Abstract
Aim
This study evaluated the clinicopathological findings of acute/active antibody-mediated rejection (AABMR) according to the Banff 2013 classification.
Methods
We analyzed 345 biopsies of 269 kidney transplant recipients. Pathological AABMR (PAABMR) was defined as histological evidence of acute tissue injury and endothelial injury by light microscopy regardless of donor-specific antibodies (DSAs).
Results
Among the 345 biopsies, 29 (8.4%) were diagnosed as PAABMR. The mean g score was 1.17 ± 0.60, the mean ptc score was 1.97 ± 1.32, and DSA positivity was found in 69% of PAABMR. The mean duration after transplantation was 22.9 ± 26.7 months. Among 3 groups (DSA-high, mean fluorescence intensity (MFI) ≥ 5,000; DSA-low, MFI < 5,000 to ≥1,000; below cutoff), ABO incompatibility in DSA-high was significantly lower and second transplantation in DSA-high was significantly higher. We found 83% of PAABMR by the protocol biopsy (subclinical AABMR [SAABMR]). The short-term clinical and light microscopical changes in 8 cases of SAABMR did not show worsening during follow-up period (9–24 months). However, ultrastructural finding, including glomerular endothelial swelling, subendothelial electron-lucent widening, and early glomerular basement duplication, were found by electron microscopy (EM) in the first biopsies, and half of the SAABMR cases developed de novo circular peritubular capillary multilayering in the follow-up biopsies.
Conclusion
PAABMR was mainly found by the protocol biopsy. The short-term follow-up of SAABMR patients did not show worsening clinically and light microscopically, but ultrastructural examination by EM was useful to detect early lesions of endothelial injury and progression of glomerular and peritubular capillary basement membrane alterations.
Keywords: Acute/active antibody-mediated rejection, Allograft, Banff 2013 classification, Electron microscopy, Kidney transplantation
Introduction
The pathological diagnostic criteria for acute/active antibody-mediated rejection (AABMR) were revised at the Banff 2013 meeting [1]. According to this meeting report, histological evidence of acute tissue injury, evidence of an interaction between antibodies and the vascular endothelium, and serological evidence of donor-specific antibodies (DSAs) are essential for the diagnosis of AABMR. In addition, evaluation by both electron microscopy (EM) and light microscopy (LM) is required to exclude chronic lesions in patients with chronic antibody-mediated rejection [1].
Haas and Mirocha [2] reported a trend toward a lower incidence of developing overt transplant glomerulopathy in recipients who were treated for moderate microvascular inflammation (g + ptc ≥ 2) in the presence of DSAs than in those who were untreated. In another study, recipients with antibody-mediated rejection showed a significantly higher DSA mean fluorescence intensity (MFI) [3]. However, the MFI cutoff value for DSAs is not defined in the Banff 2013 classification [1]. Several reports have argued the significance of EM evaluation to diagnose AABMR. Wavamunno et al. [4] reported that endothelial and subendothelial ultrastructural abnormalities in glomeruli are early markers of transplant glomerulopathy. Haas and Mirocha [2] reported that ultrastructural changes, including glomerular endothelial swelling, subendothelial widening, and early glomerular basement membrane (GBM) duplication, were detected in biopsy specimens (BS) of antibody-mediated rejection patients within 3 months. At the Banff 2013 meeting, evaluation of early chronic cg lesions by EM was determined to be required [1]. However, the relationship between EM and clinical findings has not been fully elucidated. The aim of this study was to evaluate the clinicopathological features and activity of AABMR and subsequent early chronic lesions, like duplication of GBM and multilayering of peritubular capillary basement membrane (PTCBM), according to the Banff 2013 classification [1].
Materials and Methods
Patients
Protocol biopsies at Toho University Omori Medical Center are usually performed at 1 hour, 3 months, 1 year, 3 years, and 7 years, and, for some recipients, 10 years after transplantation. We cross-sectionally analyzed 345 BS of 269 kidney transplant recipients performed at Toho University Omori Medical Center from January 2016 to December 2017. Among the recipients diagnosed with subclinical AABMR (SAABMR) by the protocol biopsy from January 2016 to December 2017, we evaluated the clinical and histological changes of recipients in whom follow-up BS evaluable by EM was performed until June 2018. The basic regimen of immunosuppressive therapy at Toho University Omori Medical Center included methylprednisolone, mycophenolate mofetil or azathioprine, tacrolimus (Tac) or cyclosporine (CsA), basiliximab, and anti-thymocyte globulin for preformed DSA cases.
Histopathology
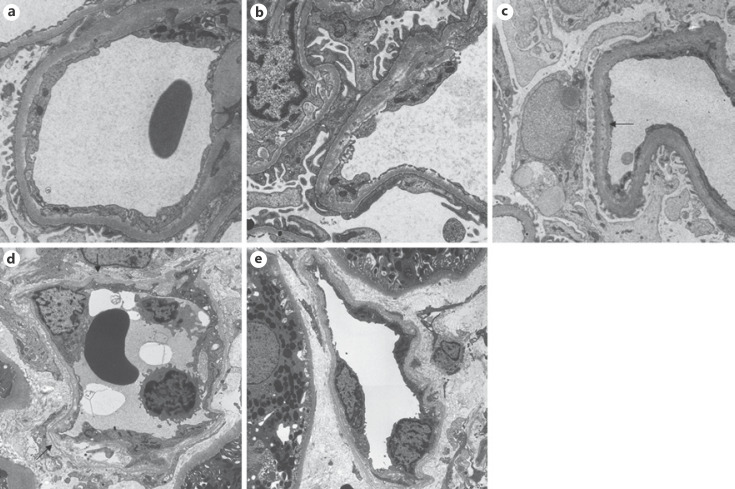
Histological evaluation of allograft biopsies at Toho University Omori Medical Center was performed by hematoxylin and eosin, periodic acid-Schiff, periodic acid methenamine silver, and Masson trichrome staining. Histological evidence of acute tissue injury, evidence of an interaction between antibodies and the vascular endothelium, and serological evidence of DSAs were essential to diagnose AABMR according to the Banff 2013 classification, and evaluation by both EM and LM was required to exclude early chronic lesions like GBM duplication and multilayering of PTCBM with chronic antibody-mediated rejection [1]. In the present study, we defined a biopsy finding of AABMR (pathological AABMR [PAABMR]) as histological evidence of acute tissue injury and endothelial injury (microvascular inflammation identified by LM or C4d deposition in peritubular capillaries identified by immunohistochemistry). We defined a biopsy finding of PAABMR as relevant findings by only LM regardless of the presence of DSAs. The presence of DSAs was determined using the LABScreen Single Antigen test. We defined an MFI of ≥1,000 as DSA positivity in accordance with the cutoff value used by Toho University Omori Medical Center. Immunohistochemistry of C4d was performed on frozen tissue using an anti-C4d antibody (Quidel, Athens, OH, USA). The tissue was fixed in 2.5% glutaraldehyde and embedded in epoxy resin for EM. Semi-thin sections were stained with toluidine blue, and the best sections containing both the glomerulus and peritubular capillaries were subjected to ultrathin sectioning. We analyzed glomerular endothelial swelling, subendothelial electron-lucent widening, early GBM duplication, partial PTCBM multilayering, and circumferential PTCBM multilayering using EM [2]. The following findings were examined as present or absent: glomerular endothelial swelling, defined as a solid mass of endothelial cell cytoplasm covering the GBM by ≥3 quarters of the 1 capillary circumference in at least 3 glomerular capillaries (Fig. 1a); subendothelial electron-lucent widening in at least 3 glomerular capillaries (Fig. 1b); early GBM duplication in at least 3 glomerular capillaries (Fig. 1c); partial PTCBM multilayering, defined as 4 or more partial basement membrane layers (Fig. 1d); circumferential PTCBM multilayering defined as 4 or more circumferential basement membrane layers (Fig. 1e).
Fig. 1.
Representative images evaluated by EM show glomerular endothelial cell swelling (a), subendothelial electron-lucent widening (b), early duplication/multilayering of the GBM (c), and early PTCBM multilayering (d, e). EM, electron microscopy; GBM, glomerular basement membrane; PTCBM, peritubular capillary basement membrane.
Statistical Analysis
All data were analyzed with SPSS software version 23.0 for Windows (IBM Japan, Tokyo, Japan). We considered p values of <0.05 to be statistically significant. The Mann-Whitney U test, Student's t test, McNemar's test, χ2 test, and Fisher's exact test were used to compare variables. Wilcoxon's signed-rank test and the paired t test were used between the diagnosis of subclinical (SAABMR) and follow-up biopsy. All data are described as the number and mean ± standard deviation.
Results
Clinicopathological Characteristics of PAABMR
Table 1 shows the clinicopathological characteristics of PAABMR. The male/female ratio was 18/11. The mean recipient age at transplantation was 41.8 ± 15.2 years. Of the 29 BS of PAABMR, 12 BS were ABO incompatible and 10 BS were from a second transplantation. The living/cadaveric ratio was 29/0, and the protocol/episode ratio was 24/5. Fifteen BS showed preformed DSAs before transplantation, and 20 BS were positive for DSAs (MFI ≥ 1,000) at the time of biopsy. Nine BS were negative for DSAs (MFI < 1,000), and these BS were highly suspected of AABMR. In this study, both DSA-positive and DSA-negative BS were defined as PAABMR for convenience. The mean duration after transplantation was 22.9 ± 26.7 months, and the mean serum Cr level was 1.53 ± 1.11 mg/dL. The mean g and ptc scores were 1.17 ± 0.60 and 1.97 ± 1.32, respectively. The mean g score was 1.06 ± 0.68 in the ptc ≥ 3 group and 1.31 ± 0.48 in the ptc < 3 group without a significant difference (p = 0.32) (these data are not shown in Table 1). Sixteen BS were in the ptc ≥ 3 group and 13 BS were in the ptc < 3 group. A g + ptc score of ≥2 was observed in 23 BS (79.3%), and a C4d score of ≥2 was observed in 20 BS (69.0%). The mean C4d, t, and i scores were 1.97 ± 1.21, 0.55 ± 0.99, and 0.34 ± 0.72, respectively. The mean ct and ci scores were 0.66 ± 0.61 and 0.45 ± 0.57, respectively. No cv lesions were observed in the 29 BS. We surveyed T-cell-mediated rejection (TCMR) events preceding the diagnosis of PAABMR. There were 29 BS without a preceding TCMR event, but 2 showed simultaneous TCMR and PAABMR.
Table 1.
Clinicopathological characteristics of PAABMR
| PAABMR (n = 29) | |
|---|---|
| Male/female, n | 18/11 |
| Recipient age at transplantation, years | 41.8±15.2 |
| ABO incompatible, n (%) | 12 (41.4) |
| 2nd transplantation, n (%) | 10 (34.5) |
| Living/cadaver | 29/0 |
| Protocol biopsy, n (%) | 24 (83) |
| Preformed DSA, n (%) | 15 (51.7) |
| DSA positive (MFI ≥1,000) at the time of biopsy, n (%) | 20 (69.0) |
| Duration after transplantation, months | 22.9±26.7 |
| Serum Cr, mg/dL | 1.53±1.11 |
| g (score) | 1.17±0.60 |
| ptc (score) | 1.97±1.32 |
| g + ptc ≥2, n (%) | 23 (79.3) |
| C4d (score) | 1.97±1.21 |
| C4d ≥2, n (%) | 20 (69.0) |
| t (score) | 0.55±0.99 |
| i (score) | 0.34±0.72 |
| ct (score) | 0.66±0.61 |
| ci (score) | 0.45±0.57 |
| TCMR, n (%) | 2 (7) |
PAABMR, pathological acute/active antibody-mediated rejection; DSA, donor-specific antibody; MFI, mean fluorescence intensity.
Clinicopathological Characteristics of Patients with DSA-High, DSA-Low, and below Cutoff PAABMR
Table 2 shows the clinicopathological characteristics of patients with DSA-high (MFI ≥ 5,000), DSA-low (MFI < 5,000 to ≥1,000), and below cutoff (MFI < 1,000) PAABMR. ABO incompatibility in DSA-high group was significantly lower. Second transplantation in DSA-high group was significantly higher. There were no significant differences in terms of sex, protocol/episode biopsy ratio, recipient age at transplantation, donor age at transplantation, duration after transplantation, Tac trough level, CsA trough level, or serum Cr level at the time of biopsy. Although not statistically significant, there was a tendency of high DSA MFI (MFI ≥ 5,000) in younger recipients. The g, ptc, and C4d scores were not significantly different among the 3 groups. The prevalence of a g + ptc score of ≥2 and prevalence of C4d score of ≥2 were not significantly different among the 3 groups. The mean t, i, ct, and ci scores were also not significantly different among the 3 groups.
Table 2.
Clinicopathological characteristics of DSA-high (MFI ≥ 5,000), DSA-low (MFI < 5,000 to ≥1,000), and below cutoff (MFI < 1,000) groups of PAABMR
| MFI ≥5,000 (n = 13) | MFI <5,000 to ≥1,000 (n = 7) | MFI <1,000 (n = 9) | p value | |
|---|---|---|---|---|
| Male/female, n | 9/4 | 4/3 | 5/4 | 0.772 |
| ABO incompatible, n (%) | 2 (15.4)* | 4 (57.1) | 6 (66.7) | 0.035 |
| Preformed DSA, n (%) | 8 (61.5) | 4 (57.1) | 3 (33.3) | 0.406 |
| 2nd transplantation, n (%) | 8 (61.5)** | 1 (14.3) | 1 (11.1) | 0.022 |
| Protocol/episode, n | 11/2 | 6/1 | 7/2 | 0.891 |
| Recipient age at transplantation, years | 37.2±13.4 | 38.4±10.0 | 51.2±18.0 | 0.078 |
| Donor age at transplantation, years | 51.9±6.5 | 53.1±13.5 | 58.1±12.9 | 0.234 |
| Duration after transplantation, months | 26.1±31.0 | 21.6±18.2 | 19.3±27.7 | 0.555 |
| Tac trough level, ng/mL | 6.13±2.62 (n = 12) | 5.13±1.66 (n = 6) | 6.94±0.99 (n = 5) | 0.395 |
| CsA trough level, ng/mL | 106.1 (n = 1) | 332.0 (n = 1) | 101.5±73.4 (n = 4) | |
| Serum Cr, mg/dL at the time of biopsy | 1.45±1.34 | 1.24±0.26 | 1.82±0.92 | 0.210 |
| g (score) | 1.15±0.69 | 1.29±0.49 | 1.11±0.60 | 0.862 |
| ptc (score) | 2.31±1.18 | 1.14±1.46 | 2.11±1.27 | 0.162 |
| g + ptc ≥2, n (%) | 12 (92.3) | 4 (57.1) | 7 (77.8) | 0.178 |
| C4d (score) | 2.38±0.96 | 2.00±1.41 | 1.33±1.23 | 0.131 |
| C4d ≥ 2, n (%) | 11 (84.6) | 5 (71.4) | 4 (44.4) | 0.133 |
| t (score) | 0.46±0.88 | 0.57±1.13 | 0.67±1.12 | 0.959 |
| i (score) | 0.23±0.83 | 0.57±0.79 | 0.33±0.50 | 0.225 |
| ct (score) | 0.69±0.75 | 0.57±0.53 | 0.67±0.50 | 0.944 |
| ci (score) | 0.46±0.66 | 0.43±0.54 | 0.44±0.53 | 0.993 |
| TCMR, n (%) | 1 (7.7) | 1 (14.3) | 0 (0) | 0.529 |
DSA, donor-specific antibody; MFI, mean fluorescence intensity; PAABMR, pathological acute/active antibody-mediated rejection; TCMR, T cell-mediated rejection; CsA, cyclosporine; Tac, tacrolimus.
ABO incompatible in DSA-high group was significantly lower among 3 groups (DSA-high, DSA-low, and below cutoff groups).
2nd transplantation in DSA-high group was significantly higher among 3 groups.
Clinicopathological Characteristics of Patients with Early and Late Post-transplantation PAABMR
The median of the time after transplant among 29 PAABMR BS was 12 months. We defined early post-transplant period as ≤12 months and late post-transplant period as >12 months. The clinicopathological characteristics of patients with early and late post-transplantation PAABMR are shown in Table 3. There were no significant differences in sex or ABO incompatibility between the 2 groups. Preformed DSAs and second transplantation were more prevalent in the early than in the late post-transplantation period. Of the 9 BS secondary transplantations in the early post-transplantation group, 8 had preformed DSAs. There were no significant differences in recipient age, donor age at transplantation, duration after transplantation, Tac trough level, CsA trough level, or serum Cr level at the time of biopsy. The g, ptc, and C4d scores, and the prevalence of a C4d score of ≥2 were not significantly different between the 2 groups. The incidence of g + ptc ≥ 2 was more prevalent in the late than in the early post-transplantation period. The mean t, i, and ct scores were not significantly different between the 2 groups, but the mean ci score was higher in the late than in the early post-transplantation period.
Table 3.
Clinicopathological characteristics of recipients with early and late post-transplantation PAABMR
| Early* post-transplant period (n = 18) | Late** post-transplant period (n = 11) | p value | |
|---|---|---|---|
| Male/female, n | 9/9 | 9/2 | 0.092 |
| ABO incompatible, n (%) | 9 (50) | 3 (27.2) | 0.208 |
| Preformed DSA, n (%) | 13 (72.2) | 2 (18.2) | 0.005 |
| 2nd transplantation, n (%) | 9 (50) | 1 (9.1) | 0.029 |
| Protocol/episode, n | 16/2 | 8/3 | 0.266 |
| Recipient age at transplantation, years | 44.3±16.2 | 37.7±13.2 | 0.265 |
| Donor age at transplantation, years | 57.0±9.4 | 49.5±11.3 | 0.062 |
| Time after transplantation, months*** | 8.5 (0, 12) | 36.0 (13, 88) | 0.000 |
| Tac trough level, ng/mL | 6.51±2.25 (n = 15) | 5.18±1.77 (n = 8) | 0.161 |
| CsA trough level, ng/mL | 210.8±119.4 (n = 3) | 70.6±32.1 (n = 3) | 0.121 |
| Serum Cr, mg/dL at the time of biopsy | 1.35±0.61 | 1.78±1.50 | 0.805 |
| g (score) | 1.17±0.51 | 1.18±0.75 | 0.855 |
| ptc (score) | 1.83±1.43 | 2.18±1.17 | 0.708 |
| g + ptc ≥ 2, n (%) | 12 (66.7) | 11 (100) | 0.039 |
| C4d (score) | 1.94±1.26 | 2.00±1.18 | 1.000 |
| C4d ≥ 2, n (%) | 12 (66.7) | 8 (72.7) | 0.534 |
| t (score) | 0.50±0.99 | 0.64±1.03 | 0.640 |
| i (score) | 0.22±0.55 | 0.55±0.93 | 0.241 |
| ct (score) | 0.50±0.51 | 0.91±0.70 | 0.111 |
| ci (score) | 0.22±0.43 | 0.82±0.60 | 0.007 |
| TCMR, n (%) | 1 (5.6) | 1 (9.1) | 0.623 |
DSA, donor-specific antibody; PAABMR, pathological acute/active antibody-mediated rejection; CsA, cyclosporine; Tac, tacrolimus.
Early post-transplant period was defined as ≤12 months.
Late post-transplant period was defined as >12 months.
Time after transplantation was described as median (range).
Analysis of Follow-Up Biopsy for SAABMR
A total of 83% of PAABMR cases were diagnosed by the protocol biopsy (Table 1), and we defined PAABMR diagnosed by the protocol biopsy as SAABMR. Tables 4 and 5 show the clinical, LM, EM findings of 8 cases diagnosed as SAABMR from January 2016 to December 2017, and all 8 diagnoses were obtained by follow-up biopsy findings including EM analysis until June 2018. Cases 1–7 had preformed DSAs, and Case 8 had de novo DSAs. There were 19 recipients (24 BS) with subclinical AABMR, including 8 recipients (follow-up group) with follow-up biopsies including EM and 11 (no follow-up group) without follow-up biopsies including EM by June 2018. We compared the clinical features, pathological findings, and treatment between these groups at the time of diagnosis of SAABMR by allograft biopsy. Preformed DSAs were more prevalent and the duration after transplantation was shorter in the follow-up group compared with the no follow-up group, indicating that the follow-up group (8 cases) was not representative of all 19 SAABMR cases. Other clinicopathological characteristics were similar in both groups.
Table 4.
Clinical characteristics of the 8 SAABMR cases with follow-up biopsy
| Case | Age at transplantation, years | ABO incompatible | 2nd transplantation | Duration after transplantation at diagnosis of SAABMR, months | Interval of follow-up biopsy, months | Highest MFI of DSA at diagnosis of SAABMR/DSA type | sCr, mg/dL at the time of biopsy diagnosed SAABMR | sCr, mg/dL at follow-up biopsy | Upr, g/gCr at the time of biopsy diagnosed SAABMR | Upr, g/gCr at follow-up biopsy | Treatment at diagnosis of SAABMR |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 39 | Yes | No | 11 | 15 | DR4(216)/preformed | 1.37 | 1.48 | 0.4 | 0.2 | Increased MMF dose |
| 2 | 52 | No | Yes | 2 | 14 | DQ9(5442)/preformed | 1.07 | 1.24 | 0.2 | 0.5 | mPSL pulse + EVR add on |
| 3 | 27 | Yes | Yes | 3 | 9 | B71(5521)/preformed | 1.30 | 1.24 | 0.1 | 0.1 | mPSL pulse + EVR add on |
| 4 | 65 | Yes | No | 2 | 9 | DR1(188)/preformed | 1.32 | 1.17 | 0.2 | 0.1 | mPSL pulse |
| 5 | 43 | Yes | No | 3 | 9 | DR4(1651)/preformed | 1.19 | 1.36 | 0.2 | 0.1 | No change |
| 6 | 29 | No | Yes | 12 | 14 | DQ6(12065)/preformed | 0.89 | 1.54 | 0.1 | 0.2 | DSG + increased EVR dose |
| 7 | 29 | No | Yes | 3 | 10 | DQ9(13811)/preformed | 0.78 | 0.82 | 0.1 | 0.2 | mPSL pulse + EVR add on |
| 8 | 30 | No | No | i2 | 24 | DQ9(3442)/de novo | 1.72 | 1.53 | 0.4 | 0.1 | mPSL pulse, DSG, CsA to Tac conversion |
| Mean ± SD | 39.3±13.6 | 6.0±4.7 | 13.0±5.1 | 1.21±0.30 | 1.30±0.24 | 0.2±0.1 | 0.2±0.1 | ||||
SAABMR, subclinical acute/active antibody-mediated rejection; DSA, donor-specific antibody; MFI, mean fluorescence intensity; sCr, serum creatinine; Upr, urine proteinuria; mPSL pulse, methylprednisolone 500 mg × 3 days; CsA, cyclosporine; MMF, mycophenolate mofetil; Tac, tacrolimus; DSG, deoxyspergualin; EVR, everolimus.
Table 5.
Pathological characteristics of the 8 SAABMR cases with follow-up biopsy
| Case | Histological change of the Banff score by LM (diagnosis/follow) |
Histological change by EM (diagnosis/follow) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| g (score) | ptc (score) | C4d (score) | ct (score) | ci (score) | cg by LM (score) | endothelial swelling | Subendothelial electron-lucent widening | early GBM duplication | cg by EM (score) | PTCBM multilayering | |
| 1 | 1/0 | 0/0 | 3/3 | 0/2 | 0/2 | 0/0 | −/− | −/− | −/− | 0/0 | Partially/partially |
| 2 | 1/1 | 3/2 | 3/3 | 0/1 | 0/1 | 0/0 | +/− | −/+ | −/+ | 0/1a | Partially/circular |
| 3 | 1/1 | 3/0 | 1/3 | 1/0 | 0/0 | 0/0 | +/− | −/+ | −/+ | 0/1a | Partially/partially |
| 4 | 1/2 | 2/3 | 1/3 | 1/1 | 0/1 | 0/0 | +/+ | +/− | +/− | 1a/0 | Partially/circular |
| 5 | 1/1 | 0/0 | 3/2 | 0/1 | 0/0 | 0/0 | +/+ | +/− | +/− | 1a/0 | Partially/partially |
| 6 | 2/2 | 3/3 | 2/1 | 1/1 | 1/1 | 0/0 | −/− | −/+ | −/+ | 0/1a | Partially/circular |
| 7 | 2/1 | 3/3 | 3/2 | 0/0 | 0/0 | 0/0 | +/− | +/+ | +/+ | 1a/1a | Partially/circular |
| 8 | 1/1 | 3/0 | 0/0 | 1/1 | 1/0 | 0/0 | −/− | −/+ | −/+ | 0/1a | Partially/partially |
SAABMR, subclinical acute/active antibody-mediated rejection; LM, light microscopy; EM, electron microscopy; Diagnosis, at the diagnosis of SAABMR; Follow, at the follow-up biopsy; GBM, glomerular basement membrane; PTCBM, peritubular capillary basement membrane; Partially, ≥4 layers of partially lesion; Circular, ≥4 layers of circular lesion.
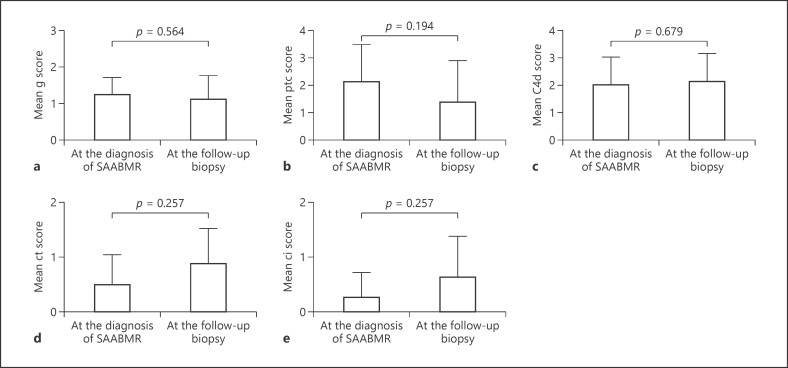
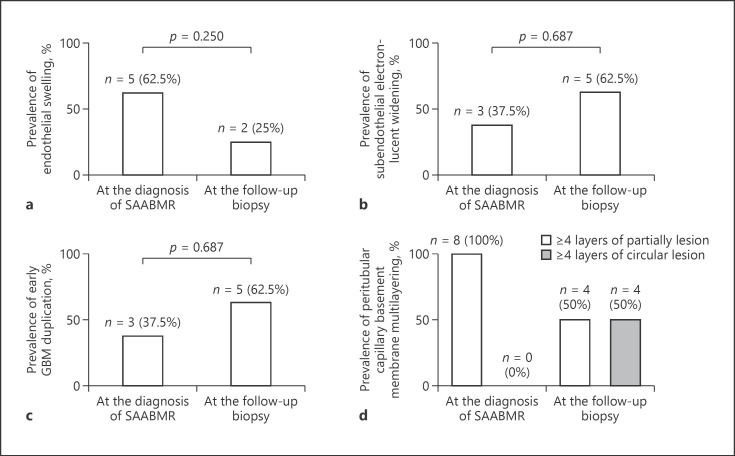
Figure 2 shows the histological change of the Banff score by LM. No significant differences in the g, ptc, c4d, ct, or ci scores were observed between diagnosis of SAABMR and the follow-up biopsy (Fig. 2). The cg score determined by LM was 0 at the diagnosis of SAABMR and the follow-up biopsy. No significant differences in the serum Cr level or severity of proteinuria were observed between diagnosis of SAABMR and the follow-up biopsy (Table 4). We further analyzed BS obtained at the diagnosis of SAABMR and the follow-up biopsy for these 8 cases using EM (Fig. 3). Glomerular endothelial swelling was found in 5 cases at the diagnosis of SAABMR and 2 cases at the follow-up biopsy. Subendothelial electron-lucent widening was found in 3 cases at the diagnosis of SAABMR and 5 cases at the follow-up biopsy. Early GBM duplication was found in 3 cases at the diagnosis of SAABMR and 5 cases at the follow-up biopsy. These cases were classified as cg1a lesion according to Banff 2013 classification [1]. Partial PTCBM multilayering was found in all cases at the diagnosis of SAABMR and in 4 cases at the follow-up biopsy. Circumferential PTCBM multilayering was not found at the diagnosis of SAABMR but was detected in 4 cases at the follow-up biopsy. No differences in these electron microscopic findings were observed between the follow-up biopsy and at the diagnosis of SAABMR (Fig. 3).
Fig. 2.
Histological change of SAABMR as evaluated by LM. The Banff g score (a), ptc score (b), C4d score (c), ct score (d), and ci score showed no significant change between the diagnosis of SAABMR and the follow-up biopsy (e). SAABMR, subclinical acute/active antibody-mediated rejection; LM, light microscopy.
Fig. 3.
Histological change of SAABMR evaluated by EM. Prevalences of glomerular endothelial cell swelling (a), subendothelial electron-lucent widening (b), early duplication of GBM (c), and partial PTCBM and circumferential PTCBM multilayering (d). SAABMR, subclinical acute/active antibody-mediated rejection; EM, electron microscopy; GBM, glomerular basement membrane; PTCBM, peritubular capillary basement membrane.
Discussion
This study aimed to clarify the clinicopathological features of AABMR based on the revised criteria of the Banff 2013 classification. PAABMR presented mainly as peritubular capillary inflammation and was mostly detected by the protocol biopsy (SAABMR). Although the Banff 2013 classification suggests that electron microscopic evaluation is essential to diagnose AABMR, there have been insufficient reports of the use of EM to evaluate AABMR. Especially in the case of SAABMR, few studies have reported the results of histological evaluation, including EM, and its changes over time.
In a previous study of the 1-year protocol biopsy, 14% of cases showed subclinical antibody-mediated rejection [5]. In the present study, we focused on acute antibody-mediated rejection, the diagnostic period was 22.9 ± 26.7 months after transplantation, 8.4% of cases showed PAABMR, and 83.0% of PAABMR cases involved subclinical rejection (Table 1). Sis et al. [6] reported that the g + ptc sum score is the best predictor of DSA. In the Banff 2013 classification, a g + ptc score of ≥2 is evidence of a current/recent interaction between antibodies and the vascular endothelium [1]. In our study, 79.5% of PAABMR cases had a g + ptc score of ≥2. Therefore, 20.5% did not have a g + ptc score of ≥2, and most PAABMR cases involved subclinical rejection. One study showed that the g and ptc scores were significantly lower in subclinical than clinical antibody-mediated rejection [7]. Another report has suggested that peritubular capillaritis is an early marker for the risk of chronic antibody-mediated rejection [8]. In our histological analysis, peritubular capillaritis rather than glomerulitis was the main pathological lesion of PAABMR.
A recent study showed that assessment of the peak MFI of DSAs adds predictive accuracy for patients with antibody-mediated rejection [3]. Bachelet et al. [9] reported that discrepancies between histological findings and serological DSA results are common and suggested that the presence of intragraft DSAs is a marker of the severity of the antibody-mediated pathological process. In the present study, DSA positivity (peak MFI ≥ 1,000) at the time of biopsy was 69%, and there was no significant difference in the MVI score among DSA-high (MFI ≥ 5,000), DSA-low (MFI < 5,000 to ≥1,000), and below cutoff (MFI < 1,000) PAABMR. In liver transplantation, absorption and elimination of preformed DSAs lead to a low rate of graft loss [10]. In the present study, 51.7% of PAABMR had preformed DSAs. Therefore, graft absorption and elimination of preformed DSAs might have contributed to the lack of a difference in the microvascular inflammation as evaluated by the MFI cutoff value at Toho University Omori Medical Center.
We found that the ABO incompatibility in DSA-high group was significantly lower, and the second transplantation was significantly higher in DSA-high group (Table 2). The use of rituximab in ABO-incompatible kidney transplantations may have been associated with a lower MFI value of DSAs. Furthermore, in terms of secondary transplantation, it is possible that DSAs with a high MFI may have been due to sensitization at the time of primary kidney transplantation. Furthermore, we found that preformed DSAs and second transplantation were more prevalent in the early than late post-transplantation period (Table 3), and of the 9 BS secondary transplants in the early post-transplantation group, 8 BS had preformed DSAs. These data indicate that preformed DSAs are likely to cause PAABMR in the early post-transplantation period.
Loupy et al. [5] reported that SAABMR at 1 year after transplantation is associated with graft loss and that SAABMR is associated with more rapid progression to transplant glomerulopathy. Haas and Mirocha [2] reported ultrastructural changes, including glomerular endothelial swelling, subendothelial widening, and early GBM duplication, in early biopsies within 3 months after transplantation in patients showing AABMR, and treatment for AABMR after the early biopsies significantly decreased subsequent development of overt transplant glomerulopathy. Therefore, among the recipients with SAABMR, the clinicopathological changes of 8 recipients who underwent follow-up biopsy after SAABMR diagnosis were analyzed by EM. The clinical changes, including graft function and urine protein, were not different between the diagnosis of SAABMR and the follow-up biopsy (Table 4). The histological Banff score was not different between the diagnosis of SAABMR and the follow-up biopsy by LM (Fig. 2). Although the GBM double counter was not observed at the time of diagnosis of SAABMR or the follow-up biopsy by LM, early GBM duplication was already observed when diagnosis was performed by EM. In the current study, the prevalence of glomerular endothelial swelling decreased from 62.5 to 25%, and subendothelial widening and early GBM duplication conversely increased from 37.5 to 62.5% at the follow-up biopsy. We speculate that endothelial injury resulted in glomerular endothelial swelling followed by subendothelial widening and early GBM duplication. Circumferential peritubular capillary membrane multilayering (≥4 basement membrane layers) was newly observed at the follow-up biopsy. In our study, SAABMR showed predominantly peritubular capillary inflammation. We considered that sustained peritubular capillaritis caused de novo circumferential peritubular capillary membrane multilayering, while early multilayering of peritubular capillaries was not circumferential but partly multilayered. We speculate that early partial multilayering progresses to circumferential multilayering. However, there were no differences in electron microscopic findings between the follow-up biopsy and at the time of diagnosis of SAABMR. Further studies are needed to consider these issues.
The treatment of SAABMR is a very important clinical issue. One report has suggested that the time of subclinical antibody-mediated rejection before manifestation of graft dysfunction may be a critical period in which to reverse antibody-mediated rejection [11]. Other studies showed that eculizumab decreases the incidence of early antibody-mediated rejection [12, 13]. At Toho University Omori Medical Center, we mainly treat SAABMR with steroid pulse therapy. Although de novo chronic lesions were observed by EM in the present study, the clinical and histological changes evaluated by LM were relatively good, at least in the short term. However, over-immunosuppression is a risk factor for infection, including BK virus nephropathy, leading to poor graft outcomes. The main limitation of this study was that we could not evaluate the difference in clinicopathological changes because of the differences in immunosuppressive treatments. Furthermore, this study had a small sample size and short-term follow-up. A long term follow-up and additional prospective studies are needed to clarify the clinical and histological significance of SAABMR, including EM analysis.
In conclusion, the cases of PAABMR during the 2-year study period were mainly found by the protocol biopsy (SAABMR) and showed predominantly peritubular capillary inflammation. There were significant differences in the prevalences of ABO incompatibility and second transplantation among the DSA-high, DSA-low, and below cutoff groups of PAABMR. ABO incompatibility in DSA-high group was significantly lower, and second transplantation in DSA-high group was significantly higher in the PAABMR. The short-term follow-up period (9–24 months) of the SAABMR patients did not reveal worsening according to clinical and light microscopical characteristics, but ultrastructural assessment was beneficial to evaluate early lesions of endothelial injury and progression of glomerular and PTCBM alterations.
Statement of Ethics
This study was approved by Toho University Omori Medical Center Ethics Committee (approval number: M18149).
Conflict of Interest Statement
The authors have no conflicts of interest to disclose.
Author Contibutions
T.A. designed the study, collected clinicopathological data, and wrote the paper. H.O. designed the study, analyzed the data, and performed pathological evaluation. K. Shinoda contributed to clinical data acquisition, the statistical analysis and participated in drafting the work. K. Sakurabayashi contributed to clinical data acquisition and participated in drafting the work. T.M. and Y.I. performed pathological evaluation and participated in drafting the work. K. Sakai contributed to design of this study and participated in drafting the work.
Acknowledgements
We appreciate the kidney transplantation team in Department of Nephrology, Toho University Faculty of Medicine for contribution to execution or planning or design of this study. We appreciate Dr. Yutaka Yamaguchi from Yamaguchi's Pathology Laboratory for helpful advice regarding pathological evaluation. We appreciate Dr. Kazutoshi Shibuya from the Department of Surgical Pathology for managing the preparation of allograft biopsy specimens. We are grateful to Ms. Mayumi Ishii from the Department of Surgical Pathology, Toho University Faculty of Medicine, for helpful comments regarding diagnosis by EM.
References
- 1.Haas M, Sis B, Racusen LC, Solez K, Glotz D, Colvin RB, et al. Banff 2013 meeting report: inclusion of c4d-negative antibody-mediated rejection and antibody-associated arterial lesions. Am J Transplant. 2014;14((2)):272–83. doi: 10.1111/ajt.12590. [DOI] [PubMed] [Google Scholar]
- 2.Haas M, Mirocha J. Early ultrastructural changes in renal allografts: correlation with antibody-mediated rejection and transplant glomerulopathy. Am J Transplant. 2011 Oct;11((10)):2123–31. doi: 10.1111/j.1600-6143.2011.03647.x. [DOI] [PubMed] [Google Scholar]
- 3.Eskandary F, Bond G, Kozakowski N, Regele H, Marinova L, Wahrmann M, et al. Diagnostic contribution of donor-specific antibody characteristics to uncover late silent antibody-mediated rejection-results of a cross-sectional screening study. Transplantation. 2017;101((3)):631–41. doi: 10.1097/TP.0000000000001195. [DOI] [PubMed] [Google Scholar]
- 4.Wavamunno MD, O'Connell PJ, Vitalone M, Fung CL, Allen RD, Chapman JR, et al. Transplant glomerulopathy: ultrastructural abnormalities occur early in longitudinal analysis of protocol biopsies. Am J Transplant. 2007;7((12)):2757–68. doi: 10.1111/j.1600-6143.2007.01995.x. [DOI] [PubMed] [Google Scholar]
- 5.Loupy A, Vernerey D, Tinel C, Aubert O, Duong van Huyen JP, Rabant M, et al. Subclinical rejection phenotypes at 1 year post-transplant and outcome of kidney allografts. J Am Soc Nephrol. 2015 Jul;26((7)):1721–31. doi: 10.1681/ASN.2014040399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sis B, Jhangri GS, Riopel J, Chang J, de Freitas DG, Hidalgo L, et al. A new diagnostic algorithm for antibody-mediated microcirculation inflammation in kidney transplants. Am J Transplant. 2012;12((5)):1168–79. doi: 10.1111/j.1600-6143.2011.03931.x. [DOI] [PubMed] [Google Scholar]
- 7.Papadimitriou JC, Drachenberg CB, Ramos E, Kukuruga D, Klassen DK, Ugarte R, et al. Antibody-mediated allograft rejection: morphologic spectrum and serologic correlations in surveillance and for cause biopsies. Transplantation. 2013;95((1)):128–36. doi: 10.1097/TP.0b013e3182777f28. [DOI] [PubMed] [Google Scholar]
- 8.Lerut E, Naesens M, Kuypers DR, Vanrenterghem Y, Van Damme B. Subclinical peritubular capillaritis at 3 months is associated with chronic rejection at 1 year. Transplantation. 2007 Jun 15;83((11)):1416–22. doi: 10.1097/01.tp.0000266676.10550.70. [DOI] [PubMed] [Google Scholar]
- 9.Bachelet T, Couzi L, Lepreux S, Legeret M, Pariscoat G, Guidicelli G, et al. Kidney intragraft donor-specific antibodies as determinant of antibody-mediated lesions and poor graft outcome. Am J Transplant. 2013;13((11)):2855–64. doi: 10.1111/ajt.12438. [DOI] [PubMed] [Google Scholar]
- 10.Cuadrado A, San Segundo D, López-Hoyos M, Crespo J, Fábrega E. Clinical significance of donor-specific human leukocyte antigen antibodies in liver transplantation. World J Gastroenterol. 2015 Oct 21;21((39)):11016–26. doi: 10.3748/wjg.v21.i39.11016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morozumi K, Takeda A, Otsuka Y, Horike K, Gotoh N, Narumi S, et al. Reviewing the pathogenesis of antibody-mediated rejection and renal graft pathology after kidney transplantation. Nephrology. 2016;21((Suppl 1)):4–8. doi: 10.1111/nep.12777. [DOI] [PubMed] [Google Scholar]
- 12.Stegall MD, Diwan T, Raghavaiah S, Cornell LD, Burns J, Dean PG, et al. Terminal complement inhibition decreases antibody-mediated rejection in sensitized renal transplant recipients. Am J Transplant. 2011;11((11)):2405–13. doi: 10.1111/j.1600-6143.2011.03757.x. [DOI] [PubMed] [Google Scholar]
- 13.Stegall MD, Chedid MF, Cornell LD. The role of complement in antibody-mediated rejection in kidney transplantation. Nat Rev Nephrol. 2012;8((11)):670–8. doi: 10.1038/nrneph.2012.212. [DOI] [PubMed] [Google Scholar]