDT and increase expression of vascular endothelial growth factor receptor 2 in a dose‐dependent manner.9
Although MDT is known for almost 100 years, it is still a method of low acceptance and dissemination in Germany. In contrast to other techniques in wound care as negative pressure therapy that is used in 129 269 hospitalised cases in 2014,10 MDT is used in only 5017 in hospitalised cases in 2016. Data from other countries are not published. In a Canadian foot and leg ulcer clinic, 68 patients were treated between January 2001 and June 200611 and in Hadassah Hospital in Jerusalem, Israel, 435 patients in 16 departments between 1996 and 2009.12 These figures of use in specialised clinics are low and not representative for the whole country. Possible reasons for low acceptance of MDT can be anxiety and pain. Since a full debridement requires an average of two to three maggot cycles, which last 3‐5 days, and since a large percentage of patients treated with MDT complain of pain that may last throughout the therapy period, it is deemed worthwhile and even essential to titrate analgesics as needed and be prepared to treat patients even with potent analgesics, such as opioids.13
MDT has been shown to decrease bacterial burden of a wound. A randomised controlled trial evaluated the efficacy of MDT compared to surgical debridement and topical application of silver sulfadiazine in 19 patients for 4 weeks.14 A significant difference was observed in the reduction of bacterial burden in favour of the MDT group. Our data also show a high rate of infections documented in the cases treated with MDT. Local wound infection might be a specific indication for the use of MDT, but we do not have separate data. Our data show a high number of cases with bacterial load. A randomised controlled trial showed that MDT can significantly reduce Staphylococcus aureus after 48 hours and P. aeruginosa after 96 hours on.15
6. LIMITATIONS
Our analysis is just descriptive and some limitation of the given data should be considered. Although routine data in the electronic patient record is frequently used for secondary purposes, there is currently no systematic analysis of coding quality in Germany.16, 17 Whether coding matches reality as a prerequisite for further use of the data in medicine and health policy requires quality assurance verification. Furthermore, our analyses are limited because they are not based on personal records, but on DRG‐data and on cases and not on individual patients. Finally, we do not have any information about the benefit of MDT for the affected patients. We cannot say whether it was used in the right way and in correct indication and whether wound healing was really achieved.
7. CONCLUSION
Our analysis shows a limited use of MDT with a small increase only in the last 6 years in German hospitals. MDT is predominately used for treatment of foot or leg ulcer associated with PAD, diabetes mellitus, and chronic venous insufficiency. It is frequently used in addition to NPWT and surgical debridement strategies. Even though evidence for each indication is lacking, there seems to be a clinical need for MDT in a small number of patients.
von Beckerath O, Kanya S, Gäbel G, Kröger K, Juntermanns B. Use of maggot debridement therapy in hospitalised patients in Germany. Int Wound J. 2020;17:10–15. 10.1111/iwj.13204
REFERENCES
- 1. Dumville JC, Worthy G, Bland JM, et al. Larvaltherapy for leg ulcers (VenUS II): randomised controlled trial. BMJ. 2009;338:b773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mumcuoglu KY. Clinical applications for maggots in wound care. Am J Clin Dermatol. 2001;2:219‐227. [DOI] [PubMed] [Google Scholar]
- 3. Gray M. Is larval debridement effective for removal of necrotic tissue from chronic wounds? J Wound Ostomy Continence Nurs. 2008;35:378‐384. [DOI] [PubMed] [Google Scholar]
- 4. Sun X, Jiang K, Chen J, et al. A systematic review of maggot debridement therapy for chronically infected wounds and ulcers. Int J Infect Dis. 2014;25:32‐37. [DOI] [PubMed] [Google Scholar]
- 5. Zarchi K, Jemec GB. The efficacy of maggot debridement therapy—a review of comparative clinical trials. Int Wound J. 2012;9:469‐477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Opletalová K, Blaizot X, Mourgeon B, et al. Maggot therapy for wound debridement: a randomized multicenter. Arch Dermatol. 2012;148:432‐438. [DOI] [PubMed] [Google Scholar]
- 7. Edwards J, Stapley S. Debridement of diabetic foot ulcers. Cochrane Database Syst Rev. 2010;1:CD003556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mudge E, Price P, Walkley N, Harding KG. A randomized controlled trial of larval therapy for the debridement of leg ulcers: results of a multicenter, randomized, controlled, open, observer blind, parallel group study. Wound Repair Regen. 2014;22:43‐51. [DOI] [PubMed] [Google Scholar]
- 9. Sun X, Chen J, Zhang J, Wang W, Sun J, Wang A. Maggot debridement therapy promotes diabetic foot wound healing by up‐regulating endothelial cell activity. J Diabetes Complications. 2016. Mar;30:318‐322. [DOI] [PubMed] [Google Scholar]
- 10. Beckerath v O, Zapenko A, Dissemond J, Kröger K, Initiative Chronische Wunden (ICW) e.V . Ten‐year analyses of the German DRG data about negative pressure wound therapy. Int Wound J. 2017;14:501‐507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Campbell N, Campbell D. A retrospective, quality improvement review of maggot debridement therapy outcomes in a foot and leg ulcer clinic. Ostomy Wound Manage. 2014;60:16‐25. [PubMed] [Google Scholar]
- 12. Gilead L, Mumcuoglu KY, Ingber A. The use of maggot debridement therapy in the treatment of chronic wounds in hospitalised and ambulatory patients. J Wound Care. 2012;21:78, 80, 82‐78, 80, 85. [DOI] [PubMed] [Google Scholar]
- 13. Mumcuoglu KY, Davidson E, Avidan A, Gilead L. Pain related to maggot debridement therapy. J Wound Care. 2012;21:400, 402:404‐405. [DOI] [PubMed] [Google Scholar]
- 14. Contreras‐Ruiz J, Fuentes‐Suárez A, Arroyo‐Escalante S, et al. Comparative study of the efficacy of larva therapy for debridement and control of bacterial burden compared to surgical debridement and topical application of an antimicrobial. Gac Med Mex. 2016;152(2):78‐87. [PubMed] [Google Scholar]
- 15. Malekian A, Esmaeeli Djavid G, Akbarzadeh K, et al. Efficacy of maggot therapy on Staphylococcus aureus and Pseudomonas aeruginosa in diabetic foot ulcers: a randomized controlled trial. J Wound Ostomy Continence Nurs. 2019;46:25‐29. [DOI] [PubMed] [Google Scholar]
- 16. Statistisches Bundesamt , Fachserie 12, Reihe June 4, 2005. Fallpauschalenbezogene Krankenhausstatistik (DRG‐Statistik).
- 17. Stausberg J. Quality of coding in acute inpatient care. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2007;50:1039‐1046. [DOI] [PubMed] [Google Scholar]


