Abstract
Surgical site infection (SSI) is a challenging complication after intertrochanteric fracture surgery but without a large‐sample size study to investigate the incidence and risk factors of it. The present study was to investigate the incidence and risk factors of SSI after intertrochanteric fracture surgery. A total of 1941 patients underwent intertrochanteric fracture surgery between October 2014 and December 2018 were included. Demographic data, surgical variables, and preoperative laboratory indexes were obtained from a prospective database and reviewed by hospital records. The optimum cut‐off value for quantitative data was detected by receiver operating characteristic analysis. The univariate analysis and multivariable analysis were conducted to analyse the risk factors. In total, 25 patients (1.3%) developed SSI, including 22(1.1%) superficial infection and 3(0.2%) deep infection. After adjustment of multiple variables, gender (odds ratio[OR] 2.64, P = .024), time to surgery>4 days (OR 2.41, P = .046), implant (intramedullary or extramedullary devices) (OR 2.96, P = .036), ALB<35 g/L (OR 2.88, P = .031) remained significant factors. In conclusion, the incidence of SSI after intertrochanteric fractures surgery was 1.3%, with 1.1% for superficial and 0.2% for deep infection. Gender, time to surgery>4 days, the implant (intramedullary or extramedullary devices), and ALB<35 g/L were independent risk factors for the rate of SSI.
Keywords: incidence, intertrochanteric fracture, risk factors, surgical site infection
1. INTRODUCTION
Intertrochanteric fracture is a frequent condition with significant mortality and morbidity, especially for the geriatric population. 1 , 2 It reported that the 30‐day and 1‐year mortality rates of intertrochanteric fractures were, respectively, up to 7.7% and 26% after surgery. 3 The substantial mortality is often associated with older age, male, comorbidities, and postoperative complications. 3 , 4 In previous studies, postoperative complications have been attributed to be one of the leading causes of death in hip fractures patients. 5 Surgical site infection (SSI) is a challenging postoperative complication for the patient and hospital, the rate of which following hip fractures is between 2.7% and 14.9%. 6 , 7 , 8 It not only leads to more hospital stay, poor functional outcomes, and greater costs but also results in a substantially increased mortality risk. 9 , 10
A variety of risk factors of SSI were documented, including age, comorbidities, obesity, the experience of the surgeon, haematoma, surgical duration, increased duration of anaesthesia, body mass index (BMI), current smoking, preoperative hospital stay, serum albumin, warfarin treatment, and so forth. 6 , 11 , 12 As reported by Harrison, 13 the method of fracture fixation was also significantly associated with the SSI. However, most of the authors combined the rates of infection for femoral neck fractures and intertrochanteric fractures, in which different methods of internal fixation were used. 13 , 14 And, there are significant differences in surgical methods, operative time, particular nature of the fracture, the patient's health, postoperative activity levels, and the surgeon's experience between intertrochanteric fractures and femoral neck fractures, which may lead to large differences in the rate of SSI. While, only a few studies investigated the incidence and risk factors of SSI after intertrochanteric fracture surgery alone, in which the sample size or the number of included risk factors was small. 15 , 16 Shinet et al 17 conducted a retrospective study to investigate the relationship between the perioperative C‐reactive protein (CRP) value and postoperative complications after an intertrochanteric femoral fracture. Ekström et al 18 found that 2 of 109 patients developed SSI after intertrochanteric fracture in their study.
Therefore, the purpose of this study was to clarify the incidence and risk factors of SSI after intertrochanteric fracture surgery, which would help to take actively intervention to prevent SSI and improve the prognosis.
2. PATIENTS AND METHODS
The study was conducted at a single, academic, Level‐1 trauma centre. A total of 2068 patients underwent surgical treatment for an intertrochanteric fracture between October 2014 and December 2018 were reviewed, of which 1941 patients were further analysed according to the exclusion criteria (Figure 1). The exclusion criteria:(a) pathologic fracture, (b) old fractures (>21 days), (c) periprosthetic fractures, (d) patients who received conservative treatment, (e) age < 18 years, (f) patients whose information was incomplete data, (g) patients who received SSI treatment in our hospital but did not undergo initial surgery. Our study was ratified by our institutional ethics committee and adhered to the principles outlined in the Helsinki Declaration. The baseline characteristics and clinical data, such as demographic data, surgical variables, and preoperative laboratory indexes, were obtained from a prospective database and reviewed by hospital records. According to the occurrence of SSI, the patients were divided into two groups, with SSI or without SSI.
FIGURE 1.
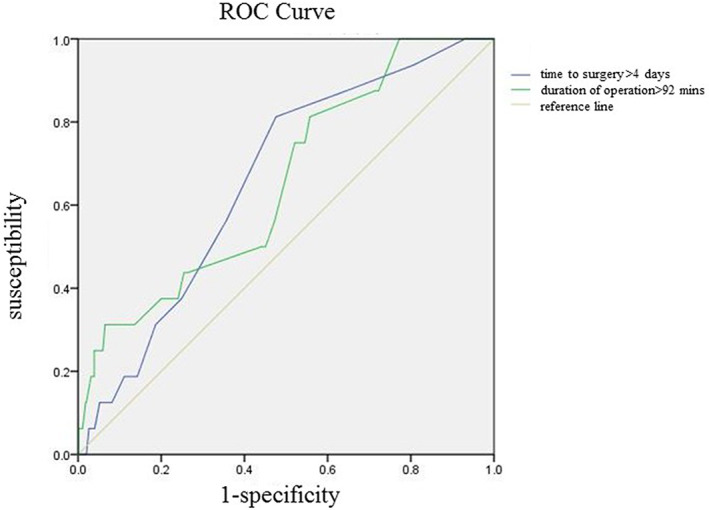
The optimum cut‐off value of some continuous variables associated with SSI were detected by ROC analysis
FIGURE 2.
The flow chart for the selection of study participants
Based on previous studies, we selected over 65 variables that could be prognostic factors associated with SSI, including demographic variables, surgical variables, preoperative laboratory indexes. The demographic variables, including age, gender, residential location (rural or urban), BMI, medical comorbidities (such as hypertension, diabetes mellitus, cardiac and cerebrovascular disease, respiratory disorders, liver, and kidney diseases), American Society of Anaesthesiologists (ASA) score, tumours (benign or malignant), and so forth, were collected. The number of comorbidities, the sum of the above major comorbidities, was also recorded. The surgical variables included: time to surgery (from admission to surgery), duration of surgery, type of anaesthesia, the implant (intramedullary devices or extramedullary devices), reduction methods, type of operating surgeon, intraoperative blood loss, and so forth. The preoperative laboratory indexes consisted of complete blood counts and biochemical analyses at the time of admission, including white blood cell (WBC), red blood cell (RBC), neutrophile granulocyte (NEUT), lymphocyte (LYM), haemoglobin (HGB), platelet (PLT), serum total protein (TP), serum albumin (ALB), alanine transaminase (ALT), and Serum globulin (GLU), and so forth.
Based on the criteria of the United States Centres for Disease Control and Prevention (CDC criteria), 19 we defined an infection developing within 30 to 90 days post‐operatively as SSI, including superficial infection and deep infection. Superficial infection is the infection of the skin or subcutaneous tissue occurring within 30 days post‐operatively, with at least one more symptom involving: localised pain; purulent discharge; spontaneous incision dehiscence; and positive results of bacterial culture. The deep infection was diagnosed if an infection were associated with fascial and muscular layers occurring within 90 days, combined with at least one of the abovementioned symptoms.
Standard antibiotic prophylaxis was 1 to 3 g Cefazolin intravenously 30 minutes pre‐operatively and 24 hours post‐operatively. All surgeries were conducted in laminar airflow theatres. All operations were conducted by dedicated orthopaedic trauma surgeons, the experience of which was recorded. The follow up of patients was performed by telephone or outpatient review. If an infection was suspected, we would require the patient for further treatment.
3. STATISTICAL ANALYSIS
The optimum cut‐off value for quantitative data, such as BMI, time to surgery, duration of surgery and anaesthesia, and intraoperative blood loss, and so forth were detected by receiver operating characteristic (ROC) analysis. Continuous variables corresponding to a normal distribution were compared by Student's t test, and those do not conform to a normal distribution were adopted by the Mann–Whitney U test. Pearson chi‐square test/ Fisher' exact test was applied to categorical variables. Categorical variables were described as frequency and percentage, while continuous variables were described as mean ± SD/median with quartile. Risk factors having statistical differences (P < .05) were entered into a multivariable logistic regression model to identify independent predictors of SSI. The P‐value <.05 indicated statistical significance. The Hosmer–Lemeshow test was applied to assess the goodness of fit, and acceptable fitness was accepted for P‐values <.05. All statistical analyses were performed by the SPSS 23.0 software (IBM, Armonk, New York).
4. RESULTS
In total, 1941 patients were included in this study, of which 25 patients (1.3%) developed SSI, including 22 (1.1%) superficial infection and 3 (0.2%) deep infection.
The optimum cut‐off value of some continuous variables associated with SSI were detected by ROC analysis, and some of the results were shown in Table 1 and Figure 2. In the univariate analysis, gender (P = .029), the number of comorbidities (P = .012), reduction methods (P = .039), time to surgery>4 days (P = .042), implant (intramedullary devices or extramedullary devices, P = .007), ALB<35 g/L (P = .036), and HGB < 110/120 g/L (P = .026) were identified as significant risk factors for the development of SSI (Table 2). Other demographic data, surgical variables, and preoperative laboratory indexes had no statistical differences between the two groups. In the multivariate logistic regression analysis model, all of the abovementioned factors were included. In the final multivariable analysis results, gender (OR 2.64, 95% CI 1.13–6.13, P = .024), time to surgery>4 days(OR 2.41, 95% CI 1.02–5.71, P = .046), implant (intramedullary devices or extramedullary devices) (OR 2.96, 95% CI 1.07–8.32, P = .036), ALB<35 g/L(OR 2.88, 95% CI 1.10–7.52, P = .031) remained significant factors (Table 3).
TABLE 1.
Optimum cut‐off value of continuous variables detected by the ROC analysis
| Variables | Cut‐off value | Area under the ROC curve (AUC) | P‐value | 95% CI |
|---|---|---|---|---|
| Time to surgery | 4 | 0.66 | .027 | 0.547‐0.773 |
| duration of surgery | 92 | 0.653 | .034 | 0.525‐0.782 |
Abbreviations: CI, confidence interval; ROC, receiver operating characteristic.
TABLE 2.
Univariable analyses of risk and prognostic factors
| Variables | Total patients (N = 1941) | Without SSI (N = 1916) | With SSI (N = 25) | P‐value |
|---|---|---|---|---|
| Intraoperative blood loss (ml), n (%) | .605 | |||
| ≤200 | 1164 (60.0) | 1151 (60.1) | 13 (52.0) | |
| 201‐400 | 505 (26.0) | 498 (26.0) | 7 (28.0) | |
| 401‐600 | 165 (8.5) | 163 (8.5) | 2 (8.0) | |
| 801‐1000 | 50 (2.6) | 49 (2.6) | 1 (4.0) | |
| >1000 | 57 (2.9) | 55 (2.9) | 2 (8.0) | |
| Intraoperative blood transfusion (ml), mean (SD) | 140.547 ± 338.21 | 139.9 ± 338.5 | 192.0 ± 318.1 | .444 |
| Age (years), mean (SD) | 72.92 ± 14.45 | 73.0 ± 14.4 | 68.0 ± 17.6 | .087 |
| Hypertension, n (%) | 858 (44.2) | 850 (44.4) | 8 (32.0) | .226 |
| Diabetes, n (%) | 386 (19.9) | 383 (20.0) | 3 (12.0) | .320 |
| Cerebrovascular disease, n (%) | 576 (29.7) | 574 (29.8) | 2 (12.5) | .131 |
| Cardiovascular disease, n (%) | 633 (32.6) | 626 (32.7) | 7 (28.0) | .621 |
| Chronic respiratory disease, n (%) | 96 (4.9) | 95 (5.0) | 1 (4.0) | .826 |
| Pneumonia, n (%) | 95 (4.9) | 94 (4.9) | 1 (4.0) | .835 |
| Tumours, n (%) | 42 (2.2) | 42 (2.2) | 0 (0.0) | .454 |
| Liver disease, n (%) | 40 (2.1) | 40 (2.1) | 0 (0.0) | .465 |
| Renal disease, n (%) | 60 (3.1) | 59 (3.1) | 1 (4.0) | .792 |
| Urinary tract infection, n (%) | 12 (0.6) | 12 (0.6) | 0 (0.0) | .691 |
| Comorbidities, no, n (%) | .012 | |||
| 0 | 380 (19.6) | 375 (19.6) | 5 (20.0) | |
| 1 | 465 (24.0) | 453 (23.6) | 12 (48.0) | |
| >2 | 1096 (56.5) | 1088 (56.5) | 8 (32.0) | |
| Residential location (urban), n (%) | 840 (43.3) | 829 (43.3) | 11 (44.0) | .941 |
| Injury mechanism (high energy), n (%) | 181 (9.3) | 176 (9.2) | 5 (20.0) | .065 |
| Reduction methods (open reduction), n (%) | 269 (13.9) | 262 (13.7) | 7 (28.0) | .039 |
| Surgeon (Deputy Chief Physician), n (%) | 1697 (87.4) | 1674 (86.8) | 23 (92.0) | .448 |
| Time to surgery (>4 days), n (%) | 929 (47.9) | 912 (47.6) | 17 (68.0) | .042 |
| Type of anaesthesia (general), n (%) | 820 (42.2) | 809 (42.2) | 11 (44.0) | .837 |
| Side (left), n (%) | 1003 (51.7) | 990 (51.7) | 13 (52.0) | .974 |
| Implant, n (%) | .007 | |||
| intramedullary devices | 1812 (93.4) | 1792 (93.5) | 20 (80.0) | |
| extramedullary devices | 129 (6.7) | 124 (6.5) | 5 (20.0) | |
| Duration of surgery (>92 minutes), n (%) | 1086 (56.0) | 1069 (55.7) | 17 (68.0) | .222 |
| ASA3‐4, n (%) | 914 (47.1) | 905 (47.2) | 9 (36.0) | .131 |
| Gender (male), n (%) | 826 (42.6) | 810 (42.3) | 16 (64.0) | .029 |
| BMI, n (%) | .863 | |||
| <18.5 | 106 (5.5) | 105 (5.5) | 1 (4.0) | |
| 18.5‐23.9 | 1173 (60.4) | 1158 (60.4) | 15 (60.0) | |
| 24‐27.9 | 494 (25.5) | 488 (25.5) | 6 (24.0) | |
| 28‐31.9 | 140 (7.2) | 137 (7.2) | 3 (12.0) | |
| ≥32 | 28 (1.4) | 28 (1.5) | 0 (0.0) | |
| Tp < 65 g/L, n (%) | 1562 (80.5) | 1541 (80.5) | 21 (84.0) | .654 |
| ALB<35 g/L, n (%) | 1072 (55.2) | 1053 (55.0) | 19 (76.0) | .036 |
| GLOB (references 20‐40 g/L), n (%) | .406 | |||
| <20 | 319 (16.4) | 313 (16.3) | 6 (24.0) | |
| >40 | 51 (2.6) | 51 (2.7) | 0 (0) | |
| A/G (references 1.2‐2.4), n (%) | .337 | |||
| <1.2 | 405 (20.9) | 397 (20.7) | 8 (32.0) | |
| >2.4 | 28 (1.4) | 28 (1.5) | 0 (0) | |
| ALT (references 9‐50 U/L), n (%) | .230 | |||
| <9 | 186 (9.6) | 183 (9.6) | 3 (12.0) | |
| >50 | 146 (7.5) | 142 (7.4) | 4 (16.0) | |
| AST (references 15‐40 U/L), n (%) | .233 | |||
| <15 | 343 (17.7) | 341 (17.8) | 2 (8.0) | |
| >40 | 224 (11.5) | 219 (11.4) | 5 (20.0) | |
| TBIL (>26), n (%) | 279 (14.4) | 275 (14.4) | 4 (16.0) | .816 |
| DBIL (>6), n (%) | 873 (45.0) | 863 (45.0) | 10 (40.0) | .581 |
| IBIL (>14), n (%) | 371 (19.1) | 368 (19.2) | 3 (12.0) | .631 |
| ALP (references 45‐125 U/L), n (%) | .784 | |||
| <45 | 231 (12.0) | 227 (11.8) | 4 (16.0) | |
| >125 | 134 (6.9) | 132 (6.9) | 2 (8.0) | |
| GGT (references 10‐60 U/L), n (%) | .508 | |||
| <10 | 98 (5.0) | 98 (5.1) | 0 (0.0) | |
| >60 | 212 (10.9) | 209 (10.9) | 3 (12.0) | |
| CHE (references 5‐12 U/L), n (%) | .266 | |||
| <2 | 783 (40.3) | 769 (40.1) | 14 (56.0) | |
| >12 | 9 (0.5) | 9 (0.5) | 0 (0) | |
| TBA (references 1‐10 umol/L), n (%) | .597 | |||
| <1 | 131 (6.7) | 130 (6.8) | 1 (4.0) | |
| >10 | 204 (10.5) | 200 (10.4) | 4 (16.0) | |
| HCRP (>8), n (%) | 1623 (83.6) | 1604 (83.7) | 19 (76.0) | .300 |
| CK (>), n (%) | 479 (24.7) | 472 (24.6) | 7 (28.0) | .698 |
| CKMB (>), n (%) | 322 (16.6) | 318 (16.6) | 4 (16.0) | .936 |
| LDH (>), n (%) | 642 (33.1) | 634 (33.1) | 8 (32.0) | .908 |
| HBDH (>), n (%) | 497 (25.6) | 491 (25.6) | 6 (24.0) | .853 |
| TC (>), n (%) | 121 (6.2) | 120 (6.3) | 1 (4.0) | .642 |
| TG (>), n (%) | 165 (8.5) | 163 (8.5) | 2 (8.0) | .928 |
| Na (references 137‐147 mmol/L), n (%) | .922 | |||
| <137 | 875 (45.1) | 864 (45.1) | 11 (44.0) | |
| >147 | 11 (0.6) | 11 (0.6) | 0 (0) | .947 |
| K (references 3.5‐5.3 mmol/L), n (%) | .356 | |||
| <3.5 | 251 (12.9) | 250 (13.0) | 1 (4.0) | |
| >5.3 | 17 (0.9) | 17 (0.9) | 0 (0) | |
| CL (references 99‐110 mmol/L), n (%) | .060 | |||
| <99 | 313 (16.1) | 305 (15.9) | 8 (32.0) | |
| >110 | 95 (4.9) | 93 (4.9) | 2 (8.0) | |
| TCO2 (references 20‐30 mmol/L), n (%) | .546 | |||
| <20 | 85 (4.4) | 85 (4.4) | 0 (0.0) | |
| >30 | 92 (4.7) | 91 (4.7) | 1 (4.0) | |
| GLU (>6.1), n (%) | 1147 (59.1) | 1136 (59.3) | 11 (44.0) | .278 |
| UREA (>8), n (%) | 404 (20.8) | 396 (20.7) | 5 (20.8) | .663 |
| CREA (references 57‐97 mmol/L), n (%) | .936 | |||
| <57 | 835 (43.0) | 824 (43.1) | 11 (44.0) | |
| (>97) | 110 (5.7) | 109 (5.7) | 1 (4.0) | |
| UA (references 208‐428 mmol/L), n (%) | .367 | |||
| <208 | 940 (48.4) | 925 (48.3) | 15 (60.0) | |
| >428 | 76 (3.9) | 76 (4.0) | 0 (0.0) | |
| WBC (references 3.5‐9.5109/L), n (%) | .770 | |||
| <3.5 | 14 (0.7) | 14 (0.7) | 0 (0.0) | |
| >9.5 | 740 (38.1) | 729 (38.0) | 11 (44.0) | |
| NEU (references 2.8‐6.3 109/L), n (%) | .959 | |||
| <1.8 | 5 (0.3) | 5 (0.3) | 0 (0.0) | |
| >6.3 | 1058 (54.5) | 1044 (54.5) | 14 (56.0) | |
| LYM (references 1.1‐3.2 109/L), n (%) | .063 | |||
| <1.1 | 950 (48.9) | 937 (48.9) | 13 (52.0) | |
| >3.2 | 11 (0.6) | 10 (0.5) | 1 (4.0) | |
| MON (references 0.1‐0.6 109/L), n (%) | .969 | |||
| <0. 1 | 3 (0.2) | 3 (0.2) | 0 (0) | |
| >0.6 | 1211 (62.4) | 1195 (62.4) | 16 (64.0) | |
| EOS (references 0.02‐0.05 109/L), n (%) | .925 | |||
| <0.02 | 495 (25.5) | 488 (25.5) | 7 (28.0) | |
| >0.52 | 6 (0.3) | 6 (0.3) | 0 (0) | |
| BAS (>0.06), n (%) | 120 (6.2) | 118 (6.2) | 2 (8.0) | .704 |
| RBC (>5.8), n (%) | 159 (8.2) | 159 (8.3) | 0 (0) | |
| HGB (<110/120), n (%) | 1304 (67.2) | 1282 (66.9) | 22 (88.0) | .026 |
| HCT (references 40%‐50%), n (%) | .574 | |||
| <40 | 1816 (93.6) | 1791 (93.5) | 25 (100.0) | |
| >50 | 1 (0.1) | 1 (0.1) | 0 (0.0) | |
| MCV (references 82‐100 fL), n (%) | .208 | |||
| <82 | 43 (2.2) | 43 (2.2) | 0 (0.0) | |
| >100 | 146 (7.5) | 142 (7.4) | 4 (16.0) | |
| MCH (references 27‐34pg), n (%) | .605 | |||
| <27 | 48 (2.5) | 48 (2.5) | 0 (0.0) | |
| >34 | 133 (6.9) | 132 (6.9) | 1 (4.0) | |
| MCHC (references 316‐354 g/L), n (%) | .495 | |||
| <316 | 49 (2.5) | 48 (2.5) | 1 (4.0) | |
| >354 | 89 (4.6) | 89 (4.6) | 0 (0.0) | |
| PLT (references 125‐350 109/L), n (%) | .052 | |||
| <125 | 170 (8.8) | 168 (8.8 | 2 (8.0) | |
| >250 | 281 (14.5) | 138 (7.2) | 143 (7.4) | |
| MPV (references 7.4‐11.0 fL), n (%) | .559 | |||
| <7.4 | 311 (16.0) | 306 (16.0) | 5 (20.0) | |
| >11.0 | 70 (3.6) | 70 (3.7) | 0 (0.0) | |
| ICU, n (%) | 51 (2.6) | 51 (2.7) | 0 (0.0) | .408 |
Note: RBC, red blood cell, reference range: female, 3.5–5.0*1012/L; males, 4.0–5.5*1012/L. HGB, haemoglobin, reference range: females, 110‐150 g/L; males, 120‐160 g/L; HCT, haematocrit, 40%‐50%; MCV, mean corpuscular volume; MCH, mean corpuscular haemoglobin; MCHC, mean corpuscular haemoglobin concentration; PLT, platelet, 100–300*109/L; MPV, mean platelet volume; ICU, intensive care unit.
Abbreviations: A/G values, albumin/globulin; ALB, albumin; ALP, alkaline phosphatase; ALT, alanine transaminase; ASA, American Society of Anaesthesiologists; AST, aspartate aminotransferase; BAS, basophilic granulocyte; BMI, body mass index; CHE, cholinesterase; DBIL, direct bilirubin; EOS, eosinophilic granulocyte; GGT, γ‐glutamyl transpeptidase; GLOB, globulin; GLU, glucose; HBDH, hydroxybutyrate dehydrogenase; HCRP, hypersensitive c‐reactive protein; IBIL, indirect bilirubin; LDH, lactate dehydrogenase; LYM, lymphocyte; MON, mononuclear cell; NEUT, neutrophile; TBA, total bile acid; TBIL, total bilirubin; TC, total cholesterol; TG, triglyceride; TP, total protein; UA, Uric acid; UREA, serum urea, CREA, Creatinine; WBC, white blood cell.
TABLE 3.
OR, 95% CI, and P‐value for independent risk factors in the multivariable logistic regression analysis of SSI
| Variable | Odds radio | 95%CI | P‐value |
|---|---|---|---|
| Gender (male) | 2.64 | 1.13–6.13 | .024 |
| Time to surgery (>4 days) | 2.41 | 1.02–5.72 | .046 |
| Implant (extramedullary devices) | 2.99 | 1.07–8.32 | .036 |
| ALB (<35 g/L) | 2.88 | 1.10–7.52 | .031 |
Abbreviations: ALB, albumin; Anaesthesiologists; CI, confidence interval.
5. DISCUSSION
In the present study, the incidence of SSI after intertrochanteric fractures surgery was 1.3%, with 1.1% for superficial infection and 0.2% for deep infection, which is comparable to previous studies. 15 , 16 , 18 , 20 To our knowledge, our analysis is the first large‐sample cohort study to detect the incidence and risk factors of SSI after intertrochanteric fracture surgery alone. In this study, we found that gender, time to surgery>4 days, the implant (intramedullary devices or extramedullary devices), ALB<35 g/L were potentially remediable factors for the rate of SSI after intertrochanteric fractures surgery.
Gender was proved to be a vital factor affecting the rate of SSI in the present study. And, the rate of SSI in the male gender was 2.7 times than that of the female gender (OR 2.64, 95% CI 1.13–6.13, P = .024). Inconsistent with our study, Harrison et al 13 found that gender was not associated with the rate of SSI after hip fractures. Based on previous studies, smoking is an independent risk factor of SSI. Ma et al found that the incidence of SSI in patients with current smoking was 4.26 times than that without current smoking. 11 And Liang et al found similar results in their study. 21 Therefore, we insist that Tobacco smoking was a significant factor that resulted in this difference between females and males in our study.
Time to surgery following hip fracture played an important role in the prognosis of patients. 22 Some recent studies have suggested that delay in surgery after hip fracture increased the risk of in‐hospital complications. 23 , 24 While some guidelines recommend that hip fracture surgery should be conducted within 24–48 hours, the optimal cut‐off time for time to surgery is still controversial. 23 In the present study, time to surgery >4 days has been demonstrated a significant risk factor for SSI after intertrochanteric fractures surgery (OR2.41, 95% CI 1.02–5.71, P = .046). The correlation between time to surgery and the rate of SSI after hip fracture had been reported in previous studies. 7 , 25 According to Cordero, 25 delay in surgery for more than 24 hours was a significant risk factor for wound infection after hip fractures. Lau et al show that the time to surgery >7 days was associated with the rate of SSIs in hip hemiarthroplasty. 26 What resulted in surgical delay were that the sicker and frailer patients always needed medical adjustments to tolerate surgery. These patients were often accompanied with older age and more than one medical complication, explaining a higher risk of SSI.
The preferred implant for intertrochanteric fractures has been converted from the sliding hip screw to the intramedullary devices in recent years. 27 , 28 In our hospital, the treatment of intertrochanteric fractures is mainly with intramedullary devices. And the extramedullary devices include the sliding hip screw, proximal femoral locking compression plate, and hemiarthroplasty. Our study found that the extramedullary devices were associated with a higher risk for SSI after intertrochanteric fractures, compared with the intramedullary devices (Table 3). Harrison et al 13 found a similar result in their study of the incidence of SSI after hip fracture surgery. According to their data, the incidence of SSI after hip fractures in extramedullary fixation was significantly higher than that in intramedullary fixation (0.78% vs 0.00%, P = .002). Compared with the extramedullary devices, some advantages of the intramedullary devices might result in a lower rate of SSI in this study, including small incision with less disruption to deep tissues, shorter operative time with less exposure, and further from the skin incision.
In recent years, the correlation between preoperative malnutrition and the prognosis of orthopaedic procedures has been reported in many studies. 29 , 30 Serum albumin is one of the most commonly used serum markers of nutritional status. It has been demonstrated that serum albumin is an independent risk factor for poor outcomes after hip fractures, such as postoperative complications and mortality. 14 , 31 Daniel et al 14 found that hypoalbuminemia was significantly associated with higher rates of death, sepsis, unplanned intubation, a longer mean length of stay, compared with normal albumin concentration. According to the study of Daniel et al and some previous studies, the serum albumin concentration < 35 g/L was considered to be malnutrition (hypoalbuminemia). 11 The prevalence of hypoalbuminemia in our study was 55.2%, which was similar to previous founding reported from 45.9% to 55.4%. 32 , 33 The current study revealed that a serum albumin concentration < 35 g/L is a risk factor for SSI after intertrochanteric fractures. The results of our study were similar to that of Ma et al, 6 in which hypoalbuminemia increased the risk of SSI after hip fractures. Therefore, we should pay more attention to the nutritional status of patients with a hip fracture for the timely nutritional supplementation may reduce the incidence of poor outcomes.
In previous studies, most of the authors described the risk factors for the rate of SSI after hip fracture surgery, combining the femoral neck fracture and intertrochanteric fracture together despite different fractures characteristics and surgical characteristics between them. 6 , 10 , 12 , 13 , 25 , 34 Compared with previous studies with a small simple size and few risk factors, 15 , 16 , 17 , 18 , 20 , 35 our analysis is the first large‐sample cohort study to detect the incidence and risk factors of SSI after intertrochanteric fracture surgery alone. In addition, the present study had selected over 70 variables, including demographic variables, surgical variables, preoperative laboratory indexes. Besides, ROC analysis was conducted to find a better sensitive cut‐off value. Last, all of the patients were chosen from a consecutive intertrochanteric fracture database so that the selection bias could be prevented. We acknowledge some limitations should be recognised in our study. The number of patients with SSI was small and our study was conducted in a single centre. Thus, we will conduct multi‐centre studies to expand the sample size and avoid admission bias in the future. Besides, some other confounding factors still remain, including the experience of the surgeon, residential status, the type of fracture, and so forth.
In conclusion, first, we found the incidence of SSI after intertrochanteric fractures surgery was 1.3%, with 1.1% for superficial infection and 0.2% for deep infection. Second, gender, time to surgery>4 days, the implant (intramedullary devices or extramedullary devices), and ALB<35 g/L were significant risk factors associated with the rate of SSI after intertrochanteric fractures surgery. Last, we suggest that individual treatment should be applied for patients with sensitivity factors and corresponding preventive measures should be taken to mitigate the interference of modifiable factors.
CONFLICT OF INTEREST
All authors declare that they have no conflicts of interest.
ACKNOWLEDGEMENTS
We are grateful to Z Jin and RP Zhang of the Department of Orthopaedics, and to YC Yin of the Department of statistics and applications for their kind assistance.
Zhao K, Zhang J, Li J, et al. Incidence and risk factors of surgical site infection after intertrochanteric fracture surgery: A prospective cohort study. Int Wound J. 2020;17:1871–1880. 10.1111/iwj.13477
DATA AVAILABILITY STATEMENT
Research data are not shared.
REFERENCES
- 1. Zhang Y. Clinical Epidemiology of Orthopaedic Trauma. Stuttgart, Germany: Thieme; 2016. [Google Scholar]
- 2. Klop C, Welsing PM, Cooper C, et al. Mortality in British hip fracture patients, 2000‐2010: a population‐based retrospective cohort study. Bone. 2014;66:171‐177. [DOI] [PubMed] [Google Scholar]
- 3. Mattisson L, Bojan A, Enocson A. Epidemiology, treatment and mortality of trochanteric and subtrochanteric hip fractures: data from the Swedish fracture register. BMC Musculoskelet Disord. 2018;19(1):369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pedersen SJ, Borgbjerg FM, Schousboe B, et al. A comprehensive hip fracture program reduces complication rates and mortality. J Am Geriatr Soc. 2008;56(10):1831‐1838. [DOI] [PubMed] [Google Scholar]
- 5. Chatterton BD, Moores TS, Ahmad S, Cattell A, Roberts PJ. Cause of death and factors associated with early in‐hospital mortality after hip fracture. Bone Joint J. 2015;97‐B(2):246‐251. 10.1302/0301-620X.97B2.35248 [DOI] [PubMed] [Google Scholar]
- 6. Liu X, Dong Z, Li J, et al. Factors affecting the incidence of surgical site infection after geriatric hip fracture surgery: a retrospective multicenter study. J Orthop Surg Res. 2019;14(1):382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. de Jong L., TMALK, Kuijper T. M., Roukema G. R.. Factors affecting the rate of surgical site infection inpatients after hemiarth. Bone Joint J 2017;99‐B:1088–94. [DOI] [PubMed] [Google Scholar]
- 8. Le J, Dong Z, Liang J, et al. Surgical site infection following traumatic orthopaedic surgeries in geriatric patients: incidence and prognostic risk factors. Int Wound J. 2020;17(1):206‐213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kjorholt KE, Kristensen NR, Prieto‐Alhambra D, Johnsen SP, Pedersen AB. Increased risk of mortality after postoperative infection in hip fracture patients. Bone. 2019;127:563‐570. [DOI] [PubMed] [Google Scholar]
- 10. Edwards AC C, Boulton C, Moran CG. Early infection after hip fracture surgery risk factors, costs and outcome. J Bone Joint Surg Br. 2008;90‐B:770‐777. [DOI] [PubMed] [Google Scholar]
- 11. Ma T, Lu K, Song L, et al. Modifiable factors as current smoking, hypoalbumin, and elevated fasting blood glucose level increased the SSI risk following elderly hip fracture surgery. J Investig Surg. 2019;3(19):1‐9. [DOI] [PubMed] [Google Scholar]
- 12. Frenkel Rutenberg T, Vitenberg M, Yahav D, Spectre G, Velkes S. Surgical site infections in elderly fragility hip fractures patients undergoing warfarin treatment. J Orthop Trauma. 2019;33(10):518‐524. [DOI] [PubMed] [Google Scholar]
- 13. Harrison PR T, Cook A, Parker MJ. Factors affecting the incidence of deep wound infection after hip fracture surgery. J Bone Joint Surg Br. 2012;94‐B:237‐240. [DOI] [PubMed] [Google Scholar]
- 14. Bohl DD, Shen MR, Hannon CP, Fillingham YA, Darrith B, Della Valle CJ. Serum albumin predicts survival and postoperative course following surgery for geriatric hip fracture. J Bone Joint Surg Am. 2017;99(24):2110‐2118. [DOI] [PubMed] [Google Scholar]
- 15. Yu X, Wang H, Duan X, Liu M, Xiang Z. Intramedullary versus extramedullary internal fixation for unstable intertrochanteric fracture, a meta‐analysis. Acta Orthop Traumatol Turc. 2018;52(4):299‐307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zou J, Xu Y, Yang H. A comparison of proximal femoral nail antirotation and dynamic hip screw devices in trochanteric fractures. J Int Med Res. 2009;37(4):1057‐1064. [DOI] [PubMed] [Google Scholar]
- 17. Shin WC, Do MU, Woo SH, Choi SH, Moon NH, Suh KT. C‐reactive protein for early detection of postoperative systemic infections in intertrochanteric femoral fractures. Injury. 2018;49(10):1859‐1864. [DOI] [PubMed] [Google Scholar]
- 18. Miedel R, Ponzer S, Törnkvist H, Söderqvist A, Tidermark J. The standard gamma nail or the Medoff sliding plate for unstable trochanteric and subtrochanteric fractures. A randomised, controlled trial. J Bone Joint Surg Br. 2005;87(1):68‐75. [PubMed] [Google Scholar]
- 19. Horan TC, Gaynes RP, Martone WJ, Jarvis WR, Emori TG. CDC definitions of nosocomial surgical site infections, 1992: a modification of CDC definitions of surgical wound infections. Infect Control Hosp Epidemiol. 1992;13(10):606‐608. [PubMed] [Google Scholar]
- 20. Verettas DA, Ifantidis P, Chatzipapas CN, et al. Systematic effects of surgical treatment of hip fractures: gliding screw‐plating vs intramedullary nailing. Injury. 2010;41(3):279‐284. [DOI] [PubMed] [Google Scholar]
- 21. Liang Z, Rong K, Gu W, et al. Surgical site infection following elective orthopaedic surgeries in geriatric patients: incidence and associated risk factors. Int Wound J. 2019;16(3):773‐780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Beaupre LA, Khong H, Smith C, et al. The impact of time to surgery after hip fracture on mortality at 30‐ and 90‐days: does a single benchmark apply to all? Injury. 2019;50(4):950‐955. [DOI] [PubMed] [Google Scholar]
- 23. Kelly‐Pettersson P, Samuelsson B, Muren O, et al. Waiting time to surgery is correlated with an increased risk of serious adverse events during hospital stay in patients with hip‐fracture: a cohort study. Int J Nurs Stud. 2017;69:91‐97. [DOI] [PubMed] [Google Scholar]
- 24. Eva N Glassou KKK, Hansen TB, Pedersen AB. Delay in surgery, risk of hospital‐treated infections and the prognostic impact of comorbidity in hip fracture patients. A Danish nationwide cohort study, 2005–2016. Clin Epidemiol. 2019;11:383‐395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cordero J, Maldonado A, Iborra S. Surgical delay as a risk factor for wound infection after a hip fracture. Injury. 2016;47:S56‐S60. [DOI] [PubMed] [Google Scholar]
- 26. Lau AC, Neo GH, Lee HC. Risk factors of surgical site infections in hip hemiarthroplasty: a single‐institution experience over nine years. Singap Med J. 2014;55(10):535‐538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Niu E, Yang A, Harris AH, Bishop J. Which fixation device is preferred for surgical treatment of intertrochanteric hip fractures in the United States? A survey of Orthopaedic surgeons. Clin Orthop Relat Res. 2015;473(11):3647‐3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Socci ARCN, Leslie MP, Baumgaertner MR. Implant options for the treatment of intertrochanteric fractures of the hip: rationale, evidence, and recommendations. Bone Joint J. 2017;99‐B(1):128‐133. [DOI] [PubMed] [Google Scholar]
- 29. Bohl DD, Shen MR, Kayupov E, Della Valle CJ. Hypoalbuminemia independently predicts surgical site infection, pneumonia, length of stay, and readmission after Total joint arthroplasty. J Arthroplast. 2016;31(1):15‐21. [DOI] [PubMed] [Google Scholar]
- 30. Bohl DD, Shen MR, Mayo BC, et al. Malnutrition predicts infectious and wound complications following posterior lumbar spinal fusion. Spine (Phila Pa 1976). 2016;41(21):1693‐1699. [DOI] [PubMed] [Google Scholar]
- 31. Lv H, Yin P, Long A, et al. Clinical characteristics and risk factors of postoperative pneumonia after hip fracture surgery: a prospective cohort study. Osteoporos Int. 2016;27(10):3001‐3009. [DOI] [PubMed] [Google Scholar]
- 32. Ozturk A, Ozkan Y, Akgoz S, Yalcyn N, Ozdemir RM, Aykut S. The risk factors for mortality in elderly patients with hip fractures: postoperative one‐year results. Singap Med J. 2010;51(2):137‐143. [PubMed] [Google Scholar]
- 33. Pimlott BJ, Jones CA, Beaupre LA, Johnston DW, Majumdar SR. Prognostic impact of pre‐operative albumin on short‐term mortality and complications in patients with hip fracture. Arch Gerontol Geriatr. 2011;53(1):90‐94. [DOI] [PubMed] [Google Scholar]
- 34. Grau L, Zachwieja E, Summers SH, et al. Hepatitis C is an independent risk factor for perioperative complications and nonroutine discharge in patients treated surgically for hip fractures. J Orthop Trauma. 2018;32(11):565‐572. [DOI] [PubMed] [Google Scholar]
- 35. Ekström W, Karlsson‐Thur C, Larsson S, Ragnarsson B, Alberts KA. Functional outcome in treatment of unstable trochanteric and subtrochanteric fractures with the proximal femoral nail and the Medoff sliding plate. J Orthop Trauma. 2007;21(1):18‐25. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Research data are not shared.


