Abstract
Numerous studies have demonstrated the various medicinal properties of Panax ginseng, including angiogenic, immuno‐stimulating, antimicrobial, and anti‐inflammatory activities, which can be helpful in chronic wound healing. However, a direct role for P. ginseng in chronic wound healing has not been demonstrated. The present study was designed to evaluate the effects of P. ginseng extract on diabetic fibroblasts in vitro. Human diabetic fibroblasts were cultured in the presence of Ginsenoside Rb1 (G‐Rb1), the active component in P. ginseng (10 ng/mL), and untreated diabetic fibroblasts were used as controls. Cell proliferation, collagen synthesis, the production of various growth factors (basic fibroblast growth factor [bFGF]; vascular endothelial growth factor [VEGF]; and transforming growth factor‐β1 [TGF‐β1]), and the synthesis of matrix metalloproteinase 1 (MMP‐1) and tissue inhibitor of metalloproteinases 1 (TIMP‐1) were compared using enzyme‐linked immunosorbent assay and immunofluorescence staining. Compared with the control group, G‐Rb1‐treated fibroblasts showed significantly (P < 0.05) higher levels of cell proliferation, collagen synthesis, VEGF, TGF‐β1, and TIMP‐1. However, no significant differences in bFGF and MMP‐1 levels were observed between the two groups. These results suggest that P. ginseng treatment may stimulate the wound‐healing activity of diabetic fibroblasts in vitro.
Keywords: diabetic fibroblast, Panax ginseng, wound healing
1. INTRODUCTION
Ginseng has been used for a wide range of preventative and therapeutic purposes in traditional herbal medicine for over 4000 years. Panax ginseng is the most commonly used medicinal ginseng because its efficacy is higher than that of other ginsengs.1 The pharmacological effects of P. ginseng were discovered through extensive research. P. ginseng dilates blood vessels; controls cholesterol levels; and has anti‐cancer, anti‐aging, and anti‐allergy properties.2, 3, 4, 5, 6 P. ginseng also benefits the cardiovascular and dermatological systems.7, 8, 9 In particular, current studies demonstrate that P. ginseng has anti‐inflammatory, antioxidant, antimicrobial, and immunomodulating effects.10, 11, 12, 13, 14, 15, 16, 17, 18 In addition, P. ginseng improves protein synthesis, neovascularisation, and angiogenesis.16, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29
Considered together, these features suggest that P. ginseng may play an important role in the healing process of chronic wounds with attenuated fibroblast activity and a prolonged inflammatory phase. Despite the theoretical potential of P. ginseng in treating chronic wounds, there have been few studies addressing the effect of P. ginseng on chronic wound healing.
In a previous preliminary study, we demonstrated that P. ginseng stimulated the proliferation and collagen synthesis of human healthy fibroblasts, and the most effective concentration was found to be 10 ng/mL.30 However, these previous results were not sufficient to confirm the effect of P. ginseng on chronic wound healing.
Thus, the purpose of this study was to evaluate the effects of P. ginseng extract on the activity of diabetic fibroblasts in vitro. In particular, this study was focused on cell proliferation, collagen synthesis, growth factor (basic fibroblast growth factor [bFGF]; vascular endothelial growth factor [VEGF]; transforming growth factor‐β1 [TGF‐β1]) production, matrix metalloproteinase 1 (MMP‐1) synthesis, and tissue inhibitor of metalloproteinase 1 (TIMP‐1) synthesis, which are important contributing factors for wound healing.
2. MATERIALS AND METHODS
2.1. Human diabetic fibroblast culture
Diabetic fibroblasts were obtained from cryopreserved cells derived from the dermis of patients with diabetic foot ulcers who had undergone debridement (n = 10). The cell donors provided informed consent for the use of their cells in this research.
The cells were cultured in Dulbecco's modified Eagle medium/Ham's F‐12 nutrient (DMEM/F‐12; Gibco; Grand Island, Ney York) containing 10% foetal bovine serum (FBS; Gibco) and 25 μg/mL gentamycin at 37°C in a 5% CO2/95% air atmosphere at 100% humidity. The fibroblasts were dissociated using trypsin, diluted 2.7‐fold with Mg2+‐ and Ca2+‐free Dulbecco's phosphate‐buffered saline (DPBS; Gibco), and collected by centrifugation at 450g for 17 minutes. The cells were washed twice in 10 mL DMEM/F‐12 and re‐suspended in 3 mL DMEM/F‐12. Cell density was determined using a haemocytometer, and cell viability was assessed using the trypan blue dye exclusion assay. Third‐passage cells were used in the experiments.
2.1.1. P. ginseng treatment
Ginsenoside Rb1 (G‐Rb1), which is the active component in P. ginseng, was used for this study. The G‐Rb1 reagent was purchased from the Sigma‐Aldrich Chemical Company Inc. (St. Louis, Missouri). G‐Rb1 was diluted with DMEM/F‐12 to a concentration of 10 ng/mL for the treatment group.
2.2. Enzyme‐linked immunosorbent assay
Cultured fibroblasts were seeded in a 24‐well culture plate at 2 × 104 cells in 500 μL of DMEM/F‐12 per well containing 5% FBS. No‐G‐Rb1‐treated diabetic fibroblasts were used as the control. The cells were incubated for 72 hours at 37°C in 5% CO2 with 100% humidity.
2.2.1. Cell proliferation assay
Cell proliferation was determined using a colorimetric assay with 2‐(4‐iodophenyl)‐3‐(4‐nitrophenyl)‐5‐(2,4‐disulfophenyl)‐2H‐tetrazolium, monosodium salt (WST‐1; Roche Molecular Biochemicals, Meylan, France). Briefly, 20 μL of the WST‐1 reagent was added to a 200‐μL cell monolayer in each of the 24 wells, and the plate was incubated for 3 hours at 37°C. Absorbance was measured at a test wavelength of 450 nm using an enzyme‐linked immunosorbent assay (ELISA), with a reference wavelength of 600 nm to evaluate cell proliferation. Each sample was analysed in triplicate and averaged.
2.2.2. Collagen synthesis assay
A collagen type I carboxy‐terminal propeptide enzyme immunoassay (CICP) was performed using a Metra CICP kit (Quidel, San Diego, California) according to the manufacturer's instructions. Briefly, 100 μL of diluted culture supernatant was added to each well of a monoclonal anti‐CICP antibody‐coated plate, and the plate was incubated at room temperature for 2 hours. A 100‐μL aliquot of rabbit anti‐CICP antisera was added for 50 minutes, followed by 100 μL of goat anti‐rabbit alkaline phosphatase conjugate for 50 minutes. Subsequently, the p‐nitrophenyl phosphate substrate was added for 30 minutes to quantify the CICP. The reaction was stopped, and collagen synthesis was measured at 405 nm.
2.2.3. Growth factor level assay
The levels of VEGF, bFGF, and TGF‐β1 were measured in culture media using an ELISA kit (R&D Systems, Minneapolis, Minnesota) according to the manufacturer's instructions. Briefly, the samples and standards were diluted appropriately in the respective assay diluent and then incubated at room temperature in wells pre‐coated with the specific capturing antibodies. After washing, the respective detection antibody conjugate was added and then incubated at room temperature before washing again. The addition of substrate solution then resulted in colour development. After the addition of a stop solution, the colour intensity was measured in an ELISA reader at the appropriate wavelength. The measured TGF‐β1 in this study included both active and latent forms.
2.2.4. MMP‐1 synthesis assay
The levels of MMP‐1 were measured in culture media using a Human Total MMP‐1 ELISA kit (R&D Systems, Minneapolis, Minnesota) according to the manufacturer's instructions. Briefly, 100 μL of diluted culture supernatant was added to each well of a monoclonal Total MMP‐1 antibody‐coated plate that was immediately coated and blocked before using. The plate was then incubated at room temperature for 2 hours. A 100‐μL aliquot of Human Total MMP‐1 detection antibody was added and incubated for 2 hours followed by 100 μL of Streptavidin‐horseradish peroxidase, which was incubated for 20 minutes in the dark. Subsequently, substrate solution was added for 20 minutes to quantify Total MMP‐1. The reaction was stopped, and Pro‐Matrix Metalloproteinase synthesis was determined by measuring the absorbance at 450 nm.
2.2.5. TIMP‐1 synthesis assay
The levels of human TIMP‐1 were measured in culture media using the Quantikine Human TIMP‐1 ELISA kit (R&D Systems, Minneapolis, Minnesota) according to the manufacturer's instructions. Briefly, 100‐μL aliquots of diluted culture supernatant were added to each well of monoclonal anti‐TIMP‐1 antibody‐coated plates and incubated at room temperature for 2 hours. Each well was washed and incubated with 200 μL of anti‐human TIMP‐1 conjugates. Following a wash to remove any unbound antibody‐enzyme reagent, 200 μL of substrate solution was added to the wells and incubated for 30 minutes at room temperature in the dark. The reaction was then stopped, and TIMP‐1 synthesis was determined by measuring the absorbance at 450 nm.
2.3. Immunocytochemistry analysis
For immunocytochemistry, cultured diabetic fibroblasts were seeded into an 8‐well culture slide at a density of 5 × 103 cells/well in 500 μL of DMEM/F‐12 containing 5% FBS and 10 ng/mL G‐Rb1 per well. No‐G‐Rb1‐treated diabetic fibroblasts were used as the control. The cells were incubated for 72 hours at 37°C in 5% CO2 with 100% humidity. The culture medium was then removed, and the cells were gently washed thrice with ice‐cold DPBS. Cells were fixed by adding a volume of 4% formaldehyde in DPBS equal to the original volume of the culture medium for 30 minutes on ice. The fixative was removed, and cells were washed thrice for 5 minutes, each with DPBS. Cells were incubated with 0.1 to 0.25% Triton X‐100 in DPBS for 10 minutes to permeabilise the membranes and were then washed in DPBS thrice, with each wash lasting 5 minutes. Cells were then incubated for 5 minutes in 3% H2O2 in DPBS to attenuate endogenous peroxidase activity. The fixed cells were washed thrice for 5 minutes each with DPBS. A sufficient volume of diluted appropriate primary antibodies (anti‐collagen I, anti‐VEGF, anti‐bFGF, anti‐TGF‐β1, anti‐MMP‐1, anti‐TIMP‐1 antibodies; Abcam, Cambridge, Massachusetts) was added to the cells. Cells were incubated with the primary antibody for 60 minutes at room temperature. Then, primary antibody solutions were removed, and cells were washed in DPBS thrice, with each wash lasting 5 minutes. These cells were then incubated with the appropriate fluorescent dye‐conjugated secondary antibody (anti‐rabbit‐ fluorescein isothiocyanate (FITC), Abcam, Cambridge, Massachusetts) at 37°C for 1 hour in the dark and mounted in 25 μL VECTASHIELD Mounting Medium with 4′‐6‐diamidino‐2‐phenylindole (DAPI; Vector Laboratories, Burlingame, California). As a final step, the nucleus was counter‐stained with DAPI (Sigma Aldrich, St. Louis, MO, USA) for cell proliferation analysis. The stained cells were analysed using a fluorescent microscope (BX‐53, Olympus Inc., Tokyo, Japan) and photographed using a microscope digital camera (DP‐71 CCD, Olympus Inc., Tokyo, Japan). All immunofluorescence staining images taken were of the exact centre of each well (×100 magnification).
2.4. Statistical analyses
All experiments were performed in triplicate, and the average value was used as a datum for each subject. Results are expressed as the mean ± SD. Statistical analyses were performed using the Wilcoxon signed‐rank test. Statistical significance was accepted at P‐values <0.05. The data were analysed using SPSS 20.0 software (SPSS, Inc., Chicago, Illinois).
3. RESULTS
3.1. Enzyme‐linked immunosorbent assay
3.1.1. Cell proliferation
ELISA analysis demonstrated a significant difference in cell proliferation between the two groups. The cell proliferation in the G‐Rb1‐treated group was 48% greater than that in the control group (P < 0.05, Figure 1).
Figure 1.
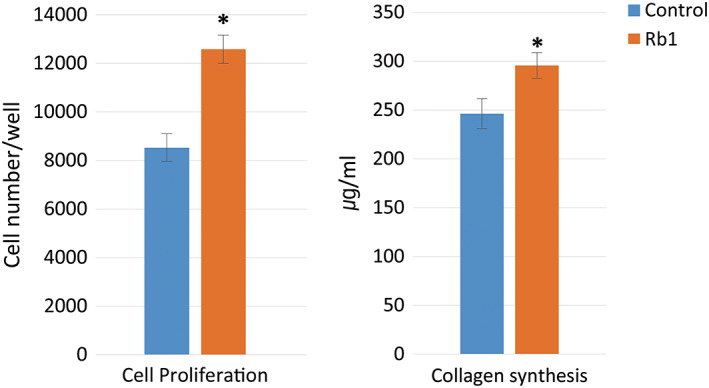
Cell proliferation and collagen synthesis of the two groups in the enzyme‐linked immunosorbent assay analysis (control: not treated diabetic fibroblast group, Rb1: G‐Rb1‐treated diabetic fibroblast group; *P < 0.05, Wilcoxon signed‐rank test)
3.1.2. Collagen synthesis
Collagen synthesis in the G‐Rb1‐treated group significantly increased when compared with that of the control group, with an increase of 20% (P < 0.05, Figure 1).
3.1.3. Growth factor synthesis
Synthesis of VEGF and TGF‐β1 significantly increased in the G‐Rb1‐treated group when compared with that of the control group, with increases of 47% and 14%, respectively (P < 0.05, Figure 2). However, the bFGF level of the G‐Rb1‐treated group was slightly lower than that of the control group by 13%, and this difference was not statistically significant (P = 0.07, Figure 2).
Figure 2.
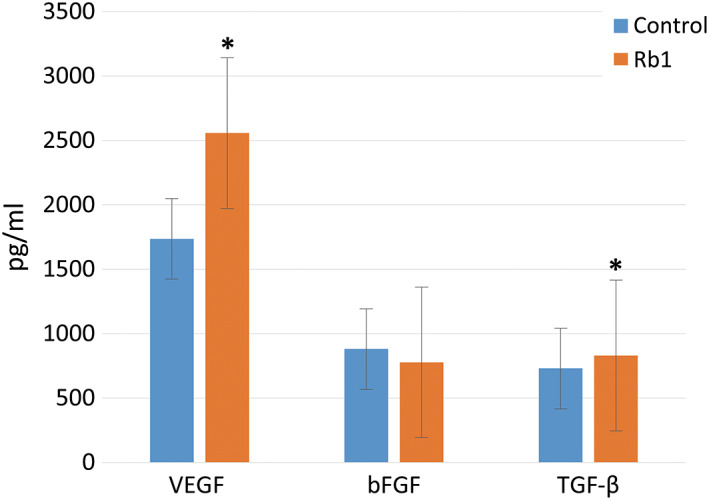
The growth factor (vascular endothelial growth factor, basic fibroblast growth factor, and transforming growth factor‐β) levels of the two groups in the enzyme‐linked immunosorbent assay analysis (*P < 0.05, Wilcoxon signed‐rank test)
3.1.4. MMP‐1 synthesis
The MMP‐1 level in the control group was slightly higher (6%) than that detected in the G‐Rb1‐treated group. However, this difference was not statistically significant (P = 0.58, Figure 3).
Figure 3.
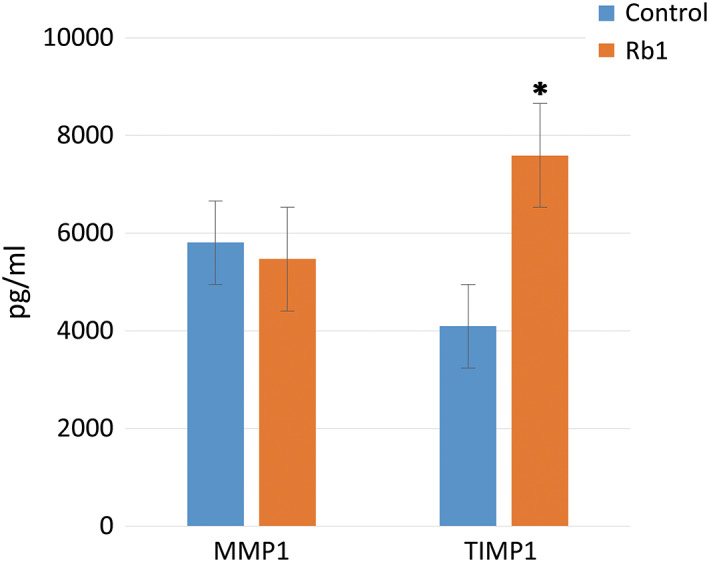
The matrix metalloproteinase 1 and tissue inhibitor of metalloproteinases 1 levels of the two groups in the enzyme‐linked immunosorbent assay analysis (*P < 0.05, Wilcoxon signed‐rank test)
3.1.5. TIMP‐1 synthesis
The TIMP‐1 level in the G‐Rb1‐treated group was 85% greater than that in the control group (P < 0.05, Figure 3).
3.2. Immunocytochemistry analysis
3.2.1. Cell proliferation
In the immunocytochemistry analysis, G‐Rb1 treatment induced a significant increase in cell proliferation, with an increase of 34% (P < 0.05, Figures 4 and 5).
Figure 4.
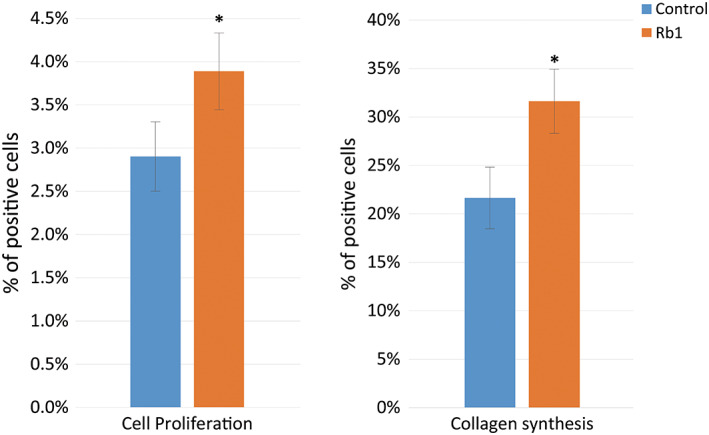
Immunocytochemistry analysis for cell proliferation and collagen synthesis. Colour intensity of the two groups (*P < 0.05, Wilcoxon signed‐rank test)
Figure 5.

Immunocytochemistry images for cell proliferation and collagen synthesis Nuclei were stained with 4'‐6‐diamidino‐2‐phenylindole (DAPI) (blue), and collagen type I was labelled with fluorescein isothiocyanate (FITC) (green). ×100 magnification
3.2.2. Collagen synthesis
G‐Rb1 treatment also significantly increased collagen synthesis by 46% (P < 0.05, Figures 4 and 5).
3.2.3. Growth factor synthesis
As shown in Figures 6 and 7, G‐Rb1 treatment induced a significant increase in the expression of VEGF and TGF‐β1, with increases of 80% and 72%, respectively (P < 0.05). However, the bFGF level of the G‐Rb1‐treated group was 1.4% higher than that of the control group, but this difference was not statistically significant (P = 0.39, Figures 6 and 7).
Figure 6.
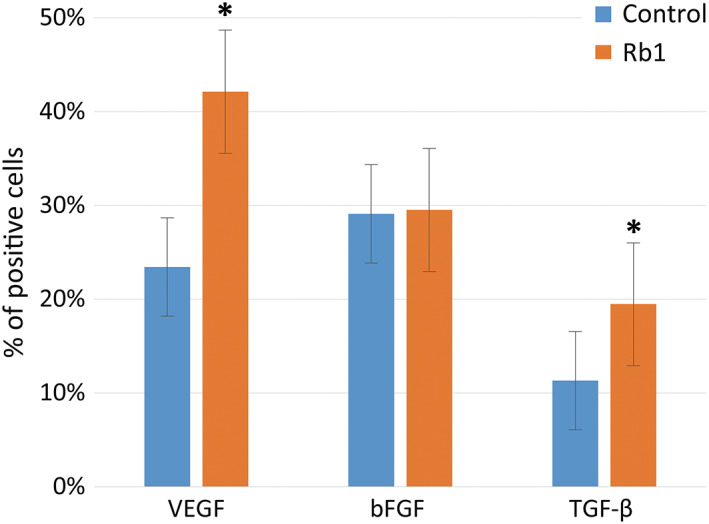
Immunocytochemistry analysis for the production of growth factors (vascular endothelial growth factor, basic fibroblast growth factor, and transforming growth factor‐β1). Colour intensity of the two groups (*P < 0.05, Wilcoxon signed‐rank test)
Figure 7.

Immunocytochemistry images for the growth factors (vascular endothelial growth factor, basic fibroblast growth factor, and transforming growth factor‐β1) production. Growth factors were labelled with fluorescein isothiocyanate (FITC) (green). ×100 magnification
3.2.4. MMP‐1 synthesis
The MMP‐1 levels in the control group were higher (23%) than those detected in the G‐Rb1‐treated group, but this difference was not statistically significant (P = 0.11, Figures 8 and 9).
Figure 8.
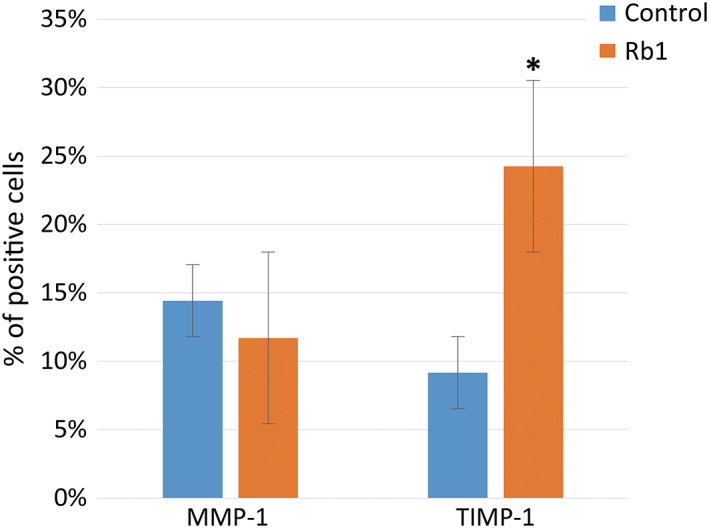
Immunocytochemistry analysis for the matrix metalloproteinase 1 and tissue inhibitor of metalloproteinases 1 production. Colour intensity of the two groups (*P < 0.05, Wilcoxon signed‐rank test)
Figure 9.
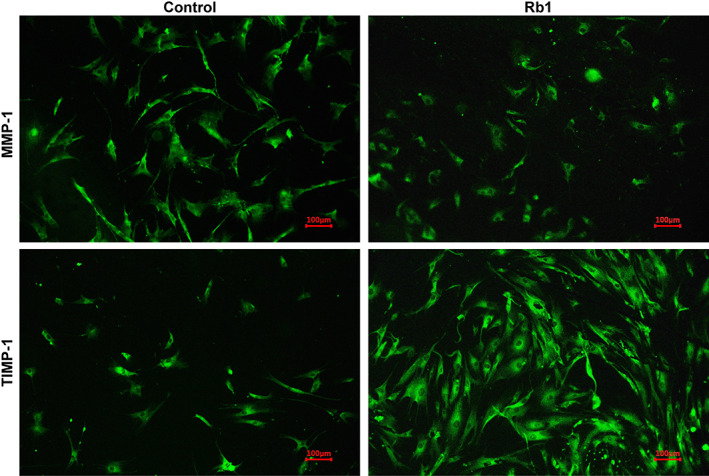
Immunocytochemistry images for MMP‐1 and TIMP‐1 production. MMP‐1 and TIMP‐1 were labelled with fluorescein isothiocyanate (FITC) (green). ×100 magnification. Abbreviations: MMP‐1, matrix metalloproteinase 1; TIMP‐1, tissue inhibitor of metalloproteinases 1
3.2.5. TIMP‐1 synthesis
The TIMP‐1 levels in the G‐Rb1‐treated group were 2.6‐fold greater than those in the fibroblast group (P < 0.05, Figures 8 and 9).
4. DISCUSSION
Despite extensive research on the pharmacological effects of P. ginseng, there have been few studies addressing the effects of P. ginseng extract on the activity of fibroblasts, which arguably are the single most important cell type in not only the proliferative phase but throughout the wound‐healing process. Fibroblasts play a pivotal role in mediating successful wound healing, ranging from the synthesis and remodelling of the extracellular matrix (ECM) to the production of growth factors.31 Collagen comprises approximately 80% of the ECM, contributing to its strength and facilitating skin elasticity, tightening, and cell integrity.32 Therefore, fibroblast proliferation and its collagen synthesis capacity are essential for wound healing. The results of the present study have demonstrated that G‐Rb1‐treated diabetic fibroblasts show significantly higher proliferation and collagen synthesis than those of untreated cells.
It is possible that the increase in collagen synthesis of G‐Rb1‐treated diabetic fibroblasts is related to the activities of TGF‐β1 and TIMP‐1 given that the levels of TGF‐β1 and TIMP‐1 in the G‐Rb1 group were higher than those in the control group. The expression of TGF‐β1, a pivotal regulator of cell proliferation and ECM production, was up‐regulated when cells were treated with G‐Rb1 (Figures 2, 4 and 5), which could lead to a significant increase in collagen. Moreover, an especially striking observation was noted with regard to TIMP‐1 levels: they were 2.6‐fold greater in G‐Rb1‐treated cells in the immunofluorescence staining test and 1.9‐fold greater in the ELISA. TIMPs act as local inhibitors of MMPs and thus control the MMP‐induced breakdown of the extracellular matrices, an excess of which may lead to chronic inflammation.
Wound healing requires a balance between the accumulation of collagenous and non‐collagenous ECM components and their remodelling by MMPs and TIMPs. In particular, MMPs are the main protease family involved in this remodelling of ECM.33, 34 At least 24 MMPs have been identified in humans.35 MMPs are divided into subgroups depending on their substrate specificity. Collagenases (MMP‐1 and MMP‐8) and gelatinases (MMP‐2 and MMP‐9) play a major role in the healing process.34 In particular, Lobman et al have found that the levels of MMP‐1, MMP‐8, and MMP‐9 were significantly higher in diabetic foot ulcers,36 and Muller et al have demonstrated that MMP‐1 is the major collagenase involved in wound healing.34 Furthermore, it has been suggested that an adapted regulation of MMP‐1 is essential for the proper progression of the healing process33 and for the specific proteolysis of type I collagen, which is a structural component of the dermis.37, 38 Therefore, the current study mainly focused on the synthesis of MMP‐1, among other members of the MMP family.
The activities of MMPs are inhibited by TIMPs in a specific manner. Currently, the TIMP family comprises four members, TIMP‐1, −2, −3, and −4, that inhibit MMPs by binding to their active site in a 1:1 stoichiometric ratio.39 With few exceptions, TIMPs are capable of inhibiting all MMPs, although TIMP‐1 has a higher affinity for MMP‐1 and MMP‐9 and TIMP‐2 for MMP‐2 and MMP‐8.
In addition, the results of this study suggest that G‐Rb1‐treated diabetic fibroblasts can actively secrete VEGF to a much greater extent than untreated cells. VEGF, as an endothelial cell mitogen and inducer of vascular permeability, is unique in its effects on multiple components of the wound‐healing cascade, particularly for angiogenesis.40, 41, 42, 43 Angiogenesis is a critical step in the wound‐healing process, especially for chronic wound healing. Neovascularisation, the formation of new blood vessels, is necessary to sustain the newly formed granulation tissues and the survival of keratinocytes. This study showed that there might be potential for the use of G‐Rb1 in angiogenesis.
Therefore, we can expect that G‐Rb1 treatment may be beneficial in healing chronic diabetic wounds, which are commonly characterised by fibroblast dysfunction and a prolonged inflammatory phase. Fibroblasts isolated from diabetic wounds have lower proliferative potential and cause changes associated with cellular senescence because of high glucose concentrations.44 In addition, previous studies have shown that collagen deposition and angiogenesis are impaired.45, 46, 47, 48 The production of key growth factors, including bFGF, VEGF, and TGF‐β, is decreased.46, 47, 49 Furthermore, there is an excess of MMPs and a decrease in their natural inhibitors, TIMPs. All of these can lead to a breakdown of growth factors and ECM components.50 The current study has demonstrated that G‐Rb1 treatment significantly increased cell proliferation, collagen synthesis, VEGF production, TGF‐β1 production, and TIMP‐1 synthesis in diabetic fibroblasts. In addition, as previously mentioned, several clinical and experimental studies have confirmed that P. ginseng has a variety of positive effects in humans, including immuno‐stimulation, antimicrobial, and anti‐inflammatory actions. Considered together, P. ginseng may be a good treatment option for healing diabetic wounds.
Ginseng is comprised of saponins and non‐saponins. Saponins are a class of chemical compounds found abundantly in various plant species. Ginseng saponin has a different chemical structure than that of other plant saponins and has extensive biological activities. Therefore, this distinct saponin is called a ginsenoside. Ginsenosides are found nearly exclusively in Panax species (ginseng) and are often split into the Rb1 group (characterised by the presence of protopanaxadiols: Rb1, Rb2, Rc, and Rd) and the Rg1 group (protopanaxatriols: Rg1, Re, Rf, and Rg2). The remaining components are called non‐saponins, which include polysaccharides, polyacetylenes, peptides, and amino acids. G‐Rb1 is the active P. ginseng component with a variety of biological activities, including anti‐inflammatory, antioxidant, and antimicrobial effects. G‐Rb1 also improves protein synthesis, neovascularisation or angiogenesis, and immunity.
Our results could be clinically applicable to wound dressings. Various dressing materials have been commercialised with the aim of supplementing the shortfalls of conventional gauze dressings. If P. ginseng has a positive effect on chronic wound healing, it could be added to dressing material to accelerate healing. In a bench‐to‐bedside effort, a recent trial examined the potential therapeutic benefit of MMP inhibitor‐impregnated dressing for treating chronic wounds, with encouraging results.36 In particular, P. ginseng may be more likely to facilitate healing in older patients with chronic wounds because they commonly have poor blood circulation, a weak immune system, deficient nutritional factors, and decreased cell activities. In addition, chronic wounds usually persist in the inflammatory stage for a long time. As mentioned earlier, several clinical and experimental studies have confirmed that P. ginseng has a variety of positive effects in humans, including angiogenesis activation, immuno‐stimulation, antimicrobial functions, and anti‐inflammatory actions. Considering these effects and the results of the present study, P. ginseng might have the potential to be used for healing chronic wounds.
Generally, P. ginseng is well tolerated. Adverse effects of P. ginseng have been reported in cases where it was administered orally, including the use of capsules, liquids, or powders; however, these effects were mild and reversible.1, 51, 52 Shergis et al have conducted a systematic review to evaluate randomised controlled trials for P. ginseng in patients with any type of disease or in healthy individuals.52 Of 40 studies reporting the effects of the administration of P. ginseng, 16 studies reported no adverse events, and 24 studies showed 135 minor events. The minor events included diarrhoea, sleeplessness, palpitations, headache, nausea, and mild hepatic dysfunction. No severe adverse events were reported from any study. In addition, there have been no reports demonstrating the side effects of P. ginseng when it was administered topically.
There are limitations to the present study. It was difficult to assess the effect of P. ginseng on the overall wound‐healing process. in vivo wound healing is a complex process involving multiple factors. Future studies regarding how P. ginseng treatment assists the wound‐healing process in vivo are necessary. Based on the present results, we are proceeding to the next step of performing in vivo studies. In addition, this study focused only on the possibility of using P. ginseng to increase diabetic fibroblast activities in vitro. The basic mechanism of action should be further investigated, including biochemical analyses, to confirm the effect of ginseng treatment. Nevertheless, to the authors' knowledge, this is the first study demonstrating the effects of P. ginseng extract on the activity of fibroblasts, particularly focusing on cell proliferation; collagen synthesis; and the production of VEGF, bFGF, TGF‐β1, MMP‐1, and TIMP‐1, which are essential for diabetic wound healing.
5. CONCLUSIONS
P. ginseng treatment may stimulate the wound‐healing activity of diabetic fibroblasts in vitro.
CONFLICTS OF INTEREST
The authors have no conflicts of interest to report.
ACKNOWLEDGEMENTS
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education (grant number 2018R1D1A1B07050442).
Namgoong S, Lee H, Han S‐K, Lee H‐W, Jeong S‐H, Dhong E‐S. Effect of Panax ginseng extract on the activity of diabetic fibroblasts in vitro. Int Wound J. 2019;16:737–745. 10.1111/iwj.13091
Funding information Ministry of Education, Grant/Award Number: 2018R1D1A1B07050442
REFERENCES
- 1. Kiefer D, Pantuso T. Panax ginseng. Am. Fam. Physician. 2003;68:1539‐1542. [PubMed] [Google Scholar]
- 2. Metori K, Furutsu M, Takahashi S. The preventive effect of ginseng with Du‐Zhong leaf of protein metabolism in aging. Biol. Pharm. Bull. 1997;20:237‐242. [DOI] [PubMed] [Google Scholar]
- 3. Park E‐K, Choo M‐K, Kim E‐J, Han MJ, Kim D‐H. Antiallergic activity of ginsenoside Rh2. Biol. Pharm. Bull. 2003;26:1581‐1584. [DOI] [PubMed] [Google Scholar]
- 4. Xiaoguang C, Hongyan L, Xiaohong L, et al. Cancer chemopreventive and therapeutic activities of red ginseng. J. Ethnopharmacol. 1998;60:71‐78. [DOI] [PubMed] [Google Scholar]
- 5. Yamamoto M, Uemura T, Nakama S, Uemiya M, Kumagai A. Serum HDL‐cholesterol‐increasing and fatty liver‐improving actions of Panax ginseng in high cholesterol diet‐fed rats with clinical effect on hyperlipidemia in man. Am. J. Chin. Med. 1983;11:96‐101. [DOI] [PubMed] [Google Scholar]
- 6. Yang F, Zheng Y, Li D, Deng W. Effect of Shenfu injection on microcirculation. J. Biomed. Eng. 2003;20:91‐4, 100. [PubMed] [Google Scholar]
- 7. Hinz B. Formation and function of the myofibroblast during tissue repair. J Invest Dermatol. 2007;127:526‐537. [DOI] [PubMed] [Google Scholar]
- 8. Jun HK, Chen X, Gillis CN. Ginsenosides protect pulmonary vascular endothelium against free radical—induced injury. Biochem. Biophys. Res. Commun. 1992;189:670‐676. [DOI] [PubMed] [Google Scholar]
- 9. Kanzaki T, Morisaki N, Shiina R, Saito Y. Role of transforming growth factor‐β pathway in the mechanism of wound healing by saponin from Ginseng Radix rubra. Br. J. Pharmacol. 1998;125:255‐262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ahn JY, Song JY, Yun YS, Jeong G, Choi IS. Protection of Staphylococcus aureus‐infected septic mice by suppression of early acute inflammation and enhanced antimicrobial activity by ginsan. FEMS Immunol. Med. Microbiol. 2006;46:187‐197. [DOI] [PubMed] [Google Scholar]
- 11. de Oliveira ACC, Perez AC, Merino G, Prieto JG, Alvarez AI. Protective effects of Panax ginseng on muscle injury and inflammation after eccentric exercise. Comp Biochem Physiol C Toxicol Pharmacol. 2001;130:369‐377. [DOI] [PubMed] [Google Scholar]
- 12. Hong C‐E, Lyu S‐Y. Anti‐inflammatory and anti‐oxidative effects of Korean red ginseng extract in human keratinocytes. Immune Netw. 2011;11:42‐49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jie YH, Cammisuli S, Baggiolini M. Immunomodulatory effects of Panax ginseng CA Meyer in the mouse. Inflamm. Res. 1984;15:386‐391. [DOI] [PubMed] [Google Scholar]
- 14. Kim HA, Kim S, Chang SH, Hwang HJ, Choi Y‐N. Anti‐arthritic effect of ginsenoside Rb1 on collagen induced arthritis in mice. Int. Immunopharmacol. 2007;7:1286‐1291. [DOI] [PubMed] [Google Scholar]
- 15. Kim SH, Park KS, Chang MJ, Sung JH. Effects of Panax ginseng extract on exercise‐induced oxidative stress. J. Sports Med. Phys. Fitness. 2005;45:178‐182. [PubMed] [Google Scholar]
- 16. Kim YS, Cho I‐H, Jeong M‐J, et al. Therapeutic effect of total ginseng saponin on skin wound healing. J. Ginseng Res. 2011;35:360‐367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Leung KW, Pon YL, Wong RN, Wong AS. Ginsenoside‐Rg1 induces vascular endothelial growth factor expression through the glucocorticoid receptor‐related phosphatidylinositol 3‐kinase/Akt and β‐catenin/T‐cell factor‐dependent pathway in human endothelial cells. J. Biol. Chem. 2006;281:36280‐36288. [DOI] [PubMed] [Google Scholar]
- 18. Park J‐S, Park E‐M, Kim D‐H, et al. Anti‐inflammatory mechanism of ginseng saponins in activated microglia. J. Neuroimmunol. 2009;209:40‐49. [DOI] [PubMed] [Google Scholar]
- 19. Chan L, Yue PY, Wong Y, Wong RN. MicroRNA‐15b contributes to ginsenoside‐Rg 1‐induced angiogenesis through increased expression of VEGFR‐2. Biochem. Pharmacol. 2013;86:392‐400. [DOI] [PubMed] [Google Scholar]
- 20. Cheung LW, Leung KW, Wong CK, Wong RN, Wong AS. Ginsenoside‐Rg1 induces angiogenesis via non‐genomic crosstalk of glucocorticoid receptor and fibroblast growth factor receptor‐1. Cardiovasc. Res. 2010;89:419‐425. [DOI] [PubMed] [Google Scholar]
- 21. Cho J‐S, Moon Y‐M, Um J‐Y, Moon J‐H, Park I‐H, Lee H‐M. Inhibitory effect of ginsenoside Rg1 on extracellular matrix production via extracellular signal‐regulated protein kinase/activator protein 1 pathway in nasal polyp‐derived fibroblasts. Exp. Biol. Med. 2012;237:663‐669. [DOI] [PubMed] [Google Scholar]
- 22. Kimura Y, Sumiyoshi M, Kawahira K, Sakanaka M. Effects of ginseng saponins isolated from Red Ginseng roots on burn wound healing in mice. Br. J. Pharmacol. 2006;148:860‐870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lee J, Jung E, Lee J, et al. Panax ginseng induces human Type I collagen synthesis through activation of Smad signaling. J. Ethnopharmacol. 2007;109:29‐34. [DOI] [PubMed] [Google Scholar]
- 24. Liang H‐C, Chen C‐T, Chang Y, Huang Y‐C, Chen S‐C, Sung H‐W. Loading of a novel angiogenic agent, ginsenoside Rg1 in an acellular biological tissue for tissue regeneration. Tissue Eng. 2005;11:835‐846. [DOI] [PubMed] [Google Scholar]
- 25. Lu Z‐Q, Dice JF. Ginseng extract inhibits protein degradation and stimulates protein synthesis in human fibroblasts. Biochem. Biophys. Res. Commun. 1985;126:636‐640. [DOI] [PubMed] [Google Scholar]
- 26. Morisaki N, Watanabe S, Tezuka M, et al. Mechanism of angiogenic effects of saponin from ginseng Radix rubra in human umbilical vein endothelial cells. Br. J. Pharmacol. 1995;115:1188‐1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sengupta S, Toh S‐A, Sellers LA, et al. Modulating angiogenesis. Circulation. 2004;110:1219‐1225. [DOI] [PubMed] [Google Scholar]
- 28. Song KC, Chang T‐S, Lee H, Kim J, Park JH, Hwang GS. Processed Panax ginseng, sun ginseng increases type I collagen by regulating MMP‐1 and TIMP‐1 expression in human dermal fibroblasts. J. Ginseng Res. 2012;36:61‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yang N, Chen P, Tao Z, et al. Beneficial effects of ginsenoside‐Rg1 on ischemia‐induced angiogenesis in diabetic mice. Acta Biochim. Biophys. Sin. 2012;44:999‐1005. [DOI] [PubMed] [Google Scholar]
- 30. Lee GY, Park KG, Namgoong S, et al. Effects of Panax ginseng extract on human dermal fibroblast proliferation and collagen synthesis. Int. Wound J. 2016;13:42‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cook H, Stephens P, Davies KJ, Thomas DW, Harding KG. Defective extracellular matrix reorganization by chronic wound fibroblasts is associated with alterations in TIMP‐1, TIMP‐2, and MMP‐2 activity. J Invest Dermatol. 2000;115:225‐233. [DOI] [PubMed] [Google Scholar]
- 32. Gordon MK, Hahn RA. Collagens. Cell Tissue Res. 2010;339:247‐257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ravanti L, Kähäri V‐M. Matrix metalloproteinases in wound repair. Int. J. Mol. Med. 2000;6:391‐798. [PubMed] [Google Scholar]
- 34. Muller M, Trocme C, Lardy B, Morel F, Halimi S, Benhamou PY. Matrix metalloproteinases and diabetic foot ulcers: the ratio of MMP‐1 to TIMP‐1 is a predictor of wound healing. Diabet. Med. 2008;25:419‐426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ugalde AP, Ordóñez GR, Quirós PM, Puente XS, López‐Otín C. Metalloproteases and the Degradome. In: Clark I. (ed.). Matrix Metalloproteinase Protocols. Methods in Molecular Biology (Methods and Protocols), vol 622. Totowa, NJ: Humana Press. [DOI] [PubMed] [Google Scholar]
- 36. Lobmann R, Zemlin C, Motzkau M, Reschke K, Lehnert H. Expression of matrix metalloproteinases and growth factors in diabetic foot wounds treated with a protease absorbent dressing. J. Diabetes Complications. 2006;20:329‐335. [DOI] [PubMed] [Google Scholar]
- 37. Pilcher BK, Dumin JA, Sudbeck BD, Krane SM, Welgus HG, Parks WC. The activity of collagenase‐1 is required for keratinocyte migration on a type I collagen matrix. J. Cell Biol. 1997;137:1445‐1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Stamenkovic I. Extracellular matrix remodelling: the role of matrix metalloproteinases. J Pathol. 2003;200:448‐464. [DOI] [PubMed] [Google Scholar]
- 39. Gomez D, Alonso D, Yoshiji H, Thorgeirsson U. Tissue inhibitors of metalloproteinases: structure, regulation and biological functions. Eur. J. Cell Biol. 1997;74:111‐122. [PubMed] [Google Scholar]
- 40. Bao P, Kodra A, Tomic‐Canic M, Golinko MS, Ehrlich HP, Brem H. The role of vascular endothelial growth factor in wound healing. J. Surg. Res. 2009;153:347‐358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yang R, Thomas GR, Bunting S, et al. Effects of vascular endothelial growth factor on hemodynamics and cardiac performance. J. Cardiovasc. Pharmacol. 1996;27:838‐844. [DOI] [PubMed] [Google Scholar]
- 42. Zhang F, Oswald T, Lin S, et al. Vascular endothelial growth factor (VEGF) expression and the effect of exogenous VEGF on survival of a random flap in the rat. Br. J. Plast. Surg. 2003;56:653‐659. [DOI] [PubMed] [Google Scholar]
- 43. Wilkins B, Jones D. Immunohistochemical characterization of intact stromal layers in long‐term cultures of human bone marrow. Br. J. Haematol. 1995;90:757‐766. [DOI] [PubMed] [Google Scholar]
- 44. Han S‐K, Choi K‐J, Kim W‐K. Clinical application of fresh fibroblast allografts for the treatment of diabetic foot ulcers: a pilot study. Plast. Reconstr. Surg. 2004;114:1783‐1789. [DOI] [PubMed] [Google Scholar]
- 45. Goodson WH, Hunt TK. Deficient collagen formation by obese mice in a standard wound model. Am J Surg. 1979;138:692‐694. [DOI] [PubMed] [Google Scholar]
- 46. Hansen SL, Young DM, Boudreau NJ. HoxD3 expression and collagen synthesis in diabetic fibroblasts. Wound Repair Regen. 2003;11:474‐480. [DOI] [PubMed] [Google Scholar]
- 47. Pierce GF. Inflammation in nonhealing diabetic wounds: the space‐time continuum does matter. Am. J. Pathol. 2001;159:399‐403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Frank S, Hübner G, Breier G, Longaker MT, Greenhalgh DG, Werner S. Regulation of vascular endothelial growth factor expression in cultured keratinocytes. Implications for normal and impaired wound healing. J. Biol. Chem. 1995;270:12607‐12613. [DOI] [PubMed] [Google Scholar]
- 49. Schultz GS, Sibbald RG, Falanga V, et al. Wound bed preparation: a systematic approach to wound management. Wound Repair Regen. 2003;11:S1‐S28. [DOI] [PubMed] [Google Scholar]
- 50. Robson MC. Cytokine manipulation of the wound. Clin. Plast. Surg. 2003;30:57‐65. [DOI] [PubMed] [Google Scholar]
- 51. Coon JT, Ernst E. Panax ginseng. Drug Saf. 2002;25:323‐344. [DOI] [PubMed] [Google Scholar]
- 52. Shergis JL, Zhang AL, Zhou W, Xue CC. Panax ginseng in randomised controlled trials: a systematic review. Phytother. Res. 2013;27:949‐965. [DOI] [PubMed] [Google Scholar]


