Abstract
When plants are exposed to high-light conditions, the potentially harmful excess energy is dissipated as heat, a process called non-photochemical quenching. Efficient energy dissipation can also be induced in the major light-harvesting complex of photosystem II (LHCII) in vitro, by altering the structure and interactions of several bound cofactors. In both cases, the extent of quenching has been correlated with conformational changes (twisting) affecting two bound carotenoids, neoxanthin, and one of the two luteins (in site L1). This lutein is directly involved in the quenching process, whereas neoxanthin senses the overall change in state without playing a direct role in energy dissipation. Here we describe the isolation of an intermediate state of LHCII, using the detergent n-dodecyl-α-D-maltoside, which exhibits the twisting of neoxanthin (along with changes in chlorophyll–protein interactions), in the absence of the L1 change or corresponding quenching. We demonstrate that neoxanthin is actually a reporter of the LHCII environment—probably reflecting a large-scale conformational change in the protein—whereas the appearance of excitation energy quenching is concomitant with the configuration change of the L1 carotenoid only, reflecting changes on a smaller scale. This unquenched LHCII intermediate, described here for the first time, provides for a deeper understanding of the molecular mechanism of quenching.
Keywords: light-harvesting complex, NPQ, photoprotection, resonance Raman, LHCII
Abbreviations: α-DM, n-dodecyl-α-D-maltoside; β-DM, n-dodecyl-β-D-maltoside; α-DM-LHCII, LHCII purified with n-dodecyl-α-D-maltoside; β-DM-LHCII, LHCII purified with n-dodecyl-β-D-maltoside; Chl a, chlorophyll a; Chl b, chlorophyll b; LHC, light harvesting complex; LHCII, light harvesting complex II; NPQ, non-photochemical quenching; qE, energy dependent component of NPQ; Qy, light absorption due to electronic transition along the y-axis of the chlorophyll molecule
During the first steps of the photosynthetic process, solar photons are absorbed by specialized light-harvesting complexes (LHCs), and the resulting excitation energy is transferred to reaction center pigments, where it is converted into a chemical potential. In low-light conditions, most of the photons absorbed lead to a charge separation event at the reaction center (1, 2). However, when the absorbed energy is in excess of that which can be used for energy transduction, the overaccumulation of excited states can result in damage to the photosynthetic membrane, in particular, via the production of reactive oxygen species. In high-light conditions, the antenna system of plants and algae reorganizes reversibly, creating energy traps that dissipate the excess excitation as heat (3, 4, 5). This regulatory mechanism is known as non-photochemical quenching of chlorophyll fluorescence (NPQ). NPQ is a multi-component phenomenon whose fastest phase, qE (energy-dependent quenching), is triggered by the ΔpH across the thylakoid membrane, itself resulting from photosynthetic activity. qE induction and relaxation occurs in seconds, and it cannot involve de novo protein synthesis but rather corresponds to a reorganization of the existing photosynthetic membrane.
Over the last two decades, a large number of studies have been performed to gain insight into the molecular mechanisms underlying qE (6, 7, 8, 9, 10, 11). Proteins of the LHC family have the in-built capacity to quench excitation energy (6, 12, 13, 14, 15). LHCII, the major antenna complex in higher plants, occurs as a trimer of nearly identical monomers. Each monomer binds 14 chlorophyll molecules (8 Chl a and 6 Chl b), three tightly-bound carotenoids (two luteins and one 9-cis neoxanthin), and one weakly-bound violaxanthin carotenoid (16). Self-association of this protein upon detergent removal causes a significant quenching of excitation energy, first reported some four decades ago (17). Femtosecond transient absorption measurements conducted on LHCII aggregates showed that excitation quenching occurs through energy transfer from Chl a to the S1 excited state of a lutein molecule (6), more precisely the LHCII-bound lutein absorbing at 495 nm, bound to the protein site termed L1 (18, 19). The short lifetime of the carotenoid S1 excited state ensures an efficient dissipation of the excitation energy as heat (6, 20). More recently a similar quenching mechanism was observed in several isolated high-light-inducible proteins (21), members of the LHC superfamily that are in a permanently-quenched state and that are believed to be the ancestors of LHC antenna proteins (22, 23). Excitation energy transfers from Chls to different carotenoid states have been observed in quenched monomeric LHCII (10), quenched LHCII trimers (24, 25), the minor antenna CP29 (15), and in thylakoid membranes exposed to high light (26).
The formation of the quenching site in LHCII involves structural changes in a number of bound cofactors, revealed by resonance Raman spectroscopy (12). Changes in the interaction state of several chlorophylls with the protein host were observed, as well as in the configuration of the neoxanthin molecule (27), and more recently, in that of L1 lutein (14), consistent with the role of L1 as the quenching species. The amplitude of the neoxanthin change is strictly correlated with the extent of quenching in LHCII as well as in intact chloroplasts and leaves (6), and this carotenoid thus appears as a reporter of structural changes leading to quenching, a proposition that has recently been supported by molecular dynamics simulations (28). LHC proteins were proposed to be the major site of quenching in plants, which would occur through a subtle equilibrium between two LHC conformations (29, 30).
The LHCII structure is very sensitive to its environment (12, 24, 25, 27, 28, 31, 32). LHCII purified in the presence of either α- or β-dodecyl-D-maltoside (α-DM or β-DM) displays slightly different electronic properties (31, 33), and the H-bonding interactions of two bound chlorophyll a molecules are sensitive to the detergent used (34). In this work, we have studied the vibrational properties of the different pigments bound to LHCII in the presence of α-DM or β-DM. We show that the Raman signals of Chls a and b and neoxanthin that were previously associated with LHCII quenching are already present in LHCII purified in the presence of α-DM—even though this preparation is unquenched—while aggregation-induced quenching affects the L1 carotenoid-binding site alone. The molecular structure of Chl a, Chl b, lutein and neoxanthin pigments and those of the detergents used for the purification of LHCII are dispayed in Figure 1.
Figure 1.
Molecular structures of the LHCII tightly bound pigments and the detergents used for LHCII purification. Molecular structures of chlorophyll a, chlorophyll b, 9′-cis neoxanthin, all-trans lutein, α-dodecyl-D-maltoside (α-DM), and β-dodecyl-D-maltoside (β-DM).
Results
Influence of the stereochemistry of dodecyl-maltoside on LHCII electronic properties
As already reported (31, 33, 34), both the carotenoid and chlorophyll absorption regions of LHCII exhibit differences between the α-DM-solubilized versus the β-DM-solubilized protein (Fig. 2A). In the blue region, the Soret absorption transition of Chl is perturbed—in α-DM, an increase in intensity is seen at 432 nm, concomitant with a loss at 438 nm. The carotenoid contributions appear to lose intensity at 480 and 472 nm and gain intensity at 457 nm. In the Chl Qy region, a new band is present at 660 nm in LHCII isolated in α-DM, while the intensity of the transitions at 676 and 672 nm is reduced. All these changes are more easily observed in the difference spectrum (Fig. 2B). None of these changes in absorption induces any observable differences in fluorescence properties (e.g., Fig. 2C).
Figure 2.
Absorption and time-resolved fluorescence spectra of LHCII.A, absorption spectra at 4.2 K of LHCII in α-DM and β-DM (blue and red, respectively). B, difference spectrum “α-DM-LHCII minus β-DM-LHCII.” C, time-resolved fluorescence of α-DM-LHCII (blue) and β-DM-LHCII (red), excited at 405 nm with emission recorded at 680 nm. The grayed region represents instrumental response function in time domain.
Influence of DM stereochemistry on LHCII chlorophylls
Resonance Raman spectroscopy has seen extensive application to the assessment of pigment structure and interactions in photosynthetic proteins (35, 36, 37) and played a vital role in revealing the modifications to cofactor structure associated with the LHCII “conformational change” model of NPQ (12, 27). The Raman spectra of chlorophyll molecules are particularly rich, containing a number of bands that are sensitive to the chlorophyll conformation and to its interactions with the immediate environment. Upon aggregation-induced quenching in LHCII, two bound Chl a molecules lose a hydrogen bond to their conjugated keto carbonyl group on position C131, while one or two Chls b gain H-bonds to their conjugated formyl group at position C7 (observed for excitations at 413.1 and 441.6 nm, respectively [27]).
Chlorophyll resonance Raman spectra of the two LHCII preparations at 77 K are presented in Figure 3. Comparing α-DM-LHCII relative to β-DM-LHCII for the Chl a excitation at 413.1 nm (Fig. 3A), there is a clear increase in contributions on the high-frequency side of the envelope of bands in the 1660 to 1700 cm−1 region, which corresponds to stretching modes of Chl a keto groups conjugated with the macrocycle. This increase around 1690 cm−1 is accompanied by a corresponding decrease at lower frequency at ∼1670 cm−1 (shown by black arrow heads in Fig. 3A). As discussed elsewhere (34), this reflects the loss of an H-bond to probably two LHCII-bound Chl a molecules, at the level of their conjugated keto carbonyl group.
Figure 3.
High-frequency region of resonance Raman spectra of LHCII. Resonance Raman spectra at 77 K in the 1540 to 1720 cm−1 region for α-DM-LHCII (blue), β-DM-LHCII (red), and LHCII aggregates (black) excited at (A) 413.1 and (B) 441.6 nm.
Excitation at 441.6 nm yields spectra in which Chl b vibrational modes dominate. The high-frequency region thus corresponds to stretching modes of both conjugated carbonyl groups of Chl b – C7-formyl and C131-keto, in the 1620 to 1660 and 1660 to 1700 cm−1 ranges, respectively. When the two preparations are compared at this wavelength (Fig. 3B), an intense contribution is observed for α-DM-LHCII at 1637 cm−1 that is not present in β-DM-LHCII, accompanied by a decrease in the intensity of the contribution at 1666 cm−1 (shown by black arrow heads in Fig. 3B). This indicates that the formyl carbonyl of at least one Chl b, which is free from interactions in β-DM-LHCII, becomes strongly H-bonded in α-DM-LHCII. It is particularly interesting to note that the changes in Chl a and b interactions observed for α-DM-LHCII are strikingly similar to those seen upon aggregation-induced quenching in LHCII (27) (see spectra of aggregates in Fig. 3).
Influence of DM stereochemistry on LHCII-bound neoxanthin
Carotenoid resonance Raman spectra are mainly composed of four groups of bands, termed ν1–ν4. The ν1 band above 1500 cm−1 arises from stretching vibrations of C=C double bonds, and its frequency depends on the length of the π-electron conjugated chain and on the molecular configuration of the carotenoid (38, 39, 40, 41, 42, 43). The ν2 band at 1160 cm−1 contains contributions from stretching vibrations of C–C single bonds, coupled with C–H in-plane bending modes. This ν2 region is a fingerprint for the assignment of carotenoid isomerization states (40, 44). At 1000 cm−1, the ν3 band arises from in-plane rocking vibrations of the methyl groups attached to the conjugated chain, coupled with in-plane bending modes of the adjacent C–H's (38). It was recently shown to be a fingerprint of the conjugated end-cycle configuration (45, 46) and sensitive to the presence of a conjugated allene group (47, 48). Finally, the ν4 band, around 960 cm−1, arises from C–H out-of-plane wagging motions coupled with C=C torsional modes (38). When the carotenoid conjugated system is planar, these out-of-plane modes are not coupled with the electronic transition and thus exhibit little intensity. However, distortions around C–C single bonds increase the coupling of (some of) these modes with the electronic transition, resulting in an increase in their intensity (49).
Excitation of LHCII at 488.0 nm yields Raman spectra in which contributions of the bound neoxanthin molecule dominate (19). The spectra of α-DM- and β-DM-LHCII excited at 488.0 nm at 77 K do not exhibit substantial differences in their ν1 and ν2 regions (data not shown). Hence, this indicates that the conjugation length of Neo and its configuration (cis) are not altered by purification with different detergents. However, significant differences are observed in the ν3 and ν4 bands (Fig. 4). In the ν4 region, both samples display a band at 963 cm−1, whereas an additional component at 952 cm−1 gains intensity for α-DM-LHCI, indicating that neoxanthin undergoes a twist in the presence of this detergent, which is not present in β-DM-LHCII. This is accompanied by a change in the structure of the ν3 band. In the latter region, α-DM- and β-DM-LHCII both show a doublet characteristic of allene-containing carotenoids. However, the relative intensity of the two components at 1003.3 and 1006.6 cm−1 is different, consistent with a structural change of the neoxanthin between the two samples. Once again, the changes observed for α-DM-LHCII are similar to those seen in the quenched state of LHCII (see spectrum of LHCII aggregates in Fig. 4). However, the spectrum for aggregated LHCII displays additional components at 956 and 971 cm−1, which are absent in the spectrum of LHCII in α-DM. Note that LHCII aggregation produces identical spectra, whether the starting point is α-DM- or β-DM-LHCII. These specific features at 956 and 971 cm−1 have previously been assigned to a distortion of the lutein1 carotenoid (14), the pigment identified as the quenching species in aggregated LHCII (6).
Figure 4.
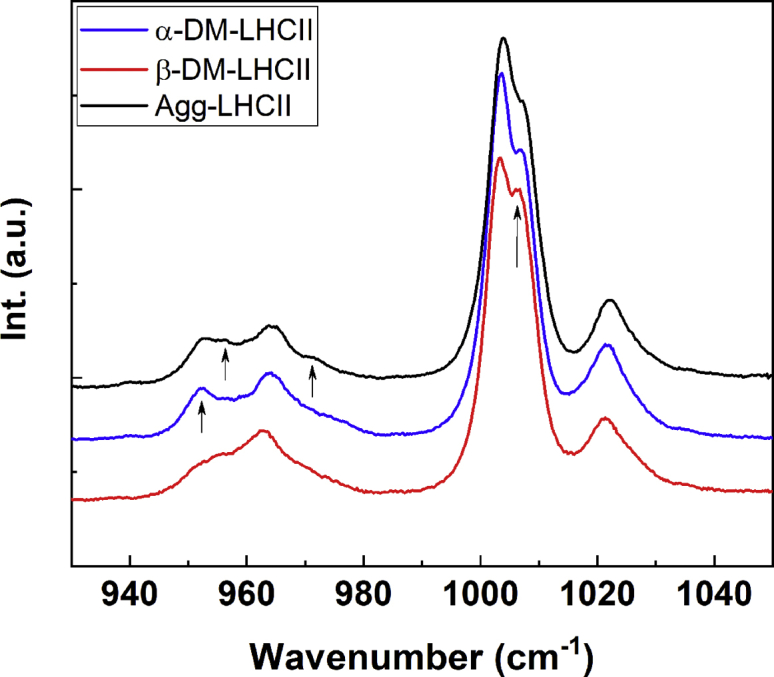
ν3and ν4regions of resonance Raman spectra of LHCII. Resonance Raman spectra at 77 K in the 930 to 1050 cm−1 region for α-DM-LHCII (blue), β-DM-LHCII (red), and LHCII aggregates (black) excited at 488.0 nm.
Structure of the lutein1 carotenoid
As discussed, the resonance Raman spectra obtained for LHCII when solubilized in α-DM or in the aggregated form display no significant differences at 413.1 and 441.6 nm, where Chl a and b molecules contribute, respectively. Excitation at 488.0 nm generates very similar spectra between these two samples, but with small differences in the ν4 region. A zoom of this region is presented in Figure 5, for excitation at 488.0 and 496.5 nm (dominated by neoxanthin and lutein1, respectively) for LHCII in β-DM, in α-DM, and upon aggregation by detergent removal. The spectra of α-DM-LHCII are globally similar to the quenched aggregates, reflecting the major twisting of the neoxanthin carotenoid, whereas aggregated LHCII exhibits additional components at 956 and 971 cm−1, which are absent for α-DM-LHCII. These bands are more clearly seen for excitation at 496.5 nm, corresponding to the absorption peak of lutein1 (18, 19).
Figure 5.

ν3region of LHCII resonance Raman spectra of LHCII. Resonance Raman spectra at 77 K in the ν4 region for LHCII in α-DM (red), β-DM (blue), and in the aggregated form (black), for excitation at 488.0 (A) and 496.5 nm (B).
These differences between α-DM-LHCII and the quenched aggregates are strikingly similar to those observed upon aggregation of LHCII purified from the npq2 mutant, which lacks neoxanthin. In the latter case, the spectral changes were attributed to a change in conformation of the lutein1 carotenoid, which in the WT complex is considerably masked by the larger changes in neoxanthin signal (14).
Discussion
Changes in pigment conformation in the major plant antenna protein LHCII, associated with the appearance of fluorescence quenching, have been documented for some 25 years (27). Removal of the solubilizing detergent in β-DM-purified LHCII leads to a tenfold or more decrease in its fluorescence level, along with structural changes in the binding pockets of two Chls a, one or two Chls b, and the neoxanthin and lutein1 carotenoids. Here we show that LHCII purified using the stereoisomer α-DM exhibits intermediate properties, where all of the quenching-associated changes are already present apart from that in L1, even though this LHCII preparation is unquenched (see fluorescence lifetimes in Fig 2C). Aggregation of α-DM-LHCII induces quenching, with the change in the L1 carotenoid now present. Note that the presence of additional LHCII conformations (i.e., more than two) has already been inferred from single-molecule and time-resolved fluorescence experiments on LHCII, as well as molecular dynamics simulations (13, 29).
All these pigment structural changes were up to now directly associated with the appearance of quenching. The ability of the detergent α-DM to stabilize an unquenched intermediate, which has not been observed previously, allows us to disentangle the molecular changes leading to quenching in LHCII. Indeed, the only change directly associated with quenching is that occurring in the L1 binding pocket. A careful analysis of the 488.0-nm resonance Raman spectra obtained previously for quenched LHCII, whether in aggregates, crystals, or gels (6, 12, 14, 27), reveals that all the samples display the additional components at 956 and 971 cm−1 in the ν4 region. This indicates that, in each case, the change in L1 configuration associated with quenching has indeed occurred. Thus the configuration of the L1 carotenoid is intrinsically linked with the quenching process, consistent with its attribution as the site of quenching (6, 15).
The significance of the changes affecting Chls a and b and neoxanthin, upon aggregation of β-DM-LHCII, may thus be questioned. It could be argued that β-DM stabilizes LHCII in a non-native state, which relaxes upon detergent removal, and that α-DM-LHCII and the aggregates represent the only two states observed in vivo (in unquenched and quenched conditions, respectively). However, it was shown that the changes occurring upon qE induction in chloroplasts and leaves do indeed involve twisting of the bound neoxanthin, and this twisting signal is proportional to the extent of quenching (6), just as for aggregation of β-DM-LHCII (and not of α-DM-LHCII). This rather suggests a two-step mechanism for qE: a state similar to β-DM-LHCII is the major light-harvesting state, and this converts to the quenched (aggregate-like) state via an unquenched intermediate similar to α-DM-LHCII. The fact that the “α-DM effect” has not been observed before tends to suggest that this intermediate is at least relatively rare. However, given the complexity of these kinds of measurements on intact chloroplasts and leaves, and the small sizes of the changes involved, they have generally been performed in fully-unquenched and fully-quenched states, and not so much at intermediate levels of quenching. It is therefore possible that conditions do exist where a structure similar to α-DM-LHCII is present in vivo to a more significant extent. It could also be hypothesized that this intermediate state is involved in an as-yet-unknown aspect of qE or its regulation and/or in a different regulatory process requiring tuning of the pigment structure of LHCII. It is worth noting, in this regard, that qE in vivo is tightly regulated, and in a somewhat complex manner (e.g., zeaxanthin, produced during exposure of leaves to high light, accelerates the onset of qE upon a second illumination (50)), whereas other (non-qE) phases of NPQ may also involve LHCII quenching (51). Further investigations along these lines should lead to a more profound understanding of the remarkable flexibility exhibited by the LHCII protein.
Experimental procedures
α-DM- and β-DM-LHCII were isolated from Arabidopsis thaliana plants as described (52, 53). Thylakoid membranes at a Chl concentration of 1 mg/ml were solubilized by adding the same volume of buffer containing 1.2% α-DM or 2% β-DM, respectively. The mixture was gently vortexed for a few seconds, and unsolubilized material was removed by centrifugation at 17,000g for 10 min. The supernatant was then loaded onto a 0 to 0.1 M sucrose density gradient containing 10 mM Hepes pH 7.5, with 0.03% α-DM or 0.06% β-DM, respectively. The LHCII band was collected after overnight ultracentrifugation at 280,000g.
Quenched LHCII was prepared by detergent removal using SM-2 bioabsorbent beads (Bio-Rad), allowing for a tenfold reduction in fluorescence yield as determined by a mini PAM-I fluorimeter (Heinz Walz).
Time-resolved photoluminescence decay curves were acquired on an EI fluorescence plate reader (Edinburgh Instruments) using 4000 detection bins of 2 μs integration time. Excitation was with an Edinburgh EPL 405-nm picosecond diode laser, with a repetition rate of 5 MHz, and a 716/40 bandpass filter was placed between the sample and the detection system. All samples were measured in black 96-well plates with an optimal working volume of 150 μl. photoluminescence decay curves were mathematically fitted using FAST software (Edinburgh Instruments).
UV-visible absorption spectra were measured using a Varian Cary E5 double-beam scanning spectrophotometer, with a 1.0-cm pathlength cuvette. Samples were maintained at low temperature in a helium bath cryostat (Maico Metriks); 60% glycerol (v/v) was added to the sample to prevent devitrification.
Resonance Raman spectra at 77 K were obtained in a liquid nitrogen flow cryostat (Air Liquide), using a Jobin-Yvon U1000 Raman spectrophotometer equipped with a liquid-nitrogen-cooled, charge-coupled-device detector (Spectrum One, Jobin-Yvon). Laser excitations at 488.0, 496.5, and 501.7 nm, and 413.1 nm, were obtained with Coherent argon (Sabre) and krypton (Innova 90) lasers, respectively. Excitation at 441.6 nm was obtained with a Liconix helium–cadmium laser.
Data availability
All data are contained within the article. Additional raw data are available upon request to the corresponding author.
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article.
Acknowledgments
The authors wish to thank Dr Niko Hildebrandt for the use of the time-resolved fluorescence setup.
Author contributions
F. L., C. L., and P. X. resources; F. L., C. L., P. X., M. J. L-P., S. S., and A. A. P. investigation; F. L., C. L., and S. S. visualization; C. I., M. J. L-P., A. A. P., and B. R. formal analysis; C. I., M. J. L-P., A. A. P., and B. R. validation; C. I., M. J. L-P., A. A. P., and B. R. writing - original draft; A. A. P., C. I., R. C., C. Y., and B. R. conceptualization; R. C., C. Y., and B. R. funding acquisition; F. L., C. L., S. S., C. Y., M. J. L. P., C. I., A. A. P., R. C., and B. R. writing - review & editing. All authors approved the final version of the manuscript.
Funding and additional information
This work was supported by the French Infrastructure for Integrated Structural Biology (FRISBI) ANR-10-INBS-05 and the “Infrastructures en Biologie Santé et Agronomie” (IBiSA), Sino-French Cai Yuanpei Programme, Ou Liu Jin [2016] 6313, the National Natural Science Foundation of China (Grant No. 31570236), and De Nederlandse Organisatie voor Wetenschappelijk Onderzoek (NWO) through a Vici grant (86510013) to R. C. F. L. was recipient of a China Scholarship Council grant (CSC NO. 201604490129).
Edited by Joseph Jez
Contributor Information
Cristian Ilioaia, Email: cristian.ilioaia@cea.fr.
Bruno Robert, Email: bruno.robert@cea.fr.
References
- 1.Scholes G.D., Fleming G.R., Olaya-Castro A., van Grondelle R. Lessons from nature about solar light harvesting. Nat. Chem. 2011;3:763–774. doi: 10.1038/nchem.1145. [DOI] [PubMed] [Google Scholar]
- 2.Croce R., van Amerongen H. Light harvesting in oxygenic photosynthesis: Structural biology meets spectroscopy. Science. 2020;369 doi: 10.1126/science.aay2058. [DOI] [PubMed] [Google Scholar]
- 3.Niyogi K.K., Truong T.B. Evolution of flexible non-photochemical quenching mechanisms that regulate light harvesting in oxygenic photosynthesis. Curr. Opin. Plant Biol. 2013;16:307–314. doi: 10.1016/j.pbi.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 4.Pinnola A., Bassi R. Molecular mechanisms involved in plant photoprotection. Biochem. Soc. Trans. 2018;46:467–482. doi: 10.1042/BST20170307. [DOI] [PubMed] [Google Scholar]
- 5.Ruban A.V. Nonphotochemical chlorophyll fluorescence quenching: Mechanism and effectiveness in protecting plants from photodamage. Plant Physiol. 2016;170:1903–1916. doi: 10.1104/pp.15.01935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruban A.V., Berera R., Ilioaia C., van Stokkum I.H.M., Kennis J.T.M., Pascal A.A., van Amerongen H., Robert B., Horton P., van Grondelle R. Identification of a mechanism of photoprotective energy dissipation in higher plants. Nature. 2007;450:575–578. doi: 10.1038/nature06262. [DOI] [PubMed] [Google Scholar]
- 7.Holt N.E., Zigmantas D., Valkunas L., Li X.-P., Niyogi K.K., Fleming G.R. Carotenoid cation formation and the regulation of photosynthetic light harvesting. Science. 2005;307:433–436. doi: 10.1126/science.1105833. [DOI] [PubMed] [Google Scholar]
- 8.Ahn T.K., Avenson T.J., Ballottari M., Cheng Y.-C., Niyogi K.K., Bassi R., Fleming G.R. Architecture of a charge-transfer state regulating light harvesting in a plant antenna protein. Science. 2008;320:794–797. doi: 10.1126/science.1154800. [DOI] [PubMed] [Google Scholar]
- 9.Holzwarth A.R., Miloslavina Y., Nilkens M., Jahns P. Identification of two quenching sites active in the regulation of photosynthetic light-harvesting studied by time-resolved fluorescence. Chem. Phys. Lett. 2009;483:262–267. [Google Scholar]
- 10.Liguori N., Xu P., van Stokkum I.H.M., van Oort B., Lu Y., Karcher D., Bock R., Croce R. Different carotenoid conformations have distinct functions in light-harvesting regulation in plants. Nat. Commun. 2017;8:1994. doi: 10.1038/s41467-017-02239-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bode S., Quentmeier C.C., Liao P.-N., Hafi N., Barros T., Wilk L., Bittner F., Walla P.J. On the regulation of photosynthesis by excitonic interactions between carotenoids and chlorophylls. Proc. Natl. Acad. Sci. U. S. A. 2009;106:12311–12316. doi: 10.1073/pnas.0903536106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pascal A.A., Liu Z., Broess K., van Oort B., van Amerongen H., Wang C., Horton P., Robert B., Chang W., Ruban A. Molecular basis of photoprotection and control of photosynthetic light-harvesting. Nature. 2005;436:134–137. doi: 10.1038/nature03795. [DOI] [PubMed] [Google Scholar]
- 13.Krüger T.P.J., Ilioaia C., Johnson M.P., Ruban A.V., Papagiannakis E., Horton P., van Grondelle R. Controlled disorder in plant light-harvesting complex II explains its photoprotective role. Biophys. J. 2012;102:2669–2676. doi: 10.1016/j.bpj.2012.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ilioaia C., Johnson M.P., Liao P.-N., Pascal A.A., van Grondelle R., Walla P.J., Ruban A.V., Robert B. Photoprotection in plants involves a change in lutein 1 binding domain in the major light-harvesting complex of photosystem II. J. Biol. Chem. 2011;286:27247–27254. doi: 10.1074/jbc.M111.234617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mascoli V., Liguori N., Xu P., Roy L.M., van Stokkum I.H.M., Croce R. Capturing the quenching mechanism of light-harvesting complexes of plants by zooming in on the Ensemble. Chem. 2019;5:2900–2912. [Google Scholar]
- 16.Liu Z., Yan H., Wang K., Kuang T., Zhang J., Gui L., An X., Chang W. Crystal structure of spinach major light-harvesting complex at 2.72 A resolution. Nature. 2004;428:287–292. doi: 10.1038/nature02373. [DOI] [PubMed] [Google Scholar]
- 17.Mullet J.E., Arntzen C.J. Simulation of grana stacking in a model membrane system. Mediation by a purified light-harvesting pigment-protein complex from chloroplasts. Biochim. Biophys. Acta Bioenerg. 1980;589:100–117. doi: 10.1016/0005-2728(80)90135-8. [DOI] [PubMed] [Google Scholar]
- 18.Lampoura S.S., Barzda V., Owen G.M., Hoff A.J., van Amerongen H. Aggregation of LHCII leads to a redistribution of the triplets over the central xanthophylls in LHCII. Biochemistry. 2002;41:9139–9144. doi: 10.1021/bi025724x. [DOI] [PubMed] [Google Scholar]
- 19.Ruban A.V., Pascal A.A., Robert B. Xanthophylls of the major photosynthetic light-harvesting complex of plants: Identification, conformation and dynamics. FEBS Lett. 2000;477:181–185. doi: 10.1016/s0014-5793(00)01799-3. [DOI] [PubMed] [Google Scholar]
- 20.Fox K.F., Balevičius V., Chmeliov J., Valkunas L., Ruban A.V., Duffy C.D.P. The carotenoid pathway: What is important for excitation quenching in plant antenna complexes? Phys. Chem. Chem. Phys. 2017;19:22957–22968. doi: 10.1039/c7cp03535g. [DOI] [PubMed] [Google Scholar]
- 21.Staleva H., Komenda J., Shukla M.K., Šlouf V., Kaňa R., Polívka T., Sobotka R. Mechanism of photoprotection in the cyanobacterial ancestor of plant antenna proteins. Nat. Chem. Biol. 2015;11:287–291. doi: 10.1038/nchembio.1755. [DOI] [PubMed] [Google Scholar]
- 22.Dolganov N.A., Bhaya D., Grossman A.R. Cyanobacterial protein with similarity to the chlorophyll a/b binding proteins of higher plants: Evolution and regulation. Proc. Natl. Acad. Sci. U. S. A. 1995;92:636–640. doi: 10.1073/pnas.92.2.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Engelken J., Brinkmann H., Adamska I. Taxonomic distribution and origins of the extended LHC (light-harvesting complex) antenna protein superfamily. BMC Evol. Biol. 2010;10:1–15. doi: 10.1186/1471-2148-10-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Son M., Pinnola A., Gordon S.C., Bassi R., Schlau-Cohen G.S. Observation of dissipative chlorophyll-to-carotenoid energy transfer in light-harvesting complex II in membrane nanodiscs. Nat. Commun. 2020;11:1295. doi: 10.1038/s41467-020-15074-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saccon F., Giovagnetti V., Shukla M.K., Ruban A.V. Rapid regulation of photosynthetic light harvesting in the absence of minor antenna and reaction centre complexes. J. Exp. Bot. 2020;71:3626–3637. doi: 10.1093/jxb/eraa126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park S., Fischer A.L., Steen C.J., Iwai M., Morris J.M., Walla P.J., Niyogi K.K., Fleming G.R. Chlorophyll-carotenoid excitation energy transfer in high-light-exposed thylakoid membranes investigated by Snapshot transient absorption spectroscopy. J. Am. Chem. Soc. 2018;140:11965–11973. doi: 10.1021/jacs.8b04844. [DOI] [PubMed] [Google Scholar]
- 27.Ruban A.V., Horton P., Robert B. Resonance Raman spectroscopy of the photosystem II light-harvesting complex of green plants: A comparison of trimeric and aggregated states. Biochemistry. 1995;34:2333–2337. doi: 10.1021/bi00007a029. [DOI] [PubMed] [Google Scholar]
- 28.Liguori N., Periole X., Marrink S.J., Croce R. From light-harvesting to photoprotection: Structural basis of the dynamic switch of the major antenna complex of plants (LHCII) Sci. Rep. 2015;5:15661. doi: 10.1038/srep15661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chmeliov J., Gelzinis A., Songaila E., Augulis R., Duffy C.D.P., Ruban A.V., Valkunas L. The nature of self-regulation in photosynthetic light-harvesting antenna. Nat. Plants. 2016;2:16045. doi: 10.1038/nplants.2016.45. [DOI] [PubMed] [Google Scholar]
- 30.Mascoli V., Gelzinis A., Chmeliov J., Valkunas L., Croce R. Light-harvesting complexes access analogue emissive states in different environments. Chem. Sci. 2020;11:5697–5709. doi: 10.1039/d0sc00781a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Akhtar P., Dorogi M., Pawlak K., Kovács L., Bóta A., Kiss T., Garab G., Lambrev P.H. Pigment interactions in light-harvesting complex II in different molecular environments. J. Biol. Chem. 2015;290:4877–4886. doi: 10.1074/jbc.M114.607770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crisafi E., Pandit A. Disentangling protein and lipid interactions that control a molecular switch in photosynthetic light harvesting. Biochim. Biophys. Acta Biomembr. 2017;1859:40–47. doi: 10.1016/j.bbamem.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 33.Georgakopoulou S., van der Zwan G., Bassi R., van Grondelle R., van Amerongen H., Croce R. Understanding the changes in the circular dichroism of light harvesting complex II upon varying its pigment composition and organization. Biochemistry. 2007;46:4745–4754. doi: 10.1021/bi062031y. [DOI] [PubMed] [Google Scholar]
- 34.Llansola-Portoles M.J., Li F., Xu P., Streckaite S., Ilioaia C., Yang C., Gall A., Pascal A.A., Croce R., Robert B. Tuning antenna function through hydrogen bonds to chlorophyll a. Biochim. Biophys. Acta Bioenerg. 2019;1861:148078. doi: 10.1016/j.bbabio.2019.148078. [DOI] [PubMed] [Google Scholar]
- 35.Robert B. Resonance Raman spectroscopy. Photosynth. Res. 2009;101:147–155. doi: 10.1007/s11120-009-9440-4. [DOI] [PubMed] [Google Scholar]
- 36.Llansola-Portoles M.J., Pascal A.A., Robert B. Electronic and vibrational properties of carotenoids: From in vitro to in vivo. J. R. Soc. Interface. 2017;14 doi: 10.1098/rsif.2017.0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gall A., Pascal A.A., Robert B. Vibrational techniques applied to photosynthesis: Resonance Raman and fluorescence line-narrowing. Biochim. Biophys. Acta Bioenerg. 2015;1847:12–18. doi: 10.1016/j.bbabio.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 38.Saito S., Tasumi M. Normal-coordinate analysis of β-carotene isomers and assignments of the Raman and infrared bands. J. Raman Spectrosc. 1983;14:310–321. [Google Scholar]
- 39.Koyama Y., Kito M., Takii T., Saiki K., Tsukida K., Yamashita J. Configuration of the carotenoid in the reaction centers of photosynthetic bacteria. Comparison of the resonance Raman spectrum of the reaction center of Rhodopseudomonas sphaeroides G1C with those of cis-trans isomers of β-carotene. Biochim. Biophys. Acta Bioenerg. 1982;680:109–118. [Google Scholar]
- 40.Koyama Y., Takii T., Saiki K., Tsukida K. Configuration of the carotenoid in the reaction centers of photosynthetic bacteria. 2. Comparison of the resonance Raman lines of the reaction centers with those of the 14 different cis-trans isomers of β-carotene. Photobiochem. Photobiophys. 1983;5:139–150. [Google Scholar]
- 41.Koyama Y., Fujii R. Cis-trans carotenoids in photosynthesis: Configurations, excited-state properties and physiological functions. In: Frank H.A., Young A.J., Britton G., Cogdell R.J., editors. The Photochemistry of Carotenoids. Kluwer Academic Publishers; Dordrecht, Netherlands: 1999. pp. 161–188. [Google Scholar]
- 42.Rimai L., Heyde M.E., Gill D. Vibrational spectra of some carotenoids and related linear polyenes. Raman spectroscopic study. J. Am. Chem. Soc. 1973;95:4493–4501. doi: 10.1021/ja00795a005. [DOI] [PubMed] [Google Scholar]
- 43.Koyama Y., Takatsuka I., Nakata M., Tasumi M. Raman and infrared spectra of the all-trans, 7-cis, 9-cis, 13-cis and 15-cis isomers of β-carotene: Key bands distinguishing stretched or terminal-bent configurations form central-bent configurations. J. Raman Spectrosc. 1988;19:37–49. [Google Scholar]
- 44.Salares V.R., Young N.M., Carey P.R., Bernstein H.J. Excited state (excitation) interactions in polyene aggregates. Resonance Raman and absorption spectroscopic evidence. J. Raman Spectrosc. 1977;6:282–288. [Google Scholar]
- 45.Mendes-Pinto M.M., Sansiaume E., Hashimoto H., Pascal A.A., Gall A., Robert B. Electronic absorption and ground state structure of carotenoid molecules. J. Phys. Chem. B. 2013;117:11015–11021. doi: 10.1021/jp309908r. [DOI] [PubMed] [Google Scholar]
- 46.Llansola-Portoles M.J., Sobotka R., Kish E., Shukla M.K., Pascal A.A., Polívka T., Robert B. Twisting a β-carotene, an adaptive trick from nature for dissipating energy during photoprotection. J. Biol. Chem. 2017;292:1396–1403. doi: 10.1074/jbc.M116.753723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Llansola-Portoles M.J., Litvin R., Ilioaia C., Pascal A.A., Bina D., Robert B. Pigment structure in the violaxanthin–chlorophyll-a-binding protein VCP. Photosynth. Res. 2017;134:51–58. doi: 10.1007/s11120-017-0407-6. [DOI] [PubMed] [Google Scholar]
- 48.Llansola-Portoles M.J., Uragami C., Pascal A.A., Bina D., Litvin R., Robert B. Pigment structure in the FCP-like light-harvesting complex from Chromera velia. Biochim. Biophys. Acta Bioenerg. 2016;1857:1759–1765. doi: 10.1016/j.bbabio.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 49.Lutz M., Szponarski W., Berger G., Robert B., Neumann J.-M. The stereoisomerization of bacterial, reaction-center-bound carotenoids revisited: An electronic absorption, resonance Raman and NMR study. Biochem. Biophys. Acta. 1987;894:423–433. [Google Scholar]
- 50.Horton P., Ruban, a A.V., Walters R.G. Regulation of light harvesting in green plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1996;47:655–684. doi: 10.1146/annurev.arplant.47.1.655. [DOI] [PubMed] [Google Scholar]
- 51.Ruban A.V., Horton P. An investigation of the sustained component of nonphotochemical quenching of chlorophyll fluorescence in isolated chloroplasts and leaves of spinach. Plant Physiol. 1995;108:721–726. doi: 10.1104/pp.108.2.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Caffarri S., Croce R., Breton J., Bassi R. The major antenna complex of photosystem II has a xanthophyll binding site not involved in light harvesting. J. Biol. Chem. 2001;276:35924–35933. doi: 10.1074/jbc.M105199200. [DOI] [PubMed] [Google Scholar]
- 53.Caffarri S., Kouřil R., Kereïche S., Boekema E.J., Croce R. Functional architecture of higher plant photosystem II supercomplexes. EMBO J. 2009;28:3052–3063. doi: 10.1038/emboj.2009.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are contained within the article. Additional raw data are available upon request to the corresponding author.





