Abstract
This study aimed to systematically review and identify the risk factors for the recurrence of diabetic foot ulcers (DFUs) among diabetic patients. PUBMED, EMBASE, Web of Science, Cochrane Library, China Biology Medicine (CBM), China National Knowledge Infrastructure (CNKI), WanFang, and VIP databases were electronically searched to identify eligible studies updated to January 2019 to collect case‐control studies or cohort studies on the risk factors for the recurrence of DFUs. Two reviewers independently screened the literature, extracted data, and assessed the risk of bias of included studies using the Newcastle‐Ottawa Scale. A meta‐analysis was performed using RevMan 5.3. Nine retrospective cohort studies were included, in which 1426 patients were enrolled, 542 in the DFU recurrence group and 884 in the non‐recurrent DFU group. Risk factors for the recurrence of DFUs included male gender (odds ratio [OR] = 1.38, 95% confidence interval [CI], 1.07‐1.78, P < .05), smoking (OR = 1.66, 95% CI, 1.26‐2.20, P = .0004), duration of diabetes (OR = 4.43, 95% CI, 1.96‐6.90, P = .0004), duration of past DFUs (OR = 1.02, 95% CI, 1.00‐1.03, P = .006), plantar ulcers (OR = 5.31, 95% CI, 4.93‐5.72, P <.00001), peripheral artery disease (OR = 1.65, 95% CI, 1.20‐2.28, P = .002), and diabetic peripheral neuropathy (OR = 2.15, 95% CI, 1.40‐3.30, P = .0005). No significant differences were found in age, body mass index, total cholesterol, diabetic nephropathy, diabetic retinopathy, or hypertension. Health care staff should pay attention to the identified risk factors for the recurrence of DFUs. Because of the limited quality and quantity of the included studies, rigorous studies with adequate sample sizes are needed to verify the conclusion.
Keywords: diabetic foot ulcer, meta‐analysis, recurrence, risk factor
Abbreviations
- IDF
International Diabetes Federation
- DFU
diabetic foot ulcer
- CBM
China Biology Medicine
- CNKI
China National Knowledge Infrastructure
- ORs
odds ratios
- NOS
Newcastle–Ottawa Scale
- ROB
risk of bias
- CIs
confidence intervals
- WMD
weighted mean differences
- BMI
body mass index
- PAD
peripheral artery disease
- DPN
diabetic peripheral neuropathy
- DR
diabetic retinopathy
- DN
diabetic nephropathy
- TC
total cholesterol
1. INTRODUCTION
According to the International Diabetes Federation (IDF), the number of global diabetic patients reached 41.5 billion in 2015 and is expected to reach 64.2 billion in 2040. Three‐fourth (75%) of those with diabetes were living in low‐ and middle‐income countries.1 Diabetic foot ulcers (DFUs) are one of the most serious complications in diabetic patients as they are perhaps the most common cause of diabetes‐related hospitalisation and may lead to amputation.2 It is estimated that the annual risk of developing a DFU in diabetic patients ranges from 19% to 34%. Approximately 40% of patients with DFUs experience a recurrence within 1 year after the ulcer has healed, nearly 60% within 3 years, and 65% within 5 years.3 Recurrent foot ulcerations result from various factors that have adverse effects on patients' physiological condition, mental health, and social functioning. In addition, these recurrent ulcers increase the patient's medical burden because of long‐term costs related to wound management.4 Hence, it is necessary to identify the risk factors of recurrent DFUs and provide evidence for their prevention.
Although the recurrence of DFUs has received increasing attention, there are few studies on the risk factors for the recurrence of DFUs, and the identification of the risk factors has still failed to reach a consensus. For instance, disagreements on the duration of diabetes as a risk factor for diabetic patients were found in the study of Khalifa5 and Qian et al,6 with the former study indicating that patients with a long duration of diabetes were at high risk of recurrent foot ulcers, while the latter study did not show a significant difference. Likewise, research conducted by Diouri et al7 showed that males were more prone to recurrences of DFUs than females, but the results from Dubský et al4 did not show a significant difference between males and females. In addition, there is still no meta‐analysis available to help synthesise these data and provide vigorous evidence for the prevention of DFU recurrence.
Thus, we performed this meta‐analysis to identify the risk factors for the recurrence of DFUs and to potentially provide robust evidence to guide clinical practice.
2. MATERIALS AND METHODS
2.1. Literature search strategy
PUBMED, EMBASE, Web of Science, Cochrane Library, China Biology Medicine (CBM), China National Knowledge Infrastructure (CNKI), WanFang, and VIP databases were electronically searched to collect case‐control studies or cohort studies about the risk factors for recurrence of DFUs from their inception to January 2019 by using the following search terms: diabetic foot ulcer, foot ulcer, recurrence, reulceration, recurrent ulceration, risk factor, and predicted factor. In addition, all references to the retrieved studies were checked to ensure that the eligible studies were included. The entire process was independently completed by two researchers.
2.2. Study inclusion and exclusion criteria
Studies were included if the following criteria were met: (a) diabetic patients with healed foot ulcerations, (b) case‐control study or cohort study, (c) comparison groups of recurrence and non‐recurrence, (d) data on the risk factors for recurrence of DFUs reported as odds ratios (ORs) with 95% confidence intervals (95% CI), and (e) English or Chinese article. Editorials, reviews, letters, and comments were excluded from this analysis.
2.3. Data abstraction and quality appraisal
A data extraction form was designed for the included studies. The following data were independently extracted by two researchers: first author, year of publication, location of study, type of study design, population size (recurrence/non‐recurrence), sample ages, follow‐up time, and risk factors. We also contacted the authors about unclear or missing information when necessary.
The methodological quality of included studies was independently assessed using the validated Newcastle–Ottawa Scale (NOS). The NOS is based on an accumulative score in each of three categories: selection, comparability, and exposure or outcome. The NOS scores range between 0 and 9 stars. Studies with 6 to 9 stars were considered to be at low risk of bias (ROB), studies with 4 to 5 stars were considered to be at medium ROB, and studies with 1 to 3 stars were considered to be at high ROB. Two researchers independently performed the quality assessment for included studies, and disagreement was resolved by discussion. Detailed results of the NOS quality appraisal are summarised in Table 1.
Table 1.
Summary of the detailed results of Newcastle–Ottawa Scale (NOS) quality appraisal
| Author/year | Selection of population | Comparability | Evaluation of exposure or outcome | NOS scores | |||||
|---|---|---|---|---|---|---|---|---|---|
| Hu et al, 20148 | * | * | * | * | ** | * | * | * | 8 |
| Chang et al, 20179 | * | * | * | * | ** | * | * | * | 9 |
| Qian et al, 20136 | * | * | 0 | * | 0 | * | 0 | * | 5 |
| Mo et al, 201810 | * | * | * | * | ** | * | * | * | 9 |
| Xie et al, 201811 | * | * | * | * | ** | * | * | * | 9 |
| Waaijman et al., 201412 | * | 0 | * | * | ** | * | 0 | * | 8 |
| Peters et al, 200713 | * | * | * | * | ** | * | * | * | 9 |
| Khalifa 20185 | * | * | * | * | ** | * | * | * | 9 |
| Dubský et al., 20134 | * | * | * | * | ** | * | * | * | 9 |
2.4. Statistical analysis
All statistical analyses were performed using the Review Manager Software (RevMan5.3, Microsoft, Redmond, Washington). If two or more studies reported the same risk factor, pooled OR estimates with corresponding 95% CIs were calculated. For discontinuous outcomes, an OR with 95% CIs was calculated. In continuous outcomes, weighted mean differences (WMD) with 95% CIs were assessed. Heterogeneity among studies was assessed by using the χ² and I 2 tests. A fixed‐effects model was adopted when there was no statistically significant heterogeneity among the studies (P 2 > .10 and I 2 < 50%); otherwise, a random‐effects model was applied. If the OR was not reported, it was calculated from the original data. If data could not be synthesised, or risk factors were identified in only one study, the results were presented in a descriptive manner. In addition, publication bias was assessed by using a funnel plot.
3. RESULTS
3.1. Search process
An initial search identified 522 studies, and 213 studies remained after removing duplicates, of which 196 studied were excluded after assessing the titles and abstracts because they were not related to our research. Then, 17 studies were further reviewed, of which eight studies were excluded and nine were included. The entire process was independently completed by two researchers, and disagreements were negotiated and resolved with a third researcher (Figure 1).
Figure 1.
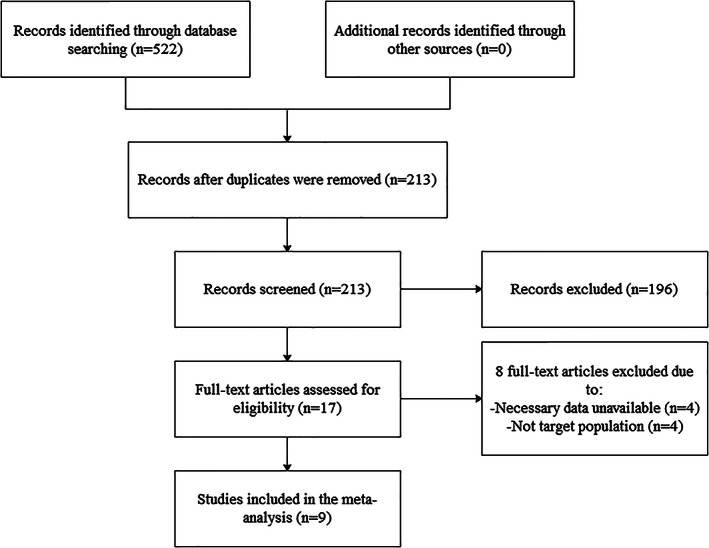
Flow diagram of the literature search
3.2. Characteristics of the included studies
The nine studies included in the systematic review and meta‐analysis were retrospective cohort studies published between 2007 and 2018. A total of 1426 patients were involved, with 542 with DFU recurrence and 884 with DFU non‐recurrence. The sample sizes of all included studies ranged from 73 to 282, follow‐up duration varied from 12 to 62 months, and mean ages ranged from 42 to 78 years. Two studies4, 13 failed to provide a mean age for participants. Regarding the study location, five6, 8, 9, 10, 11 were conducted in China, two13 in the Netherlands, one5 in Egypt, and one4 in Czechoslovakia. The characteristics of the included studies are listed in Table 2.
Table 2.
Characteristics of the included studies
| Author/year | Nation | Study design | Sample (T/C) | Age range (mean) | Follow up (months) | Risk factors |
|---|---|---|---|---|---|---|
| Hu et al, 20148 | China | R | 57/174 | 58.3 ± 10.2 | 36 | (1) (2) (4) (5) (8) |
| Chang et al, 20179 | China | R | 97/185 | 65 | 37.14 ± 17.60 | (1) (2) (3) (4) (5) (6) (8) (10) (11) (12) |
| Qian et al, 20136 | China | R | 33/108 | 68 ± 10 | 12 | (13) |
| Mo et al, 201810 | China | R | 85/104 | 66.60 ± 9.80 | 44.83 ± 16.69 | (2) (3) (8) (9) (10) (11) (12) |
| Xie et al, 201811 | China | R | 51/114 | 72.6 ± 8.0 | 24 | (2) (3) (8) (12) |
| Waaijman et al, 201412 | The Netherlands | R | 71/100 | 63.3 | 18 | (2) (3) (6) (8) (9) |
| Peters et al, 200713 | Netherlands | R | 49/32 | NR | 27.1 ± 9.2 | (7) (8) (9) (10) (11) |
| Khalifa, 20185 | Egypt | R | 57/36 | 51.73 ± 9.45 | 24 | (2) (3) (4) (5) (8) (9) (10) (11) (12) (13) |
| Dubský et al., 20134 | Czech | R | 42/31 | NR | 36 | (2) (3) (7) (8) (10) |
Note: NR: no report; R: retrospective; T: recurrence; C: non‐recurrence; Risk factors: (1) Age; (2) Gender; (3) Smoking; (4) BMI; (5) Duration of diabetes; (6) Duration of past diabetic foot ulcers; (7) Plantar ulcers; (8) peripheral artery disease; (9) diabetic peripheral neuropathy; (10) diabetic nephropathy; (11) diabetic retinopathy; (12) hypertension; (13) total cholesterol.
3.3. Synthesis of the results
Fourteen factors were ultimately analysed quantitatively: age, gender, smoking, body mass index (BMI), duration of diabetes, duration of past DFUs, plantar ulcers, peripheral artery disease (PAD), diabetic peripheral neuropathy (DPN), diabetic retinopathy (DR), diabetic nephropathy (DN), hypertension, and total cholesterol (TC). Nine factors (gender, smoking, duration of past DFUs, plantar ulcers, PAD, DPN, DR, DN, and hypertension) were not observed to have statistically significant heterogeneity, and a fixed‐effects model was applied. As the remaining factors (age, BMI, duration of diabetes, TC) possessed statistically significant heterogeneity, a random‐effects model was applied. The overall results are summarised in Table 3.
Table 3.
Meta‐analysis of the pooled risk factors for DFUs
| Risk factors | Heterogeneity test I 2(%) P | Model | Combined OR/WMD | 95% CI | P | |
|---|---|---|---|---|---|---|
| Age8, 9 | 95 | <.01 | Random | 0.91 | (0.74‐1.12) | .36 |
| Gender4, 5, 8, 9, 10, 11, 12 | 18 | .29 | Fixed | 1.38 | (1.07‐1.38) | .01 |
| Smoking4, 5, 9, 10, 11, 12 | 10 | .35 | Fixed | 1.66 | (1.26‐2.20) | <.01 |
| BMI5, 8, 9 | 73 | .02 | Random | −0.19 | (−1.32~0.94) | .74 |
| Duration of diabetes5, 8, 9 | 85 | <.01 | Random | 4.43 | (1.96‐6.90) | .004 |
| Duration of past diabetic foot ulcer9, 12 | 5 | .30 | Fixed | 1.02 | (1.00‐1.03) | .006 |
| Plantar ulcer4, 13 | 0 | .049 | Fixed | 5.31 | (4.93‐5.72) | <.01 |
| PAD4, 5, 9, 10, 11 | 46 | .11 | Fixed | 1.65 | (1.20‐2.28) | .002 |
| DPN5, 10, 12, 13 | 0 | .40 | Fixed | 2.15 | (1.40‐3.30) | <.01 |
| DN4, 5, 9, 10, 13 | 0 | .88 | Fixed | 1.32 | (0.95‐1.82) | .10 |
| DR5, 9, 10, 13 | 0 | .79 | Fixed | 1.08 | (0.77‐1.52) | .65 |
| Hypertension5, 9, 11, 13 | 0 | .93 | Fixed | 1.23 | (0.90‐1.68) | .20 |
| TC5, 6 | 73 | .05 | Random | 1.34 | (0.57‐3.14) | .50 |
Abbreviations: BMI, body mass index; DFU, diabetic foot ulcer; DPN, diabetic peripheral neuropathy; DN, diabetic nephropathy; DR, diabetic retinopathy; PAD, peripheral artery disease; TC, total cholesterol.
3.4. Demographic factors for the recurrence of DFUs
3.4.1. Age
Two studies8, 9 reported the relationship between age and the risk of DFU recurrence. However, obvious heterogeneity was found among the included studies (I 2 = 95%, P < .0001), and no significant difference was found in the estimate of the study effect size (OR = 0.91, 95% CI, 0.74‐1.12, P = .36) (Figure 2).
Figure 2.

Forest plot: effect of age on diabetic foot ulcer recurrence
3.4.2. Gender
Seven studies4, 5, 8, 9, 10, 11, 12 that included a total of 1207 patients provided eligible data for demonstrating the relationship between gender and DFU recurrence. The pooled results showed that males had a higher risk of developing DFU recurrence than females (OR = 1.38, 95% CI, 1.07‐1.78, P < .05) (Figure 3).
Figure 3.
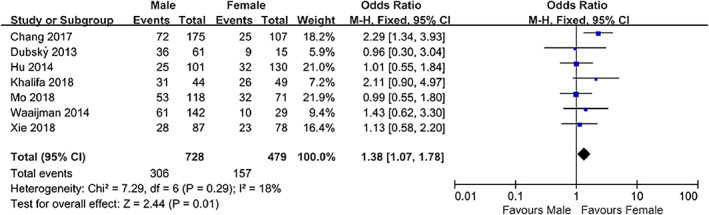
Forest plot: effect of gender on diabetic foot ulcer recurrence
3.4.3. Smoking
Six studies4, 5, 9, 10, 11, 12 that included a total of 973 patients provided available data on the association between smoking and DFU recurrence. The pooled results showed that smoking was associated with an increased incidence of DFU recurrence (OR = 1.66, 95% CI, 1.26‐2.20, P = .0004) (Figure 4).
Figure 4.
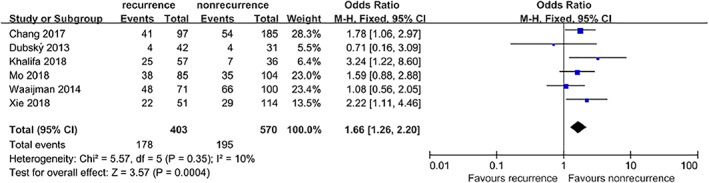
Forest plot: effect of smoking on diabetic foot ulcer recurrence
3.4.4. Body mass index
Three studies5, 8, 9 provided available data on the association between BMI and DFU recurrence. However, moderate heterogeneity was found among the included studies (I 2 = 73%, P = .02), and no significant difference was found between the combined estimates (OR = −0.19, 95% CI, −1.32‐0.94, P = .74) (Figure 5).
Figure 5.
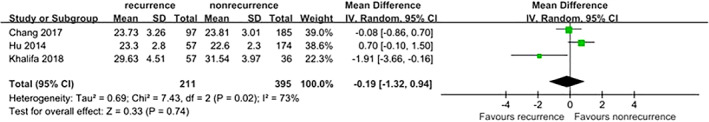
Forest plot: effect of body mass index on diabetic foot ulcer recurrence
3.5. Duration of diabetes
Three studies5, 8, 9 provided extractable data to analyse the duration of diabetes. The fixed‐effects model showed that patients with a long duration of diabetes were at a significant risk of DFU recurrence. However, because of the high heterogeneity (I 2 = 85%, P = .001), the random‐effects model was applied to analyse the pooled MD with an outcome of 4.43 (95% CI, 1.96‐6.90, P = .0004) (Figure 6).
Figure 6.
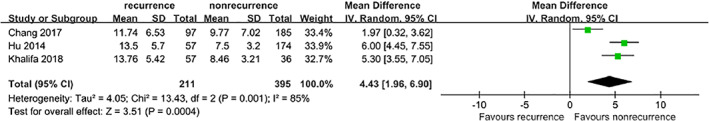
Forest plot: effect of the duration of diabetes on diabetic foot ulcer recurrence
3.6. Duration of past DFUs
Two studies9, 12 reported the relationship between the duration of past DFUs and the risk of DFU recurrence. Pooled results obtained using a fixed‐effects model suggest that patients with a long duration of past DFUs were at a higher risk of DFU recurrence (OR = 1.02, 95% CI, 1.00‐1.03, P = .006) (Figure 7).
Figure 7.

Forest plot: effect of the long duration of past diabetic foot ulcers (DFUs) on DFU recurrence
3.7. Plantar ulcers
Two studies4, 13 reported the relationship between the plantar ulcers and the risk of DFU recurrence. The results showed that patients with previous plantar ulcers were at a higher risk of DFU recurrence (OR = 5.31, 95% CI, 4.93‐5.72, P <.00001) (Figure 8).
Figure 8.

Forest plot: effect of the plantar ulcers on diabetic foot ulcer recurrence
3.8. Clinical factors for the recurrence of DFUs
3.8.1. Peripheral artery disease
Five studies4, 5, 9, 10, 11 provided extractable data to analyse the association between the risk of DFU recurrence and PAD. The fixed‐effects model showed that patients with PAD were at a significant risk of DFU recurrence (OR = 1.65, 95% CI, 1.20‐2.28, P = .002) (Figure 9).
Figure 9.
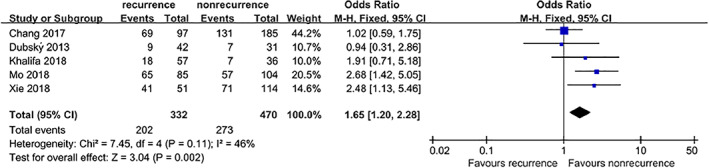
Forest plot: effect of peripheral artery disease on diabetic foot ulcer recurrence
3.8.2. Diabetic peripheral neuropathy
Four studies5, 10, 12, 13 that included a total of 534 patients provided available data on the association between DPN and DFU recurrence. The pooled results suggested that DPN was associated with an increased incidence of DFU recurrence (OR = 2.15, 95% CI, 1.40‐3.30, P = .0005) (Figure 10).
Figure 10.
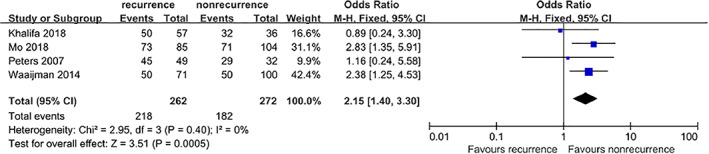
Forest plot: effect of diabetic peripheral neuropathy on diabetic foot ulcer recurrence
3.8.3. Diabetic nephropathy
Five studies4, 5, 9, 10, 13 that included a total of 718 patients provided available data on the association between DN and DFU recurrence. However, no significant difference was found between the combined estimates (OR = 1.32, 95% CI, 0.95‐1.82, P = .10) (Figure 11).
Figure 11.
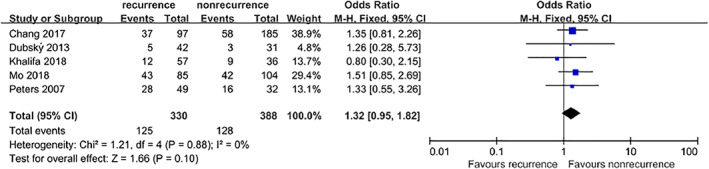
Forest plot: effect of diabetic nephropathy on diabetic foot ulcer recurrence
3.8.4. Diabetic retinopathy
Four studies5, 9, 10, 13 that included a total of 645 patients provided available data on the association between DR and DFU recurrence. However, no significant difference was found between the combined estimates (OR = 1.08, 95% CI, 0.77‐1.52, P = .65) (Figure 12).
Figure 12.
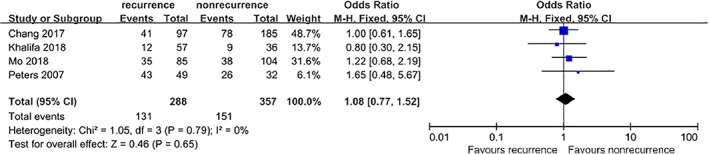
Forest plot: effect of diabetic retinopathy on diabetic foot ulcer recurrence
3.8.5. Hypertension
Four studies5, 9, 10, 11 that included a total of 729 patients provided available data on the association between hypertension and DFU recurrence. However, no significant difference was found between the combined estimates. (OR = 1.23, 95% CI, 0.90‐1.68, P = .20) (Figure 13).
Figure 13.
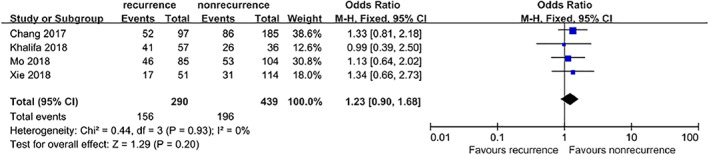
Forest plot: effect of hypertension on diabetic foot ulcer recurrence
3.8.6. Total cholesterol
Two studies5, 6 reported the relationship between TC and the risk of DFU recurrence. However, distinct heterogeneity was found among the included studies (I 2 = 73%, P < .05), and no significant difference was found in the estimate of the study effect size (OR = 1.34, 95% CI, 0.57‐3.14, P = .50) (Figure 14).
Figure 14.

Forest plot: effect of TC on diabetic foot ulcer recurrence
4. DISCUSSION
In our meta‐analysis, risk factors for the recurrence of DFUs included male gender, smoking, long duration of diabetes, long duration of past DFUs, plantar ulcers, PAD, and DPN. No significant differences were found in age, BMI, TC, DN, DR, or hypertension.
Meta‐analysis results showed that the risk of DFU recurrence in male patients was 1.38 times higher than that in female patients (OR = 1.38, 95% CI, 1.07‐1.78, P < .05), which was consistent with the results of a previous study.7 However, among the studies included in this study, Dubský et al4 and Khalifa5 found that DFU recurrence was not related to gender, but the authors did not give any explanation for this negative result, possibly because of the small sample size.
In the included studies, some5, 11 reported a higher risk of DFU recurrence in patients with a history of smoking. Our result is consistent with these studies (OR = 1.66, 95% CI, 1.26‐2.20, P = .0004). Although several studies4, 9, 10, 12 reported that smoking was not a risk factor of DFU recurrence, these studies were limited in their small sample size, while our result was based on six studies, in which 44.2% (178/403) of patients in the DFU recurrence group had a history of smoking compared with 34.2% (195/570) in DFU non‐recurrence group, and low heterogeneity was identified among them. According to previous studies, smoking affects the control of blood glucose in diabetic patients, which is closely related to the occurrence of DFUs.14 In addition, smoking can cause vasoconstriction and blood flow obstruction, leading to ischaemia and affecting the repair of ulcers.15
Our study also found that, with the progression of diabetes, the risk of DFU recurrence increases (OR = 4.43, 95% CI, 1.96‐6.90, P = .0004), which is consistent with the studies of Qian et al6 and Hu et al,8 but studies conducted by Chang et al9 and Khalifa5 found that the duration of diabetes was not an independent risk factor for DFU recurrence; however, the authors did not give any explanation for this negative result.
In addition, duration of past DFUs was confirmed as a risk factor of DFU recurrence in this meta‐analysis. This might be explained by a study which showed that the risk of recurrent ulcerations in patients with a DFU ≥2 months at the first visit was 1.93 times higher than that in patients with a DFU ≤2 months, which was related to the delayed visit and the improper treatment of wounds in patients with DFUs,16 suggesting that early and proper treatment should be carried out in the care of DFU patients for preventing recurrent ulcerations. However, because of limited available data for analysis, this conclusion was made based on only two studies; hence, more research is necessary before a definite conclusion can be drawn.
Moreover, DFU patients with initial ulcers at the plantar site had a 5.31 times higher risk of recurrence of DFUs than those with initial ulcers at other sites (OR = 1.02, 95% CI, 1.00‐1.03, P = .006), which was related to the fact that the plantar site is under more pressure than other sites.4 However, this conclusion was reached based on only two studies as others did not provide available data for analysis, and more research should be conducted to validate our conclusion.
As is commonly acknowledged, PAD can cause abnormalities in the microcirculation of the foot, resulting in poor blood supply; hence, the recurrence rate of DFUs in patients with PAD is high.17, 18 The meta‐analysis demonstrated that the recurrence risk of foot ulcers in DFU patients with PAD was 2.41 times than that in patients without PAD, suggesting that early and timely assessment and identification of PAD should be carried out in the care of DFU patients.
DFU patients with DPN were at a higher risk of DFU recurrence (OR = 2.15, 95% CI, 1.40‐3.30, P = .0005), which was consistent with the results of a previous study.19 DFU patients with DPN may experience feelings of abnormal temperature or pain sensations in their feet, and their perception of external stimuli will be weakened and easily damaged. Furthermore, sweat glands will be demineralized in case of autonomic neuropathy, which will make the skin on the foot dry, chapped, and prone to ulcers. In motor neuropathy, foot muscle atrophy leads to foot malformations, and foot compression imbalance is also prone to damage.20 The results of this study indicated that DPN is a risk factor for the recurrence of DFUs, and the recognition of DPN should be evaluated as early as possible in clinical practice.
There are several limitations to this study. First, some of the research data needed to be recalculated and extracted, and there may be errors in the data conversion. Second, because of limitations of the included data, we did not conduct subgroup analyses and funnel plots. Some of our results show significant heterogeneity, and it is difficult to identify the possible causes of heterogeneity. Third, only the Chinese and English literature was retrieved, and there may be incomplete retrieval. Another potential limitation is the number of existing studies; hence, further research should be conducted to validate our conclusion.
5. CONCLUSION
The results of this meta‐analysis showed that gender, smoking, duration of diabetes, duration of past DFUs, plantar ulcers, PAD, and DPN were risk factors for DFU recurrence. By identifying these factors, health care staff could focus on the identified risk factors for the recurrence; hence, patients with a relatively higher risk of DFU recurrence could be treated in a more timely manner. However, because of the limited quantity and quality of the included studies, the research evidence of risk factors for DFU recurrence is still insufficient. The above conclusions may be biased, and more studies are needed to verify the correlation between the above risk factors and DFU recurrence.
Huang Z‐H, Li S‐Q, Kou Y, Huang L, Yu T, Hu A. Risk factors for the recurrence of diabetic foot ulcers among diabetic patients: a meta‐analysis. Int Wound J. 2019;16:1373–1382. 10.1111/iwj.13200
Contributor Information
Ting Yu, Email: 524208040@qq.com.
Ailing Hu, Email: h-ailing@163.com.
REFERENCES
- 1. Ogurtsova K, da Rocha Fernandes JD, Huang Y, et al. IDF diabetes atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract. 2017;128:40‐50. [DOI] [PubMed] [Google Scholar]
- 2. Del Pino‐Sedeno T, Trujillo‐Martin MM, Andia I, et al. Platelet‐rich plasma for the treatment of diabetic foot ulcers: a meta‐analysis. Wound Repair Regen. 2018;27(2):170‐182. [DOI] [PubMed] [Google Scholar]
- 3. Armstrong DG, Boulton AJM, Bus SA. Diabetic foot ulcers and their recurrence. N Engl J Med. 2017;376(24):2367‐2375. [DOI] [PubMed] [Google Scholar]
- 4. Dubský M, Jirkovská A, Bem R, et al. Risk factors for recurrence of diabetic foot ulcers: prospective follow‐up analysis in the Eurodiale subgroup. Int Wound J. 2013;10(5):555‐561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Khalifa WA. Risk factors for diabetic foot ulcer recurrence: a prospective 2‐year follow‐up study in Egypt. Foot (Edinb). 2018;35:11‐15. [DOI] [PubMed] [Google Scholar]
- 6. Qian H, Xu L, Zhang S, et al. Disease course of patients with diabetic foot ulcer during one‐year follow‐up. J Intern Med Cocepts Pract. 2013;8(02):124‐127. [Google Scholar]
- 7. Diouri A, Slaoui Z, Chadli A, et al. Incidence of factors favoring recurrent foot ulcers in diabetic patients. Ann Endocrinol (Paris). 2002;63(6 Pt 1):491‐496. [PubMed] [Google Scholar]
- 8. Hu X, Shi L, Liu S. Analysis of risk factors for recurrence of foot ulcer in type 2 diabetes mellitus. Today Nurse. 2014;22(04):23‐24. [Google Scholar]
- 9. Chang X, Yuan L, Wang F, et al. Analysis of clinical outcomes and related factors in patients with diabetic foot after discharge. Chinese Nursing Research. 2017;31(36):4634‐4637. [Google Scholar]
- 10. Mo Z, Chen D, Gao Y, et al. Risk factors for foot ulcer recurrence in diabetic patients with new‐onset foot ulcers. China Tropical Nedicine. 2018;18(07):716‐719. [Google Scholar]
- 11. Xie C, Chen Y, Xiong Y, Chen P, Yang X. Influencing factors of recurrences of the healed diabetic foot ulcers. Chin J Mult Organ Dis Elderly. 2018;17(07):501‐504. [Google Scholar]
- 12. Waaijman R, de Haart M, Arts ML, et al. Risk factors for plantar foot ulcer recurrence in neuropathic diabetic patients. Diabetes Care. 2014;37(6):1697‐1705. [DOI] [PubMed] [Google Scholar]
- 13. Peters EJ, Armstrong DG, Lavery LA. Risk factors for recurrent diabetic foot ulcers: site matters. Diabetes Care. 2007;30(8):2077‐2079. [DOI] [PubMed] [Google Scholar]
- 14. Nilsson PM, Gudbjornsdottir S, Eliasson B, et al. Smoking is associated with increased HbA1c values and microalbuminuria in patients with diabetes‐‐data from the National Diabetes Register in Sweden. Diabetes Metab. 2004;30(3):261‐268. [DOI] [PubMed] [Google Scholar]
- 15. Darling JD, McCallum JC, Soden PA, et al. Predictive ability of the Society for Vascular Surgery Wound, ischemia, and foot infection (WIfI) classification system following infrapopliteal endovascular interventions for critical limb ischemia. J Vasc Surg. 2016;64(3):616‐622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mai L, Li Y, Zhang L, et al. Five‐year recurrence rate of ulcer in patients with new‐onset diabetic foot ulcer: risk factors. J Nurs Sci. 2016;31(11):18‐21. [Google Scholar]
- 17. Mantey I, Foster AV, Spencer S, et al. Why do foot ulcers recur in diabetic patients? Diabet Med. 1999;16(3):245‐249. [DOI] [PubMed] [Google Scholar]
- 18. Ciccone MM, Marchese A, Generali A, et al. Interventional therapy in diabetic foot: risk factors, clinical events and prognosis at one year follow‐up (a study of 103 cases). Pak J Biol Sci. 2012;15(16):789‐794. [DOI] [PubMed] [Google Scholar]
- 19. Connor H, Mahdi OZ. Repetitive ulceration in neuropathic patients. Diabetes Metab Res Rev. 2004;20(Suppl 1):S23‐S28. [DOI] [PubMed] [Google Scholar]
- 20. Ni P, Yuan L. Research Progress of influencing factors on recurrence of diabetic foot ulcer. J Nurs (China). 2017;24(09):35‐38. [Google Scholar]


