Abstract
Hypergranulation tissue formation is a common complication after gastrostomy tube (G‐tube) placement, occurring in 44%–68% of children. Hydrocolloid dressings are often used in the treatment of hypergranulation tissue but have not been studied for the prevention of postoperative hypergranulation tissue. An institutional review board (IRB)‐approved, prospective, randomised study was performed in paediatric patients who underwent G‐tube placement at a single, large children's hospital from January 2011 to November 2016. After placement, patients were randomly assigned to (1) standard postoperative G‐tube care, (2) standard hydrocolloid G‐tube dressing, or (3) silver‐impregnated hydrocolloid G‐tube dressing, and the incidences of postoperative hypergranulation tissue formation, tube dislodgement, infection, and emergency department use were compared. A total of 171 patients were enrolled; 128 patients (75%) had at least 4 months of follow up and were included in the analyses. Eighty‐nine patients (69.5%) developed hypergranulation tissue during the postoperative period, with no significant differences in incidence among the three treatment arms. Of those who developed hypergranulation tissue, 46 (56%) visited the emergency department, compared with 6 of the 39 patients (19%) who did not develop hypergranulation tissue. Hydrocolloid dressings (standard or silver‐impregnated) do not prevent the development of hypergranulation tissue or other complications after G‐tube placement in paediatric patients.
Keywords: children, gastrostomy tube, hydrocolloid dressings, hypergranulation tissue
1. INTRODUCTION
Gastrostomy tubes (G‐tubes) are widely used within the paediatric population for both short‐term and long‐term access for enteral feeds and medications. G‐tube placement is associated with various early and late complications, including tube dislodgement, hypergranulation tissue formation, skin infections around the tube, gastroesophageal reflux, and persistent gastrocutaneous fistula after removal.1 The procedure is also associated with a high number of visits to outpatient clinics and the emergency department (ED) for complications, which increases health care resource utilisation and procedural costs.2 The number of G‐tube procedures is increasing annually in the United States, with an average increase of 0.15 procedures per year (Slope 0.50), from 10 procedures per 100 000 patients in 1997 to 11.9 procedures per 100 000 patients in 2009.3 As the postoperative care of the G‐tube in paediatric patients is usually the responsibility of the parents, the increase in the number of procedures has led to considerable familial burden in addition to increased health care costs to treat complications.3
Hypergranulation tissue formation is the most common G‐tube complication, seen in 44%–68% of patients.1, 2, 4, 5 Hypergranulation tissue is excessive, vascularised scar tissue that builds up in response to chronic irritation within a wound during the early granulation and epithelialisation stages of wound healing. It can lead to bleeding from the wound site, chronic skin breakdown, and leaking of feeds and gastric acid onto skin and clothing. The treatment of hypergranulation tissue complications includes chemical cautery with silver nitrate, polyurethane foam, laser ablation, washcloth abrasion, and topical corticosteroids.6 A hydrocolloid dressing (HD) is recommended during the granulation and epithelialisation stages of wound healing.7, 8, 9 HD is a non‐occlusive polyester mesh containing carboxymethylcellulose particles and petrolatum. HD is non‐adherent, painless to change or remove, and rapidly absorbs wound exudate.8 A recent change to standard HD is the addition of silver sulphate, impregnated into the HD mesh (Silver HD). The silver ions are bactericidal, inducing bacterial DNA replication errors that lead to cell death.10, 11 The silver ions are not absorbed into the wound tissue, and there is no systemic absorption of silver from the dressing. More rapid wound healing has been demonstrated in open‐label randomised trials of silver HD compared with standard HD.12 While HD and silver HD are often used to treat wounds and have been shown to reduce hypergranulation tissue after it develops, the effect of these dressings on the prevention of hypergranulation tissue has not been previously studied.
We conducted a prospective, randomised study to compare the effect of (1) standard G‐tube care, (2) standard HD treatment, and (3) silver HD treatment after G‐tube placement in children on the postoperative formation of hypergranulation tissue, tube dislodgement, infection, and ED use. We hypothesised that silver HD would reduce the incidence of G‐tube‐associated postoperative hypergranulation tissue development by 50% compared with standard G‐tube care.
2. METHODS
2.1. Study participants
We performed a prospective, randomised study to compare the effects of standard care and treatment with standard HD or silver HD on preventing postoperative G‐tube complications. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki, as reflected in the approval given by the institutional review board of the Ann & Robert H. Lurie Children's Hospital of Chicago. Informed consent was obtained from the parent or legal authorised representative of each participant prior to enrolment.
Children were eligible for enrollment if they were between 1 month and 17 years of age and underwent G‐tube placement by a paediatric surgeon from January 2011 to November 2016. Children less than 30 days of age or children with mothers who were pregnant or breastfeeding were excluded. Silver sulphate‐impregnated hydrocolloid has not been studied in children under 30 days of life or in mothers who are pregnant or breastfeeding. Additional exclusion criteria were patients who underwent G‐tube placement by gastroenterology or interventional radiology at the same institution. A sample size of 171 patients (57 in each arm) was calculated using a power of 0.8 and a significance level of 0.05, predicting a possible 5% loss to follow up.
2.2. Randomisation
Children were randomised by random number generator for digits at least three numerals in length. The final digit of the random number was used for assignment. A last number of 1–3 was assigned to standard care, 4–6 was assigned to HD, and 7–9 was assigned to silver HD. Any number ending in zero was removed from the random number list.
2.3. Intervention
Patients were randomly assigned to one of the three groups: (1) standard postoperative G‐tube care, (2) standard HD G‐tube wound treatment, or (3) silver HD G‐tube wound treatment, all of which are considered standard of care. Dressings were provided free of charge by Halyard Health (Alpharetta, Georgia). For patients assigned to HD or silver HD, the dressing was placed around the G‐tube on postoperative day 1, at the time of parent education in the proper use and care of the G‐tube. Parents were instructed to change the dressing once every 2 days and to continue for a total of 30 days. At the end of the 30‐day study period, parents were instructed on routine G‐tube care, including daily washing with soap and water.
2.4. Follow up and study outcomes
De‐identified data were collected from the medical record, including patient demographics, comorbidities, operative technique, postoperative complications, and treatment assignment. Patients were routinely seen for a postoperative visit 2 weeks after G‐tube placement, where they were assessed for the presence or absence of hypergranulation tissue, tube dislodgment, and infection. Visits to the ED for G‐tube‐related complications were also noted. If no complications occurred, the next visit was scheduled for 3 months later for the first G‐tube change. Additional visits were performed as needed for complications or routine G‐tube evaluations.
2.5. Statistical analysis
The χ 2 test and t‐test were used to compare differences in outcomes between the three treatment groups. A P value < 0.05 was considered statistically significant. Data were analysed in SAS 9.3.
3. RESULTS
A total of 171 patients were enrolled. Forty‐three (25%) patients were excluded; 22 were lost to follow up, 12 did not meet the inclusion criteria, 6 had incomplete data, and 3 died during the study, leaving 128 (75%) patients for subsequent analyses (Figure 1). The study cohort had a median age of 1.5 years (range: 0–18.5 years; Table 1). The age distribution was unimodal. Gender was equally distributed, with 65 (51%) males and 63 (49%) females. Ten patient families (7.8%) reported a preference for speaking a language other than English. Most patients had private insurance (68.8%), with approximately one‐third covered by Medicaid (31.3%). A laparoscopic technique for G‐tube placement was used in 80 patients, and an open technique was used in 48 patients. There was no difference in the incidence of hypergranulation tissue between the laparoscopic and open surgical groups. The most common comorbidity seen in the patient cohort was developmental delay (57.8%), followed by gastroesophageal reflux disease (47.7%). Cardiac anomalies were present in 30.5% of the patients.
Figure 1.
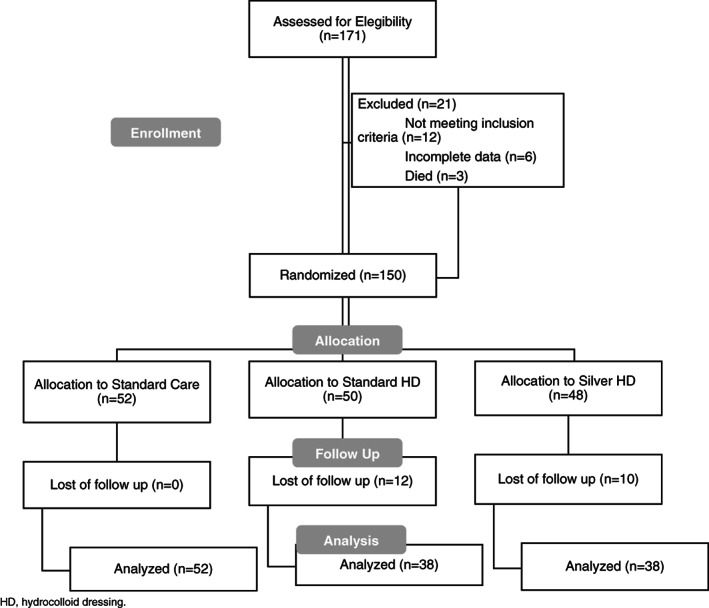
Consort flow diagram (n = 171)
Table 1.
Baseline demographics (N = 128)
| n (%) | |
|---|---|
| Gender | |
| Male | 65 (50.7%) |
| Female | 63 (49.2%) |
| Age (y) | |
| Median | 1.5 (range: 0–18.5) |
| 0–6 mo | 33 (25.8%) |
| 6–12 mo | 20 (15.6%) |
| 1–2 y | 32 (25%) |
| 3–5 y | 19 (14.8%) |
| 6–10 y | 12 (9.4%) |
| 11–15 y | 10 (7.8%) |
| 16–20 y | 2 (1.6%) |
| Race/ethnicity | |
| White | 84 (65.6%) |
| Hispanic/Latino | 23 (17.9%) |
| Black/African American | 14 (10.9%) |
| Other | 7 (5.5%) |
| Primary language | |
| English | 118 (92.2%) |
| Spanish | 8 (6.2%) |
| Other | 2 (1.6%) |
| Insurance | |
| Medicaid | 40 (31.3%) |
| Private | 80 (68.8%) |
| Procedure | |
| Open | 48 (37.5%) |
| Laparoscopic | 80 (62.5%) |
| Comorbidities | |
| Developmental delay | 74 (57.8%) |
| GERD | 61 (47.65%) |
| Cardiac anomaly | 39 (30.46%) |
| Other | 18 (14.06%) |
GERD, gastroesophageal reflux disease.
Hypergranulation tissue was the most common complication, occurring in 89 of 171 patients (69.5%; Table 2). Additional complications included accidental tube dislodgment (25.9%) and infection of the surgical site (8.9%).
Table 2.
Postoperative complications in patients with a gastronomy tube (N = 128)
| Postoperative complications | n (%) | ED visits (%) |
|---|---|---|
| Hypergranulation tissue | 89 (69.5%) | 46 (51.6%) |
| Tube dislodgment | 33 (25.98%) | 27 (72.97%) |
| Infection | 12 (9.6%) | 2 (8.33%) |
ED, emergency department.
Of the 89 patients who developed hypergranulation tissue, 36 were assigned to standard care, 24 to standard HD, and 20 to silver HD, with an overall incidence of 69%, 63%, and 76%, respectively. There was no difference among the three treatment arms in the incidence of hypergranulation tissue at the three different time points analysed: (1) <30 days after G‐tube placement (standard care, HD, or silver HD); (2) >30 days after G‐tube placement (routine care); and (3) 0–120 days (entire study period; Table 3). Among the patients who developed hypergranulation tissue, 13 (14.6%) had recurrent hypergranulation tissue during the study period. Seven (54%) patients had more than one episode, and six had a single episode. The average time to the first recurrence was 10 weeks. Of the patients who developed hypergranulation tissue, 46 of 89 visited the ED (56%), while only 6 of 39 patients without hypergranulation tissue visited the ED (19%). Of all patients who visited the ED, 80% were for complications related to the G‐tube, while the remainder were for unrelated causes (Table 4).
Table 3.
Incidence of gastrostomy‐related postoperative hypergranulation tissue
| No (%) | Yes (%) | n | P‐value | |
|---|---|---|---|---|
| Hypergranulation tissue < 30 d after gastronomy tube placement/dressing placement | ||||
| Standard care | 69 | 31 | 52 | 0.59 |
| Standard HD | 66 | 34 | 38 | |
| Silver HD | 76 | 24 | 38 | |
| Other | 68 | 32 | 38 | 0.33 |
| Silver HD | 76 | 24 | 128 | |
| Standard care | 69 | 31 | 52 | 0.82 |
| Any HD | 71 | 29 | 76 | |
| Hypergranulation tissue > 30 d after gastronomy tube placement/dressing placement | ||||
| Standard care | 62 | 38 | 52 | 0.10 |
| Standard HD | 71 | 29 | 38 | |
| Silver HD | 47 | 53 | 38 | |
| Other | 66 | 34 | 90 | 0.055 |
| Silver HD | 47 | 53 | 38 | |
| Standard care | 62 | 38 | 52 | 0.79 |
| Any HD | 59 | 41 | 76 | |
| Hypergranulation tissue during the entire study length (0–120 d) | ||||
| Standard care | 31 | 69 | 52 | 0.46 |
| Standard HD | 37 | 63 | 38 | |
| Silver HD | 24 | 76 | 38 | |
| Other | 33 | 67 | 90 | 0.28 |
| Silver HD | 24 | 76 | 38 | |
| Standard care | 31 | 69 | 52 | 0.95 |
| Any HD | 30 | 70 | 76 | |
HD, hydrocolloid dressing.
Other: Standard care + standard HD.
Table 4.
Relationship between ED visits and gastrostomy‐related postoperative hypergranulation tissue
| Hypergranulation tissue formation | n | P‐value | |||
|---|---|---|---|---|---|
| No (%) | Yes (%) | ||||
| ED visit | No | 39 | 61 | 71 | 0.013* |
| Yes | 19 | 81 | 57 | ||
| ED for G‐tube complication | No | 33 | 67 | 93 | 0.166 |
| Yes | 20 | 80 | 30 | ||
P‐value < 0.05.
4. DISCUSSION
In our study population, hypergranulation tissue was the most common complication in patients after a G‐tube placement. These data are consistent with numerous other studies.1, 2, 4, 5 The use of HDs, either standard or silver‐impregnated, did not prevent the development of hypergranulation tissue after G‐tube placement.
The prevalence of hypergranulation tissue during the study was higher compared with previous studies (69.5% versus 44–68%; 1,2,4,5). The increased incidence is likely secondary to increased vigilance for the occurrence of hypergranulation tissue relative to the study objectives. As prospective studies are more accurate tools for data collection,13 the true incidence in this study was therefore expected to be higher than the majority of published data. While the prevalence of hypergranulation tissue was slightly lower in the intervention study period (30% early occurrence versus 40% late occurrence), this difference was not associated with any of the study interventions. Complications related to the G‐tube were the most common cause of visits to the ED among this population of children. In addition to hypergranulation tissue, tube dislodgment and infection of the surgical site were also very common. Saavedra et al. previously reported tube dislodgment as the most common G‐tube‐related complaint in the ED (62% of study participants14). Our data demonstrate a lower, but equally concerning, rate of tube dislodgement.
Current treatment for hypergranulation tissue has no established gold standard. Multiple treatments have been used, including topical corticosteroids, chemical cautery with silver nitrate, polyurethane foam, laser ablation, and washcloth abrasion.6, 15 More recently, the use of salt (Hypertonic environment16) has been demonstrated to be effective in reducing oedematous hypergranulation tissue; this inexpensive and uncomplicated approach is reproducible in a home environment if required. Moody et al. showed successful resolution of hypergranulation tissue in four adult patients by combining HD dressing with pulsed dye laser but not with either treatment alone.17 The lack of clearly defined guidelines for the treatment of hypergranulation tissue combined with a high incidence and significant health care and familial burden make the prevention of hypergranulation tissue a prime target for interventional studies. Unfortunately, in the current study, we were unable to prevent the appearance of hypergranulation tissue with early application of HDs (standard or silver‐impregnated) immediately after G‐tube placement. These data suggest that neither excess fluid nor local skin irritation from the tube is involved in the pathogenesis of hypergranulation tissue formation after G‐tube placement.
There are limitations related to the interpretation and application of the findings from this study. First, the study was powered for a total of 171 patients, assuming a 5% loss to follow up. The loss to follow up was far higher in this study. However, given the overall higher incidence of hypergranulation tissue compared with that reported in previous studies, we do not believe that the high loss to follow up changed the negative results of the study. Second, we did not assess adherence to hydrocolloid (standard or silver‐impregnated) beyond the 2‐week postop check. Finally, because of limited to supplies of the product for use in the study, we were not able to continue the use of the HDs (standard and silver‐impregnated) beyond the 30‐day intervention period. As the rate of hypergranulation tissue was not different between assigned groups for either intervention, it is unlikely that an extension of the study period would have changed the results of the study.
5. CONCLUSION
Gastrostomy‐related postoperative hypergranulation tissue is a significant concern in the paediatric population. HDs (standard or silver‐impregnated) did not prevent the postoperative development of hypergranulation tissue after G‐tube placement. Future studies should examine approaches to prevent G‐tube complications such as hypergranulation tissue development and reduce the associated clinical and economic burdens for patients and caregivers.
León AH, Hebal F, Stake C, Baldwin K, Barsness KA. Prevention of hypergranulation tissue after gastrostomy tube placement: A randomised controlled trial of hydrocolloid dressings. Int Wound J. 2019;16:41–46. 10.1111/iwj.12978
REFERENCES
- 1. Naiditch JA, Lautz T, Barsness KA. Postoperative complications in children undergoing gastrostomy tube placement. J Laparoendosc Adv Surg Tech A. 2010;20(9):781‐785. [DOI] [PubMed] [Google Scholar]
- 2. Crosby J, Duerksen D. A retrospective survey of tube‐related complications in patients receiving long‐term home enteral nutrition. Dig Dis Sci. 2005;50(9):1712‐1717. [DOI] [PubMed] [Google Scholar]
- 3. Fox D, Campagna E, Friedlander J, Partrick D, Ress D, Kempe A. National trends and outcomes of pediatric gastrostomy tube placement. J Pediatr Gastroenterol Nutr. 2014;59(5):582‐588. [DOI] [PubMed] [Google Scholar]
- 4. Franken J, Mauritz FA, Suksamanapun N, Hulsker CC, van der Zee DC, van Herwaarden‐Lindeboom MY. Efficacy and adverse events of laparoscopic gastrostomy placement in children: results of a large cohort study. Surg Endosc. 2015;29(6):1545‐1552. [DOI] [PubMed] [Google Scholar]
- 5. Goldberg E, Barton S, Xanthopoulos MS, Stettler N, Liacouras CA. A descriptive study of complications of gastrostomy tubes in children. J Pediatr Nurs. 2010;25(2):72‐80. [DOI] [PubMed] [Google Scholar]
- 6. McShane D, Bellet J. Treatment of hypergranulation tissue with high potency topical corticosteroids in children. Pediatr Dermatol. 2012;29(5):675‐678. [DOI] [PubMed] [Google Scholar]
- 7. Vaneau M, Chaby G, Guillot B, et al. Consensus panel recommendations for chronic and acute wound dressings. Arch Dermatol. 2007;143(10):1291‐1294. [DOI] [PubMed] [Google Scholar]
- 8. Pott FS, Meier MJ, Stocco JG, Crozeta K, Ribas JD. The effectiveness of hydrocolloid dressings versus other dressings in the healing of pressure ulcers in adults and older adults: a systematic review and meta‐analysis. Rev Lat Am Enfermagem. 2014;22(3):511‐520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Harris A, Rolstad BS. Hypergranulation tissue: a nontraumatic method of management. Ostomy Wound Manage. 1994;40(5):20‐22. 24, 26–30. [PubMed] [Google Scholar]
- 10. Coutts P, Sibbald RG. The effect of a silver‐containing hydrofiber dressing on superficial wound bed and bacterial balance of chronic wounds. Int Wound J. 2005;2(4):348‐356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lansdown AB, Williams A, Chandler S, Bendfield S. Silver absorption and antibacterial efficacy of silver dressings. J Wound Care. 2005;14(4):155‐160. [DOI] [PubMed] [Google Scholar]
- 12. Jorgensen B, Price P, Andersen KE, et al. The silver‐releasing foam dressing, Contreet foam, promotes faster healing of critically colonised venous leg ulcers: a randomised, controlled trial. Int Wound J. 2005;2(1):64‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Euser AM, Zoccali C, Jager KJ, Dekker FW. Cohort studies: prospective versus retrospective. Nephron Clin Pract. 2009;113(3):c214‐c217. [DOI] [PubMed] [Google Scholar]
- 14. Saavedra H, Losek J, Shanley L, Titus O. Gastrostomy tube‐related complaints in the pediatric emergency department. Pediatr Emerg Care. 2009;25(11):728‐732. [DOI] [PubMed] [Google Scholar]
- 15. Widgerow AD, Leak K. Hypergranulation tissue: evolution, control and potential elimination. Wound Healing South. Afr. 2010;3(2):7‐9. [Google Scholar]
- 16. Tanaka H, Arai K, Fujino A, et al. Treatment for hypergranulation at gastrostomy sites with sprinkling salt in paediatric patients. J Wound Care. 2013;22(1):17‐18. 20. [DOI] [PubMed] [Google Scholar]
- 17. Moody M, Landau J, Goldberg L, Marquez D. 595 nm long pulsed dye laser with a hydrocolloid dressing for the treatment of hypergranulation tissue on the scalp in postsurgical defects. Dermatol Online J. 2011;17(7):2. [PubMed] [Google Scholar]


