Abstract
Patients who are stationary endure prolonged pressures and shear loads at contact areas between their body and the support surface, which over time may cause pressure ulcers (PUs). Donut‐shaped gel head supports are commonly used to protect the occiput, which is among the most common anatomical sites for PUs; however, the biomechanical efficacy of these devices is unclear. To investigate their effects on scalp tissues, we have used our three‐dimensional anatomically realistic finite element model of an adult head, to which we have added a donut‐shaped gel head support. We then compared the occipital scalp tissue loads' occurrence while the donut‐shaped gel head support is in use with those associated with a fluidised head positioner and a standard medical foam. The donut‐shaped gel head support inflicted the greatest exposure to tissue mechanical stresses, particularly to the high (and therefore dangerous) stress domain, when compared to the other positioners. We concluded that while the donut‐shaped gel head support is designed to avert tissue loads away from the occiput and disperse them to the surroundings, in practice, it fails to do so. In fact, the donut‐shaped gel head support imposes the head‐weight forces to transfer through a relatively narrow ring of scalp tissues, hence increasing the risk of developing occipital PUs.
Keywords: deep tissue injury, finite element modelling, head support, positioner, prophylaxis
Key Messages.
a donut‐shaped gel head support is meant to reduce the occurrence of pressure ulcers in scalp tissues
to investigate the effects of donut‐shaped gel head support on scalp tissues, we have used our anatomically realistic computational model of an adult head
the donut‐shaped gel head support imposes the head‐weight forces to transfer through a relatively narrow ring of scalp tissues
the highly distorted and deformed tissues at that ring are at a high risk for injury
1. INTRODUCTION
A pressure ulcer (PU), also called a pressure injury, is localised damage in soft tissues that are subjected to sustained mechanical loading, often by bodyweight forces.1 Patients who are stationary, for example, paralysed or under anaesthesia, endure prolonged pressures and shear loads at contact areas between their body and the support surface, which over time, may cause PUs.2, 3 The back of the head is among the most common anatomical sites for PUs associated with a supine body position.4
Frequent repositioning of patients has been shown to reduce the risk of PUs, but a typical question in this regard is how often one needs to reposition a patient on a certain support surface.5, 6 This problem becomes even more complicated in operation rooms (ORs). During surgery, it is difficult, dangerous, and, in many cases, impossible to reposition. Various limitations on repositioning or the ability to move patients in general may exist, such as with regard to the required body posture during operation, mechanical ventilation, and connection to other life‐support equipment.5, 6, 7, 8 Head positioning, specifically, is required for maintaining midline and/or the chin upwards, for immediate access to the airways in an anaesthetised, ventilated patient, as well as for the distribution of head loads at the occipital region in the supine posture.8, 9
Common positioning techniques that nursing teams are employing often include the use of items that are not specifically designed for the purpose of therapeutic positioning, such as rolled towels and blankets that flatten and will not maintain a set position, and may also heat up.8 Dedicated head supports also exist. Medical claims presented by manufacturers of such products often refer to reduction of the occurrence of PUs in scalp tissues and the ears; however, quantitative assessments of the biomechanical or clinical efficacies of commercial head supports are overall poor.
Donut‐shaped gel head supports are one such specific medical device, which is commonly used in adult and paediatric surgery to protect the occipital scalp tissues in the OR, during recovery from surgery or in intensive care units (ICUs) by so‐called “off‐loading” the occiput, that is, shifting the head‐weight forces from the central occipital region to other more peripheral head regions. A more modern head positioner technology is the fluidised positioner (Z‐Flo by Mölnlycke Health Care, Gothenburg, Sweden), which is made of a viscoelastic material with shape memory properties. Our published research and Barakat‐Johnson et al's work demonstrated that the fluidised positioner is considerably more effective in alleviating superficial and internal soft tissue loads at the back of the head compared to a simple medical foam,10, 11 however, we did not evaluate the commonly used donut‐shaped products. This study builds upon our published work10 and determines, for the first time in the literature, the magnitudes and distributions of occipital soft tissue loads while using donut‐shaped gel head supports against the fluidized positioner and a foam support. For this purpose, we have used our three‐dimensional (3D) anatomically realistic finite element (FE) model of an adult head and related computational methodology of analysis, which is consistent with our previous work.10
2. METHODS
2.1. Geometry
To determine the magnitudes and distributions of mechanical loads that are formed in the soft tissues of the back of the head while in contact with a donut‐shaped gel head support, compared to the medical foam and Z‐Flo positioners analysed in our earlier published work, we employed our FE modelling framework and computational methodology as previously reported.10 Briefly, this 3D anatomically realistic adult head modelling framework (Figures 1, 2) was built based on the Visible Human (male) Project image database.12 Tissues in each transverse slice of this head model were segmented and then unified to create the 3D head reconstruction, using the Scan‐IP module of the Simpleware segmentation software package.13 Although the anatomical details of the brain, sinuses, optic nerves, and other soft tissue structures which are present within the skull have been included in the modelling for completeness, they do not influence scalp tissue loads other than applying gravitational (tissue weight) forces. Hence, we do not provide information regarding mechanical behaviour and properties of soft tissue structures that are contained in the skull hereinafter. The dimensions of the head in our modelling were 16.5 cm ear‐to‐ear and 21.5 cm occiput‐to‐forehead.12
Figure 1.
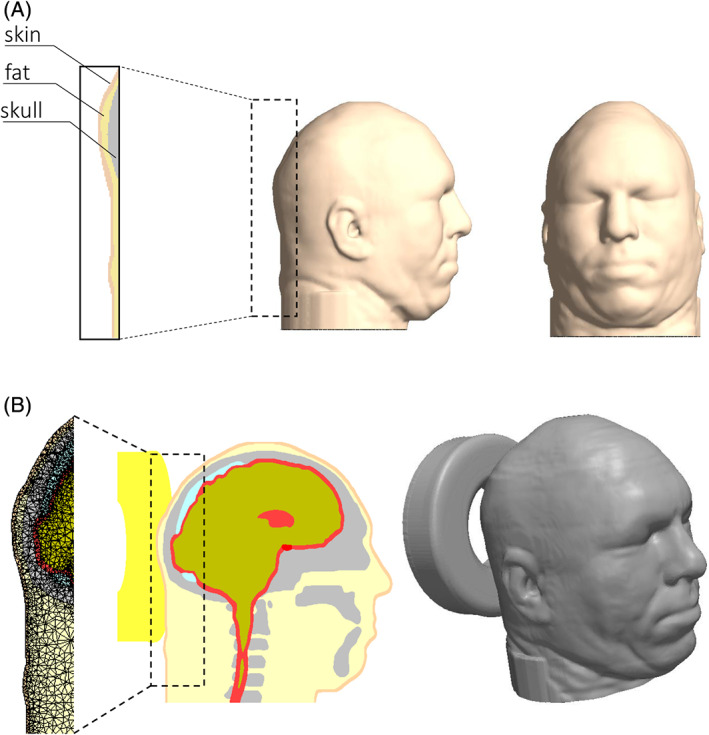
Model geometry: A, side (left frame) and frontal (right frame) views of the head model, with magnification of the tissues at the back of the head (zoom‐in from the left frame). B, Mid‐sagittal cross section through the head model (left frame) and its three‐dimensional reconstruction when supported by the donut‐shaped gel head support (right frame); a magnification of the finite element mesh shows meshing at the occipital scalp region (zoom‐in from the left frame)
Figure 2.
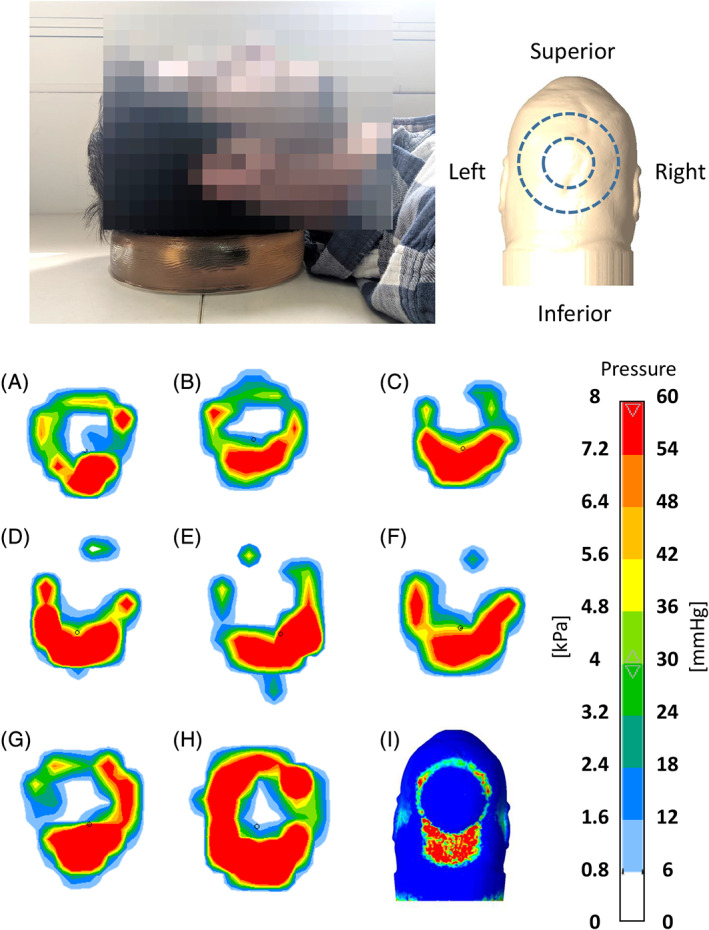
Pressure maps measured under the rested head when positioned on a donut‐shaped gel head support (as shown in the photograph in the top frame and as further depicted in the top right panel) for eight different subjects (A‐H). The corresponding model‐predicted pressure distribution at the back of the head for the same configuration is shown in panel (I). Similarity to the empirical pressure maps, particularly concerning the highly loaded site at the inferior head/neck region, is evident. The orientations of the experimental and computational pressure maps are all as illustrated in the top right panel
A donut‐shaped gel head support was further added to the modelling to support the above virtual head reconstruction. The donut‐shaped gel head support was also reconstructed by means of the Scan‐IP module of Simpleware. We modelled a generic donut‐shaped gel head support representative of many gel‐made commercial products that are commonly used in clinical practice, with height of 5 cm and outer and inner diameters of 20 cm and 10 cm, respectively.
2.2. Mechanical behaviour and properties of model components
The mechanical behaviour and properties of the skin and fat tissues of the scalp and those of skull bone were adopted from literature reports. Specifically, the skull was assumed to be isotropic and linear elastic with an elastic modulus of 6484 MPa and Poisson's ratio of 0.2.14 Skin and fat were assumed to be homogeneous, isotropic, nonlinearly elastic, and compressible materials, which undergo large deformations under the weight of the head. Accordingly, each of these tissue types was represented by the Mooney‐Rivlin constitutive model:
| (1) |
where W is the strain energy density, I 1 is the first invariant of the right Cauchy‐Green deformation tensor, J is the determinant of the deformation gradient tensor, G is the shear modulus, and λ is Lamé's first parameter that can be converted to the other commonly used elastic properties reported in Table 1, namely, the elastic modulus E (Equation 2), the bulk modulus K (Equation 3), and Poisson's ratio ν (Equation 4), as follows:
| (2) |
| (3) |
| (4) |
Table 1.
Mechanical properties and element data of the finite element (FE) model variants
| Model component | Shear modulus, G (MPa) | Bulk modulus, K (MPa) | Elastic modulus, E (MPa) | Poisson's ratio, v (MPa) | Number of elements in FE mesh |
|---|---|---|---|---|---|
| Skina | 0.0319 | 3.1794 | – | – | 123 826 |
| Fatb , c | 0.000286 | 0.0285 | – | – | 370 572 |
| Skulld | – | – | 6483.6 | 0.2 | 169 928 |
| Vertebraee | – | – | 10 000 | 0.2 | 56 799 |
| Donut‐shaped gel head support | – | – | 0.03 | 0.49 | 50 543 |
The mechanical properties of the gel material of the donut‐shaped gel head support were measured in our laboratory through indentation tests conducted using an electromechanical testing apparatus (Instron Model 5944, Norwood, Massachusetts). Given the well‐known viscoelastic nature of gels used in PU prevention equipment, we focused on the long‐term elastic gel properties. For the tested gel material, the long‐term properties have been achieved in our experiments after approximately 2 minutes of the ramp‐and‐hold indentation protocol (as explained below). This duration is remarkably shorter than any possibly relevant real‐world surgical procedure prior to which the head is positioned in clinical practice. Hence, the long‐term gel properties were used for the quasi‐static FE analyses. A spherical rigid indenter with radius R = 3 mm was mounted on the load cell of the testing machine for spherical indentations into the gel material to a depth δ = 2.95 mm at a displacement rate of 1 mm/s. We conducted four trials and calculated the mean long‐term relaxation force F L, which is extracted from the following relaxation force function F(t) at time t that is long enough (approximately 2 min) so that the relaxation force is plateaued (ie, changes in the measured force did not exceed 5%):
| (5) |
The constants F 1, F 2 and τ 1, τ 2 are computed from the best curve fitting to each experimental force‐relaxation dataset, using a Matlab code (MathWorks, Natick, Massachusetts) written for this purpose. The long‐term shear modulus of the donut‐shaped gel head support G L is then calculated from the long‐term relaxation force F L, using the Hertz contact formulation:
| (6) |
where δ and R are the aforementioned indentation depth and radius of the indenter, respectively. The calculated long‐term mechanical properties of the gel material of the donut‐shaped gel head support, based on our above experiments, are also reported in Table 1.
2.3. Boundary conditions and numerical method
The boundary conditions in the present FE modelling were chosen to simulate weight bearing of the head on the donut‐shaped gel head support during supine lying, as occurs, for example, in ORs. The inferior surfaces of the donut‐shaped gel head support were fixed for all translations and rotations (Figure 1). We applied a vertical displacement of the modelled head towards the head support, which resulted in a reaction force of approximately 4 kg.
The aforementioned model geometries were meshed into FEs using the Scan‐IP module of Simpleware. All elements were of the tetrahedral type; the numbers of elements in each model component are specified in Table 1. The FE simulations were set up using Preview (version 1.19, University of Utah, Salt Lake City, Utah),19, 20, 23, 24 solved using the Pardiso FE solver (version 2.5) and post‐processed using PostView (version 1.9.1), which are all modules of the FEBio software package (University of Utah, Salt Lake City, Utah).21, 22 The runtime of each simulation was approximately 17 hours using a 64‐bit Windows 10 Pro‐based workstation with a CPU comprising Intel Xeon E5‐2620 2 GHz (two processors) and 64 GB RAM.
2.4. Validation and outcome measures
For validation purposes, we measured the interface pressures between the head and donut‐shaped gel head support using a pressure mat (M‐flex; Vista Medical Europe B.V., the Netherlands). We repeated these measurements for eight different healthy adult subjects, whose characteristics are listed in Table 2. These empirical pressure maps are in good agreement with the corresponding model‐predicted (calculated) contact pressure distribution, visually, quantitatively and in locations of the highest pressure points (Figure 2). Importantly, all pressure maps—the experimental maps and the one map generated by the modelling—indicated that the greatest pressures develop at the inferior head/neck region (Figure 2).
Table 2.
Subject characteristics for the group of participants in the pressure mapping studies
| Participate | Gender | Weight (kg) | Height (m) | BMI |
|---|---|---|---|---|
| a | Female | 43 | 1.59 | 17 |
| b | Female | 63 | 1.64 | 23.4 |
| c | Female | 55 | 1.55 | 22.9 |
| d | Female | 52 | 1.65 | 19.1 |
| e | Female | 54 | 1.56 | 22.2 |
| f | Male | 70 | 1.8 | 21.6 |
| g | Male | 65 | 1.8 | 20.1 |
| h | Male | 72 | 1.74 | 23.8 |
To analyse internal scalp tissue exposures to mechanical loads, we calculated the effective and shear (Cauchy) stresses in skin and fat tissues, separately for each of these tissue types. The values and volumetric distributions of intensities of the aforementioned outcome measures were plotted and quantitatively compared across the head support cases, consistent with the methodology of published, robust data analyses developed by our group for standardised computational efficacy research of PU prevention technologies.10 We further compared the “total stress concentration exposure” (TSCE) for a given tissue type across the head support devices. The TSCE has been defined here as the area bounded between the corresponding stress curve in Figure 5 and the horizontal (stress) axis, for the highest quartile of the calculated stress range (to focus on exposures to elevated or focal tissue stresses). The aforementioned TSCE has been calculated separately for the effective stress curves (TSCE effective) and for the shear stress curves (TSCE shear).10, 23, 24, 25, 26, 27
Figure 5.
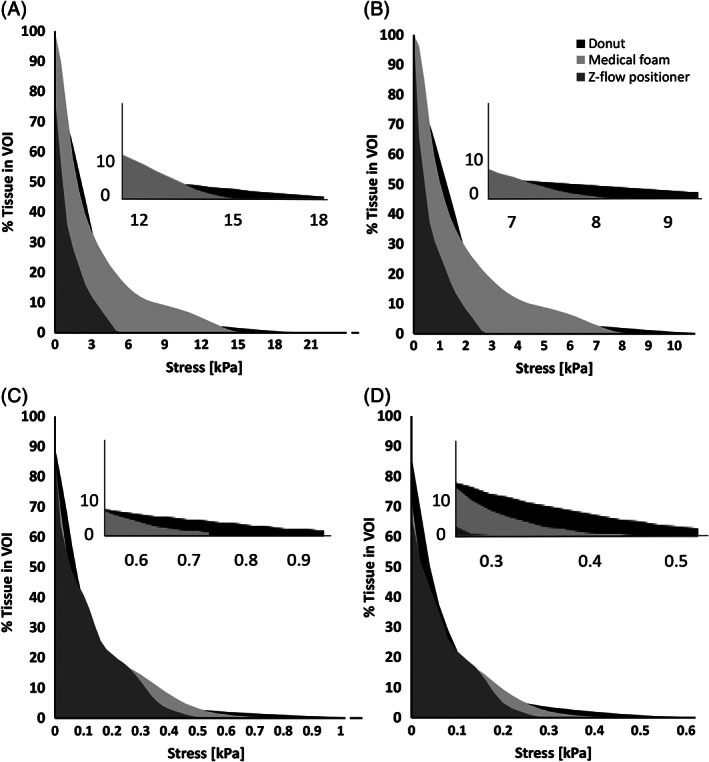
Cumulative percentage of skin (A,B) and subcutaneous fat (C,D) exposures to effective (left column) and shear (right column) stress levels when the head is rested on the donut‐shaped gel head support, on the Z‐Flo head positioner (Mölnlycke Health Care, Gothenburg, Sweden), and on a standard medical foam support
3. RESULTS
Distributions of effective and shear soft tissue stresses that develop in the occipital scalp when the head is resting on a donut‐shaped gel head support are shown in a cross‐sectional view (Figure 3) and in views of the back of the head (Figure 4), depicting skin stresses (top frames in Figure 4) and subcutaneous fat stresses (bottom frames in Figure 4; skin tissue has been artificially removed to depict fat stress patterns). Skin tissue stresses were at the scale of zero to 4 kPa, whereas fat tissue stresses were at the 0–0.5 kPa range (Figures 3 and 4). Stresses in skin and fat are shown to form in a roughly circular shape and to the peak at the inferior head/neck region, with evident focal tissue distortions caused by contact with the “step” in the donut‐shaped gel head support (Figures 3 and 4). Skin tissue is generally subjected to greater stress values than fat (Figures 3 and 4).
Figure 3.
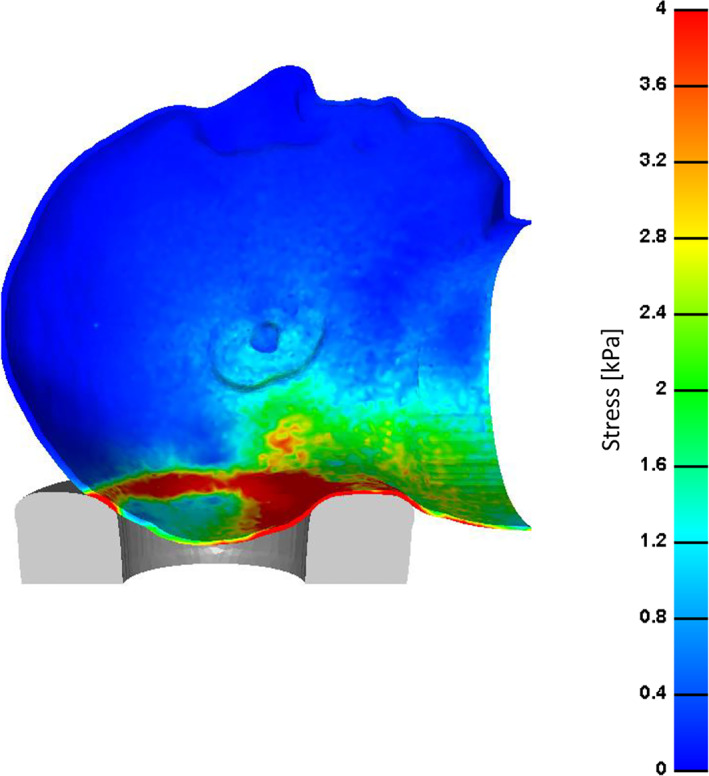
Effective stress distribution on the skin of the scalp through a cross‐sectional view when the simulated head is rested on a donut‐shaped gel head support
Figure 4.
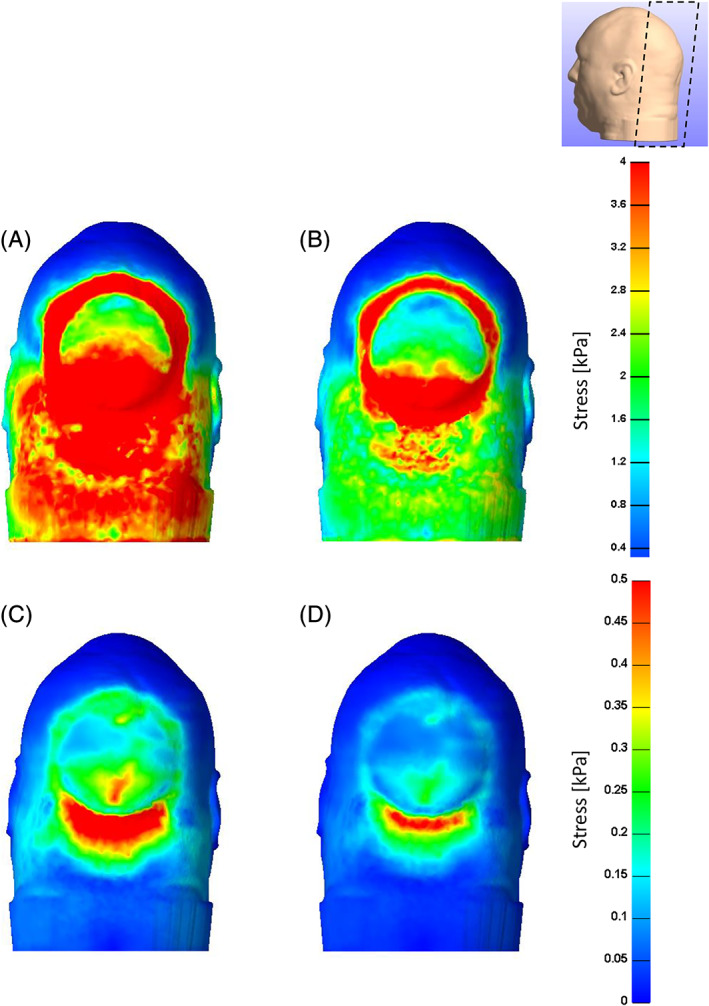
Effective (left column) and shear (right column) stresses on the skin (A,B) and subcutaneous fat (C,D) when the simulated head is rested on a donut‐shaped gel head support
A comparison of the volumetric tissue exposures to stresses in the occipital scalp across the donut‐shaped gel head support, Z‐Flo fluidized head positioner, and standard medical foam is presented in Figure 5.
For skin tissue, the volumetric exposure to stresses was the lowest, and for all stress levels, when the head was resting on the fluidized head positioner (Figure 5). The greatest exposure to tissue stresses, particularly to the high (and therefore dangerous) stress domain, was caused by the donut‐shaped gel head support (Figure 5A,B). The medical foam was more protective against stress exposure than the donut‐shaped gel head support, but was considerably less protective than the fluidized head positioner (Figure 5A,B).
For fat tissue, differences between volumetric stress exposures across the different head support cases were mostly apparent for the high‐stress (hazardous) levels (Figure 5C,D). The fluidized positioner was again associated with the lowest stress exposure, in contrast to the donut‐shaped gel head support, which caused the greatest stress exposure in fat (Figure 5C,D).
Consistent with the above results, the values of the TSCE‐effective and TSCE‐shear measures of scalp tissue stress exposure calculated for the fluidized positioner were zero for both the skin and fat layers of the occipital scalp. Further corresponding to the above data (shown in Figure 5), the TSCE‐effective and TSCE‐shear values for the donut‐shaped gel head support were the greatest for skin and fat (Figure 6).
Figure 6.
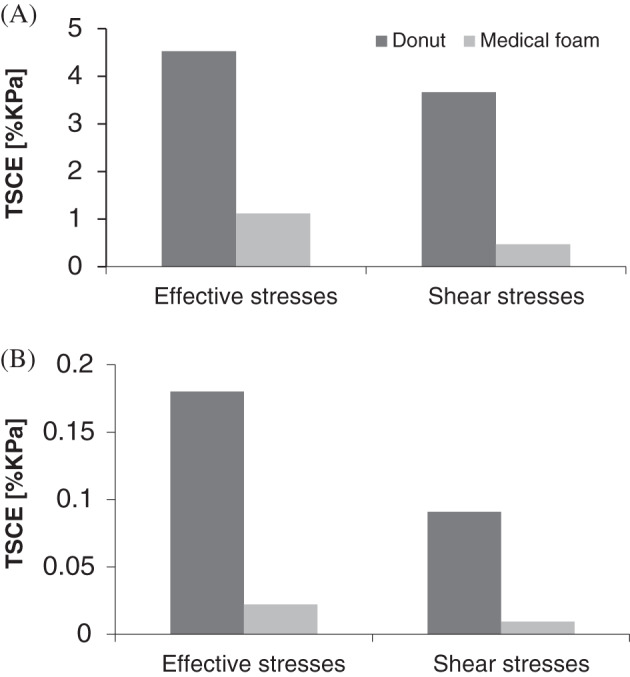
Comparisons of total tissue stress concentration exposures (TSCE) derived from the histogram curves in Figure 5, per tissue type, that is, skin (A) or fat (B), and for each stress measure, that is, effective (left bars) or shear (right bars). The TSCE for a given tissue type in the scalp has been defined here as the area bounded between the corresponding stress curve in Figure 5 and the horizontal (stress) axis for the highest quartile of the calculated stress range (to focus on exposures to elevated or focal tissue stresses). All TSCE values were zero for the Z‐Flo head positioner (ie, for both skin and fat and for effective as well as shear stress data) [Correction added on6 January 2020, after first online publication: Figure 6 has been updated in this version.]
Taken together, the TSCE measures have demonstrated that the donut‐shaped gel head support functions worse than simple medical foam (in three out of the four TSCE stress exposure measures). Based on this TSCE analysis, the fluidized positioner was not even at the same scale of stress‐exposure‐associated risk to scalp tissue health (Figures 5 and 6).
4. DISCUSSION
Donut‐shaped gel head supports are typically made of gel materials and are used to protect the head, neck, and ears during motionless treatments by off‐loading the occiput, often in the OR during surgical procedures conducted in supine patients, as well as during recovery from operation or in the ICU.8, 9 In the present work, we compared the state of mechanical loads in scalp tissues of the occipital region while using a donut‐shaped gel head support, a Z‐Flo head positioner, or a standard medical foam support for the same virtual adult head using our FE modelling framework, which was validated against pressure mapping. Our results demonstrated that a standard, flat medical foam is more successful than a donut‐shaped gel head support in dispersing scalp tissue loads, but the foam is still limited in conformability relative to the Z‐Flo fluidised positioner. Of all the three tested head supports, the Z‐Flo maximises head envelopment, and hence, the head contact area, resulting in the lowest exposure to surface and internal scalp tissue stresses.
Although the donut‐shaped gel head support is designed to avert tissue loads away from the occiput and disperse them to the surroundings, in practice it fails to do so. In fact, the donut‐shaped gel head support imposes the head‐weight forces to transfer through a relatively narrow ring of scalp tissues, which is highly distorted and deformed, hence increasing the risk of developing occipital PUs in ORs where this type of device is being used (Figures 3 and 4). It appears that the curved (geometrically irregular) “step‐shaped” superior surface of the donut‐shaped gel head support is causing these stress concentrations in scalp tissues.
Levy et al (2017) simulated tissue stresses in the scalp of a newborn lying supine on a donut‐shaped gel head support. They showed that the use of the donut‐shaped support is associated with elevated stress exposure of scalp tissues, particularly due to localised shear stresses that result from the off‐loading.25 Their work focusing on the head agrees with patient guides and clinical practice guidelines where it is strongly recommended not to use ring cushions, which, in fact, increase the risk for sitting‐acquired PUs.26, 27
As in all in silico modelling studies, there are some limitations to note. First, we do need to mention that the specific mechanical state of the scalp tissues is determined by the particular geometrical design and mechanical properties of the material of a given donut‐shaped gel head support. The selections made in this work do not represent the entire variety of commercially available device designs, and their interactions with all possible head sizes and shapes. Additionally, in some cases, donut‐shaped gel head supports are designed to have a contoured top surface, rather than a round top curve, or even a “centre dish” designed to conform around the occipital region and provide extra support there. The aforementioned variant designs, which offer a potentially increased head contact area, may perform better in terms of scalp exposure to sustained internal tissue loads, but this would require additional research to determine. Second, we can presume that the level of geometrical fit between the device and the patient‐specific occipital shape is also an important factor in this biomechanical problem. Accordingly, non‐adjustable donut or other fixed‐shape supports may induce injury risks depending on the extent of the mismatch in the head‐support fit at each individual patient case. Third, and related to the previous point, it is also worth noting that the head anatomy used in our present modelling (which has been acquired from the male Visible Human database) has subject‐specific anatomical features such as a sturdy neck (ie, each potentially selected head anatomy would be different). This sturdy neck resulted in the relatively high and concentrated pressures calculated at the neck of our virtual subject (Figure 2i). However, in all the empirical pressure maps acquired from subjects with a variety of neck structures—both males and females—the same phenomenon of high pressures concentrated at the neck has been consistently observed (Figure 2). Accordingly, the high pressure points at the neck are not only due to the sturdy neck of the person used for our modelling, but are also associated with the posture of any neck on the donut‐shaped gel head support. The last point, which is a potential limitation of our work, is that the mechanical properties assigned to the tissues in the modelling are adopted from animal studies because of the lack of specific literature describing human scalp tissue properties under sustained, quasi‐static loading conditions as relevant to PU research. Furthermore, the head anatomy and the biomechanical properties of tissues represent healthy conditions, and do not account for pathologies such as existing wounds, scars, abnormally fragile, diabetic, or otherwise unhealthy skin, which is characteristic to an end‐of‐life stage or to inherited diseases such as epidermolysis bullosa. With all that said, many surgical patients would have healthy scalp tissues (that will still require protection during surgery in a supine posture), but for aged, fragile, or unhealthy scalp tissues, breakdown is expected to occur sooner or under lower sustained deformations and stresses.
In conclusion, we have employed a biomechanical modelling framework, which was originally developed by our group10 for evaluating adult head supports that are often used in the OR, during recovery from surgery or in intensive care, and possibly in other medical settings as well. We found that the donut‐shaped gel head support, which is meant to reduce the risk of PU formation, may actually increase it.
ACKNOWLEDGEMENTS
The study was supported by an unrestricted educational grant from Mölnlycke Health Care, Gothenburg, Sweden.
Katzengold R, Gefen A. Modelling an adult human head on a donut‐shaped gel head support for pressure ulcer prevention. Int Wound J. 2019;16:1398–1407. 10.1111/iwj.13203
Submitted to the International Wound Journal
Funding information Mölnlycke Health Care, Gothenburg, Sweden
REFERENCES
- 1. European Pressure Ulcer Advisory Panel (EPUAP) , National Pressure Ulcer Advisory Panel (NPUAP) and the Pan‐Pacific Alliance (PPA), International Pressure Ulcer Guidelines, 2014. www.epuap.org/pu‐guidelines/#2014guidelines&qrg. Accessed June 2019.
- 2. Gefen A. The biomechanics of sitting‐acquired pressure ulcers in patients with spinal cord injury or lesions. Int Wound J. 2007;4:222‐231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Manorama A, Meyer R, Wiseman R, Bush TR. Quantifying the effects of external shear loads on arterial and venous blood flow: implications for pressure ulcer development. Clin Biomech (Bristol, Avon). 2013;28(5):574‐578. [DOI] [PubMed] [Google Scholar]
- 4. Giuglea C, Marinescu S, Florescu IP, Jecan C. Pressure sores‐a constant problem for plegic patients and a permanent challenge for plastic surgery. J Med Life. 2010;3(2):149‐153. [PMC free article] [PubMed] [Google Scholar]
- 5. Gillespie BM, Chaboyer WP, McInnes E, Kent B, Whitty JA, Thalib L. Repositioning for pressure ulcer prevention in adults. Cochrane Database Syst Rev. 2014;(4):CD009958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vanderwee K, Grypdonck MH, De Bacquer D, Defloor T. Effectiveness of turning with unequal time intervals on the incidence of pressure ulcer lesions. J Adv Nurs. 2007;57(1):59‐68. [DOI] [PubMed] [Google Scholar]
- 7. Spruce L, Van Wicklin SA. Back to basics: positioning the patient. AORN J. 2014;100(3):298‐305. [DOI] [PubMed] [Google Scholar]
- 8. Waters T, Short M, Lloyd J, et al. AORN ergonomic tool 2: positioning and repositioning the supine patient on the OR bed. AORN J. 2011;93(4):445‐449. [DOI] [PubMed] [Google Scholar]
- 9. Burlingame BL. Guideline implementation: positioning the patient. AORN J. 2017;106(3):227‐237. [DOI] [PubMed] [Google Scholar]
- 10. Katzengold R, Gefen A. What makes a good head positioner for preventing occipital pressure ulcers. Int Wound J. 2018;15(2):243‐249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Barakat‐Johnson M, Lai M, Gefen A, Coyer F. Evaluation of a fluidised positioner to reduce occipital pressure injuries in intensive care patients: a pilot study. Int Wound J. 2019;16(2):424‐432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Visible human project gallery, US Department of Health & Human Services, National Library of Medicine (NLM) . Visible human project. https://www.nlm.nih.gov/databases/download/vhp.html. 1994. Accessed August 20, 2019.
- 13. Simpleware® Ltd . ScanIP, +FE, +NURBS and +CAD Reference Guide ver. http://www.simpleware.com/software/ Accessed January 5, 2012.
- 14. Moore DF, Jérusalem A, Nyein M, Noels L, Jaffee MS, Radovitzky RA. Computational biology‐ modeling of primary blast effects on the central nervous system. NeuroImage. 2009;47(2):T10‐T20. [DOI] [PubMed] [Google Scholar]
- 15. Linder‐Ganz E, Shabahin N, Itzchak Y, Gefen A. Assessment of mechanical conditions in sub‐dermal tissues during sitting: a combined experimental‐MRI and finite element approach. J Biomech. 2007;40(7):1443‐1454. [DOI] [PubMed] [Google Scholar]
- 16. Sopher R, Nixon J, Gorecki C, Gefen A. Exposure to internal muscle tissue loads under the ischial tuberosities during sitting is elevated at abnormally high or low body mass indices. J Biomech. 2010;43(2):280e6. [DOI] [PubMed] [Google Scholar]
- 17. Gefen A, Haberman E. Viscoelastic properties of ovine adipose tissue covering the gluteus muscles. J Biomech Eng. 2007;129(6):924‐930. [DOI] [PubMed] [Google Scholar]
- 18. Greaves CY, Gadala M, Oxland TR. A three‐dimensional finite element model of the cervical spine with spinal cord: an investigation of three injury mechanisms. Ann Biomed Eng. 2008;36(3):396‐405. [DOI] [PubMed] [Google Scholar]
- 19. Peko Cohen L, Ovadia‐Blechman Z, Hoffer O, Gefen A. Dressings cut to shape alleviate facial tissue loads while using an oxygen mask. Int Wound J. 2019;16(3):813‐826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Levy A, Gefen A. Assessment of the biomechanical effects of prophylactic sacral dressings on tissue loads: a computational modeling analysis. Ostomy Wound Manage. 2017;63(10):48‐55. [PubMed] [Google Scholar]
- 21. Maas SA, Ellis BJ, Ateshian GA, Weiss JA. FEBio: finite elements for biomechanics. J Biomech Eng. 2012;134(1):5‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. FEBio Finite Element for Biomechanics, Theory Manual ver. 1.5. http://mrl.sci.utah.edu/software/febio. 2012. Accessed August 20, 2019.
- 23. Schwartz D, Levy A, Gefen A. A computer modeling study to assess the durability of prophylactic dressings subjected to moisture in biomechanical pressure injury prevention. Ostomy Wound Manage. 2018;64(7):18‐26. [PubMed] [Google Scholar]
- 24. Schwartz D, Gefen A. The biomechanical protective effects of a treatment dressing on the soft tissues surrounding a non‐offloaded sacral pressure ulcer. Int Wound J. 2019;16(3):684‐695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Levy A, Kopplin K, Gefen A. Device‐related pressure ulcers from a biomechanical perspective. J Tissue Viability. 2017;26(1):57‐68. [DOI] [PubMed] [Google Scholar]
- 26. Consortium for Spinal Cord Medicine Member Organizations—Clinical Practice Guidelines . Pressure Ulcer Prevention and Treatment Following Spinal Cord Injury: A Clinical Practice Guideline for Health‐Care Professionals. 2nd ed. Paralyzed Veterans of America; Washington, D.C., United States; 2014. [Google Scholar]
- 27. National Health Services (NHS) Foundation Trust (Central and North West London, UK) . How to prevent pressure ulcers: a guide for patients and carers, February 2015, Ref: CNWLDN004_FEB2015.


