Abstract
The antibody-binding crystallizable fragment (Fc) γ receptors (FcγRs) are expressed by leukocytes and activate or suppress a cellular response once engaged with an antibody-coated target. Therapeutic mAbs that require FcγR binding for therapeutic efficacy are now frontline treatments for multiple diseases. However, substantially fewer development efforts are focused on the FcγRs, despite accounting for half of the antibody–receptor complex. The recent success of engineered cell-based immunotherapies now provides a mechanism to introduce modified FcγRs into the clinic. FcγRs are highly heterogeneous because of multiple functionally distinct alleles for many genes, the presence of membrane-tethered and soluble forms, and a high degree of post-translational modification, notably asparagine-linked glycans. One significant factor limiting FcγR improvement is the fundamental lack of knowledge regarding endogenous receptor forms present in the human body. This review describes the composition of FcγRs isolated from primary human leukocytes, summarizes recent efforts to engineer FcγRs, and concludes with a description of potential FcγR features to enrich for enhanced function. Further understanding FcγR biology could accelerate the development of new clinical therapies targeting immune-related disease.
Keywords: Fc receptor, antibody, immunotherapy, glycobiology, glycoprotein
Abbreviations: Fc, crystallizable fragment; FcγRs, Fc γ receptors; IgG, immunoglobulin G; LacNAc, N-acetyllactosamine; MHC, major histocompatibility complex; NK, natural killer
Fc γ receptors as warheads for cell-based immunotherapies
Crystallizable fragment (Fc) γ receptors (FcγRs) bind to immunoglobulin G (IgG) antibodies at the surface of a white blood cell (leukocyte) and are required for the efficacy of many antibody-based drugs used to treat diseases (termed therapeutic mAbs). Thus, FcγRs link the target-binding specificity of antibodies to the cytotoxic properties of leukocytes, with an individual FcγR type contributing to the treatment of multiple diseases (Fig. 1). In general, the FcγRs bind the IgG1 and IgG3 subclasses with greater affinity but show lower or negligible affinity for IgG2 and IgG4 (1). The rapid proliferation of mAbs of mostly the IgG1 subclass has focused on binding new targets to treat different diseases and more recently enhancing the FcγR-binding affinity to improve efficacy. Although mAbs are currently drugs, it is theoretically possible to improve affinity by engineering either the antibody or the FcγR. Creating drugs from engineered FcγRs was previously impractical because of the lack of appropriate cell-based therapies, but recent advances in engineered leukocyte therapies provide a vehicle to deploy modified receptors in the clinic.
Figure 1.
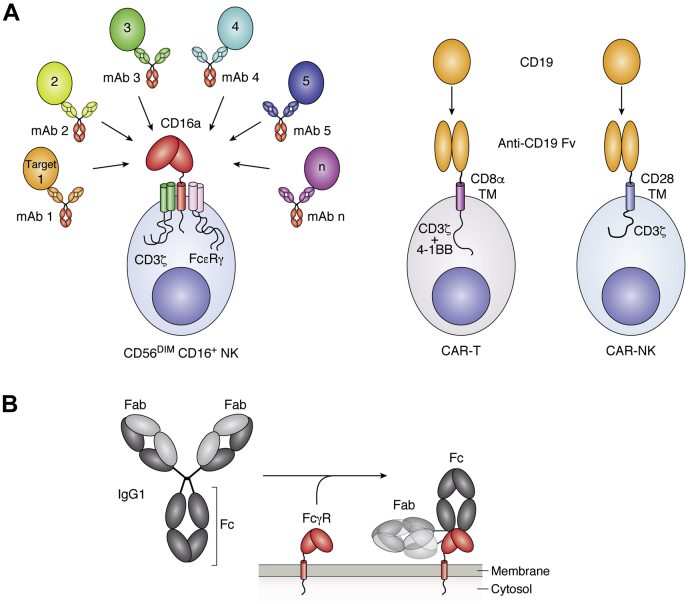
NK cells naturally target multiple antigens by binding the conserved crystallizable fragment (Fc) of IgG, unlike chimeric antigen receptor (CAR)–T cells or CAR–NK cells that are programmed to recognize a single antigen.A, NK cell Fc γ receptor IIIa/CD16a associates with either CD3ζ or the Fc ε receptor γ chain to activate an NK cell after ligation to an antibody-bound target (70, 71). The CAR-T example shown represents the tisagenlecleucel (Kymriah) therapy that targets CD19 on B-cell lymphoma. This CAR–NK construct likewise recently showed success (3, 72). Both CARs contain custom transmembrane and activation domains. B, Fc γ receptors bind to the crystallizable fragment of IgG. IgG, immunoglobulin G; NK, natural killer.
The recent cell-based immunotherapy revolution has demonstrated the therapeutic and commercial viability of genetically modified lymphocytes to combat even late-stage disease. Lymphocytes are a subset of leukocytes that include antibody-producing B cells, T cells that can kill diseased tissue directly or recruit an immune response by activating other cell types, and natural killer (NK) cells that can destroy antibody-coated tissue and other foreign tissues. Chimeric antigen receptor (CAR) T-cell therapies, including Kymriah (tisagenlecleucel) and Yescarta (axicabtagene ciloleucel), treat B-cell lymphoma by reprogramming the patient’s own T cells to express an engineered CD19-binding receptor to destroy cancerous tissue (Fig. 1). CAR T limitations include cost at ∼$400k/treatment, the near-absolute requirement for autologous transplantation (using the patient’s own tissue) to avoid graft versus host disease, cytokine release syndrome, and the narrow therapeutic benefit of a single CAR (2). NK cells offer an alternative CAR expression platform that promises rapid deployability and off-the-shelf availability. Furthermore, NK cells also express a potent FcγR at high levels: FcγRIIIa/CD16a. CD16a binds antibodies coated on the surface of a target cell to trigger a cytotoxic NK cell response (Fig. 1B), and it is well established that increased antibody binding increases cytotoxicity and the therapeutic efficacy of mAbs (discussed below). Thus, in addition to hosting CARs, NK cells are suitable hosts for engineered FcγRs.
Unlike T cells, allogeneic NK cell transplants (from a genetically mismatched donor) are tolerated and can be developed from cord blood (3) or cultured cells (4, 5) or transferred from donors without matching major histocompatibility complex (MHC) loci (6). This property allows a single NK cell source to be used to treat multiple patients, in contrast to T-cell therapy production, which is individualized because of the strict requirement for MHC matching. Interestingly, autologous NK cell transplants often exhibit limited efficacy because of reduced NK cell function; however, allogeneic transplantation leads to favorable graft versus tumor effects. Thus, allogeneic NK cells promise well-tolerated and effective off-the-shelf treatments with reduced cost and side effects.
As indicated above, future lymphocyte-based treatments are poised to expand beyond CAR incorporation to leverage innate NK cell features. NK cells adopt a vital role in surveillance and clearing diseased tissue. NK cell engineering efforts focus on enhancing these natural functions. Multiple NK cell engineering avenues are being pursued, including CAR NK cells with dramatic recent success (3), NK cells with increased expression of FcγRIIIa/CD16a (Artiva Biotherapeutics; clinical trials as a combination therapy planned for 2020), and cultured NK92 cells (13 current food & drug administration-registered trials as of July 2020) including multiple CD16a-expressing variants.
The importance of FcγRs in current immunotherapies is well established as discussed below with multiple contemporary efforts aimed at improving immunotherapies through FcγR engineering, whether at the amino acid level or by increasing the expression of activating FcγRs on leukocytes. This article will focus on recent definitions of the specific FcγR forms found in the human body, many of which are highly variable because of extensive post-translational modification. Each individual receptor form potentially exhibits distinct characteristics, and certain forms may provide substantial therapeutic benefit after enrichment. This article will also summarize recent efforts to improve NK cell function through FcγR engineering at the amino acid level and identify motifs for future FcγR engineering.
Antibody-binding FcγRs
The canonical FcγRs are expressed on a variety of leukocytes and are subdivided into activating receptors (FcγRI/CD64, FcγRIIa/CD32a, FcγRIIc/CD32c, FcγRIIIa/CD16a, and FcγRIIIb/CD16b) and inhibitory receptors (FcγRIIb/CD32b). These receptors all bind IgG subclasses, however, with different affinities (Table 1 (7, 8)). CD16a is the primary receptor for anticancer mAbs and is the only FcγR expressed on NK cells for 85 to 93% of the population; the remainder express low levels of CD32c (9). CD16a is also expressed by macrophages and some circulating monocytes that adopt a critical role in clearing antibody-coated targets (10). CD16b is a related receptor that is expressed at very high levels on neutrophils, although the role of CD16b is unclear and it may both promote and inhibit a cellular response (11). CD32a is an activating receptor widely expressed on all leukocytes with the exception of T and B lymphocytes. CD32b is similar to CD32a but is mainly expressed on B cells and functions to inhibit B-cell maturation. CD64 is inducible on monocytes, macrophages, neutrophils, and dendritic cells and binds IgG1 with high affinity (∼1 nM), unlike the other receptors that are considered “low affinity” with dissociation constants ranging from low nM to low μM (1, 12).
Table 1.
Properties of human Fc γ receptors
| FcγR | Function | Affinity | Major alleles | N-glycan sites | Expressed by |
|---|---|---|---|---|---|
| CD16a/FcγRIIIa | Activation | IgG3>IgG1>>IgG4>IgG2 | V158/F158 | 5 | NK, Mona, MΦa, Ta, Baa, Ma |
| CD16b/FcγRIIIb | Activation | IgG3>IgG1 | NA1/NA2/SH | 4/6/6 | N, Baa, Eb |
| CD32a/FcγRIIa | Activation | IgG1>IgG3>IgG2=IgG4 | H131/R131 | 2 | MΦ, N, P, M, E |
| CD32b/FcγRIIb | Inhibition | IgG1=IgG3=IgG4>IgG2 | na | 3 | B, MΦ, D, Ba, Mona, Na |
| CD32c/FcγRIIc | Activation | IgG1=IgG3=IgG4>IgG2 | na | 3 | Ba, NKa |
| CD64/FcγRI | Activation | IgG1=IgG3>IgG4 | na | 7 | MΦ, Mon, D, M, Nb |
B, B cells; Ba, basophils; D, dendritic cells; E, eosinophils; M, mast cells; MΦ, macrophages; Mon, monocytes; N, neutrophils; na, not applicable; NK, natural killer cells; P, platelets; T, T cells.
Indicates expression in a subset of cells.
Indicates inducible expression.
It is well established that increasing Fc-mediated binding by activating FcγRs improves cytotoxicity (13, 14). For example, the anti-CD20 mAb Gazyva (obinutuzumab) increased progression-free survival in non-Hodgkin’s lymphoma by 50% over the standard Rituxan (rituximab) therapy (15). Gazyva and Rituxan bind overlapping CD20 epitopes; however, Gazyva binds CD16a with a 7-fold greater affinity (16, 17, 18). Supported by this example and many others, antibody engineering through amino acid substitutions or glycan engineering promises to increase mAb efficacy (19). Alternatively, affinity can likewise be improved through modifying the receptor. One naturally occurring example exists: patients expressing the CD16a allele encoding a Val residue at position 158 as opposed to the more common Phe had binding of IgG1 2- to 4-fold tighter and showed a substantially improved clinical response to mAb therapies (1, 20, 21, 22, 23, 24).
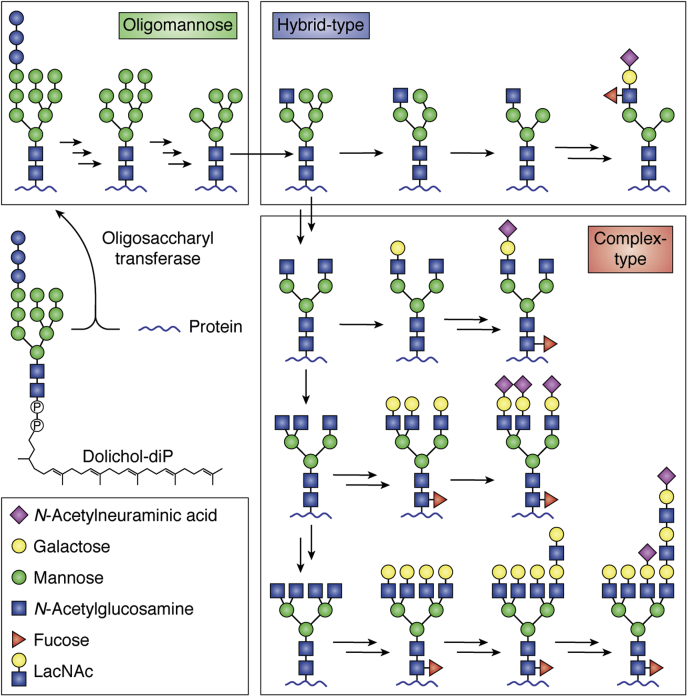
One key source of protein heterogeneity is the attachment of complex carbohydrates (glycans) during protein expression and secretion with some glycans extending to dozens of residues. Glycans are common modifications on endoplasmic reticulum, Golgi, and secreted proteins (including serum proteins) that mediate protein folding, oligomerization, stability, and protein function as well as harbor motifs recognized by receptors to name a few key roles although many others exist. Glycans attached to Asn residues (N-glycans) or Ser and Thr residues (O-glycans) are particularly prevalent. N-Glycans are remodeled through extension and trimming reactions in the endoplasmic reticulum and Golgi during protein secretion to form three main classes: minimally remodeled oligomannose forms, hybrid glycans with intermediate processing, and complex types that are heavily modified and often contain terminal N-acetylneuraminic acid caps at the nonreducing branch termini (see Fig. 2 (25)).
Figure 2.
Simplified mammalian N-glycan processing scheme showing the three main N-glycan types and heterogeneity found on secreted proteins. Multiple arrows indicate multiple processing steps. Note that remodeling reactions occur without a template or proof-reading mechanisms. N-glycan, glycans attached to Asn residues.
FcγR modifications affect antibody-binding affinity and therefore represent a viable engineering target. All FcγRs are heavily modified with N-glycans during protein folding (11). Two groups independently noted that removing sialic acid residues from CD16a increased antibody-binding affinity (26, 27). At the same time, Patel et al. (28) reported that changing the composition of CD16a N-glycans from complex to oligomannose types increased antibody-binding affinity by 12-fold. Cambay et al. (29) reported a similar result, although with a lower magnitude of less than 2-fold. Further observations indicated that CD16a was the only “low-affinity” FcγR to exhibit this composition-dependent property in vitro, and composition of the CD16a N162 glycan primarily affected antibody-binding affinity (30, 31). Although clearly implicating glycan engineering as one mechanism to improve antibody-binding affinity, the immediate impact of these observations was unclear because endogenous FcγR glycan composition, particularly of CD16a, was unknown. This deficit was largely due to technical limitations including small FcγR amounts in the body, multiple N-glycosylation sites, and challenges with purifying and analyzing integral membrane proteins. The sections below will evaluate the endogenous composition of FcγRs. These studies identified a mixture of compositions present on primary leukocytes, including those forms that promote strong interactions and some that bind with lower affinity to highlight the potential for FcγR improvements.
Functional differences in FcγR alleles
Multiple variables contribute to FcγR variability including multiple alleles for some genes with distinct correlations to post-translational processing and antibody binding. Two predominant CD32a alleles encode either an R131 or H131 allotype; the latter exhibits greater IgG1-binding affinity (1). Two major CD16a alleles encode either a tighter-binding V158 allotype or the weaker F158 as discussed in the previous section. Minor CD16a forms include H48 (8%) or R48 (6%) in place of the more common L48 (86%) (32). Three CD16b alleles, NA1, NA2, or SH, are expressed as glycosylphosphatidylinositol-anchored proteins after proteolysis during expression, displaying negligible differences in antibody-binding affinity (1). Variability in major and minor FcγR alleles coupled with gene copy-number differences contribute to a highly dynamic and variable landscape of antibody-binding capability at the surface of an FcγR-expressing cell (27, 33). The nature and impact of these mixed receptor communities is unknown, but there is a potential for FcγR allotypes to interact in heretofore unpredicted ways, increasing the functional heterogeneity of the FcγR-expressing leukocytes from individual donors.
The high serum antibody concentration likewise impacts FcγRs at the cell surface. The in vitro equilibrium dissociation constants for IgG1 (1 nM–20 μM) are much lower than those of the IgG1 concentration in the serum (33–100 μM), suggesting all receptors are mostly to almost completely bound by antibodies in the serum. How do ligated receptors then bind immune complexes? Dissociation rates on the order of a second to minutes predict a reasonable level of ligand turnover for the “low-affinity” receptors that must first dissociate to bind immune complexes. Because the immune complexes have a higher affinity for the cell surface because of avidity effects, immune complex binding is favored. CD64 is a notable “high-affinity” FcγR that displays a very slow dissociation rate with a t1/2 ∼ 30 min although it is unclear how the slow kinetics impact cell activation. One benefit of this slow dissociation rate is that engineered CD64+ cells complexed with an antibody before infusion might retain the antibody for long enough to affect an antibody-mediated response in the body after infusion (34).
Antibodies are likewise glycoproteins with a distribution of glycoforms in the serum that provides a distribution of FcγR-binding affinities. Primary human NK cells retained a high percentage of tight-binding IgG1 glycoforms on the surface, likely selecting the tightest binding ligand from the pool of IgG1 in serum that contains much lower levels of this specific antibody glycoform (33). The tight-binding IgG1 glycoforms lack a fucose modification to the N-linked N-acetylglucosamine residue (fucose is a red triangle in Fig. 2; (35)). This enrichment indicates NK cells are potentially prioritizing responses to targets identified by antigen-specific antibodies lacking fucose found in patients with immune thrombocytopenia, HIV, and dengue and a likelihood of similar responses in other diseases (36, 37, 38).
Variable processing
In addition to different allotypes, post-translational processing introduces significant FcγR heterogeneity. Substantial advances in mass spectrometry including glycopeptide separations and analysis that allow a characterization of FcγRs from primary tissue highlight how each cell type specifically modifies each FcγR. The remainder of this section will review glycoproteomic characterizations of FcγRs purified from primary tissue that have only become available in the last 3 years to reveal forms present in the human body. These recent advances prove that individual cell type and environment lead to unique processing that is not recapitulated with recombinant expression systems. These differences include a much higher degree of sialylation and hybrid forms, as well as substantial branch elongation (11). This critical observation underscores the importance of using primary cells because it is impossible to discover novel functional roles for individual N-glycans if the endogenous glycoforms are not present in vitro.
Characterizing endogenous FcγRs, as discussed below, identified individual substrates present on cells that potentially impact cell function. In some cases, clear connections are already available, including the connection of CD32a N-glycans and inhibitory cis-interactions with Mac1 or the presence of high-affinity CD16a glycoforms on NK cells but not monocytes. There are many other features that remain to be explored, including extended complex-type branching that may serve as docking sites for cross-linking proteins at the cell surface. It is likely that many of these modifications will provide unique features for targeted improvements to FcγR-mediated therapies.
FcγRIIIa/CD16a
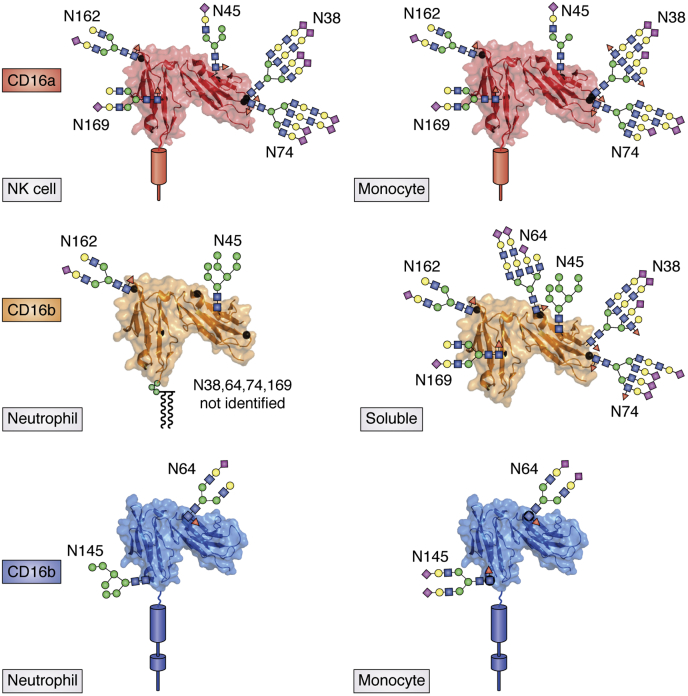
CD16a occupies a central role in many mAb therapies as the primary receptor initiating a cytotoxic cell–mediated response (39, 40). Furthermore, as noted above, CD16a processing affects antibody-binding affinity and potentially mAb efficacy; therefore, the characterization of CD16a processing is expected to inform mAb-based therapy development. It is not yet known, however, if CD16a glycoforms that bind tightly in vitro enhance cell activity or mAb efficacy. CD16a has five N-glycosylation sites, and composition at the N162 site directly impacts affinity in vitro (Fig. 3) (30).
Figure 3.
The predominant Fc γ receptor glycoforms identified in the tissue or serum from healthy donors. The N-glycans are scaled roughly to the appropriate size. The NK cell N-glycans increase the mass of the extracellular domain by 40%. The CD16 N45 glycan contributes to protein stability, the CD16a N162 glycan composition impacts antibody-binding affinity, and the CD32a N64 glycan inhibits antibody binding through cis-interactions on the neutrophil surface. N-glycan, glycans attached to Asn residues; NK, natural killer.
N162
The composition of the N-glycan at N162, located near the IgG1 Fc-binding interface, influenced binding affinity in vitro with oligomannose forms promoting the tightest interactions (28). Primary NK cells from five healthy donors revealed a wide distribution of N-glycans found at this site, unlike monocytes with no apparent variability between donors (33, 41). One V158/F158 heterozygous NK cell donor expressed exclusively oligomannose forms at N162 on the V158 allotype, although the predominant glycan found across all NK cell donors was a core-fucosylated complex-type structure with two branches and a single N-acetylneuraminic acid (Fig. 3). The other four NK cell donors expressed 5 to 40% of minimally processed hybrid- and oligomannose-type N-glycans at this site. If the in vitro affinity measurements represent binding in vivo, NK cells express CD16a with a range of antibody-binding affinities and cells with a higher proportion of tight-binding oligomannose N162 glycoforms potentially bind IgG1 with greater affinity. It is possible that protein processing is responsive to extracellular signals and cells have the ability to dynamically modulate receptor-binding affinity, although this hypothesis remains untested. Furthermore, N162 was the only site that showed substantial composition differences between donors.
N45
Characterizations of recombinantly expressed CD16a indicated a high proportion of oligomannose-type N-glycans, likely because of the formation of substantial contacts between sugars at this site and amino acid side chains (42, 43, 44). It was therefore surprising that N45 from primary NK cells contained predominantly hybrid-type N-glycans (27, 33). Monocytes showed a higher percentage of oligomannose forms, but hybrid types remained predominant (41). Hybrid forms are found on secreted proteins but are less common than complex or oligomannose types. This finding is supported by an earlier glycomics study showing a high percentage of hybrid N-glycans on CD16a from primary NK cells of three geriatric donors (28). It is likely that restricted processing is a product of intramolecular contacts and does not appear to impact antibody-binding affinity, stabilizing the protein.
A single amino acid substitution found in a small percentage of donors impacts N45 processing and is related to NK cell dysfunction. Roughly 8% of the population expresses CD16a H48, instead of the more common L48 allotype (32). Homozygosity of the H48-encoding allele is believed to be responsible for an NK cell–related immunodeficiency (45). N45 from NK cell CD16a H48 in a heterozygous donor revealed greater processing, unlike the comparable processing at the four other N-glycosylation sites (46). It is possible that these N45-glycan differences are attributable to H48 disrupting CD2 binding and exposing the N45 site for further glycan processing (45).
Another consistent theme of FcγR N45 N-glycans from primary cells is a high degree of capping at the termini of complex-type branches, in particular with N-acetylneuraminic acid.
N38 and N74
Two N-glycosylated loops opposite the antibody-binding site display the most highly processed sites on CD16a expressed by primary NK cells and monocytes (33, 41). These are predominantly tetra-antennary complex-type N-glycans (containing four branches) with four sialic acid residues plus the addition of N-acetyllactosamine (LacNAc) repeats that extend the complex-type branches (Fig. 2). LacNAc repeats are potent galectin ligands and are important for T-cell and B-cell function, although it is not clear how these features impact NK cells and monocytes (47, 48). Monocytes expressed glycans at these sites with slightly less N-acetylneuraminic acid and a greater degree of branch fucosylation that may bind to different crosslinking factors.
N169
Comparable to N162, the N169 site contained predominantly a complex-type biantennary N-glycan with a single N-acetylneuraminic acid; however, highly processed tetra-antennary N-glycans with LacNAc repeats appeared at low levels in NK cells and monocytes (33, 41). It is unclear why N38 and N74 are processed to a high extent but N169 and N162 experience less processing. N162 forms observable contacts with the protein surface; however, these are markedly weaker than those formed by N45; N169 does not appear restricted (43). It is likewise unclear how N169 glycan composition impacts CD16a properties as it is dispensable for antibody binding (30).
FcγRIIIb/CD16b
CD16b is highly homologous to CD16a at the amino acid level and accordingly shares many features with CD16a (Fig. 3). CD16b results from a gene duplication in humans after the split with chimps and is highly expressed as a glycosylphosphatidylinositol-anchored protein on neutrophils. CD16b is highly similar to CD16a at the amino acid level, with only a handful of differences in the extracellular domain when compared with CD16a in the sequence of the extracellular domain. There are, however, a few notable differences as discussed below in addition to two common allotypes: the CD16b NA2 and SH allotypes include an additional N-glycosylation site at N64 (six total sites), and the NA1 allele is not glycosylated at N45 because of a S47N substitution (four sites).
N38 and N74
N38 and N74 showed high levels of processing with branch sialylation and LacNAc repeats comparable with CD16a (49, 50).
N45
The CD16b NA2 N45 glycosylation site contains predominantly oligomannose-type N-glycans identified in contrast to the hybrid forms found on CD16a, although two reports differ in the proportion. One study using CD16b isolated from serum reported only oligomannose forms (49), whereas two others reported 80 to 90% oligomannose forms from neutrophils (27, 50). One report also identified lower percentages of oligomannose forms in soluble CD16 (40–60%) likely because of the presence of both CD16a and CD16b in the solution (27). Donor differences possibly explain the incongruent observations. It is unclear if cell- or protein-specific factors contribute to the significant processing differences when compared with the CD16a N45-glycan. Hetero-oligomers formed in the Golgi would limit processing of glycans buried during complexation, and glycan processing is a sensitive reporter of surface-exposed epitopes.
N64
Yagi et al. (49) reported high levels of N64 glycan processing for the soluble form of the NA2 allele, comparable with N38 and N74 but with less fucosylation and sialylation in contrast to Washburn et al. and Wojcik et al. who noted limited glycan occupancy at this site (27, 50, 51). It is unclear if N64 glycosylation impacts CD16b function.
N162
The primary CD16b N162-glycan was a core-fucosylated complex-type structure with two branches and a single α2-6 N-acetylneuraminic acid, consistent with CD16a, although no evidence for less-processed hybrid or oligomannose forms was found and one report analyzed material from 50 donors (27, 49, 50). The comparison with CD16a is notable because the IgG1 Fc binding affinity for the CD16b NA2 allotype, despite a high sequence identify to CD16a, is not affected by changing its N-glycan composition, unlike CD16a (30). Interestingly, the N-glycan sensitivity was introduced to CD16b NA2 through a D129G substitution, based on the residue found in CD16a that also increased affinity (31). Thus, a mechanism to modulate CD16a affinity that is specifically preserved does not appear to be present for CD16b. Finally, N162 processing on the NA1 allotype showed greater processing, potentially because of the loss of N45 glycosylation that serves to stabilize the two extracellular domains (27, 43). It is unclear if this increased processing affects function, but it is possible that adding sialic acid residues reduces antibody-binding affinity (26).
N169
This N-glycan shows substantial processing with a high degree of complex-type forms but less branching than glycans at N38 and N74 (49, 50).
FcγRIIa/CD32a
CD32a contains two N-glycosylation sites, and both sites are occupied at high levels. A sialylated N64 glycan is required to interact with Mac1 and inhibits CD32a on the neutrophil surface (51).
N64
Two separate studies identified predominantly sialylated complex-type N64-glycans with a monosialylated form predominant on neutrophils and a disialylated form on monocytes (41, 51) (Fig. 3). These reports identified biantennary and triantennary structures on both cell types with some occupancy of oligomannose forms on neutrophils and hybrid forms on monocytes. In either case, sialylated N-glycans are present to form interactions with Mac1.
N145
N145 showed comparable though slightly less processing than the N64 site on CD32a isolated from monocytes. These sites contain the same predominant sialylated biantennary glycans but a few more hybrid forms at lower abundance were present on N145. The N145 site of CD32a isolated from neutrophils, however, showed dramatically less processing with >75% oligomannose and hybrid forms. This difference in processing by two different cell types may indicate the presence of an additional factor in neutrophils that prevents N145 processing, comparable with the association of CD16a and CD2 in NK cells. These differences again suggest the possibility of cell type–specific functional diversification through N-glycan processing.
Soluble FcγRs
Activated leukocytes shed the receptor extracellular domains in the serum after proteolysis, with the exception of CD64. Soluble forms modulate multiple processes although it is not clear whether soluble receptor forms are simply inert products of activated cells or adopt specific anti-inflammatory roles (11). In principle, soluble receptors could compete with soluble antibodies and prevent FcγR-mediated cell activation, although antibodies greatly outnumber receptors in the peripheral compartment.
On NK cell activation induced by an antibody-coated target or artificially through a protein kinase C agonist, CD16a is shed after ADAM17-mediated proteolysis at S197 (52). Shedding likely increases serial engagement of target cells (one NK cell killing more than one target cell) and increases NK cell survival (53). ADAM17 is likewise responsible for shedding CD16b in neutrophils (54).
Conclusions from FcγR processing
Characterizing the products of endogenous FcγR processing identified the substrates to define the role of these individual modifications in receptor function and cell activity. Links between composition and function are known for sialylated CD32a N64 N-glycans from neutrophils that mediate Mac-1 interactions, and there is a possible role of CD16a N162 glycoforms in tuning antibody-binding affinity. The function of many other distinct features is not known, including LacNAc repeats on CD16 N38 and N74 that are found on at least 3 cell types. This compositional conservation strongly suggests a role in FcγR function. It is also possible that individual modifications, including branch fucosylation, impact complexes formed by receptors. Any functionally relevant modification has the potential to impact FcγR-dependent therapies, and it is likely that multiple critical glycan-mediated processes remain undiscovered. It is also important to note donor-, cell type-, and FcγR-specific differences in processing. It is clear that each site of each FcγR exists in an individual environment, and the individual processing must be studied at this level of resolution.
FcγR engineering
Antibodies have been the focus of many engineering efforts likely because of the rapidly deployable and highly generalizable nature of mAbs with relatively little attention paid to FcγRs themselves (55). Although FcγRs provide useful modules for chimeric protein design, these designs generally do not enhance FcγR functionality and, with one exception, will not be discussed here (a few examples are included (56, 57, 58, 59)). These early FcγR engineering reports provide a strong justification for further engineering to increase affinity and increase the activity of engineered leukocytes.
Engineering NK cells to express the stronger antibody binding CD16a V158 allotype benefits cancer treatment. CD16a V158 was reintroduced to cultured NK92 cells, originally derived from a 50-year-old patient but lack CD16 expression and antibody-mediated cytotoxicity (60, 61). After infusion, these cells showed greater efficacy toward the tumor than the patient’s endogenous NK cells. It remains to be seen if generating greater CD16a expression levels will lead to further increases in efficacy.
FcγR engineering to limit shedding is designed to improve cell function. The thought is that leukocytes that do not shed FcγRs will maintain an ability to engage new targets. CD16a is shed after ADAM17-mediated proteolysis at S197 (52). The CD16a S197P variant prevented proteolysis and shedding while improving the antitumor activity of human induced pluripotent stem cell-derived NK cells (62). Negative aspects of eliminating shedding include decreased serial engagement of target cells (one NK cell killing more than one target cell) and decreased NK cell survival (53).
One chimeric FcγR construct combined the antibody-binding affinity of the CD64 extracellular domain with the CD16a signaling interactions (34). This construct enhanced antibody-dependent cytotoxicity in NK92 and human induced pluripotent stem cell-derived NK cells. These cells also exhibited an increase in natural cytotoxicity, which is somewhat surprising, given a role for the CD16a extracellular domain in natural cytotoxicity (45). These cells likewise retained mAb to a greater extent after washes that may prove beneficial for ex vivo ligation followed by infiltration. It is unclear, however, if these cells retain the activity in the serum that may prevent immune complex binding by occupying the CD64 extracellular antibody-binding domain.
Engineering potential
Improving the FcγR function may be achieved by targeting the polypeptide or carbohydrate components. The recent developments in lymphocyte engineering described above provide a suitable platform to deploy engineered receptors.
The potency of carbohydrate manipulation as a strategy to improve biopharmaceutical efficacy is demonstrated by afucosylated mAbs (including Gazyva, discussed above) showing improved cytotoxicity and efficacy. Multiple glycoengineered antibodies are in various stages of development and likely represent the next generation of mAbs. FcγR glycoengineering would require targeted host cell manipulation of the known glycosyltransferase and glycosylhydrolase enzymes. Potential caveats include changing cell glycosylation to modify only the FcγR of interest without impacting other glycan-related processes. A worthy design goal for CD16a and CD32a includes reducing/eliminating sialylation to increase antibody-binding affinity in CD16a and prevent Mac-1–mediated inhibition of CD32a on neutrophils. Alternative approaches may include modifying the CD16a N162-glycan composition as oligomannose forms bind antibodies with greater affinity. Alternative, truncated forms at this site are possible through engineered site-specific glycosylhydrolases (63). Truncating the CD16a N162-glycan to a single N-acetylglucosamine residue promises to improve affinity to a greater extent than an oligomannose N-glycan; in vitro studies demonstrated that CD16a with truncated N-glycans bound IgG1 Fc tighter than CD16a with unmodified oligomannose N-glycans (44).
Many of the challenges associated with glycoengineering leukocytes to express customized FcγRs may be alleviated by engineering extracellular domains for immune system modulation as soluble, serum-borne fragments. These proteins are expected to dampen antibody-mediated mechanisms as is proposed for their natural soluble counterparts.
Engineering the polypeptide fraction of FcγRs represents a substantial challenge, but one with a considerable payoff considering the impact of engineered IgG Fcs that were developed from computational design and directed evolution (64). FcγRs could be engineered to enhance favorable properties, including increased antibody-binding affinity or greater clustering, residence time, and so forth. One challenge of directed evolution techniques is preparing proteins with appropriate processing. Although yeast strains producing mammalian glycans are available, expression yield is generally low and the glycan repertoires are limited (65). Surface display using a mammalian expression host may provide a more tractable engineering platform (66, 67, 68, 69).
Conclusions
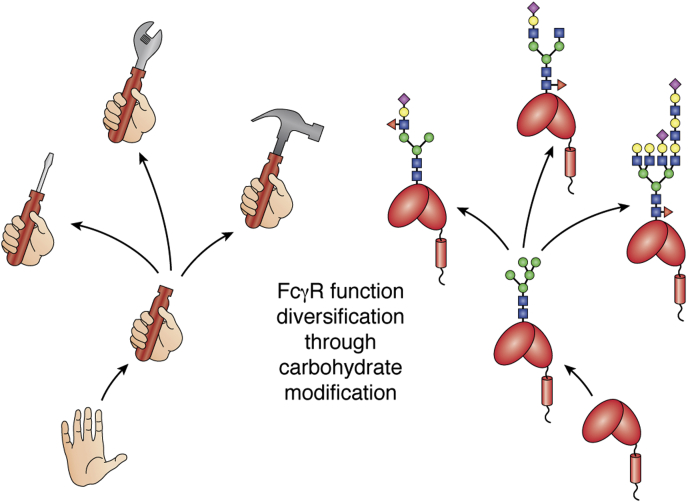
The FcγRs promote or suppress immune responses and represent an important motif for engineering to increase the efficacy of antibody-mediated therapies. These surface cell receptors are highly heterogeneous, resulting from different genes, alleles, and differential processing. Processing in the form of extensive carbohydrate additions provides different functions to each receptor, although this area is only lightly explored and there is a distinct possibility that multiple functions remain undefined (Fig. 4). In addition, selecting/enhancing FcγR forms with superior properties from the pool available in the body or engineering these receptors to increase function promises new treatments to many diseases.
Figure 4.
Extensive carbohydrate modifications provide FcγRs with distinct functions. Some enhance antibody-binding affinity, some repress activity, and some likely bind cell matrix proteins. FcγRs, Fc γ receptors.
Conflict of interest
The author declares that they have no conflicts of interest with the contents of this article.
Acknowledgments
Author contributions
All contributions made by A. W. B.
Funding and additional information
This study was supported by the National Institutes of Health under Award No. R01 GM115489 (National Institute of General Medical Sciences) and R21 AI142122 (National Institute of Allergy and Infectious Diseases) (to A. W. B.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Edited by Peter Cresswell
References
- 1.Bruhns P., Iannascoli B., England P., Mancardi D.A., Fernandez N., Jorieux S., Daeron M. Specificity and affinity of human Fcgamma receptors and their polymorphic variants for human IgG subclasses. Blood. 2009;113:3716–3725. doi: 10.1182/blood-2008-09-179754. [DOI] [PubMed] [Google Scholar]
- 2.Bonifant C.L., Jackson H.J., Brentjens R.J., Curran K.J. Toxicity and management in CAR T-cell therapy. Mol. Ther. Oncolytics. 2016;3:16011. doi: 10.1038/mto.2016.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu E., Marin D., Banerjee P., Macapinlac H.A., Thompson P., Basar R., Nassif Kerbauy L., Overman B., Thall P., Kaplan M., Nandivada V., Kaur I., Nunez Cortes A., Cao K., Daher M. Use of CAR-transduced natural killer cells in CD19-positive lymphoid tumors. N. Engl. J. Med. 2020;382:545–553. doi: 10.1056/NEJMoa1910607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rezvani K., Rouce R.H. The application of natural killer cell immunotherapy for the treatment of cancer. Front. Immunol. 2015;6:578. doi: 10.3389/fimmu.2015.00578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang C., Oberoi P., Oelsner S., Waldmann A., Lindner A., Tonn T., Wels W.S. Chimeric antigen receptor-engineered NK-92 cells: an off-the-shelf cellular therapeutic for targeted elimination of cancer cells and induction of protective antitumor immunity. Front. Immunol. 2017;8:533. doi: 10.3389/fimmu.2017.00533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klingemann H., Grodman C., Cutler E., Duque M., Kadidlo D., Klein A.K., Sprague K.A., Miller K.B., Comenzo R.L., Kewalramani T., Yu N., Van Etten R.A., McKenna D.H. Autologous stem cell transplant recipients tolerate haploidentical related-donor natural killer cell-enriched infusions. Transfusion. 2013;53:412–418. doi: 10.1111/j.1537-2995.2012.03764.x. quiz 411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nimmerjahn F., Ravetch J.V. FcgammaRs in health and disease. Curr. Top Microbiol. Immunol. 2011;350:105–125. doi: 10.1007/82_2010_86. [DOI] [PubMed] [Google Scholar]
- 8.Chenoweth A.M., Wines B.D., Anania J.C., Mark Hogarth P. Harnessing the immune system via FcgammaR function in immune therapy: a pathway to next-gen mAbs. Immunol. Cell Biol. 2020;98:287–304. doi: 10.1111/imcb.12326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li X., Wu J., Ptacek T., Redden D.T., Brown E.E., Alarcon G.S., Ramsey-Goldman R., Petri M.A., Reveille J.D., Kaslow R.A., Kimberly R.P., Edberg J.C. Allelic-dependent expression of an activating Fc receptor on B cells enhances humoral immune responses. Sci. Transl. Med. 2013;5 doi: 10.1126/scitranslmed.3007097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yeap W.H., Wong K.L., Shimasaki N., Teo E.C., Quek J.K., Yong H.X., Diong C.P., Bertoletti A., Linn Y.C., Wong S.C. CD16 is indispensable for antibody-dependent cellular cytotoxicity by human monocytes. Sci. Rep. 2016;6:34310. doi: 10.1038/srep34310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patel K.R., Roberts J.T., Barb A.W. Multiple variables at the leukocyte cell surface impact Fc gamma receptor-dependent mechanisms. Front. Immunol. 2019;10:223. doi: 10.3389/fimmu.2019.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Subedi G.P., Barb A.W. The immunoglobulin G1 N-glycan composition affects binding to each low affinity Fc gamma receptor. MAbs. 2016;8:1512–1524. doi: 10.1080/19420862.2016.1218586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Romain G., Senyukov V., Rey-Villamizar N., Merouane A., Kelton W., Liadi I., Mahendra A., Charab W., Georgiou G., Roysam B., Lee D.A., Varadarajan N. Antibody Fc engineering improves frequency and promotes kinetic boosting of serial killing mediated by NK cells. Blood. 2014;124:3241–3249. doi: 10.1182/blood-2014-04-569061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clynes R.A., Towers T.L., Presta L.G., Ravetch J.V. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat. Med. 2000;6:443–446. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- 15.Sehn L.H., Chua N., Mayer J., Dueck G., Trneny M., Bouabdallah K., Fowler N., Delwail V., Press O., Salles G., Gribben J., Lennard A., Lugtenburg P.J., Dimier N., Wassner-Fritsch E. Obinutuzumab plus bendamustine versus bendamustine monotherapy in patients with rituximab-refractory indolent non-Hodgkin lymphoma (GADOLIN): a randomised, controlled, open-label, multicentre, phase 3 trial. Lancet Oncol. 2016;17:1081–1093. doi: 10.1016/S1470-2045(16)30097-3. [DOI] [PubMed] [Google Scholar]
- 16.Niederfellner G., Lammens A., Mundigl O., Georges G.J., Schaefer W., Schwaiger M., Franke A., Wiechmann K., Jenewein S., Slootstra J.W., Timmerman P., Brannstrom A., Lindstrom F., Mossner E., Umana P. Epitope characterization and crystal structure of GA101 provide insights into the molecular basis for type I/II distinction of CD20 antibodies. Blood. 2011;118:358–367. doi: 10.1182/blood-2010-09-305847. [DOI] [PubMed] [Google Scholar]
- 17.Salles G., Morschhauser F., Lamy T., Milpied N., Thieblemont C., Tilly H., Bieska G., Asikanius E., Carlile D., Birkett J., Pisa P., Cartron G. Phase 1 study results of the type II glycoengineered humanized anti-CD20 monoclonal antibody obinutuzumab (GA101) in B-cell lymphoma patients. Blood. 2012;119:5126–5132. doi: 10.1182/blood-2012-01-404368. [DOI] [PubMed] [Google Scholar]
- 18.Mossner E., Brunker P., Moser S., Puntener U., Schmidt C., Herter S., Grau R., Gerdes C., Nopora A., van Puijenbroek E., Ferrara C., Sondermann P., Jager C., Strein P., Fertig G. Increasing the efficacy of CD20 antibody therapy through the engineering of a new type II anti-CD20 antibody with enhanced direct and immune effector cell-mediated B-cell cytotoxicity. Blood. 2010;115:4393–4402. doi: 10.1182/blood-2009-06-225979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamaguchi Y., Barb A.W. A synopsis of recent developments defining how N-glycosylation impacts immunoglobulin G structure and function. Glycobiology. 2020;30:214–225. doi: 10.1093/glycob/cwz068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bibeau F., Lopez-Crapez E., Di Fiore F., Thezenas S., Ychou M., Blanchard F., Lamy A., Penault-Llorca F., Frebourg T., Michel P., Sabourin J.C., Boissiere-Michot F. Impact of Fc{gamma}RIIa-Fc{gamma}RIIIa polymorphisms and KRAS mutations on the clinical outcome of patients with metastatic colorectal cancer treated with cetuximab plus irinotecan. J. Clin. Oncol. 2009;27:1122–1129. doi: 10.1200/JCO.2008.18.0463. [DOI] [PubMed] [Google Scholar]
- 21.Zhang W., Gordon M., Schultheis A.M., Yang D.Y., Nagashima F., Azuma M., Chang H.M., Borucka E., Lurje G., Sherrod A.E., Iqbal S., Groshen S., Lenz H.J. FCGR2A and FCGR3A polymorphisms associated with clinical outcome of epidermal growth factor receptor expressing metastatic colorectal cancer patients treated with single-agent cetuximab. J. Clin. Oncol. 2007;25:3712–3718. doi: 10.1200/JCO.2006.08.8021. [DOI] [PubMed] [Google Scholar]
- 22.Cartron G., Dacheux L., Salles G., Solal-Celigny P., Bardos P., Colombat P., Watier H. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood. 2002;99:754–758. doi: 10.1182/blood.v99.3.754. [DOI] [PubMed] [Google Scholar]
- 23.Weng W.K., Levy R. Two immunoglobulin G fragment C receptor polymorphisms independently predict response to rituximab in patients with follicular lymphoma. J. Clin. Oncol. 2003;21:3940–3947. doi: 10.1200/JCO.2003.05.013. [DOI] [PubMed] [Google Scholar]
- 24.Musolino A., Naldi N., Bortesi B., Pezzuolo D., Capelletti M., Missale G., Laccabue D., Zerbini A., Camisa R., Bisagni G., Neri T.M., Ardizzoni A. Immunoglobulin G fragment C receptor polymorphisms and clinical efficacy of trastuzumab-based therapy in patients with HER-2/neu-positive metastatic breast cancer. J. Clin. Oncol. 2008;26:1789–1796. doi: 10.1200/JCO.2007.14.8957. [DOI] [PubMed] [Google Scholar]
- 25.Moremen K.W., Tiemeyer M., Nairn A.V. Vertebrate protein glycosylation: diversity, synthesis and function. Nat. Rev. Mol. Cell Biol. 2012;13:448–462. doi: 10.1038/nrm3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hayes J.M., Frostell A., Karlsson R., Muller S., Martin S.M., Pauers M., Reuss F., Cosgrave E.F., Anneren C., Davey G.P., Rudd P.M. Identification of Fc gamma receptor glycoforms that produce differential binding kinetics for rituximab. Mol. Cell Proteomics. 2017;16:1770–1788. doi: 10.1074/mcp.M117.066944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Washburn N., Meccariello R., Duffner J., Getchell K., Holte K., Prod'homme T., Srinivasan K., Prenovitz R., Lansing J., Capila I., Kaundinya G., Manning A.M., Bosques C.J. Characterization of endogenous human FcgammaRIII by mass spectrometry reveals site, allele and sequence specific glycosylation. Mol. Cell Proteomics. 2019;18:534–545. doi: 10.1074/mcp.RA118.001142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patel K.R., Roberts J.T., Subedi G.P., Barb A.W. Restricted processing of CD16a/Fc gamma receptor IIIa N-glycans from primary human NK cells impacts structure and function. J. Biol. Chem. 2018;293:3477–3489. doi: 10.1074/jbc.RA117.001207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cambay F., Forest-Nault C., Dumoulin L., Seguin A., Henry O., Durocher Y., De Crescenzo G. Glycosylation of Fcgamma receptors influences their interaction with various IgG1 glycoforms. Mol. Immunol. 2020;121:144–158. doi: 10.1016/j.molimm.2020.03.010. [DOI] [PubMed] [Google Scholar]
- 30.Subedi G.P., Barb A.W. CD16a with oligomannose-type N-glycans is the only “low-affinity” Fc gamma receptor that binds the IgG crystallizable fragment with high affinity in vitro. J. Biol. Chem. 2018;293:16842–16850. doi: 10.1074/jbc.RA118.004998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roberts J.T., Barb A.W. A single amino acid distorts the Fc gamma receptor IIIb/CD16b structure upon binding immunoglobulin G1 and reduces affinity relative to CD16a. J. Biol. Chem. 2018;293:19899–19908. doi: 10.1074/jbc.RA118.005273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Haas M., Koene H.R., Kleijer M., de Vries E., Simsek S., van Tol M.J., Roos D., von dem Borne A.E. A triallelic Fc gamma receptor type IIIA polymorphism influences the binding of human IgG by NK cell Fc gamma RIIIa. J. Immunol. 1996;156:2948–2955. [PubMed] [Google Scholar]
- 33.Patel K.R., Nott J.D., Barb A.W. Primary human natural killer cells retain proinflammatory IgG1 at the cell surface and express CD16a glycoforms with donor-dependent variability. Mol. Cell Proteomics. 2019;18:2178–2190. doi: 10.1074/mcp.RA119.001607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Snyder K.M., Hullsiek R., Mishra H.K., Mendez D.C., Li Y., Rogich A., Kaufman D.S., Wu J., Walcheck B. Expression of a recombinant high affinity IgG Fc receptor by engineered NK cells as a docking platform for therapeutic mAbs to target cancer cells. Front. Immunol. 2018;9:2873. doi: 10.3389/fimmu.2018.02873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shields R.L., Lai J., Keck R., O'Connell L.Y., Hong K., Meng Y.G., Weikert S.H., Presta L.G. Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human Fcgamma RIII and antibody-dependent cellular toxicity. J. Biol. Chem. 2002;277:26733–26740. doi: 10.1074/jbc.M202069200. [DOI] [PubMed] [Google Scholar]
- 36.Kapur R., Kustiawan I., Vestrheim A., Koeleman C.A., Visser R., Einarsdottir H.K., Porcelijn L., Jackson D., Kumpel B., Deelder A.M., Blank D., Skogen B., Killie M.K., Michaelsen T.E., de Haas M. A prominent lack of IgG1-Fc fucosylation of platelet alloantibodies in pregnancy. Blood. 2014;123:471–480. doi: 10.1182/blood-2013-09-527978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ackerman M.E., Crispin M., Yu X., Baruah K., Boesch A.W., Harvey D.J., Dugast A.S., Heizen E.L., Ercan A., Choi I., Streeck H., Nigrovic P.A., Bailey-Kellogg C., Scanlan C., Alter G. Natural variation in Fc glycosylation of HIV-specific antibodies impacts antiviral activity. J. Clin. Invest. 2013;123:2183–2192. doi: 10.1172/JCI65708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang T.T., Sewatanon J., Memoli M.J., Wrammert J., Bournazos S., Bhaumik S.K., Pinsky B.A., Chokephaibulkit K., Onlamoon N., Pattanapanyasat K., Taubenberger J.K., Ahmed R., Ravetch J.V. IgG antibodies to dengue enhanced for FcgammaRIIIA binding determine disease severity. Science. 2017;355:395–398. doi: 10.1126/science.aai8128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Battella S., Cox M.C., Santoni A., Palmieri G. Natural killer (NK) cells and anti-tumor therapeutic mAb: unexplored interactions. J. Leukoc. Biol. 2016;99:87–96. doi: 10.1189/jlb.5VMR0415-141R. [DOI] [PubMed] [Google Scholar]
- 40.Guillerey C., Huntington N.D., Smyth M.J. Targeting natural killer cells in cancer immunotherapy. Nat. Immunol. 2016;17:1025–1036. doi: 10.1038/ni.3518. [DOI] [PubMed] [Google Scholar]
- 41.Roberts J.T., Patel K.R., Barb A.W. Site-specific N-glycan analysis of antibody-binding Fc gamma receptors from primary human monocytes. Mol. Cell Proteomics. 2020;19:362–374. doi: 10.1074/mcp.RA119.001733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zeck A., Pohlentz G., Schlothauer T., Peter-Katalinic J., Regula J.T. Cell type-specific and site directed N-glycosylation pattern of FcgammaRIIIa. J. Proteome Res. 2011;10:3031–3039. doi: 10.1021/pr1012653. [DOI] [PubMed] [Google Scholar]
- 43.Subedi G.P., Falconer D.J., Barb A.W. Carbohydrate-polypeptide contacts in the antibody receptor CD16A identified through solution NMR spectroscopy. Biochemistry. 2017;56:3174–3177. doi: 10.1021/acs.biochem.7b00392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Falconer D.J., Subedi G.P., Marcella A.M., Barb A.W. Antibody fucosylation lowers the FcgammaRIIIa/CD16a affinity by limiting the conformations sampled by the N162-glycan. ACS Chem. Biol. 2018;13:2179–2189. doi: 10.1021/acschembio.8b00342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mace E.M., Orange J.S. Genetic causes of human NK cell deficiency and their effect on NK cell subsets. Front. Immunol. 2016;7:545. doi: 10.3389/fimmu.2016.00545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Patel K.R., Roberts J.T., Barb A.W. Allotype-specific processing of the CD16a N45-glycan from primary human natural killer cells and monocytes. Glycobiology. 2020;30:427–432. doi: 10.1093/glycob/cwaa002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Giovannone N., Liang J., Antonopoulos A., Geddes Sweeney J., King S.L., Pochebit S.M., Bhattacharyya N., Lee G.S., Dell A., Widlund H.R., Haslam S.M., Dimitroff C.J. Galectin-9 suppresses B cell receptor signaling and is regulated by I-branching of N-glycans. Nat. Commun. 2018;9:3287. doi: 10.1038/s41467-018-05770-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Demetriou M., Granovsky M., Quaggin S., Dennis J.W. Negative regulation of T-cell activation and autoimmunity by Mgat5 N-glycosylation. Nature. 2001;409:733–739. doi: 10.1038/35055582. [DOI] [PubMed] [Google Scholar]
- 49.Yagi H., Takakura D., Roumenina L.T., Fridman W.H., Sautes-Fridman C., Kawasaki N., Kato K. Site-specific N-glycosylation analysis of soluble Fcgamma receptor IIIb in human serum. Sci. Rep. 2018;8:2719. doi: 10.1038/s41598-018-21145-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wojcik I., Senard T., de Graaf E.L., Janssen G.M.C., de Ru A.H., Mohammed Y., van Veelen P.A., Vidarsson G., Wuhrer M., Falck D. Site-specific glycosylation mapping of Fc gamma receptor IIIb from neutrophils of individual healthy donors. Anal. Chem. 2020;92:13172–13181. doi: 10.1021/acs.analchem.0c02342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Saggu G., Okubo K., Chen Y., Vattepu R., Tsuboi N., Rosetti F., Cullere X., Washburn N., Tahir S., Rosado A.M., Holland S.M., Anthony R.M., Sen M., Zhu C., Mayadas T.N. Cis interaction between sialylated FcgammaRIIA and the alphaI-domain of Mac-1 limits antibody-mediated neutrophil recruitment. Nat. Commun. 2018;9:5058. doi: 10.1038/s41467-018-07506-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jing Y., Ni Z., Wu J., Higgins L., Markowski T.W., Kaufman D.S., Walcheck B. Identification of an ADAM17 cleavage region in human CD16 (FcgammaRIII) and the engineering of a non-cleavable version of the receptor in NK cells. PLoS One. 2015;10 doi: 10.1371/journal.pone.0121788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Srpan K., Ambrose A., Karampatzakis A., Saeed M., Cartwright A.N.R., Guldevall K., De Matos G., Onfelt B., Davis D.M. Shedding of CD16 disassembles the NK cell immune synapse and boosts serial engagement of target cells. J. Cell Biol. 2018;217:3267–3283. doi: 10.1083/jcb.201712085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang Y., Wu J., Newton R., Bahaie N.S., Long C., Walcheck B. ADAM17 cleaves CD16b (FcgammaRIIIb) in human neutrophils. Biochim. Biophys. Acta. 2013;1833:680–685. doi: 10.1016/j.bbamcr.2012.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Saunders K.O. Conceptual approaches to modulating antibody effector functions and circulation half-life. Front. Immunol. 2019;10:1296. doi: 10.3389/fimmu.2019.01296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wiernik A., Foley B., Zhang B., Verneris M.R., Warlick E., Gleason M.K., Ross J.A., Luo X., Weisdorf D.J., Walcheck B., Vallera D.A., Miller J.S. Targeting natural killer cells to acute myeloid leukemia in vitro with a CD16 x 33 bispecific killer cell engager and ADAM17 inhibition. Clin. Cancer Res. 2013;19:3844–3855. doi: 10.1158/1078-0432.CCR-13-0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li W., Wu Y., Kong D., Yang H., Wang Y., Shao J., Feng Y., Chen W., Ma L., Ying T., Dimitrov D.S. One-domain CD4 fused to human anti-CD16 antibody domain mediates effective killing of HIV-1-Infected cells. Sci. Rep. 2017;7:9130. doi: 10.1038/s41598-017-07966-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tanaka H., Fujiwara H., Ochi F., Tanimoto K., Casey N., Okamoto S., Mineno J., Kuzushima K., Shiku H., Sugiyama T., Barrett A.J., Yasukawa M. Development of engineered T cells expressing a chimeric CD16-CD3zeta receptor to improve the clinical efficacy of mogamulizumab therapy against adult T-cell leukemia. Clin. Cancer Res. 2016;22:4405–4416. doi: 10.1158/1078-0432.CCR-15-2714. [DOI] [PubMed] [Google Scholar]
- 59.Marklin M., Hagelstein I., Koerner S.P., Rothfelder K., Pfluegler M.S., Schumacher A., Grosse-Hovest L., Jung G., Salih H.R. Bispecific NKG2D-CD3 and NKG2D-CD16 fusion proteins for induction of NK and T cell reactivity against acute myeloid leukemia. J. Immunother. Cancer. 2019;7:143. doi: 10.1186/s40425-019-0606-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gong J.H., Maki G., Klingemann H.G. Characterization of a human cell line (NK-92) with phenotypical and functional characteristics of activated natural killer cells. Leukemia. 1994;8:652–658. [PubMed] [Google Scholar]
- 61.Jochems C., Hodge J.W., Fantini M., Fujii R., Morillon Y.M., 2nd, Greiner J.W., Padget M.R., Tritsch S.R., Tsang K.Y., Campbell K.S., Klingemann H., Boissel L., Rabizadeh S., Soon-Shiong P., Schlom J. An NK cell line (haNK) expressing high levels of granzyme and engineered to express the high affinity CD16 allele. Oncotarget. 2016;7:86359–86373. doi: 10.18632/oncotarget.13411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhu H., Blum R.H., Bjordahl R., Gaidarova S., Rogers P., Lee T.T., Abujarour R., Bonello G.B., Wu J., Tsai P.F., Miller J.S., Walcheck B., Valamehr B., Kaufman D.S. Pluripotent stem cell-derived NK cells with high-affinity noncleavable CD16a mediate improved antitumor activity. Blood. 2020;135:399–410. doi: 10.1182/blood.2019000621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Umekawa M., Li C., Higashiyama T., Huang W., Ashida H., Yamamoto K., Wang L.X. Efficient glycosynthase mutant derived from Mucor hiemalis endo-beta-N-acetylglucosaminidase capable of transferring oligosaccharide from both sugar oxazoline and natural N-glycan. J. Biol. Chem. 2010;285:511–521. doi: 10.1074/jbc.M109.059832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang X., Mathieu M., Brezski R.J. IgG Fc engineering to modulate antibody effector functions. Protein Cell. 2018;9:63–73. doi: 10.1007/s13238-017-0473-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hamilton S.R., Davidson R.C., Sethuraman N., Nett J.H., Jiang Y., Rios S., Bobrowicz P., Stadheim T.A., Li H., Choi B.K., Hopkins D., Wischnewski H., Roser J., Mitchell T., Strawbridge R.R. Humanization of yeast to produce complex terminally sialylated glycoproteins. Science. 2006;313:1441–1443. doi: 10.1126/science.1130256. [DOI] [PubMed] [Google Scholar]
- 66.Choi B.K., Bobrowicz P., Davidson R.C., Hamilton S.R., Kung D.H., Li H., Miele R.G., Nett J.H., Wildt S., Gerngross T.U. Use of combinatorial genetic libraries to humanize N-linked glycosylation in the yeast Pichia pastoris. Proc. Natl. Acad. Sci. U. S. A. 2003;100:5022–5027. doi: 10.1073/pnas.0931263100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ho M., Nagata S., Pastan I. Isolation of anti-CD22 Fv with high affinity by Fv display on human cells. Proc. Natl. Acad. Sci. U. S. A. 2006;103:9637–9642. doi: 10.1073/pnas.0603653103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vervecken W., Kaigorodov V., Callewaert N., Geysens S., De Vusser K., Contreras R. In vivo synthesis of mammalian-like, hybrid-type N-glycans in Pichia pastoris. Appl. Environ. Microbiol. 2004;70:2639–2646. doi: 10.1128/AEM.70.5.2639-2646.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bobrowicz P., Davidson R.C., Li H., Potgieter T.I., Nett J.H., Hamilton S.R., Stadheim T.A., Miele R.G., Bobrowicz B., Mitchell T., Rausch S., Renfer E., Wildt S. Engineering of an artificial glycosylation pathway blocked in core oligosaccharide assembly in the yeast Pichia pastoris: production of complex humanized glycoproteins with terminal galactose. Glycobiology. 2004;14:757–766. doi: 10.1093/glycob/cwh104. [DOI] [PubMed] [Google Scholar]
- 70.Hibbs M.L., Selvaraj P., Carpen O., Springer T.A., Kuster H., Jouvin M.H., Kinet J.P. Mechanisms for regulating expression of membrane isoforms of Fc gamma RIII (CD16) Science. 1989;246:1608–1611. doi: 10.1126/science.2531918. [DOI] [PubMed] [Google Scholar]
- 71.Lanier L.L., Yu G., Phillips J.H. Co-association of CD3 zeta with a receptor (CD16) for IgG Fc on human natural killer cells. Nature. 1989;342:803–805. doi: 10.1038/342803a0. [DOI] [PubMed] [Google Scholar]
- 72.Hoyos V., Savoldo B., Quintarelli C., Mahendravada A., Zhang M., Vera J., Heslop H.E., Rooney C.M., Brenner M.K., Dotti G. Engineering CD19-specific T lymphocytes with interleukin-15 and a suicide gene to enhance their anti-lymphoma/leukemia effects and safety. Leukemia. 2010;24:1160–1170. doi: 10.1038/leu.2010.75. [DOI] [PMC free article] [PubMed] [Google Scholar]