Abstract
Autologous breast reconstructions using deep inferior epigastric perforator (DIEP) flaps create a large incision, presenting an opportunity for surgical site complications. In this pilot study, we aimed to examine outcomes in DIEP donor site incisions managed with standard dressings (control; n = 5) or closed incision negative pressure therapy (ciNPT; n = 5). We observed no significant differences between group age, body mass index, and past medical history. Both treatment groups had a similar duration of hospital stay, the number of blood transfusions, and pain scores on postoperative day 2 (P > .05). There was a trend of higher drainage (P = .251) and shorter time to incision healing (P = .067) in the ciNPT group than the control though the difference was not statistically significant. We did observe a significant improvement in scar pigmentation, vascularity, and pliability at 3, 6, and 12 months post‐surgery in the ciNPT group compared with control (P < .05). No surgical site complications were reported in the ciNPT group within the follow‐up period. In the control group, one patient developed wound edge fat necrosis requiring reoperation. In conclusion, we report that ciNPT is a useful incision management system for DIEP flap donor site incisions and it facilitated improved scar quality over standard dressings in this small pilot study. Further clinical studies are required to assess the full advantages provided by ciNPT.
Keywords: breast reconstruction, cicatrix, negative pressure wound therapy, surgical wound, wound healing
1. INTRODUCTION
Among patients undergoing mastectomy for the treatment of breast cancer, reconstruction of the breast is a common procedure that may occur immediately or after a period of delay in accordance with patient health and needs. For patients preferring autologous reconstruction, breast reconstruction using a deep inferior epigastric perforator (DIEP) flap is an option to create a natural appearance and texture.1 This procedure involves the removal of skin, fat tissue, and perforators from the lower abdomen to create a free flap, which is then transplanted to the patient's chest and shaped to create a new breast. After DIEP flap reconstruction, both the reconstructed breasts and the donor site require postoperative care and monitoring to facilitate uncomplicated incision healing and minimal scarring. Closed incision negative pressure therapy (ciNPT) is one method of incision management that has been used to promote closure and support positive outcomes.2, 3, 4, 5, 6 In this study, we aimed to identify any differences in outcomes between patients receiving postoperative abdominal incision care using ciNPT and standard occlusive dressings.
2. METHODS
This retrospective study involved 10 patients who underwent immediate bipedicled DIEP flap breast reconstruction at the Ditmanson Medical Foundation Chia‐Yi Christian Hospital between August 2017 and January 2018. Approval of the study protocol by the hospital's Institutional Review Board was sought and obtained in accordance with the Good Clinical Practice guidelines and government laws and regulations. In the control group (n = 5), the abdominal incision was covered with an occlusive hydrocolloid silver dressing (AQUACEL Ag Surgical; ConvaTec, Deeside, United Kingdom). In the ciNPT group (n = 5), patients received ciNPT (PREVENA Incision Management System with PREVENA CUSTOMIZABLE Dressing or PREVENA PEEL & PLACE Dressing; KCI, An Acelity Company; San Antonio, Texas) over the abdominal incision (Figure 1). For both groups, the dressings remained in place for 6 days postoperatively. Abdominal drains were removed when exudate was less than 15 mL per day, after which patients were discharged from the hospital.
Figure 1.
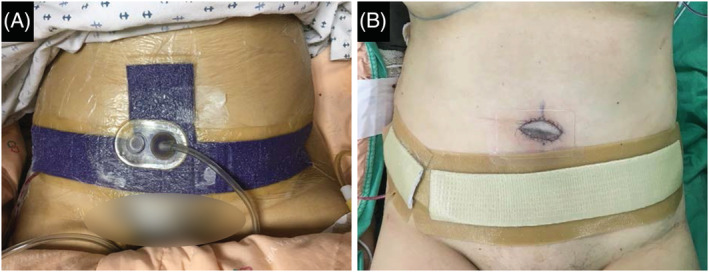
Application of closed incision negative pressure therapy (A) or occlusions dressings (B) on postoperative day 1
Incision drainage was recorded on postoperative days 1 through 6. Patient pain levels were scored according to the numeric rating scale (NRS‐11), which is an 11‐point scale for patient self‐reporting of pain.7 Surgical scar appearance was evaluated using the Vancouver Scar Score8 by a blinded plastic surgeon 3, 6, and 12 months after the operation. Subcategories such as pigmentation, vascularity, and height were scored on a 0 to 3 scale and pliability was scored on a 0 to 5 scale, with 0 representing “normal” incision healing and higher scores representing increasingly pathologic scarring characteristics.
Unless stated otherwise, all inferential statistical analyses were performed using a two‐tailed test at α = .05 significance level. All recorded data were used in the analysis, and there were no missing data. All statistical analyses were carried out using the SAS software release 9.4 (SAS Institute Inc.; Cary, North Carolina). The Wilcoxon rank‐sum test was performed for pain data, the duration of hospital stay, the number of blood transfusions, time to incision healing (defined as elapsed time from operation day to the removal of sutures), and postoperative drainage data within the 6‐day period. For postoperative scar quality data at 3, 6, and 12 months, the Friedman test was performed. Significant results for the treatment group factor from the repeated measures model were further examined using the Wilcoxon rank‐sum test at each time point without adjustment for multiplicity.
3. RESULTS
The two treatment groups did not significantly differ in age, body mass index, or past medical history (Table 1). During therapy, there were no significant differences in the duration of hospital stay or the number of blood transfusions. The median drainage volume for the ciNPT and control groups was 113 and 67 mL, respectively. The pain scores on day 2 and time to incision healing trended lower in the ciNPT group compared with the control group. However, none of these differences were statistically significant (Table 2).
Table 1.
Patient characteristics
| ciNPT (n = 5) | Control (n = 5) | P value | |
|---|---|---|---|
| Age (yr; mean, range) | 46 (43‐48) | 48 (41‐48) | .916 |
| Body mass index (mean, range) | 23.83 (23.44‐26.04) | 22.31 (20.17‐24.44) | .465 |
| Past medical history (n, %) | |||
| Smoking | 0 (0%) | 1 (20%) | .292 |
| Chemotherapy | 0 (0%) | 1 (20%) | .292 |
Abbreviation: ciNPT, closed incision negative pressure therapy.
Table 2.
Treatment and outcomes
| ciNPT (n = 5) | Control (n = 5) | P value | |
|---|---|---|---|
| Hospital stay (d; mean, range) | 8 (8‐10) | 8 (8‐8) | .239 |
| Drainage volume (mL; mean, range) | |||
| First 3 d | 92 (44‐97) | 59 (35‐61) | .175 |
| Total | 113 (62‐177) | 67 (44‐77) | .251 |
| Blood transfusions (n, %) | 3 (60%) | 1 (20%) | .524 |
| Pain score (mean, range) | 5.4 (4‐7) | 6.6 (5‐8) | .163 |
| Time to incision healing (d; mean, range) | 13 (13) | 17.2 (13‐20) | .067 |
Abbreviation: ciNPT, closed incision negative pressure therapy.
Patients were evaluated for scarring according to the Vancouver Scar Scale at 3‐, 6‐, and 12‐month follow‐ups (Figure 2). The surgical scars were evaluated for pigmentation, vascularity, pliability, and height. The scores for scars managed with ciNPT were significantly lower than controls in each category at all time points (P < .05), with the exception of height at the 12‐month follow‐up (P = .067). A comparison of scores across the entire 12‐month observation period found that scores for the ciNPT group were significantly lower than controls in all scoring categories (P < .0001; Figure 3). When the scores were totalled and compared between groups, scores for the ciNPT group were lower than the control group at 3, 6, and 12 months (P < .05).
Figure 2.
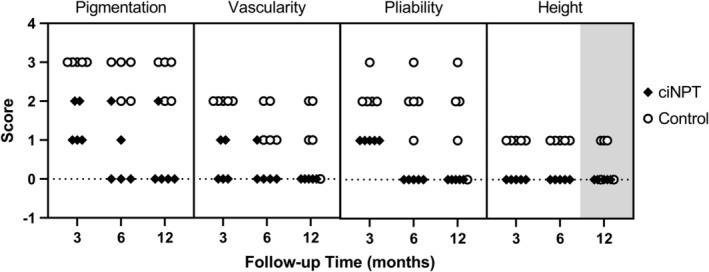
Scores of scarring in patients receiving incision care with ciNPT or control at 3‐, 6‐, and 12‐month follow‐ups. Higher scores represent more prominent scarring characteristics. Each symbol represents one patient. All differences were significant (P < .05) except for height at 12‐month follow‐up (grey box; P = .067). ciNPT, closed incision negative pressure therapy
Figure 3.
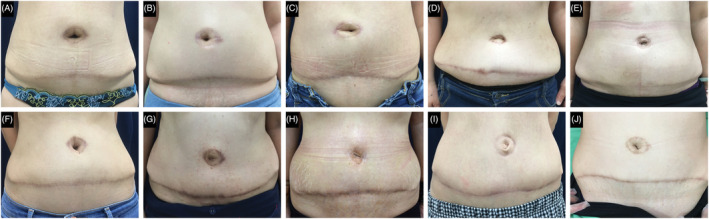
Comparison of incision appearance at 12 months after surgery in the negative pressure therapy (A–E) and control groups (F–J)
In the group receiving ciNPT, there were no instances of haematoma, seroma, flap necrosis, or surgical site infections. In a qualitative assessment of the incision edges, the attending physician noted less swelling and oedema in the ciNPT group in the first‐week post‐surgery. In the control group, one patient exhibited wound edge fat necrosis at postoperative week 3, requiring debridement and closure under local anaesthesia. In both groups, there were no instances of abdominal bulge or other late complications.
4. DISCUSSION
Mastectomy is a commonly indicated treatment for breast cancer that can leave patients with feelings of diminished femininity and psychological distress. Reconstruction is, therefore, acknowledged as an important component in reaching good patient outcomes, and a variety of techniques exist by which a natural‐looking breast shape can be obtained. Of these, autologous breast reconstruction has the benefit of generating higher rates of aesthetic patient satisfaction compared with implant‐based reconstruction.9 The use of the abdominal muscle‐sparing DIEP flap for breast reconstruction provides some advantages over other techniques, making it an appealing option for autologous reconstruction.10, 11 However, this process results in a large hip‐to‐hip incision that requires adequate postoperative care to prevent complications. Even for an incision that heals without major complication, highly visible scarring can become a source of patient dissatisfaction, requiring later revisions. Previously, our standard incisional care protocol consisted of coverage with an occlusive hydrocolloid silver dressing for 1 week followed by placement of a silicone dressing until discharge from the hospital. Although this regimen was sufficient for most cases, we had observed instances of slow incision healing and oedema due to tension at the donor site after DIEP flap harvesting. We also observed a large quantity of postoperative drainage. Due to these issues, we determined a need for a comfortable, protective dressing to promote incision healing without complications.
Application of ciNPT has been reported to help create a closed environment over the incision, hold the wound edges together, reduce oedema, promote perfusion, and remove exudate via the application of constant negative pressure. Use of ciNPT has shown positive postoperative outcomes in patient populations with closed incisions resulting from groin wounds in vascular surgery,12 sternal reconstructive surgery,2 spine surgery,13 hip arthroplasty,14 and laparotomy.15 In this pilot study of 10 DIEP flap breast reconstruction patients, we compared outcomes of patients treated with ciNPT vs standard occlusive dressings.
In the two treatment groups, there were no differences in hospital stay, the number of blood transfusions, or pain scores on postoperative day 2. The drainage volume trended higher in the ciNPT group than in the control group totalled on days 3 and 6. Although this was not a statistically significant increase, the results appear to contradict other clinical studies that have observed reduced drainage in patients treated with ciNPT compared with standard dressings. Abesamis et al reported a lower mean total fluid output within the first postoperative week after abdominoplasty and a shorter time to drain removal (output <50 mL/day) in patients receiving ciNPT.16 Chowdhry et al also observed a shorter time to drain removal (output <20 mL/day) in ciNPT‐managed sternotomies.2 The reason for this discrepancy is not immediately apparent and may be worth investigating in future studies. Notwithstanding these results, ciNPT was able to actively remove the drainage, helping to prevent the accumulation of excessive moisture at the incision site.
We also observed that the ciNPT group scored consistently better in a blinded evaluation of surgical scars at 3, 6, and 12 months after DIEP flap reconstruction. Specifically, there was a significant benefit in postoperative scar pigmentation, vascularity, and pliability, which is consistent with previously published data in the research literature. Abatangelo et al reported that abdominoplasty incisions managed with ciNPT had better 90‐day scar outcomes than incisions managed with standard dressings.17 Likewise, Ferrando et al discovered that using ciNPT over oncological breast surgical incisions was significantly associated with better scores compared with standard dressings on three different scar assessment scales: the two physician‐evaluated Observer Scar Assessment Scale and Manchester Scar Scale; and the patient‐evaluated Patient Scar Assessment Scale.18 Tanaydin et al also reported that ciNPT over inframammary incisions resulted in a significant improvement over fixation strips in scar quality and appearance, assessed using the Patient Scale and Observer Scale and the Visual Analogue Scale at 42, 90, and 180 days.19 In contrast, Svensson‐Björk et al noted no significant differences between ciNPT and standard dressings for inguinal vascular surgical scars evaluated using the Patient Scar Assessment Scale, the Stony Brook Scar Evaluation, a 10‐point‐graded numeric ranking scale, and a modified Vancouver Scar Scale.20 However, in this study population, the majority of surgical scars in both groups received the best possible scores indicating minimal baseline scarring, leaving little room for comparative differences. Our study is the first to report surgical scar assessment comparing ciNPT with standard dressings for breast reconstruction donor site incisions.
This study is limited primarily to the small sample size. By using a small patient population, the risks of this study influence from patient selection bias. Further study with a larger cohort is required to determine whether these results are representative of the general population. Additionally, the small sample size could be the source of a type II sampling error, leading to significant differences being overlooked. However, this pilot study has provided useful preliminary data on the use of ciNPT over DIEP flap donor site incisions and supports the undertaking of controlled clinical trials to establish improved best practice guidelines.
In conclusion, we report that postoperative incisional care with ciNPT was a valuable tool for actively managing the surgical site of DIEP flap harvesting up to 7 days after closure in these patients. ciNPT also helped to provide an advantage over standard dressing in surgical scar formation and quality. In our experience, application of ciNPT was painless, easy to use, and was able to provide continuous therapy and reassurance for our patients. As a result, we have incorporated ciNPT into our protocol for managing the abdominal incision after DIEP flap harvesting.
ACKNOWLEDGEMENTS
The authors thank Mikaela M. Sifuentes, PhD (Acelity), for assistance with manuscript preparation and Zhenmei Liu, MS, MSc (Acelity), for statistical analysis support.
Fang C‐L, Changchien C‐H, Chen M‐S, Hsu C‐H, Tsai C‐B. Closed incision negative pressure therapy following abdominoplasty after breast reconstruction with deep inferior epigastric perforator flaps. Int Wound J. 2020;17:326–331. 10.1111/iwj.13273
REFERENCES
- 1. Hamdi M, Rebecca A. The deep inferior epigastric artery perforator flap (DIEAP) in breast reconstruction. Semin Plast Surg. 2006;20:95‐102. [Google Scholar]
- 2. Chowdhry SA, Wilhelmi BJ. Comparing negative pressure wound therapy with instillation and conventional dressings for sternal wound reconstructions. Plast Reconstr Surg Glob Open. 2019;7:e2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gabriel A, Maxwell GP. Economic analysis based on the use of closed‐incision negative‐pressure therapy after postoperative breast reconstruction. Plast Reconstr Surg. 2019;143:36S‐40S. [DOI] [PubMed] [Google Scholar]
- 4. Singh DP, Gabriel A, Parvizi J, Gardner MJ, D'Agostino R Jr. Meta‐analysis of comparative trials evaluating a single‐use closed‐incision negative‐pressure therapy system. Plast Reconstr Surg. 2019;143:41S‐46S. [DOI] [PubMed] [Google Scholar]
- 5. Gabriel A, Sigalove S, Sigalove N, et al. The impact of closed incision negative pressure therapy on postoperative breast reconstruction outcomes. Plast Reconstr Surg Glob Open. 2018;6:e1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Muller‐Sloof E, de Laat HE, Hummelink SL, Peters JW, Ulrich DJ. The effect of postoperative closed incision negative pressure therapy on the incidence of donor site wound dehiscence in breast reconstruction patients: DEhiscence PREvention study (DEPRES), pilot randomized controlled trial. J Tissue Viability. 2018;27:262‐266. [DOI] [PubMed] [Google Scholar]
- 7. Hartrick CT, Kovan JP, Shapiro S. The numeric rating scale for clinical pain measurement: a ratio measure? Pain Pract. 2003;3:310‐316. [DOI] [PubMed] [Google Scholar]
- 8. Baryza MJ, Baryza GA. The Vancouver scar scale: an administration tool and its interrater reliability. J Burn Care Rehabil. 1995;16:535‐538. [DOI] [PubMed] [Google Scholar]
- 9. Craft RO, Colakoglu S, Curtis MS, et al. Patient satisfaction in unilateral and bilateral breast reconstruction. Plast Reconstr Surg. 2011;127:1417‐1424. [DOI] [PubMed] [Google Scholar]
- 10. Knox AD, Ho AL, Leung L, et al. Comparison of outcomes following autologous breast reconstruction using the DIEP and pedicled TRAM flap part 1: a 12 year clinical retrospective study and literature review. Plast Reconstr Surg. 2016;138(1):16‐28. [DOI] [PubMed] [Google Scholar]
- 11. Krishnan NM, Purnell C, Nahabedian MY, et al. The cost effectiveness of the DIEP flap relative to the muscle‐sparing TRAM flap in postmastectomy breast reconstruction. Plast Reconstr Surg. 2015;135:948‐958. [DOI] [PubMed] [Google Scholar]
- 12. Antoniou GA, Onwuka CC, Antoniou SA, Russell D. Meta‐analysis and trial sequential analysis of prophylactic negative pressure therapy for groin wounds in vascular surgery. J Vasc Surg. 2019;70:1700‐1710.e6. [DOI] [PubMed] [Google Scholar]
- 13. Dyck BA, Bailey CS, Steyn C, et al. Use of incisional vacuum‐assisted closure in the prevention of postoperative infection in high‐risk patients who underwent spine surgery: a proof‐of‐concept study. J Neurosurg Spine. 2019;1‐10. [DOI] [PubMed] [Google Scholar]
- 14. Pachowsky M, Gusinde J, Klein A, et al. Negative pressure wound therapy to prevent seromas and treat surgical incisions after total hip arthroplasty. Int Orthop. 2012;36:719‐722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Curran T, Alvarez D, Pastrana Del Valle J, Cataldo TE, Poylin V, Nagle D. Prophylactic closed‐incision negative‐pressure wound therapy is associated with decreased surgical site infection in high‐risk colorectal surgery laparotomy wounds. Colorectal Dis. 2019;21:110‐118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Abesamis GM, Chopra S, Vickery K, Deva AK. A comparative trial of incisional negative‐pressure wound therapy in abdominoplasty. Plast Reconstr Surg Glob Open. 2019;7:e2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Abatangelo S, Saporiti E, Giatsidis G. Closed incision negative‐pressure therapy (ciNPT) reduces minor local complications in post‐bariatric abdominoplasty body contouring: a retrospective case‐control series. Obes Surg. 2018;28:2096‐2104. [DOI] [PubMed] [Google Scholar]
- 18. Ferrando PM, Ala A, Bussone R, Beramasco L, Actis Perinetti F, Malan F. Closed incision negative pressure therapy in oncological breast surgery: comparison with standard care dressings. Plast Reconstr Surg Glob Open. 2018;6:e1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tanaydin V, Beugels J, Andriessen A, Sawor JH, van der Hulst RR. Randomized controlled study comparing disposable negative‐pressure wound therapy with standard care in bilateral breast reduction mammoplasty evaluating surgical site complications and scar quality. Aesthetic Plast Surg. 2018;42:927‐935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Svensson‐Bjork R, Hasselmann J, Acosta S. Evaluation of inguinal vascular surgical scars treated with closed incisional negative pressure wound therapy using three‐dimensional digital imaging—a randomized controlled trial on bilateral incisions. Wound Repair Regen. 2018;26:77‐86. [DOI] [PubMed] [Google Scholar]


