Abstract
Pyoderma gangrenosum (PG) is a rare ulcerative skin disease that presents a therapeutic challenge. Tumour necrosis factor alpha (TNFα) inhibitors have been reported to successfully control PG. Our aim was to systematically evaluate and compare the clinical effectiveness of TNFα inhibitors in adults with PG. A literature search including databases such as PubMed, Embase, Scopus, and Web of Science was conducted, using search terms related to PG and TNFα inhibitors. Studies and case reports were included if patients were diagnosed with PG, over the age of 18 and administered TNFα inhibitor. A total of 3212 unique citations were identified resulting in 222 articles describing 356 patients being included in our study. The study we report found an 87% (95% CI: 83%‐90%) response rate and a 67% (95% CI: 62%‐72%) complete response rate to TNFα inhibitors. No statistically significant differences in the response rates (P = 0.6159) or complete response rates (P = 0.0773) to infliximab, adalimumab, and etanercept were found. In our study TNFα inhibitors demonstrated significant effectiveness with response and complete response rates supporting the use of TNFα inhibitors to treat PG in adults. Our study suggests that there is no significant difference in effectiveness among infliximab, adalimumab, and etanercept.
Keywords: adalimumab, etanercept, infliximab, pyoderma gangrenosum, TNFα inhibitors
1. INTRODUCTION
Pyoderma gangrenosum (PG) is a rare ulcerative skin disease with an incidence of 0.3 to 1.0/100.0001 and is associated with systemic diseases and preceding trauma in 57% and 16% of cases, respectively.2 PG is a neutrophillic dermatosis characterised by skin infiltrations of polymorphonuclear leukocytes in the absence of vasculitis and infection.3 Commonly located on lower limbs, lesions typically present as tender pustules or nodules that rapidly progress to ulcers with violaceous undermined borders.4 With no uniformly accepted diagnostic criteria, PG has been a diagnosis of exclusion.1, 5 However, recent diagnostic criteria have been proposed as a result of a Delphi consensus exercise using the RAND/UCLA Appropriateness Method.6 The pathophysiology and aetiology are poorly understood, but recent studies have suggested that immune dysregulation with activation of the inflammatory cascade leads to lesions of PG, but triggers of immune dysregulation remain unknown.7
The mainstay of treatment is immunosuppression that presents a therapeutic challenge, with no acknowledged standard treatment guidelines, because of incompletely understood pathogenesis and lack of high‐quality studies. The literature is characterised by a paucity of controlled clinical trials with only two randomised controlled trials, one comparing infliximab with placebo (n = 30)8 and the other comparing prednisolone with cyclosporine (n = 112).9 Consequently, clinical management relies primarily upon case reports, case series, and local practice. TNFα inhibitors have been reported to successfully control PG. Nevertheless, to the best of our knowledge, no large systematic evaluation has been carried out.
Therefore, the aim of our study was to systematically evaluate and compare the clinical effectiveness of TNFα inhibitors in adults with PG.
2. METHODS
The review followed the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) statement.10 It was not possible to conduct the review in full accordance with the PRISMA statement, hence the name semi‐systematic review, because the data were on the individual level as a result of the literature lacking high‐quality studies and consisting predominantly of case reports and case series.
2.1. Search strategy
A literature search of citations from 1998 to 2018 was conducted in larger databases including PubMed, Embase, Web of Science, Scopus, and Cochrane Library. Grey literature was searched in NHS Evidence, OpenGrey, NICE Local Practice Case Studies, The National Technical Information Service, Greylit, Trials Register of Promoting Health Interventions, World Health Organisation International Clinical Trials Registry Platform, ClinicalTrials.gov, and UK Clinical Trials Gateway. The literature search was limited to include citations from 1998 to 2018 because the first TNFα inhibitor, infliximab, was first approved in 1998 by U.S. Food and Drug Administration.11, 12 The search strategy consisted of search terms related to PG and TNFα inhibitors. Search terms were truncated to include all variations and word endings. Complete search history, including search strings, databases, search dates, filters and hits, is available in Supporting Information Table S1. The reference lists of relevant or included studies were manually searched for additional citations.
2.2. Study selection
All citations from the search were merged, duplicates were removed and followed by a 2‐step process consisting of (a) examination of titles and abstracts to find relevant citations (b) that were full‐text read to assess their eligibility for inclusion. Articles written in other languages than English, French, and Scandinavian languages were translated.
Studies and case reports were included if patients were diagnosed with PG, over the age of 18 and administered TNFα inhibitor, and if the response of PG to the TNFα inhibitor was reported. Articles were excluded if patients had been previously reported in another publication to avoid duplication or if patients developed PG during anti‐TNFα treatment because it was not possible to distinguish whether the treatment led to a partial response, no response, or triggered PG.
A large fraction of articles were assessed by a full‐text read to find eligible patients, especially to find the patients that responded poorly to the TNFα inhibitor without the TNFα inhibitor administration being reported in the title or abstract.
2.3. Data extraction
The data extraction process was a 2‐step process with (a) an extraction of data and (b) a control to find potential errors. For each patient included, following data items were collected: age, gender, location and number of PG lesions, duration of PG, comorbidities, and treatment and response. Furthermore, we extracted data regarding previous treatment, response to previous systemic corticosteroid treatment, TNFα inhibitor regimen, time to response, time to complete healing, and reoccurrence and adverse events.
The primary outcome measure was reported as complete response (complete healing of PG ulcers or major improvement, within weeks or with almost complete healing, and without a later known response), partial response (significant improvement of lesions and symptoms), and no response (minimal improvement, no change or worsening of lesions and symptoms).
Patients administered different or multiple courses of TNFα inhibitors were reported based on the first TNFα inhibitor administration to have homogenous and TNFα inhibitor‐naive patients, as the TNFα inhibitor experienced patients may respond differently to anti‐TNFα treatment. Patients administered TNFα inhibitors resulting in no or partial response followed by an addition of immunosuppressive drugs were reported based on the initial treatment attempt because the response would best reflect the effect of TNFα inhibitors when minimising effects from other concomitant drugs.
To avoid duplicates, included articles were compared based on author names, title, date, and publisher, and patients were compared based on age, gender, comorbidity, and response to treatment.
2.4. Statistical analysis
Categorical variables were compared using Fisher's exact test and means were compared using one‐way anova. Only available data were used. A P < 0.05 was considered statistically significant. The statistical analyses were generated with SAS Studio software.
3. RESULTS
A total of 3212 unique citations were found. 1286 and 1704 citations were excluded by abstract and full‐text read, respectively, yielding 222 articles4, 8, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106, 107, 108, 109, 110, 111, 112, 113, 114, 115, 116, 117, 118, 119, 120, 121, 122, 123, 124, 125, 126, 127, 128, 129, 130, 131, 132, 133, 134, 135, 136, 137, 138, 139, 140, 141, 142, 143, 144, 145, 146, 147, 148, 149, 150, 151, 152, 153, 154, 155, 156, 157, 158, 159, 160, 161, 162, 163, 164, 165, 166, 167, 168, 169, 170, 171, 172, 173, 174, 175, 176, 177, 178, 179, 180, 181, 182, 183, 184, 185, 186, 187, 188, 189, 190, 191, 192, 193, 194, 195, 196, 197, 198, 199, 200, 201, 202, 203, 204, 205, 206, 207, 208, 209, 210, 211, 212, 213, 214, 215, 216, 217, 218, 219, 220, 221, 222, 223, 224, 225, 226, 227, 228, 229, 230, 231, 232 with 356 patients that fulfilled the eligible criteria and none of the exclusion criteria. The selection process is depicted in Supporting Information Figure S1, data of the included patients are available in Table S2 and the excluded articles with reasons for exclusion are available in Table S3.
3.1. Clinical characteristics
Patients were categorised into three groups based on infliximab, adalimumab, or etanercept administration. The groups were compared with find any differences in the distribution of clinical characteristics (Table 1). The adalimumab group was statistically significantly (P = 0.0480) younger with a mean age of 39.78 years compared with 46.10 years for the infliximab group and 47.94 years for the etanercept group. There was a statistically significant (P = 0.0001) difference of associated diseases (inflammatory bowel disease [IBD], haematological diseases, other inflammatory disorders, and no associated diseases) among the etanercept, adalimumab, and infliximab groups. Noticeably, the etanercept group had fewer patients with IBD, 19% compared with 62% for infliximab, and 56% for adalimumab, and more patients with other inflammatory diseases, 47% compared with 20% for infliximab and 28% for adalimumab.
Table 1.
Clinical characteristics of patients
| Characteristics | All | Infliximab | Adalimumab | Etanercept | P‐value |
|---|---|---|---|---|---|
| Age (y) | |||||
| Mean ± SD | 45.41 ± 16.39 | 46.10 ± 16.72 | 39.78 ± 12.55 | 47.94 ± 17.32 | 0.0480 |
| No. of missing values | 58 | 55 | 2 | 1 | |
| Gender | |||||
| Female | 60% (181) | 60% (135) | 49% (20) | 69% (24) | 0.2100 |
| Male | 40% (123) | 40% (91) | 51% (21) | 31% (11) | |
| No. of missing values | 52 | 49 | 2 | 1 | |
| Location of PG | |||||
| Lower limb(s) | 49% (133) | 45% (94) | 48% (16) | 68% (23) | 0.1357 |
| Torso | 25% (69) | 28% (59) | 21% (7) | 9% (3) | |
| Other body partsa | 6% (17) | 7% (14) | 3% (1) | 3% (1) | |
| Multiple body partsb | 21% (59) | 20% (42) | 27% (9) | 21% (7) | |
| No. of missing values | 78 | 66 | 10 | 2 | |
| Number of PG lesions | |||||
| Single | 33% (83) | 34% (62) | 25% (8) | 34% (12) | 0.6254 |
| Multiple (>1) | 67% (167) | 66% (119) | 75% (24) | 66% (23) | |
| No. of missing values | 106 | 94 | 11 | 1 | |
| Associated diseases | |||||
| IBDc | 57% (179) | 62% (146) | 56% (24) | 19% (7) | 0.0001 |
| Haematological diseasesd | 4% (14) | 4% (10) | 2% (1) | 8% (3) | |
| Other inflammatory disorderse | 24% (75) | 20% (46) | 28% (12) | 47% (17) | |
| No associated diseases | 15% (48) | 14% (33) | 14% (6) | 25% (9) | |
| No. of missing values | 40 | 40 | 0 | 0 | |
| Duration of PG | |||||
| <12 weeks | 40% (61) | 45% (49) | 28% (5) | 27% (7) | 0.1579 |
| >12 weeks | 60% (93) | 55% (61) | 72% (13) | 73% (19) | |
| No. of missing values | 201 | 165 | 25 | 10 | |
Data are given as percentage (number) of patients, unless otherwise specified. Only available data were included in the statistical analyses.
Body parts including upper limb(s), anogenital region, face and neck.
PG located at least on two of the following body parts: upper limb(s), lower limb(s), torso, anogenital area, and extracutaneous area or head/neck.
Patients with IBD were categorised as IBD despite other concomitant diseases.
Patients with haematological diseases and no IBD were categorised as haematological diseases despite other concomitant diseases.
Including primarily rheumatologic diseases.
In total, 60% were females and 40% were males, the location was predominantly on lower limbs (49%), the number of PG lesions was single in 33% of the patients and multiple (>1) in 67% of the patients and the duration of PG was more than 12 weeks in 60% of the patients and less than 12 weeks in 40% of the patients. No statistically significant differences of gender, duration of PG, location, or number of lesions were found among the infliximab, adalimumab, and etanercept groups.
3.2. Treatment and response
Of the 356 patients, 275 were treated with infliximab, 43 were treated with adalimumab, 36 were treated with etanercept, and 2 were treated with certolizumab. An 87% (95% CI: 83%‐90%) response rate and a 67% (95% CI: 62%‐72%) complete response rate to TNFα inhibitors were found (Table 2). There were no statistically significant differences in response rates (P = 0.6159) or complete response rates (P = 0.0773) to infliximab, adalimumab, and etanercept. Subgroup analyses according to the TNFα inhibitor agent, type of PG, and associated disease found no statistically significant differences in response or complete response rates.
Table 2.
Response and complete response rates to TNFα inhibitors according to TNFα inhibitor agent, associated disease, type of PG, and duration of PG
| Response rate | Complete response rate | No response rate | P‐valuea | P‐valueb | |
|---|---|---|---|---|---|
| No stratification | 87% (309) (95% CI: 83%‐90%) | 67% (240) (95% CI: 62%‐72%) | 13% (47) (95% CI: 10%‐17%) | ||
| TNFα inhibitor agent | |||||
| Infliximab | 87% (239) | 68% (187) | 13% (36) | 0.6159 | 0.0773 |
| Adalimumab | 91% (39) | 77% (33) | 9% (4) | ||
| Etanercept | 83% (30) | 53% (19) | 17% (6) | ||
| No. of missing values | 0 | 0 | 0 | ||
| Type of PG | |||||
| NPPSPG | 87% (183) | 71% (149) | 13% (27) | 0.7061 | 0.2970 |
| PPG | 83% (45) | 61% (33) | 17% (9) | ||
| PSPG | 93% (13) | 79% (11) | 7% (1) | ||
| No. of missing values | 68 | 47 | 10 | ||
| Associated diseasesc | |||||
| IBD | 91% (162) | 74% (132) | 10% (17) | 0.2136 | 0.6667 |
| Haematological diseases | 93% (13) | 64% (9) | 7% (1) | ||
| Other inflammatory disorders | 81% (61) | 68% (51) | 19% (14) | ||
| No associated diseases | 90% (43) | 69% (33) | 10% (5) | ||
| No. of missing values | 30 | 15 | 10 | ||
| Duration of PG | |||||
| <12 weeks | 93% (57) | 87% (53) | 7% (4) | 0.5678 | 0.0124 |
| >12 weeks | 89% (84) | 69% (65) | 11% (10) | ||
| No. of missing values | 168 | 122 | 33 | ||
Abbreviations: NPPSPG, non‐peristomal or non‐post surgery pyoderma gangrenosum; PPG, peristomal pyoderma gangrenosum; PSPG, postsurgery pyoderma gangrenosum.
Data are given as percentage (number) of patients, unless otherwise specified. Only available data were included in the statistical analyses.
P‐values for response rates. Calculated using Fisher's exact test. The response rate is the percentage of patients with partial and complete response.
P‐values for complete response rates. Calculated using Fisher's exact test. The complete response rate is the percentage of patients with complete response.
Same as Table 1.
The 69% complete response rate for patients with PG duration more than 12 weeks was, unlike the response rate, statistically significantly (P = 0.0124) lower than the 87% complete response rate for patients with PG duration less than 12 weeks.
3.3. Additional clinical characteristics
35 of 324 (10.8%) patients had an adverse effect including four patients with fatal outcome because of sepsis or endocarditis (Table 3). Thirty‐three of the 35 adverse events occurred with infliximab. Thirty‐five of 200 (17.5%) patients with complete response had a reoccurrence including 17 patients while off TNFα inhibitor treatment and 11 patients while on TNFα inhibitor treatment. Time to complete healing was on average 20.37 weeks.
Table 3.
Additional clinical characteristics
| Clinical characteristics | Numbers |
|---|---|
| Adverse effects | 10.8% (35/324) |
| No. of missing values | 32 |
| Reoccurrence | 17.5% (35/200) |
| No. of missing values | 40 |
| Time (weeks) to complete | 20.37 (mean) |
| Healing | 1 to 156 (range) |
| No. of missing values | 117 |
4. DISCUSSION
4.1. Key findings
The study we report found response (87%, 95% CI: 83%‐90%) and complete response rates (67%, 95% CI: 62%‐72%) to TNFα inhibitors that may be considered as clinically significant. In addition, the results showed that infliximab, adalimumab, and etanercept did not statistically significantly differ in response and complete response rates.
Although not statistically significant, it is noteworthy that patients treated with etanercept had a less‐favourable response. The response rate for etanercept was 83% compared with 91% for adalimumab and 87% for infliximab. And the complete response rate was 53% for etanercept compared with 77% for adalimumab and 68% for infliximab (Figure 1).
Figure 1.
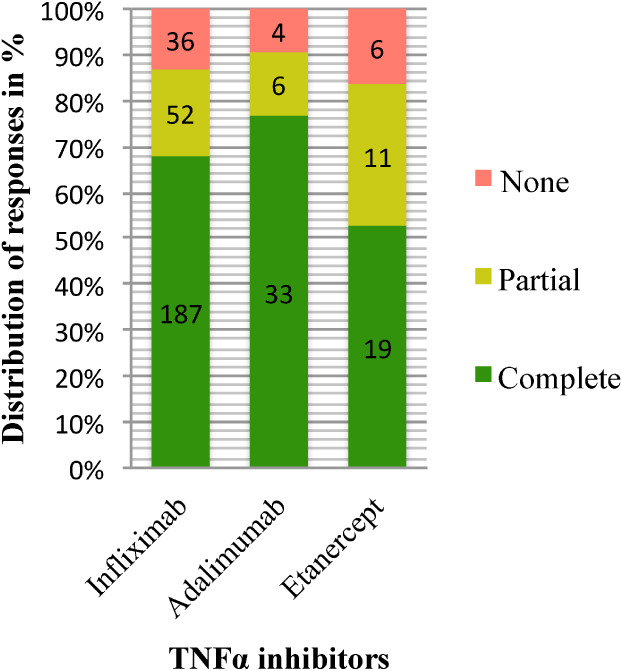
Distribution of complete, partial, and no responses to infliximab adalimumab and etanercept. The absolute frequencies of responses are shown within the histograms
4.2. Findings in relation to other studies
A randomised controlled trial by Brooklyn et al8 demonstrated a response after 2 weeks in 46% (6/13) of subjects who received a single infusion of infliximab versus 6% (1/17) of subjects who received placebo (P = 0.025). Subsequently, subjects with no response at week 2 received an open‐label infliximab infusion regardless of the allocation to infliximab or placebo group. At week 6, the response rate was 69% (20/29) and the complete response rate was 21% (6/29).
The 46% response rate to infliximab found at week 2 is substantially lower than the 87% response rate found in our study, but 2‐week observation time and a single infusion might have led to underestimation of the former response rate. The low 21% complete response rate at week 6, compared with the 68% complete response rate from our study, might also be underestimated because of inadequate dosage and observation time when considering that our study found a mean of 20.37 weeks to complete healing.
Brooklyn et al found a difference (P = 0.014) in response rates according to the duration of PG. Patients with more than 12‐week duration had a less‐favourable response than those with less than 12‐week duration (47% [7/15] versus 93% [13/14]). This might be a consequence of more cases with recalcitrant PG in the group of patients with more than 12‐week duration of PG. Yet, in the present study, we did not find a statistically significant (P = 0.5678) difference in response rates according to the duration of PG, but a statistically significant (P = 0.0124) difference in complete response rates was found, which supports Brooklyn et al's findings. Consistent with the results of our study, Brooklyn et al found no difference in response rates according to IBD status. Infliximab and adalimumab are licensed for treatment of IBD that may explain why our study found statistically significantly (P = 0.0001) fewer patients with associated IBD treated with etanercept (19%) than infliximab (62%) and adalimumab (56%).
The response and complete response rates to TNFα inhibitors in our study were slightly lower, but overall broadly similar to those found in case series or retrospective analyses with PG. Response rates ranged from 87.5% to 100% and complete response rates ranged from 37.5% to 100%.189, 211, 219, 225, 230, 233 Most patients from the randomised controlled trial by Brooklyn et al, case series, and retrospective analyses were included in this systematic review.
Another randomised controlled trial (n = 112) by Ormerod et al9 found approximately 90% response rates and 47% complete response rates to oral corticosteroid or cyclosporine after 6 months. The response rates were found in fig. 3 and the complete response rates were found in Table 3.9 This is inferior to the rates to TNFα inhibitors, suggesting that TNFα inhibitors may be more efficacious. The rates to oral corticosteroid or cyclosporine may be overestimated compared with the response and complete response rates to TNFα inhibitors from our study because patients treated with TNFα inhibitors are usually more therapy resistant and have failed multiple treatments. In our study, 60% (84/141) of the patients had a duration of PG more than 12 weeks indicating that a significant fraction of patients treated with TNFα inhibitors might have been resistant to previous therapy.
In the study, we report 4 of 356 (1.12%) patients died of infection after administration of infliximab. By comparison, 5 of 23 (21.7%) patients died of infection in a retrospective analysis of 23 patients requiring inpatient management of PG.84 These five patients were receiving immunosuppressive drugs but no TNFα inhibitors. Therefore, suggesting that a high risk of death by infectious causes is not limited to administration of TNFα inhibitors. Rather, it may be related to immunosuppression along with PG lesions being portal of entry for pathogens. Our study suggests that infliximab may be associated with more adverse events than etanercept and adalimumab, as 33 of 35 adverse events occurred with infliximab administration. However, it should be taken into consideration that more patients were treated with infliximab and not solely rely on the number of adverse events reported.
4.3. Limitations
To minimise publication bias, grey literature was searched, data were controlled for duplications and a large fraction of articles were full‐text assessed to find eligible patients without TNFα inhibitor administration being reported in the title or abstract. Nevertheless, the vast majority of data came from case reports, thus increasing the risk of publication bias that might have overestimated the found response and complete response rates. Patients might have received concomitant drugs that would overestimate the response and complete response rates, while insufficient dosages of TNFα inhibitors might have underestimated the response and complete response rates. Reoccurrences and adverse events may have been underestimated because of inconsistent reporting and too short follow‐up time. As there are no diagnostic criteria, patients misdiagnosed with PG might have been included in our semi‐systematic review.
Some of the limitations with the outcome measures are that the response rates do not specify the degree of response, and the complete response rates are susceptible to misclassification because of variable follow‐up time and no standard objective assessment of lesion response.
It is a limitation that the data were incomplete because of missing values. In addition, extracted data items including previous treatment, response to previous systemic corticosteroid treatment, concomitant drugs, TNFα inhibitor regimen, and time to response were not used in our study because the data were unreliable as a result of inconsistent and low quality of reporting in the included articles.
Infliximab, adalimumab, and etanercept did not differ significantly in response and complete response rates. This should be interpreted cautiously as differences in factors such as severity of PG, concomitant therapy, therapy resistance, TNFα inhibitor regimen, and follow‐up time among the three treatment groups could potentially have led to a type II error. The found age difference with the adalimumab group being younger, and therefore possibly had a better response, could have compromised the comparability of the groups. However, etanercept performed worse than infliximab, despite the fact that these two groups had approximately the same mean age, suggesting age might not have been a confounder.
As there was no placebo comparison in our study, it is possible that none of the TNFα inhibitors had a positive effect, but this is unlikely as Brooklyn et al8 demonstrated that infliximab was superior to placebo in the treatment of PG.
Despite the limitations, this systematic evaluation of TNFα inhibitors provides a significant contribution to the existing scarce literature on treatment options for PG that is characterised by anecdotal evidence.
5. CONCLUSION
TNFα inhibitors demonstrated significant effectiveness with an 87% response rate and a 67% complete response rate supporting the use of TNFα inhibitors to treat PG in adults. Our study suggests that there is no statistically significant difference in effectiveness among infliximab, adalimumab, and etanercept, but there is a risk of a type II error because the groups of patients treated with these TNFα inhibitors might not have been comparable. Although not statistically significant, patients had a noteworthy less‐favourable response to etanercept.
Future controlled trials comparing different TNFα inhibitors with other treatments such as systemic corticosteroid, cyclosporine, or other biologic drugs with objective and meaningful assessment of lesion response, adequate dosage, and at least 6‐month follow‐up time may validate these findings and further strengthen the evidence for the use of TNFα inhibitors in the treatment of PG.
CONFLICT OF INTEREST
The authors have no conflict of interests to declare.
Supporting information
Figure S1. Modified PRISMA flow diagram depicting the study selection process.
Table S1. Search history.
Table S2. Data of the included patients.
Table S3. The excluded articles with reason(s) for exclusion. Articles could be excluded for more than one reason. Primary patients were defined as patients not previously reported in another publication.
Ben Abdallah H, Fogh K, Bech R. Pyoderma gangrenosum and tumour necrosis factor alpha inhibitors: A semi‐systematic review. Int Wound J. 2019;16:511–521. 10.1111/iwj.13067
REFERENCES
- 1. Al Ghazal P, Herberger K, Schaller J, et al. Associated factors and comorbidities in patients with pyoderma gangrenosum in Germany: a retrospective multicentric analysis in 259 patients. Orphanet J Rare Dis. 2013;8:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kridin K, Cohen AD, Amber KT. Underlying systemic diseases in pyoderma gangrenosum: a systematic review and meta‐analysis. Am J Clin Dermatol. 2018;19:479‐487. [DOI] [PubMed] [Google Scholar]
- 3. Farhi D, Wallach D. The neutrophilic dermatoses. Dermatol Nurs. 2008;20:274‐276. 279–282. [PubMed] [Google Scholar]
- 4. Patel F, Fitzmaurice S, Duong C, et al. Effective strategies for the management of pyoderma gangrenosum: a comprehensive review. Acta Derm Venereol. 2015;95:525‐531. [DOI] [PubMed] [Google Scholar]
- 5. Patel F, Fitzmaurice S, Duong C, et al. Effective strategies for the management of pyoderma gangrenosum: a comprehensive review. Acta Derm Venereol. 2014;95:525‐531. [DOI] [PubMed] [Google Scholar]
- 6. Maverakis E, Ma C, Shinkai K, et al. Diagnostic criteria of ulcerative pyoderma gangrenosum: a Delphi consensus of international experts. JAMA Dermatol. 2018;154:461‐466. [DOI] [PubMed] [Google Scholar]
- 7. Ahn C, Negus D, Huang W. Pyoderma gangrenosum: a review of pathogenesis and treatment. Expert Rev Clin Immunol. 2018;14:225‐233. [DOI] [PubMed] [Google Scholar]
- 8. Brooklyn TN, Dunnill MG, Shetty A, et al. Infliximab for the treatment of pyoderma gangrenosum: a randomised, double blind, placebo controlled trial. Gut. 2006;55:505‐509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ormerod AD, Thomas KS, Craig FE, et al. Comparison of the two most commonly used treatments for pyoderma gangrenosum: results of the STOP GAP randomised controlled trial. BMJ. 2015;350:h2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Business Insider . One of the world's blockbuster drugs might not exist if its research hadn't flopped in a major way, 2015, 2018,
- 12. EurekAlert! : REMICADE becomes first anti‐TNF biologic therapy to treat 1 million patients worldwide, 2007, 2018.
- 13. Vekic DA, Woods J, Lin P, Cains GD. SAPHO syndrome associated with hidradenitis suppurativa and pyoderma gangrenosum successfully treated with adalimumab and methotrexate: a case report and review of the literature. Int J Dermatol. 2018;57:10‐18. [DOI] [PubMed] [Google Scholar]
- 14. Schwaiger K, Russe E, Kholosy H, et al. Reconstructive microsurgical approach for the treatment of pyoderma gangrenosum. J Plast Reconstr Aesthet Surg. 2018;71:44‐52. [DOI] [PubMed] [Google Scholar]
- 15. Marzano AV, Ortega‐Loayza AG, Ceccherini I, Cugno M. LPIN2 gene mutation in a patient with overlapping neutrophilic disease (pyoderma gangrenosum and aseptic abscess syndrome). JAAD Case Rep. 2018;4:120‐122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Leiphart PA, Lam CC, Foulke GT. Suppression of pathergy in pyoderma gangrenosum with infliximab allowing for successful tendon debridement. JAAD Case Rep. 2018;4:98‐100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lamiaux M, Dabouz F, Wantz M, et al. Successful combined antibiotic therapy with oral clindamycin and oral rifampicin for pyoderma gangrenosum in patient with PASH syndrome. JAAD Case Rep. 2018;4:17‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fleisher M, Marsal J, Lee SD, et al. Effects of Vedolizumab therapy on Extraintestinal manifestations in inflammatory bowel disease. Dig Dis Sci. 2018;63:825‐833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Almukhtar R, Armenta AM, Martin J, et al. Delayed diagnosis of post‐surgical pyoderma gangrenosum: a multicenter case series and review of literature. Int J Surg Case Rep. 2018;44:152‐156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vural S, Gundogdu M, Kundakci N, Ruzicka T. Familial Mediterranean fever patients with hidradenitis suppurativa. Int J Dermatol. 2017;56:660‐663. [DOI] [PubMed] [Google Scholar]
- 21. Velasco‐Tamariz V, Carreno‐Tarragona G, Tous‐Romero F, Gil‐de la Cruz E, Martin‐Clavero E, Rivera‐Diaz R. Dramatic resolution of disseminated pyoderma gangrenosum associated with monoclonal gammopathy after therapy with bortezomib and dexamethasone. Int Wound J. 2017;14:1382‐1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vacas AS, Torre AC, Bollea‐Garlatti ML, Warley F, Galimberti RL. Pyoderma gangrenosum: clinical characteristics, associated diseases, and responses to treatment in a retrospective cohort study of 31 patients. Int J Dermatol. 2017;56:386‐391. [DOI] [PubMed] [Google Scholar]
- 23. Sun NZ, Ro T, Jolly P, Sayed CJ. Non‐response to Interleukin‐1 antagonist Canakinumab in two patients with refractory pyoderma gangrenosum and hidradenitis Suppurativa. J Clin Aesthet Dermatol. 2017;10:36‐38. [PMC free article] [PubMed] [Google Scholar]
- 24. Stiegler JD, Lucas CT, Sami N. Pyoderma gangrenosum in pregnancy successfully treated with infliximab and prednisone. JAAD Case Rep. 2017;3:387‐389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sonbol H, Duchatelet S, Miskinyte S, Bonsang B, Hovnanian A, Misery L. PASH syndrome: a disease with genetic heterogeneity. Br J Dermatol. 2017;178(1):e17‐e18. [DOI] [PubMed] [Google Scholar]
- 26. Scherlinger M, Guillet S, Doutre MS, Beylot‐Barry M, Pham‐Ledard A. Pyoderma gangrenosum with extensive pulmonary involvement. J Eur Acad Dermatol Venereol. 2017;31:e214‐e216. [DOI] [PubMed] [Google Scholar]
- 27. Pinard J, Chiang DY, Mostaghimi A, Granter SR, Merola JF, Barkoudah E. Wounds that would not heal: pyoderma gangrenosum. Am J Med. 2017;131(4):377‐379. [DOI] [PubMed] [Google Scholar]
- 28. Mogle BT, Seabury RW, Jennings S, Cwikla GM. Severe neutrophilic dermatosis following submental deoxycholic acid administration. Clin Toxicol. 2017;55:681. [DOI] [PubMed] [Google Scholar]
- 29. Marzano AV, Damiani G, Ceccherini I, Berti E, Gattorno M, Cugno M. Autoinflammation in pyoderma gangrenosum and its syndromic form (pyoderma gangrenosum, acne and suppurative hidradenitis). Br J Dermatol. 2017;176:1588‐1598. [DOI] [PubMed] [Google Scholar]
- 30. Lee JH, Chang IK, Lee HE, et al. Treatment of recalcitrant pyoderma gangrenosum with ulcerative colitis by adalimumab injection. Ann Dermatol. 2017;29:260‐262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Koduru P, Irani M, Abraham B. Resolution of pyoderma gangrenosum with vedolizumab in a Crohn's patient. Am J Gastroenterol. 2017;112:S1091. [Google Scholar]
- 32. Karciauskiene J, Vilkickaite V, Kucinskiene V, Valiukeviciene S. The successful management of PASH syndrome with biologics: a case report. Exp Dermatol. 2017;26:19‐20. [Google Scholar]
- 33. Kanameishi S, Nakamizo S, Endo Y, et al. High level of serum human interleukin‐18 in a patient with pyogenic arthritis, pyoderma gangrenosum and acne syndrome. J Eur Acad Dermatol Venereol. 2017;31:e115‐e116. [DOI] [PubMed] [Google Scholar]
- 34. Heard LK, Richardson VN, Lewis CM, Davis LS. A case of autoinflammatory skin and bone disease flared by a change in osteoporosis management. JAAD Case Rep. 2017;3:103‐105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Haridas V, Shetty P, Dsouza LC, Dinesh US, Haridas K, Bargale A. Pyoderma gangrenosum in Sjogren's syndrome and its successful treatment with topical application of etanercept. Int J Rheum Dis. 2017;20:657‐659. [DOI] [PubMed] [Google Scholar]
- 36. Dupuis E, Zarbafian M, Asgarpour J, Parsons L, Mydlarski PR. Plasmablasticlike lymphoma arising within chronic pyoderma gangrenosum. JAAD Case Rep. 2017;3:200‐201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. De Wet J, Jordaan HF, Kannenberg SM, Tod B, Glanzmann B, Visser WI. Pyoderma gangrenosum, acne, and suppurative hidradenitis syndrome in end‐stage renal disease successfully treated with adalimumab. Dermatol Online J. 2017;23. [PubMed] [Google Scholar]
- 38. Calskan E. Er: YAG laser ablation: an adjuvant treatment for medically resistant pyoderma gangrenosum. Dermatol Surg. 2017;43(11):1405‐1407. [DOI] [PubMed] [Google Scholar]
- 39. Beynon C, Chin MF, Hunasehally P, et al. Successful treatment of autoimmune disease‐associated pyoderma gangrenosum with the IL‐1 receptor antagonist Anakinra: a case series of 3 patients. J Clin Rheumatol. 2017;23:181‐183. [DOI] [PubMed] [Google Scholar]
- 40. Baliu‐Pique C, Mascaro JM Jr. Multifocal and refractory pyoderma gangrenosum: possible role of cocaine abuse. Australas J Dermatol. 2017;58:e83‐e86. [DOI] [PubMed] [Google Scholar]
- 41. Yeo PM, Tan KW, Lim RS, Seng SC, Ong JP, Rajaratnam R. Neutrophilic dermatoses as a continuous spectrum: an illustrative case. Ann Acad Med Singapore. 2016;45:569‐571. [PubMed] [Google Scholar]
- 42. Wu C, Fang K, Jin HZ. Prednisone combined with etanercept for the treatment of psoriatic arthritis accompanied by pyoderma gangrenosum. J Clin Dermatol. 2016;45:197‐199. [Google Scholar]
- 43. Thompson C, Kulp‐Shorten C, Callen J. A case of postoperative pyoderma gangrenosum successfully treated with infliximab. J Am Acad Dermatol. 2016;74:AB38. [Google Scholar]
- 44. Sartini A, Bianchini M, Schepis F, Marzi L, De Maria N, Villa E. Complete resolution of non‐necrotizing lung granuloma and pyoderma gangrenosum after restorative proctocolectomy in a woman with severe ulcerative colitis and cytomegalovirus infection. Clin Case Rep. 2016;4:195‐202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Reynolds C, Schofer N, Zengin E, Lohse AW, Faiss S, Schmiedel S. Multiple abscesses after a cruise along the Latin American coast. Internist (Berl). 2016;57:284‐288. [DOI] [PubMed] [Google Scholar]
- 46. Rajan D, Greer JB, Regueiro MD, et al. IBD LIVE case series‐case 6. Inflamm Bowel Dis. 2016;22:2754‐2764. [DOI] [PubMed] [Google Scholar]
- 47. Pichler M, Larcher L, Holzer M, et al. Surgical treatment of pyoderma gangrenosum with negative pressure wound therapy and split thickness skin grafting under adequate immunosuppression is a valuable treatment option: case series of 15 patients. J Am Acad Dermatol. 2016;74:760‐765. [DOI] [PubMed] [Google Scholar]
- 48. Novo‐Torres A, Céspedes‐Guirao FJ, Guzmán Restituyo N, Lorda‐Barraguer E. Management of pyoderma gangrenosum with combination of systemic treatment, vacuum‐assisted closure and synthetic dermal substitute. Eur J Plast Surg. 2016;39:297‐302. [Google Scholar]
- 49. Laun J, Elston JB, Harrington MA, Payne WG. Severe bilateral lower extremity pyoderma gangrenosum. Eplasty. 2016;16:ic44. [PMC free article] [PubMed] [Google Scholar]
- 50. Kokavec J, Rajak S, Huilgol S, Selva D. Pyoderma gangrenosum of the eyelid. Can J Ophthalmol. 2016;51:e58‐e60. [DOI] [PubMed] [Google Scholar]
- 51. Khajehnoori M, O'Brien T. A case of surgically treated peristomal pyoderma gangrenosum in a patient with rheumatoid arthritis. J Surg Case Rep. 2016;2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kashiwado Y, Uchino A, Ota T, Nagano S. Intestinal Behcet's disease with pyoderma gangrenosum successfully treated with the combination therapy of adalimumab and glucocorticoids. Mod Rheumatol. 2018;28(5):901‐905. [DOI] [PubMed] [Google Scholar]
- 53. Jeffery T, Tai Y, Pepall L, Thom G. Topical crushed prednisolone use in recalcitrant peristomal pyoderma gangrenosum. Australas J Dermatol. 2016;57:57.26776229 [Google Scholar]
- 54. Hurabielle C, Schneider P, Baudry C, Bagot M, Allez M, Viguier M. Certolizumab pegol—a new therapeutic option for refractory disseminated pyoderma gangrenosum associated with Crohn's disease. J Dermatolog Treat. 2016;27:67‐69. [DOI] [PubMed] [Google Scholar]
- 55. Greb JE, Gottlieb AB, Goldminz AM. High‐dose ustekinumab for the treatment of severe, recalcitrant pyoderma gangrenosum. Dermatol Ther. 2016;29:482‐483. [DOI] [PubMed] [Google Scholar]
- 56. Galimberti RL, Vacas AS, Bollea Garlatti ML, Torre AC. The role of interleukin‐1beta in pyoderma gangrenosum. JAAD Case Rep. 2016;2:366‐368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Chatzinasiou F, Polymeros D, Panagiotou M, Theodoropoulos K, Rigopoulos D. Generalized pyoderma gangrenosum associated with ulcerative colitis: successful treatment with infliximab and azathioprine. Acta dermatovenerol Croat. 2016;24:83‐85. [PubMed] [Google Scholar]
- 58. Arivarasan K, Bhardwaj V, Sud S, Sachdeva S, Puri AS. Biologics for the treatment of pyoderma gangrenosum in ulcerative colitis. Intest Res. 2016;14:365‐368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Alvarez‐Lopez MA, Buron‐Alvarez I, Villegas‐Fernandez C. Refractory pyoderma gangrenosum treated with platelet‐rich plasma. J Eur Acad Dermatol Venereol. 2016;30:1423‐1424. [DOI] [PubMed] [Google Scholar]
- 60. Zampeli VA, Lippert U, Nikolakis G, et al. Disseminated refractory pyoderma gangraenosum during an ulcerative colitis flare. Treatment with infliximab. J Dermatol Case Rep. 2015;9:62‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Vahlquist A, Hakansson LD, Ronnblom L, et al. Recurrent pyoderma gangrenosum and cystic acne associated with leucocyte adhesion deficiency due to novel mutations in ITGB2: successful treatment with infliximab and adalimumab. Acta Derm Venereol. 2015;95:349‐351. [DOI] [PubMed] [Google Scholar]
- 62. Tolkachjov SN, Fahy AS, Wetter DA, et al. Postoperative pyoderma gangrenosum (PG): the Mayo Clinic experience of 20 years from 1994 through 2014. J Am Acad Dermatol. 2015;73:615‐622. [DOI] [PubMed] [Google Scholar]
- 63. Staub J, Pfannschmidt N, Strohal R, et al. Successful treatment of PASH syndrome with infliximab, cyclosporine and dapsone. J Eur Acad Dermatol Venereol. 2015;29:2243‐2247. [DOI] [PubMed] [Google Scholar]
- 64. Saraceno R, Babino G, Chiricozzi A, Zangrilli A, Chimenti S. PsAPASH: a new syndrome associated with hidradenitis suppurativa with response to tumor necrosis factor inhibition. J Am Acad Dermatol. 2015;72:e42‐e44. [DOI] [PubMed] [Google Scholar]
- 65. Sagami S, Ueno Y, Tanaka S, Nagai K, Hayashi R, Chayama K. Successful use of adalimumab for treating pyoderma gangrenosum with ulcerative colitis under corticosteroid‐tapering conditions. Intern Med. 2015;54:2167‐2172. [DOI] [PubMed] [Google Scholar]
- 66. Patier de la Pena JL, Moreno‐Cobo MA, Sanchez‐Conde M, Echaniz Quintana AM. Behcet disease and refractory pyoderma gangrenosum with response to infliximab. Rev Clin Esp. 2015;215:66‐67. [DOI] [PubMed] [Google Scholar]
- 67. Olmedo Martin RV, Amo Trillo V, Lopez Ortega S, Jimenez Perez M. Peristomal pyoderma gangrenosum after rectal adenocarcinoma in the context of colonic and complex perianal Crohn's disease. Gastroenterol Hepatol. 2015;39:338‐341. [DOI] [PubMed] [Google Scholar]
- 68. Ohashi T, Yasunobu K, Yamamoto T. Peristomal pyoderma gangrenosum: a report of three cases. J Dermatol. 2015;42:837‐838. [DOI] [PubMed] [Google Scholar]
- 69. Murphy B, Morrison G, Podmore P. Successful use of adalimumab to treat pyoderma gangrenosum, acne and suppurative hidradenitis (PASH syndrome) following colectomy in ulcerative colitis. Int J Colorectal Dis. 2015;30:1139‐1140. [DOI] [PubMed] [Google Scholar]
- 70. Meyersburg D. Multilocular superficial pyoderma gangrenosum or PAPA‐syndrom—successful treatment with ustekinumab. Aktuelle Derm. 2015;41:300‐303. [Google Scholar]
- 71. Meng X, Zhu X, Chen L, Wu J, Liu G, Xia B. Pyoderma gangrenosum of articulations carpi associated with ulcerative colitis: one case report. Int J Clin Exp Med. 2015;8:19184‐19187. [PMC free article] [PubMed] [Google Scholar]
- 72. McAllister BP, Williams ED, Yoo LJ, et al. Refractory peristomal pyoderma gangrenosum successfully treated with intravenous immunoglobulin: a case report. Am J Gastroenterol. 2015;110:1739‐1740. [DOI] [PubMed] [Google Scholar]
- 73. Lindwall E, Singla S, Davis WE, Quinet RJ. Novel PSTPIP1 gene mutation in a patient with pyogenic arthritis, pyoderma gangrenosum and acne (PAPA) syndrome. Semin Arthritis Rheum. 2015;45:91‐93. [DOI] [PubMed] [Google Scholar]
- 74. Lamb R, Herd R. Systemic pyoderma gangrenosum responding to treatment with infliximab and methotrexate. J Am Acad Dermatol. 2015;72:AB152. [Google Scholar]
- 75. Groleau PF, Grossberg AL, Gaspari AA. Hidradenitis suppurativa and concomitant pyoderma gangrenosum treated with infliximab. Cutis. 2015;95:337‐342. [PubMed] [Google Scholar]
- 76. Goldberg ND, Vadlamudi A, Parrish N. Treatment of refractory Crohn's disease and pyoderma gangrenosum with a combination regimen of rifaximin, gentamicin and metronidazole. Case Rep Gastroenterol. 2015;9:25‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Gameiro A, Pereira N, Cardoso JC, Goncalo M. Pyoderma gangrenosum: challenges and solutions. Clin Cosmet Invest Dermatol. 2015;8:285‐293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Gade M, Studstrup F, Andersen AK, Hilberg O, Fogh C, Bendstrup E. Pulmonary manifestations of pyoderma gangrenosum: 2 cases and a review of the literature. Respir Med. 2015;109:443‐450. [DOI] [PubMed] [Google Scholar]
- 79. Chin MF, Beynon CR, Lawson T, Hunasehally P, Bhagwandas K, Bevan M. Successful treatment of pyoderma gangrenosum with the interleukin‐1 receptor antagonist anakinra: a case series of three patients. Br J Dermatol. 2015;173:67‐68. [DOI] [PubMed] [Google Scholar]
- 80. Castaño‐González I, Vilar‐Alejo J, Fernández‐Palacios J, Carretero‐Hernández G. Infliximab como opción terapéutica en pioderma gangrenoso mamario bilateral postquirúrgico refractario. Cirugía Plástica Ibero‐Latinoamericana. 2015;41:97‐103. [Google Scholar]
- 81. Carlesimo M, Abruzzese C, Narcisi A, et al. Cutaneous manifestations and gastrointestinal disorders: report of two emblematic cases. Clin Ter. 2015;166:e269‐e272. [DOI] [PubMed] [Google Scholar]
- 82. Campanati A, Brisigotti V, Ganzetti G, et al. Finally, recurrent pyoderma gangrenosum treated with adalimumab: case report and review of the literature. J Eur Acad Dermatol Venereol. 2015;29:1245‐1247. [DOI] [PubMed] [Google Scholar]
- 83. Buhalog B, Eastman K, McDonald R. Recalcitrant pyoderma gangrenosum treated with parenteral iron sucrose therapy. JAAD Case Rep. 2015;1:54‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Ye MJ, Ye JM. Pyoderma gangrenosum: a review of clinical features and outcomes of 23 cases requiring inpatient management. Dermatol Res Pract. 2014;2014:461‐467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Teich N, Klugmann T. Rapid improvement of refractory pyoderma gangrenosum with infliximab gel in a patient with ulcerative colitis. J Crohns Colitis. 2014;8:85‐86. [DOI] [PubMed] [Google Scholar]
- 86. Teich N. Failure of sublesional infliximab injection for refractory parastomal pyoderma gangrenosum in a patient with Crohn's disease. Tech Coloproctol. 2014;18:965‐966. [DOI] [PubMed] [Google Scholar]
- 87. So BJ, Chun SH, Lee JM, Jung SK, Kim IH. A refractory case of pyoderma gangrenosum responding to infliximab. J Dermatol. 2014;41:104.24354617 [Google Scholar]
- 88. Pileri F, Abbati G, Zappia F, Conti A, Malagoli M, Pietrangelo A. The management of peristomal pyoderma gangrenosum in a patient suffering from colon Crohn's disease: a case report. Ital J Med. 2014;8:105. [Google Scholar]
- 89. Nakamura M, Ghaznavi AM, Darian V, Siddiqui A. Pyoderma gangrenosum following the revision of a breast reconstruction and abdominoplasty. Open Dermatol J. 2014;8:68‐71. [Google Scholar]
- 90. Marzano AV, Fanoni D, Antiga E, et al. Expression of cytokines, chemokines and other effector molecules in two prototypic autoinflammatory skin diseases, pyoderma gangrenosum and Sweet's syndrome. Clin Exp Immunol. 2014;178:48‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Marzano AV, Ceccherini I, Gattorno M, et al. Association of pyoderma gangrenosum, acne, and suppurative hidradenitis (PASH) shares genetic and cytokine profiles with other autoinflammatory diseases. Medicine (Baltimore). 2014;93:e187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Hill DS, Naim S, Watts AM, Morgan M, Bower C, Toms AD. A report of two patients with a history of pyoderma gangrenosum where total knee arthroplasty was performed under prophylactic immunosuppression. Eur Orthop Traumatol. 2014;5:85‐89. [Google Scholar]
- 93. Felton S, Al‐Niaimi F, Lyon C. Severe Back pain in a Young patient with pyoderma gangrenosum and Crohn's disease controlled with anti‐tumor necrosis factor therapy: sterile osteomyelitis. Dermatol Ther (Heidelb). 2014;4:137‐140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Donmez S, Pamuk ON, Gedik M, A KR, Bulut G. A case of granulomatosis with polyangiitis and pyoderma gangrenosum successfully treated with infliximab and rituximab. Int J Rheum Dis. 2014;17:471‐475. [DOI] [PubMed] [Google Scholar]
- 95. Cinotti E, Labeille B, Perrot JL, Pallot‐Prades B, Cambazard F. Certolizumab for the treatment of refractory disseminated pyoderma gangrenosum associated with rheumatoid arthritis. Clin Exp Dermatol. 2014;39:750‐751. [DOI] [PubMed] [Google Scholar]
- 96. Cerdan‐Santacruz C, Caparros‐Sanz MR, Lancharro‐Bermudez M, Mendoza‐Hernandez JL, Cerdan‐Miguel J. Peri‐ileostomy pyoderma gangrenosum. Case report Rev Esp Enferm Dig. 2014;106:285‐288. [PubMed] [Google Scholar]
- 97. Byeon YM, Lee J, Lee SJ, et al. Peritonsillar involvement in pyoderma gangrenosum associated with ulcerative colitis. Intest Res. 2014;12:153‐156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Bannura CG, Barrera EA, Melo LC. Pioderma gangrenoso gigante de curso fulminante asociado a enfermedad inflamatoria intestinal. Rev Chil Cir. 2014;66:259‐263. [Google Scholar]
- 99. Almoshawer E, Eickelmann M, Blume JH, Szeimies RM. Chronically‐ulcerating pyoderma gangrenosum: successful healing under infliximab‐therapy. J Dtsch Dermatol Ges. 2014;12:E26‐E27. [Google Scholar]
- 100. Yoo L, Elwir S, Tinsley A, Williams E. A case report of a patient with Crohn's disease complicated by pyoderma gangrenosum treated with intravenous immunoglobulin. Am J Gastroenterol. 2013;108:S421. [Google Scholar]
- 101. Simian D, Quijada MI, Lubascher J, Acuna R, Quera R. Treatment of inflammatory bowel disease with infliximab: experience in 25 patients. Rev Med Chil. 2013;141:1158‐1165. [DOI] [PubMed] [Google Scholar]
- 102. Segura Charry JS, Jaimes DA, Londoño JD. Refractory pyoderma gangrenosum: utility of combined therapy. Focus on benefits of hyperbaric therapy in its treatment. Rev Colomb Reumatol. 2013;20:171‐176. [Google Scholar]
- 103. Rizvi SM, Mork NJ, Gjersvik P. A horseshoe‐shaped wound on the back. Tidsskr Nor Laegeforen. 2013;133:2270. [DOI] [PubMed] [Google Scholar]
- 104. Rice SA, Woo PN, El‐Omar E, Keenan RA, Ormerod AD. Topical tacrolimus 0.1% ointment for treatment of cutaneous Crohn's disease. BMC Res Notes. 2013;6:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Ratnagobal S, Sinha S. Pyoderma gangrenosum: guideline for wound practitioners. J Wound Care. 2013;22:68‐73. [DOI] [PubMed] [Google Scholar]
- 106. Morete M, Rodriguez JA, Figueira M, Echarri A. Patient education on their disease as a determinant on the progression of complications. The role of specialized nursing. J Crohn's Colit. 2013;7:S302. [Google Scholar]
- 107. Mooij JE, van Rappard DC, Mekkes JR. Six patients with pyoderma gangrenosum successfully treated with infliximab. Int J Dermatol. 2013;52:1418‐1420. [DOI] [PubMed] [Google Scholar]
- 108. Lipka S, Katz S, Ginzburg L. Massive pyoderma gangrenosum in a 77 year old female with Crohn's disease responsive to adalimumab. J Crohns Colitis. 2013;7:427‐428. [DOI] [PubMed] [Google Scholar]
- 109. Li J, Chong AH, Green J, Kelly R, Baker C. Mycophenolate use in dermatology: a clinical audit. Australas J Dermatol. 2013;54:296‐302. [DOI] [PubMed] [Google Scholar]
- 110. Krüger NA, De Marchi JJ, de Souza MM. Biological therapy for pyoderma gangrenosum. J Coloproctol. 2013;33:232‐235. [Google Scholar]
- 111. Ito T, Sato N, Yamazaki H, Koike T, Emura I, Saeki T. A case of aseptic abscesses syndrome treated with corticosteroids and TNF‐alpha blockade. Mod Rheumatol. 2013;23:195‐199. [DOI] [PubMed] [Google Scholar]
- 112. Hasegawa M, Nagai Y, Sogabe Y, et al. Clinical analysis of leg ulcers and gangrene in rheumatoid arthritis. J Dermatol. 2013;40:949‐954. [DOI] [PubMed] [Google Scholar]
- 113. Gross M, Ben‐Chetrit E. Laryngeal involvement in Behcet's disease‐a challenge for treatment. Clin Rheumatol. 2013;32:75‐77. [DOI] [PubMed] [Google Scholar]
- 114. Freedberg DE, Husain S, Swaminath A. Education and imaging. Gastrointestinal: severe inflammatory bowel disease‐associated pyoderma gangrenosum. J Gastroenterol Hepatol. 2013;28:1691. [DOI] [PubMed] [Google Scholar]
- 115. Fakhar F, Memon S, Deitz D, Abramowitz R, Alpert DR. Refractory postsurgical pyoderma gangrenosum in a patient with Beckwith Wiedemann syndrome: response to multimodal therapy. BMJ Case Rep. 2013;2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Andrisani G, Guidi L, Papa A, Potenza AE, Cervelli D, Armuzzi A. A case of pyoderma gangrenosum with ulcerative colitis treated with combined approach: infliximab and surgery. J Crohns Colitis. 2013;7:421‐426. [DOI] [PubMed] [Google Scholar]
- 117. Williamson KD, Nguyen NQ. A large shin ulcer after minor trauma: please do not debride! Gastroenterology. 2012;143:e11‐e12. [DOI] [PubMed] [Google Scholar]
- 118. Walters J, Glover S. IVIG treatment for refractory pyoderma gangrenosum in patients with inflammatory bowel disease. Am J Gastroenterol. 2012;107:S503. [Google Scholar]
- 119. Ueda M, Katoh M, Tanizaki H, Tanioka M, Matsumura Y, Miyachi Y. Refractory pyoderma gangrenosum associated with ulcerative colitis successfully treated with infliximab. Dermatol Online J. 2012;18:12. [PubMed] [Google Scholar]
- 120. Suarez‐Perez JA, Herrera‐Acosta E, Lopez‐Navarro N, et al. Pyoderma gangrenosum: a report of 15 cases and review of the literature. Actas Dermosifiliogr. 2012;103:120‐126. [DOI] [PubMed] [Google Scholar]
- 121. Sinagra E, Orlando A, Renna S, Maida M, Cottone M. Multifocal pyoderma gangrenosum resistant to infliximab in active ulcerative colitis: don't forget the role of cyclosporin. Inflamm Bowel Dis. 2012;18:E1594‐E1595. [DOI] [PubMed] [Google Scholar]
- 122. Shareef MS, Munro LR, Owen RG, Highet AS. Progression of IgA gammopathy to myeloma following infliximab treatment for pyoderma gangrenosum. Clin Exp Dermatol. 2012;37:146‐148. [DOI] [PubMed] [Google Scholar]
- 123. Kroshinsky D, Hoang MP, Hasserjian RP. Case records of the Massachusetts General Hospital. case 1‐2012. An 82‐year‐old man with persistent ulcers on the hands. N Engl J Med. 2012;366:166‐174. [DOI] [PubMed] [Google Scholar]
- 124. Kim YH, Park JH, Choi CW, Lee GY, Kim WS. Pustular pyoderma gangrenosum associated with ulcerative colitis. Kor J Dermatol. 2012;50:1050‐1053. [Google Scholar]
- 125. Kim FS, Pandya AG. The use of etanercept in the treatment of peristomal pyoderma gangrenosum. Clin Exp Dermatol. 2012;37:442‐443. [DOI] [PubMed] [Google Scholar]
- 126. Kakagia D, Efremidou E, Lyratzopoulos N, Mitrakas A, Pitiakoudis M, Kouklakis G. Crohn's disease associated pyoderma gangrenosum treated with adalimumab. Balkan Med J. 2012;29:93‐95. [Google Scholar]
- 127. Huang B, Melmed GY, Shih DQ. Facial ulceration in a patient with Crohn's disease. Gastroenterology. 2012;142:1071‐1258. [DOI] [PubMed] [Google Scholar]
- 128. Hinterberger L, Muller CS, Vogt T, Pfohler C. Adalimumab: a treatment option for pyoderma gangrenosum after failure of systemic standard therapies. Dermatol Ther (Heidelb). 2012;2:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Hayashi H, Kuwabara C, Tarumi K, Makino E, Fujimoto W. Successful treatment with infliximab for refractory pyoderma gangrenosum associated with inflammatory bowel disease. J Dermatol. 2012;39:576‐578. [DOI] [PubMed] [Google Scholar]
- 130. Guedes R, Moreira A, Menezes N, Baptista A, Varela P. Treatment of thalidomide resistant pyoderma gangrenosum with etenercept. Acta Dermatovenerol Croat. 2012;20:175‐180. [PubMed] [Google Scholar]
- 131. Goldminz AM, Botto NC, Gottlieb AB. Severely recalcitrant pyoderma gangrenosum successfully treated with ustekinumab. J Am Acad Dermatol. 2012;67:e237‐e238. [DOI] [PubMed] [Google Scholar]
- 132. Durmaz Y, Bilgici A, Cil EE, Kuru O. Infliximab treatment in resistant pyoderma gangrenosum: a case report. Turkiye Fiziksel Tip ve Rehabilitasyon Dergisi. 2012;58:332‐334. [Google Scholar]
- 133. Del Giacco SR, Firinu D, Lorrai MM, et al. Idiopathic pyoderma gangrenosum: successful resolution with infliximab therapy and pro‐inflammatory cytokines assessment. Acta Derm Venereol. 2012;92:439‐440. [DOI] [PubMed] [Google Scholar]
- 134. Cordero‐Coma M, Perez‐Moreiras JV, Toribio A, et al. Refractory pyoderma gangrenosum of the orbit and the lacrimal sac. Orbit. 2012;31:249‐251. [DOI] [PubMed] [Google Scholar]
- 135. Choi SD, Lowe P, Weninger W. Successful infliximab therapy for hidradenitis suppurativa and pyoderma gangrenosum, complicated by development of palmo‐plantar pustular psoriasis: case report and literature review. Australas J Dermatol. 2012;53:31. [Google Scholar]
- 136. Carrasco Cubero C, Ruiz Tudela MM, Salaberri Maestrojuan JJ, Perez Venegas JJ. Pyoderma gangrenosum associated with inflammatory bowel disease. Report of two cases with good response to infliximab. Reumatol Clin. 2012;8:90‐92. [DOI] [PubMed] [Google Scholar]
- 137. Bruzzese V. Pyoderma gangrenosum, acne conglobata, suppurative hidradenitis, and axial spondyloarthritis: efficacy of anti‐tumor necrosis factor alpha therapy. J Clin Rheumatol. 2012;18:413‐415. [DOI] [PubMed] [Google Scholar]
- 138. Bhatti H, Khalid N, Rao B. Superficial pyoderma gangrenosum treated with infliximab: a case report. Cutis. 2012;90:297‐299. [PubMed] [Google Scholar]
- 139. Zaidan M, Lidove O, Sacre K, Klein I, Papo T. "Fulminant" Behcet disease. Presse Med. 2011;40:1087‐1089. [DOI] [PubMed] [Google Scholar]
- 140. Thornton K. A threat to life and limb: a case of sepsis and osteomyelitis in a patient with undiagnosed pyoderma gangrenosum. J Am Geriatr Soc. 2011;59:S113. [Google Scholar]
- 141. Teich N. Infliximab anaphylaxis in siblings. Inflamm Bowel Dis. 2011;17:E108. [DOI] [PubMed] [Google Scholar]
- 142. Rudolph B, Groffik A, Müller‐Brenne T, Von Stebut E, Grabbe S, Loquai C. Severe colitis with pyoderma gangrenosum after ipilimumab treatment in a melanoma patient with colostomy‐a therapeutic challenge. JDDG. 2011;9:788. [Google Scholar]
- 143. Ricketts JR, Rothe MJ, Grant‐Kels JM. Cutaneous simulants of infectious disease. Int J Dermatol. 2011;50:1043‐1057. [DOI] [PubMed] [Google Scholar]
- 144. Mazokopakis EE, Kofteridis DP, Pateromihelaki AT, Vytiniotis SD, Karastergiou PG. Improvement of ulcerative pyoderma gangrenosum with hyperbaric oxygen therapy. Dermatol Ther. 2011;24:134‐136. [DOI] [PubMed] [Google Scholar]
- 145. Lin Z, Hegarty JP, Lin T, et al. Failure of anakinra treatment of pyoderma gangrenosum in an IBD patient and relevance to the PSTPIP1 gene. Inflamm Bowel Dis. 2011;17:E41‐E42. [DOI] [PubMed] [Google Scholar]
- 146. Kleinpenning MM, Langewouters AM, Van De Kerkhof PC, Greebe RJ. Severe pyoderma gangrenosum unresponsive to etanercept and adalimumab. J Dermatolog Treat. 2011;22:261‐265. [DOI] [PubMed] [Google Scholar]
- 147. Hanafusa T, Azukizawa H, Umegaki N, Tani M, Yamaguchi Y, Katayama I. Clinical implications of leukocytapheresis using a centrifugal cell separator for steroid‐resistant pyoderma gangrenosum associated with inflammatory bowel disease. J Dermatol. 2011;38:507‐510. [DOI] [PubMed] [Google Scholar]
- 148. Goldshmid O, Dovorish Z, Zehavi T, Eisen A, Bar‐Dayan Y, Amital H. Coexistent pyoderma gangrenosum and tibialis anterior myositis as presenting manifestations of Crohn's disease: case report and review of the literature. Rheumatol Int. 2011;31:525‐527. [DOI] [PubMed] [Google Scholar]
- 149. Duchini G, Itin P, Arnold A. A case of refractory pyoderma gangrenosum treated with a combination of Apligraf and systemic immunosuppressive agents. Adv Skin Wound Care. 2011;24:217‐220. [DOI] [PubMed] [Google Scholar]
- 150. Carinanos I, Acosta MB, Domenech E. Adalimumab for pyoderma gangrenosum associated with inflammatory bowel disease. Inflamm Bowel Dis. 2011;17:E153‐E154. [DOI] [PubMed] [Google Scholar]
- 151. Bennett M, McAleer MA, Murphy M, Harney S, Bourke JF. Novel combination of intravenous immunoglobulin and rituximab in the treatment of recalcitrant pyoderma gangrenosum. Br J Dermatol. 2011;165:35.21443534 [Google Scholar]
- 152. Tofteland ND, Shaver TS. Clinical efficacy of etanercept for treatment of PAPA syndrome. J Clin Rheumatol. 2010;16:244‐245. [DOI] [PubMed] [Google Scholar]
- 153. Tada M, Nakanishi T, Hirata C, et al. Use of infliximab in a patient with pyoderma gangrenosum and rheumatoid arthritis. Mod Rheumatol. 2010;20:598‐601. [DOI] [PubMed] [Google Scholar]
- 154. Ryan A, Sheahan K, Kirby B. Parastomal pyoderma gangrenosum successfully treated with adalimumab. Br J Dermatol. 2010;163:94. [Google Scholar]
- 155. Reddick CL, Singh MN, Chalmers RJ. Successful treatment of superficial pyoderma gangrenosum associated with hidradenitis suppurativa with adalimumab. Dermatol Online J. 2010;16:15. [PubMed] [Google Scholar]
- 156. Perez‐De Pedro I, Gomez‐Moyano E, Lopez‐Carmona D, Munoz‐Roca NL. De Ramon‐Garrido E, camps‐Garcia MT: [utility of infliximab in gangrenous pyoderma not associated with inflammatory bowel disease]. Rev Clin Esp. 2010;210:367‐369. [DOI] [PubMed] [Google Scholar]
- 157. Hsiao JL, Antaya RJ, Berger T, Maurer T, Shinkai K, Leslie KS. Hidradenitis suppurativa and concomitant pyoderma gangrenosum: a case series and literature review. Arch Dermatol. 2010;146:1265‐1270. [DOI] [PubMed] [Google Scholar]
- 158. Goshtasby PH, Chami RG, Johnson RM. A novel approach to the management of pyoderma gangrenosum complicating reduction mammaplasty. Aesthet Surg J. 2010;30:186‐193. [DOI] [PubMed] [Google Scholar]
- 159. Baglieri F, Scuderi G. Therapeutic hotline. Infliximab for treatment of resistant pyoderma gangrenosum associated with ulcerative colitis and psoriasis. a case report. Dermatol Ther. 2010;23:541‐543. [DOI] [PubMed] [Google Scholar]
- 160. Zold E, Nagy A, Devenyi K, Zeher M, Barta Z. Successful use of adalimumab for treating fistulizing Crohn's disease with pyoderma gangrenosum: two birds with one stone. World J Gastroenterol. 2009;15:2293‐2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Wolbing F, Fierlbeck G, Hotzenecker W, Schaller M, Rocken M. Septic shock after treatment of pyoderma gangrenosum with infliximab. Acta Derm Venereol. 2009;89:93‐94. [DOI] [PubMed] [Google Scholar]
- 162. Rispo A, Testa A, Diaferia M, Castiglione F, Lo Presti M. Monster parastomal pyoderma gangrenosum effectively treated by topical tacrolimus. J Crohns Colitis. 2009;3:218‐219. [DOI] [PubMed] [Google Scholar]
- 163. Jung HD, Chi SG, Kim BS, Lee WJ, Lee SJ, Kim DW. A case of recalcitrant pyoderma gangrenosum treated by infliximab. Kor J Dermatol. 2009;47:343‐346. [Google Scholar]
- 164. English M, Elghannam H, Zaman T. A case of pyoderma gangrenosum of the lung. Chest. 2009;136:195. [Google Scholar]
- 165. Eaton PA, Callen JP. Mycophenolate mofetil as therapy for pyoderma gangrenosum. Arch Dermatol. 2009;145:781‐785. [DOI] [PubMed] [Google Scholar]
- 166. Alkhouri N, Hupertz V, Mahajan L. Adalimumab treatment for peristomal pyoderma gangrenosum associated with Crohn's disease. Inflamm Bowel Dis. 2009;15:803‐806. [DOI] [PubMed] [Google Scholar]
- 167. Akhras V, Sarkany R, Walsh S, Hyde N, Marsden RA. Superficial granulomatous pyoderma treated preoperatively with infliximab. Clin Exp Dermatol. 2009;34:e183‐e185. [DOI] [PubMed] [Google Scholar]
- 168. Schwartzfarb EM, Weir D, Conlan WA, Romanelli P, Kirsner RS. Pyoderma gangrenosum in a patient with Bruton's X‐linked Agammaglobulinemia: shared pathogenesis of altered tumor necrosis factor alpha? J Clin Aesthet Dermatol. 2008;1:26‐29. [PMC free article] [PubMed] [Google Scholar]
- 169. Rogge FJ, Pacifico M, Kang N. Treatment of pyoderma gangrenosum with the anti‐TNFalpha drug—etanercept. J Plast Reconstr Aesthet Surg. 2008;61:431‐433. [DOI] [PubMed] [Google Scholar]
- 170. Roche E, Martinez‐Menchon T, Sanchez‐Carazo JL, Oliver V, Alegre de Miquel V. Two cases of eruptive pyoderma gangrenosum associated with cocaine use. Actas Dermosifiliogr. 2008;99:727‐730. [PubMed] [Google Scholar]
- 171. Poritz LS, Lebo MA, Bobb AD, Ardell CM, Koltun WA. Management of peristomal pyoderma gangrenosum. J Am Coll Surg. 2008;206:311‐315. [DOI] [PubMed] [Google Scholar]
- 172. Marzano AV, Tourlaki A, Alessi E, Caputo R. Widespread idiopathic pyoderma gangrenosum evolved from ulcerative to vegetative type: a 10‐year history with a recent response to infliximab. Clin Exp Dermatol. 2008;33:156‐159. [DOI] [PubMed] [Google Scholar]
- 173. Kreuter A, Reich‐Schupke S, Stucker M, Altmeyer P, Gambichler T. Intravenous immunoglobulin for pyoderma gangrenosum. Br J Dermatol. 2008;158:856‐857. [DOI] [PubMed] [Google Scholar]
- 174. Jacob SE, Weisman RS, Kerdel FA. Pyoderma gangrenosum—rebel without a cure? Int J Dermatol. 2008;47:192‐194. [DOI] [PubMed] [Google Scholar]
- 175. Field S, Powell FC, Young V, Barnes L. Pyoderma gangrenosum manifesting as a cavitating lung lesion. Clin Exp Dermatol. 2008;33:418‐421. [DOI] [PubMed] [Google Scholar]
- 176. Fernandez A, Velasco A, Prieto V, Canueto J, Alvarez A, Rodriguez A. Response to infliximab in atypical pyoderma gangrenosum associated with ulcerative colitis. Am J Gastroenterol. 2008;103:2951‐2952. [DOI] [PubMed] [Google Scholar]
- 177. Ermis F, Ozdil S, Akyuz F, Pinarbasi B, Mungan Z. Pyoderma gangrenosum treated with infliximab in inactive ulcerative colitis. Inflamm Bowel Dis. 2008;14:1611‐1613. [DOI] [PubMed] [Google Scholar]
- 178. Adisen E, Oztas M, Gurer MA. Treatment of idiopathic pyoderma gangrenosum with infliximab: induction dosing regimen or on‐demand therapy? Dermatology. 2008;216:163‐165. [DOI] [PubMed] [Google Scholar]
- 179. Vandevyvere K, Luyten FP, Verschueren P, Lories R, Segaert S, Westhovens R. Pyoderma gangrenosum developing during therapy with TNF‐alpha antagonists in a patient with rheumatoid arthritis. Clin Rheumatol. 2007;26:2205‐2206. [DOI] [PubMed] [Google Scholar]
- 180. Pomerantz RG, Husni ME, Mody E, Qureshi AA. Adalimumab for treatment of pyoderma gangrenosum. Br J Dermatol. 2007;157:1274‐1275. [DOI] [PubMed] [Google Scholar]
- 181. Neesse A, Michl P, Kunsch S, Ellenrieder V, Gress TM, Steinkamp M. Simultaneous onset of ulcerative colitis and disseminated pyoderma gangrenosum. Case Rep Gastroenterol. 2007;1:110‐115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Moschella SL. Is there a role for infliximab in the current therapy of hidradenitis suppurativa? A report of three treated cases. Int J Dermatol. 2007;46:1287‐1291. [DOI] [PubMed] [Google Scholar]
- 183. Juillerat P, Christen‐Zach S, Troillet FX, Gallot‐Lavallee S, Pannizzon RG, Michetti P. Infliximab for the treatment of disseminated pyoderma gangrenosum associated with ulcerative colitis. Case report and literature review. Dermatology. 2007;215:245‐251. [DOI] [PubMed] [Google Scholar]
- 184. Hewitt D, Tait C. Use of infliximab in pyoderma gangrenosum. Australas J Dermatol. 2007;48:95‐98. [DOI] [PubMed] [Google Scholar]
- 185. Heffernan MP, Anadkat MJ, Smith DI. Adalimumab treatment for pyoderma gangrenosum. Arch Dermatol. 2007;143:306‐308. [DOI] [PubMed] [Google Scholar]
- 186. Dini V, Romanelli M, Bertone M, Talarico S, Bombardieri S, Barachini P. Improvement of idiopathic pyoderma gangrenosum during treatment with anti‐tumor necrosis factor alfa monoclonal antibody. Int J Low Extrem Wounds. 2007;6:108‐113. [DOI] [PubMed] [Google Scholar]
- 187. De la Morena F, Martin L, Gisbert JP, Fernandez Herrera J, Goiriz R. Refractory and infected pyoderma gangrenosum in a patient with ulcerative colitis: response to infliximab. Inflamm Bowel Dis. 2007;13:509‐510. [DOI] [PubMed] [Google Scholar]
- 188. Cocco A, Angelucci E, Viscido A, Caprilli R. Successful treatment with infliximab of refractory pyoderma gangrenosum in 2 patients with inflammatory bowel diseases. Inflamm Bowel Dis. 2007;13:1317‐1319. [DOI] [PubMed] [Google Scholar]
- 189. Charles CA, Leon A, Banta MR, Kirsner RS. Etanercept for the treatment of refractory pyoderma gangrenosum: a brief series. Int J Dermatol. 2007;46:1095‐1099. [DOI] [PubMed] [Google Scholar]
- 190. Castro‐Fernandez M, Sanchez‐Munoz D, Ruiz‐Granados E, Merchante N, Corzo J. Coexistence of pyoderma gangrenosum and Sweet's syndrome in a patient with ulcerative colitis. Am J Gastroenterol. 2007;102:2865‐2866. [DOI] [PubMed] [Google Scholar]
- 191. Shanks KP, Popek EJ, Abramson SL. Treatment of pyoderma gangrenosum (PG) with infliximab in leukocyte adhesion deficiency (LAD) type 1. J Allergy Clin Immunol. 2006;117:S282‐S283. [Google Scholar]
- 192. Roy DB, Conte ET, Cohen DJ. The treatment of pyoderma gangrenosum using etanercept. J Am Acad Dermatol. 2006;54:S128‐S134. [DOI] [PubMed] [Google Scholar]
- 193. Pitarch G, Torrijos A, Mahiques L, Sanchez‐Carazo JL, Fortea JM. Systemic absorption of topical tacrolimus in pyoderma gangrenosum. Acta Derm Venereol. 2006;86:64‐65. [DOI] [PubMed] [Google Scholar]
- 194. Pierce M, Rice M, Fellows J. Wet colostomy and peristomal skin breakdown. J Wound Ostomy Continence Nurs. 2006;33:541‐546. discussion 546–548. [DOI] [PubMed] [Google Scholar]
- 195. Pastor N, Betlloch I, Pascual JC, Blanes M, Banuls J, Silvestre JF. Pyoderma gangrenosum treated with anti‐TNF alpha therapy (etanercept). Clin Exp Dermatol. 2006;31:152‐153. [DOI] [PubMed] [Google Scholar]
- 196. Marks DJ, Rahman FZ, Novelli M, et al. An exuberant inflammatory response to E coli: implications for the pathogenesis of ulcerative colitis and pyoderma gangrenosum. Gut. 2006;55:1662‐1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Fonder MA, Cummins DL, Ehst BD, Anhalt GJ, Meyerle JH. Adalimumab therapy for recalcitrant pyoderma gangrenosum. J Burns Wounds. 2006;5:e8. [PMC free article] [PubMed] [Google Scholar]
- 198. Ferkolj I, Hocevar A, Golouh R, Dolenc Voljc M. Infliximab for treatment of resistant pyoderma gangrenosum associated with Crohn's disease. Acta Dermatovenerol Alp Pannonica Adriat. 2006;15:173‐177. [PubMed] [Google Scholar]
- 199. Drinda S, Oelzner P, Codina Canet C, Kaatz M, Wolf G, Hein G. Fatal outcome of pyoderma gangrenosum with multiple organ involvement and partially responding to infliximab. Cent Eur J Med. 2006;1:306‐312. [Google Scholar]
- 200. Uthman I, El‐Sayad J, Sharara A. Successful treatment of recalcitrant pyoderma gangrenosum with infliximab complicated by tuberculosis despite negative screening tests. Clin Exp Dermatol. 2005;30:294. [DOI] [PubMed] [Google Scholar]
- 201. Tai YJ, Kelly R. Pyoderma gangrenosum complicated by herpes simplex virus infection. Australas J Dermatol. 2005;46:161‐164. [DOI] [PubMed] [Google Scholar]
- 202. Swale VJ, Saha M, Kapur N, Hoffbrand AV, Rustin MH. Pyoderma gangrenosum outside the context of inflammatory bowel disease treated successfully with infliximab. Clin Exp Dermatol. 2005;30:134‐136. [DOI] [PubMed] [Google Scholar]
- 203. Rispo A, Scarpa R, Di Girolamo E, et al. Infliximab in the treatment of extra‐intestinal manifestations of Crohn's disease. Scand J Rheumatol. 2005;34:387‐391. [DOI] [PubMed] [Google Scholar]
- 204. Levy D, Banta MR, Kirsner RS. Refractory pyoderma gangrenosum peristomal ulcer and sinus tract treated with micronized cadaveric dermis. J Am Acad Dermatol. 2005;52:1104. [DOI] [PubMed] [Google Scholar]
- 205. Krag AA, Gjersoe P. Treatment with infliximab of peristomal pyoderma gangrenosum in ulcerative colitis. Ugeskr Laeger. 2005;167:1968‐1969. [PubMed] [Google Scholar]
- 206. Kouklakis G, Moschos J, Leontiadis GI, et al. Infliximab for treatment of pyoderma gangrenosum associated with clinically inactive Crohn's disease. A case report. Rom J Gastroenterol. 2005;14:401‐403. [PubMed] [Google Scholar]
- 207. Kaur MR, Lewis HM. Severe recalcitrant pyoderma gangrenosum treated with infliximab. Br J Dermatol. 2005;153:689‐691. [DOI] [PubMed] [Google Scholar]
- 208. Kaufman I, Caspi D, Yeshurun D, Dotan I, Yaron M, Elkayam O. The effect of infliximab on extraintestinal manifestations of Crohn's disease. Rheumatol Int. 2005;25:406‐410. [DOI] [PubMed] [Google Scholar]
- 209. Hubbard VG, Friedmann AC, Goldsmith P. Systemic pyoderma gangrenosum responding to infliximab and adalimumab. Br J Dermatol. 2005;152:1059‐1061. [DOI] [PubMed] [Google Scholar]
- 210. Goldenberg G, Jorizzo JL. Use of etanercept in treatment of pyoderma gangrenosum in a patient with autoimmune hepatitis. J Dermatolog Treat. 2005;16:347‐349. [DOI] [PubMed] [Google Scholar]
- 211. George B, Brown P, Perrin A, Travis S, Mortensen NJ. Peristomal pyoderma gangrenosum: clinical features and response to infliximab therapy. Dis Colon Rectum. 2005;48:666‐666. [Google Scholar]
- 212. Singh M, Andrew SM, Lear JT. Infliximab as a treatment for recalcitrant pyoderma gangrenosum. Clin Exp Dermatol. 2004;29:196‐197. [DOI] [PubMed] [Google Scholar]
- 213. Sapienza MS, Cohen S, Dimarino AJ. Treatment of pyoderma gangrenosum with infliximab in Crohn's disease. Dig Dis Sci. 2004;49:1454‐1457. [DOI] [PubMed] [Google Scholar]
- 214. Lopez San Roman A, Bermejo F, Aldanondo I, Carrera E, Boixeda D, Munoz Zato E. Pyoderma gangrenosum associated with ulcerative colitis: response to infliximab. Rev Esp Enferm Dig. 2004;96:420‐422. [DOI] [PubMed] [Google Scholar]
- 215. Lawrance IC. Infliximab in the management of the extra‐intestinal manifestations of Crohn's disease. J Gastroenterol Hepatol. 2004;19:1332‐1333. [DOI] [PubMed] [Google Scholar]
- 216. Jenne L, Sauter B, Thumann P, Hertl M, Schuler G. Successful treatment of therapy‐resistant chronic vegetating pyoderma gangrenosum with infliximab (chimeric antitumour necrosis factor antibody). Br J Dermatol. 2004;150:380‐382. [DOI] [PubMed] [Google Scholar]
- 217. Disla E, Quayum B, Cuppari GG, Pancorbo R. Successful use of etanercept in a patient with pyoderma gangrenosum complicating rheumatoid arthritis. J Clin Rheumatol. 2004;10:50‐52. [DOI] [PubMed] [Google Scholar]
- 218. Coelho S, Amarelo M, Ryan S, Reddy M, Sibbald RG. Rheumatoid arthritis‐associated inflammatory leg ulcers: a new treatment for recalcitrant wounds. Int Wound J. 2004;1:81‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Regueiro M, Valentine J, Plevy S, Fleisher MR, Lichtenstein GR. Infliximab for treatment of pyoderma gangrenosum associated with inflammatory bowel disease. Am J Gastroenterol. 2003;98:1821‐1826. [DOI] [PubMed] [Google Scholar]
- 220. Mimouni D, Anhalt GJ, Kouba DJ, Nousari HC. Infliximab for peristomal pyoderma gangrenosum. Br J Dermatol. 2003;148:813‐816. [DOI] [PubMed] [Google Scholar]
- 221. Geren SM, Kerdel FA, Falabella AF, Kirsner RS. Infliximab: a treatment option for ulcerative pyoderma gangrenosum. Wounds. 2003;15:49‐53. [Google Scholar]
- 222. Finkelstein W. Treatment of peristomal pyoderma gangrenosum associated with Crohn's disease with infliximab. Am J Gastroenterol. 2003;98:S175‐S175. [Google Scholar]
- 223. Triantafillidis JK, Cheracakis P, Sklavaina M, Apostolopoulou K. Favorable response to infliximab treatment in a patient with active Crohn disease and pyoderma gangrenosum. Scand J Gastroenterol. 2002;37:863‐865. [PubMed] [Google Scholar]
- 224. Romero‐Gomez M, Sanchez‐Munoz D. Infliximab induces remission of pyoderma gangrenosum. Eur J Gastroenterol Hepatol. 2002;14:907. [DOI] [PubMed] [Google Scholar]
- 225. Ljung T, Staun M, Grove O, Fausa O, Vatn MH, Hellstrom PM. Pyoderma gangrenosum associated with crohn disease: effect of TNF‐alpha blockade with infliximab. Scand J Gastroenterol. 2002;37:1108‐1110. [DOI] [PubMed] [Google Scholar]
- 226. Grange F, Djilali‐Bouzina F, Weiss AM, Polette A, Guillaume JC. Corticosteroid‐resistant pyoderma gangrenosum associated with Crohn's disease: rapid cure with infliximab. Dermatology. 2002;205:278‐280. [DOI] [PubMed] [Google Scholar]
- 227. Foster EN, Nguyen KK, Bolce RJ, Prindiville TP. Cutaneous manifestations of inflammatory bowel disease improve with infliximab therapy. Gastroenterology. 2002;122:A618‐A618. [Google Scholar]
- 228. Tan MH, Gordon M, Lebwohl O, George J, Lebwohl MG. Improvement of pyoderma gangrenosum and psoriasis associated with Crohn disease with anti‐tumor necrosis factor alpha monoclonal antibody. Arch Dermatol. 2001;137:930‐933. [PubMed] [Google Scholar]
- 229. Sheldon D, Thirlby RC, Kozarek R. Peristomal pyoderma gangrenosum. J Am Coll Surg. 2001;193:703. [DOI] [PubMed] [Google Scholar]
- 230. Hong JJ, Merel NH, Hanauer SB. Treatment of pyoderma gangrenosum (PG) complicating Crohn's disease (CD) with infliximab. Gastroenterology. 2001;120:A621‐A621. [Google Scholar]
- 231. Sheldon DG, Sawchuk LL, Kozarek RA, Thirlby RC. Twenty cases of peristomal pyoderma gangrenosum: diagnostic implications and management. Arch Surg. 2000;135:564‐568. discussion 568–569. [DOI] [PubMed] [Google Scholar]
- 232. Hughes AP, Jackson JM, Callen JP. Clinical features and treatment of peristomal pyoderma gangrenosum. JAMA. 2000;284:1546‐1548. [DOI] [PubMed] [Google Scholar]
- 233. Arguelles‐Arias F, Castro‐Laria L, Lobaton T, et al. Characteristics and treatment of pyoderma gangrenosum in inflammatory bowel disease. Dig Dis Sci. 2013;58:2949‐2954. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Modified PRISMA flow diagram depicting the study selection process.
Table S1. Search history.
Table S2. Data of the included patients.
Table S3. The excluded articles with reason(s) for exclusion. Articles could be excluded for more than one reason. Primary patients were defined as patients not previously reported in another publication.


