Abstract
Diabetic foot ulcer is one of the most frightened diabetic complications leading to amputation disability and early mortality. Diabetic wounds exhibit a complex networking of inflammatory cytokines, local proteases, and reactive oxygen and nitrogen species as a pathogenic polymicrobial biofilm, overall contributing to wound chronification and host homeostasis imbalance. Intralesional infiltration of epidermal growth factor (EGF) has emerged as a therapeutic alternative to diabetic wound healing, reaching responsive cells while avoiding the deleterious effect of proteases and the biofilm on the wound's surface. The present study shows that intralesional therapy with EGF is associated with the systemic attenuation of pro‐inflammatory markers along with redox balance recovery. A total of 11 diabetic patients with neuropathic foot ulcers were studied before and 3 weeks after starting EGF treatment. Evaluations comprised plasma levels of pro‐inflammatory, redox balance, and glycation markers. Pro‐inflammatory markers such as erythrosedimentation rate, C‐reactive protein, interleukin‐6, soluble FAS, and macrophage inflammatory protein 1‐alpha were significantly reduced by EGF therapy. Oxidative capacity, nitrite/nitrate ratio, and pentosidine were also reduced, while soluble receptor for advanced glycation end‐products significantly increased. Overall, our results indicate that the local intralesional infiltration of EGF translates in systemic anti‐inflammatory and antioxidant effects, as in attenuation of the glycation products' negative effects.
Keywords: diabetes, diabetic foot ulcer, epidermal growth factor, inflammation, oxidative stress
Abbreviations
- AGE
advanced glycation end‐products
- CRP
C‐reactive protein
- DFU
diabetic foot ulcer
- EGF
epidermal growth factor
- GM‐CSF
granulocyte‐macrophage colony‐stimulating factor
- GRZA
granzyme A
- GRZB
granzyme B
- HbA1c
glycated haemoglobin
- ICAM‐1
intercellular adhesion molecule 1
- IFN‐γ
interferon gamma
- IL
interleukin
- MIP1‐a
macrophage inflammatory protein 1‐alpha
- MIP1‐b
macrophage inflammatory protein 1‐beta
- NO
nitric oxide
- PRF
perforin
- RAGE
receptor for AGE
- sCD137
soluble cluster of differentiation 137
- T0
time zero
- T1
time one
- T2‐DM
type 2 diabetes mellitus
- TNF‐α
tumour necrosis factor alpha
1. INTRODUCTION
Type 2 diabetes mellitus (T2‐DM) is a heterogeneous and complex group of disease involving multiple pathogenic factors and multiorgans' complications. Hyperglycaemia is its most proximal, constant, and outspoken marker.1 T2‐DM has progressively expanded to pandemic magnitude accounting for 90% to 95% of all the diabetic population.2 Diabetic foot ulcer (DFU) is one of the most frightened complications leading to amputation disability, social exclusion, and early mortality. Accordingly, diabetic population still contributes to 80% of all non‐traumatic lower extremities' amputations around the world.3
Cutaneous wound repair is a multifaceted linear process in which a sequence of overlapping changes integrates to repair a lesion, comprehensively involving soluble mediators, immunoinflammatory cells, mesenchyme‐derived granulation tissue‐producing cells, and epidermal keratinocytes.4 Analogous to the multifaceted interplay between genes and environment in diabetes pathogenesis is the interaction between the wound and its host. Complex and problem wounds are a superimposed organ that establishes a constant cross‐talk with the patient, so that this reciprocal influential loop creates a dynamic bidirectional reciprocity circuit. This turns particularly relevant and clinically meaningful in severe burn injuries5 and diabetic lower extremity wounds.6
Conceptually, diabetic wounds exhibit a complex networking of inflammatory cytokines, local proteases, cytotoxic reactive oxygen and nitrogen species, and a polymicrobial biofilm that altogether contribute to wound cells' senescence, chronification, and host homeostasis imbalance.7 These wounds magnify a pre‐existing endovascular systemic, non‐specific, immuno‐inflammatory response that amplifies insulin resistance, disrupts anabolic processes, produces a systemic toxic syndrome, and delays wound healing.8 Therefore, innovative interventions are required to trigger the wound cells to resume the acute healing phenotype in a manner that could effectively lessen the systemic inflammatory repercussion. The organismal translation of this type of therapy may contribute to attenuate cellular degeneration, tissue catabolism, and ultimately insulin resistance.
Studies dating more than a decade ago have reproducibly shown the intrinsic ability of epidermal growth factor (EGF) at pharmacological concentrations to trigger biological actions required for diabetic wound healing upon its systematic infiltration into the wound edges and bottom.9 Consequently, broad pharmacovigilance studies confirm the clinical efficacy of the infiltrative procedure in terms of both safety and patients' response with 16% absolute, 71% relative reduction amputation risks, and only 5% reulcerations upon a 12‐month follow‐up period.10
The present study validates previous observations of our group,11 demonstrating the systemic impact of locally infiltrated EGF in reestablishing a pro‐physiological redox balance. Furthermore, here we show that EGF pharmacodynamic bounties also encompass the attenuation of endovascular pro‐inflammatory markers with dramatic negative impact in diabetic individuals' general homeostasis.
2. METHODS
2.1. Ethics
The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki. In addition, this protocol was reviewed and approved by the ethics committee of the National Institute of Angiology and Vascular Surgery, Havana, Cuba. All the patients approved to be involved in the study and signed an informed consent.
2.2. Study population
A total of 11 diabetic patients affected by chronic lower extremity ulcers, admitted at the angiopathy ward of the National Institute of Angiology and Vascular Surgery in Havana, Cuba, were included in this study. Inclusion criteria were T2‐DM, neuropathic úlcera de pie diabético (UPD) classified as grade 3 or 4 of the Wagner's scale,12 and age between 45 and 75 years old. All the patients were part of the national program for integral diabetes care that involved as instrumental pharmacological intervention, the local infiltration of recombinant human EGF. Under the in‐hospital regime, the patients received the systemic medical interventions required to eliminate infection, ensure metabolic control, and the concurrent limb off‐loading. Locally, wounds were cleansed with saline, sharp debrided when required, and dressed with saline‐moistened gauze.13 Wounds were clinically examined on alternate days before EGF administration, as a routine practice during a 15‐day wash‐out period. Only those patients with non‐granulating wounds were included in the study. The treatment began following the wash‐out period, when a clean wound substrate was suitable for infiltrations.14
2.3. Recombinant human EGF
Recombinant human EGF was obtained from the Center for Genetic Engineering and Biotechnology (Havana, Cuba).15 It was formulated with buffer salts as injectable, lyophilised, and is presently available in this composition (Heberprot‐P, HeberBiotec S.A., Havana, Cuba). Patients received 75 μg of EGF at each intralesional infiltration session, three times per week on alternate days. The medication, its administration requisites, and procedure have been extensively described.16
2.4. Sample collection
After 12–14 hours overnight fasting, between 7:30 and 9:00 am, and before taking any medication, 10 mL of blood was collected from each subject. “Time zero” (T0) harvesting corresponds to the blood plasma sample obtained just prior to the initial EGF infiltration when the wound bed had been fully preconditioned17 and the metabolic status of the patient had been improved. A subsequent plasma sample, obtained 3 weeks later when nine EGF infiltration sessions had been completed, was considered “time one” (T1), which allowed for paired samples of the same individual. Previous studies justify this time point as it represents the maximal outgrowth of the fibroangiogenic response under the influence of EGF in situ infiltration.18 Early morning saliva samples were also collected at T0 and T1 for EGF level quantification. Both plasma and saliva samples were aliquoted and stored at −20°C until assayed.
2.5. Haematological and haemochemical parameters
Erythrosedimentation rate, total leukocyte count, haemoglobin, glycated haemoglobin (HbA1c), glycaemia, creatinine, albumin, and total proteins were determined at T0 and T1. Blood glucose measurements were frequently indicated as for patients' metabolic control.
2.6. Biochemical determinations
All the biochemical parameters were determined by spectrophotometric methods using commercial kits. Pro‐inflammatory markers included C reactive protein (CRP) [PTX1, Human ELISA Kit, Abcam]; interleukin (IL)‐1β (IL‐1 beta Human ELISA Kit, Abcam); IL‐6 (IL‐6 Human ELISA Kit, Abcam); and intercellular adhesion molecule 1 (ICAM‐1) [CD54, Human SimpleStep ELISA Kit, Abcam]. Redox balance markers included oxidative capacity (PerOx [TOS/TOC] Kit, Immundiagnostik, Germany); antioxidant capacity (Total Antioxidant Capacity Assay Kit, Abcam); and nitrite/nitrate ratio (Nitric Oxide Assay Kit, Abcam). Components of the advanced glycation end‐products (AGE) pathway were assessed using the following commercial kits: General AGE ELISA Kit (Donglin, China); RAGE Human SimpleStep ELISA Kit (Abcam); and Human Pentosidine ELISA Kit (BlueGene, China). Salivary EGF levels were determined using the Quantikine Human EGF Immunoassay (R & D Systems). In all cases, manufacturer's instructions were followed.
2.7. Cytokine profiling
Plasma samples underwent fewer than two freeze–thaw cycles before measurements. Seventeen cytokines were determined using magnetic bead assays (HCD8MAG15K17PMX human [Millipore Sigma Corp., Billerica, Massachusetts]). Proteins evaluated included granulocyte‐macrophage colony‐stimulating factor (GM‐CSF), soluble cluster of differentiation 137 (sCD137), soluble FAS (sFAS), FAS ligand (FASL), interferon gamma (IFN‐γ), IL‐2, IL‐4, IL‐5, IL‐6, IL‐10, IL‐13, tumour necrosis factor alpha (TNF‐α), macrophage inflammatory protein 1‐alpha (MIP1‐a), macrophage inflammatory protein 1‐beta (MIP1‐b), granzyme A (GRZA), granzyme B (GRZB), and perforin (PRF). A total of 25 μL of subjects' plasma at T0 and T1 were analysed in duplicate as instructed by the manufacturer. Briefly, plasma samples and cytokine capture‐bead cocktails were incubated for 20 hours at 4°C under agitation at 600 rpm. After washing and supernatant removal, biotin‐labelled anticytokine mixtures were added for 1 hour at 20 ± 2°C followed by the addition of a streptavidin‐phycoerythrin solution. Quantitation was performed on a Luminex MAGPIX instrument using the xPONENT 4.2 software and analysed with the Milliplex Analyst software (v5.1, Millipore) with five parameter logistics and standard curves (r 2 > 0.99 in all cases). Measurements on blinded replicate samples included with test samples had coefficients of variation of 10–25%.
2.8. Statistical analyses
Statistical analyses were performed using the GraphPad Prism 6.01 software. Normal distribution was analysed using D'Agostino‐Pearson and Shapiro–Wilk normality tests. Variance homogeneity was evaluated using Brown‐Forsythe and Bartlett's tests. If data were in compliance with normal distribution and variance homogeneity, comparisons between time points were carried out using the Student's paired t test. Otherwise, Wilcoxon test was performed. Values of P < .05 indicated statistically significant differences. Cytokines/chemokine level changes at T1 were compared using a paired t test, with a significance level of α = .05. Spearman correlation was run among T0/T1 level variations of systemic markers.
3. RESULTS
3.1. Demographics
A total of 11 type 2 diabetic patients with neuropathic DFU were studied. Male gender predominated over female with 10 men (91%) vs 1 woman (9%). Patients were between 48 and 73 years old (59.5 ± 7.6 years). Diabetes evolution time ranged between 2 and 35 years (15.6 ± 9.6 years). On the other hand, wound evolution times were in the range 30–180 days (51.8 ± 46.6 days). All lesions were classified as grade 3 of Wagner's scale and grades 1B‐2D according to the Texas classification of wound.
3.2. Systemic response of patients with DFU to the intralesional treatment with EGF
Plasma samples from patients with DFU, before (T0) and after receiving nine doses of intralesional EGF (T1), were analysed regarding haematological and haemochemical parameters, pro‐inflammatory, and redox balance markers, so as that of the molecules of the AGE pathway. Haematological and haemochemical parameters are summarised in Table 1. Erythrosedimentation rate underwent a highly significant reduction (P = .0006) at T1 with respect to T0, with a mean value decrease of 28%. All the patients showed reduced erythrosedimentation rates in more than 10%. The rest blood chemistry parameters analysed did not significantly modify.
Table 1.
Haematologic and haemochemical parameters of patients before (T0) and after (T1) intralesional treatment with EGF
| Parameters | T0 | T1 | P |
|---|---|---|---|
| Erythrosedimentation (mm/h) | 65.9 ± 36.6 | 47.7 ± 30.3 | *** |
| Leukocytes (×109/L) | 9.2 ± 2.0 | 8.1 ± 1.7 | ns |
| Haemoglobin (g/L) | 117.5 ± 17.4 | 113.4 ± 19.5 | ns |
| HbA1c (%) | 10.12 ± 2.16 | 9.67 ± 2.04 | ns |
| Glycaemia (mmol/L) | 8.44 ± 2.55 | 8.28 ± 2.57 | ns |
| Creatinine (μmol/L) | 99.6 ± 24.6 | 98.6 ± 27.5 | ns |
| Albumin (g/L) | 39.6 ± 5.1 | 41.8 ± 5.0 | ns |
| Total proteins (g/L) | 69.5 ± 5.5 | 69.0 ± 7.6 | ns |
Note: Statistical analyses were performed using the Student's paired t test.
Abbreviations: HbA1c, glycated haemoglobin, ns, not significant.
P < .001.
Pro‐inflammatory markers are shown in Figure 1. CRP exhibited no statistical differences between T0 and T1 moments (P = .1826) (Figure 1A). However, the median value at T1 was 45% lower than the median value at T0. ICAM‐1 and IL‐1β levels were not significantly modified after treatment with respect to baseline (P = .0506 and P = .1266, respectively) (Figure 1B, C). Moreover, IL‐6 levels significantly decreased at T1 (P = .0049), with a median value drop of 66% (Figure 1D).
Figure 1.
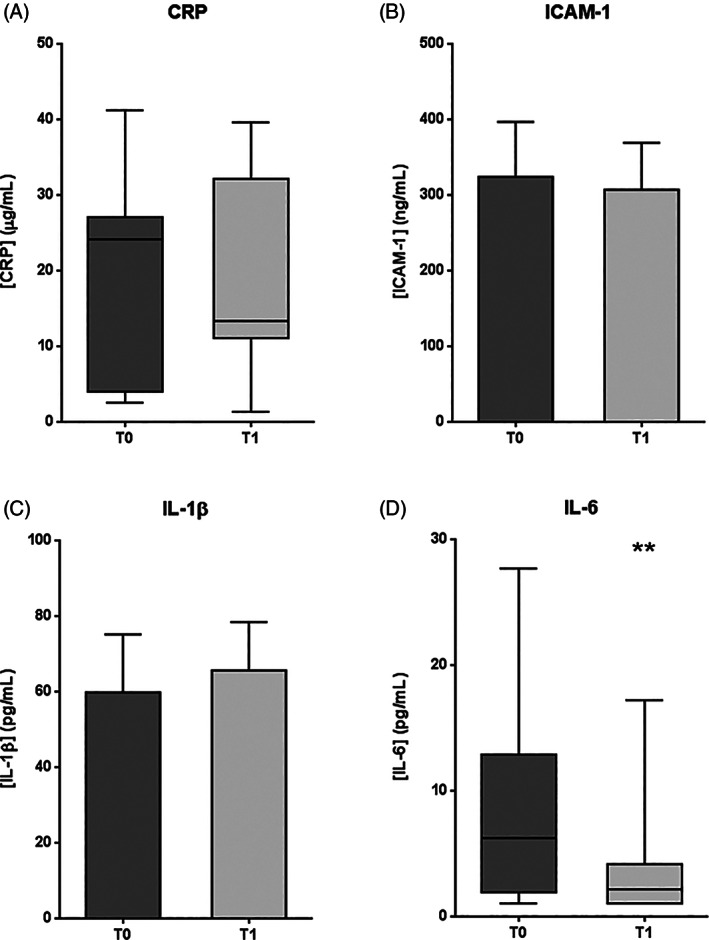
Pro‐inflammatory marker levels in plasma samples from patients with DFU, before (T0) and after (T1) intralesional EGF treatment. A, C reactive protein (CRP). B, intercellular adhesion molecule 1 (ICAM‐1). C, Interleukin‐1β (IL‐1β). D, Interleukin‐6 (IL‐6). If data are in compliance with normal distribution, they are expressed as mean ± SD. Otherwise, they are represented as the median, 25th and 75th percentiles. Statistical analyses were performed using the Student's paired t test (data with normal distribution) or the Wilcoxon test (data without normal distribution). **P < .01
A broader evaluation of inflammatory markers using a Luminex magnetic bead assay validated the reduction of IL‐6 circulating levels at T1 (P = .0273), which appeared accompanied by a significant reduction of other pro‐inflammatory mediators like MIP1‐a (P = .0273) and sFAS (P = .0371) (Figure 2). Analyses of anti‐inflammatory cytokines such as IL‐4, IL‐5, IL‐10, and IL‐13 suggested a split behaviour within the patients' cohort: (a) with a reduction parallel to the drop of the pro‐inflammatory counterpart and (b) an induction that seems to counteract the increased levels of the pro‐inflammatory arm (data not shown).
Figure 2.
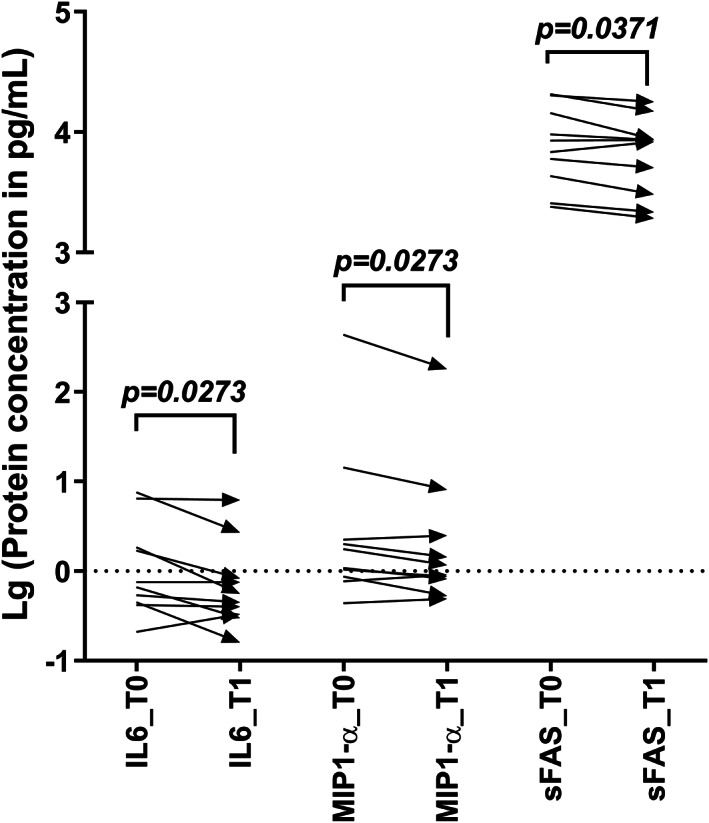
Results from Milliplex‐based evaluation of CD8‐related cytokines in plasma samples from patients with DFU. The behaviour of cytokine concentration from the baseline (T0) to 3 weeks after starting intralesional EGF treatment (T1) is represented for those factors showing significant concentration changes: interleukin‐6 (IL‐6), macrophage inflammatory protein 1‐alpha (MIP1‐a), and soluble FAS (sFAS). Data are shown as the base‐10 logarithm of protein concentration. Statistical analyses were performed using the Wilcoxon‐matched pairs‐signed rank test (P < .05)
Redox balance markers are represented in Figure 3. Total oxidative capacity exhibited a significant reduction after EGF treatment with respect to baseline (P = .0299) (Figure 3A). The mean value at T1 was 16% lower than the mean value at T0. Conversely, antioxidant capacity showed no significant variation between T0 and T1 (P = .4894) (Figure 3B). On the other hand, the nitrite/nitrate ratio was significantly lower at T1 than before treatment (P = .0123), with a mean value decrease of 57% (Figure 3C).
Figure 3.
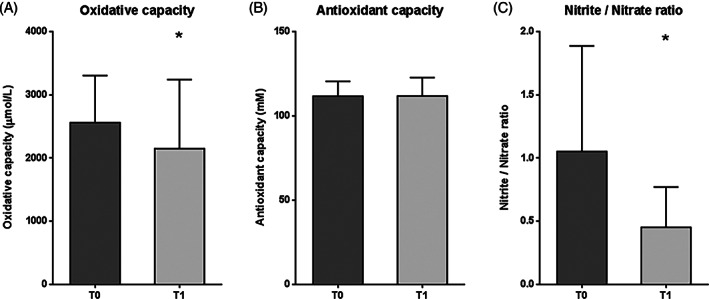
Redox balance marker levels in plasma samples from patients with DFU, before (T0) and after (T1) intralesional EGF treatment. A, Oxidative capacity. B, Antioxidant capacity. C, Nitrite/nitrate ratio. Data are expressed as mean ± SD. Statistical analyses were performed using the Student's paired t test. *P < .05
AGE levels showed no statistical differences between T0 and T1 (P = .3501) (Figure 4A). However, pentosidine levels were significantly reduced after treatment with respect to baseline (P = .0122), with a median value decrease of 19% (Figure 4B). In addition, soluble RAGE exhibited a significant increase at T1 (P = .0210), with a median value rise of 16% (Figure 4C). Salivary EGF levels were not significantly modified after treatment with respect to T0 (P = .4458), as represented in Figure 5.
Figure 4.
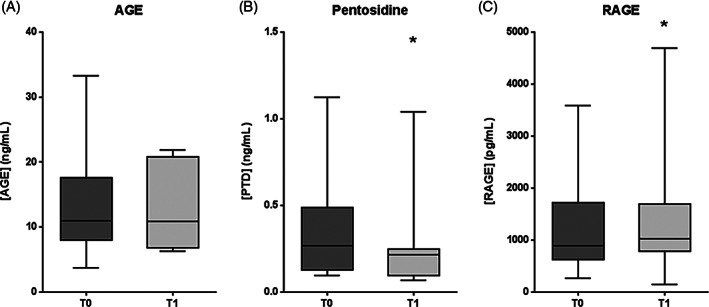
AGE pathway molecule levels in plasma samples from patients with DFU, before (T0) and after (T1) intralesional EGF treatment. A, Advanced glycation end‐products (AGE). B, Pentosidine. C, Receptor for AGE (RAGE). Data are expressed as the median, 25th, and 75th percentiles. Statistical analyses were performed using the Wilcoxon test. *P < .05
Figure 5.

Salivary EGF levels in samples from patients with DFU, before (T0) and after (T1) intralesional EGF treatment. Data are expressed as mean ± SD. Statistical analysis was performed using the Student's paired t test
4. DISCUSSION
The present study provides preliminary evidence that locally infiltrated EGF into neuropathic diabetic lower extremity wounds, translates in the reduction of some pro‐inflammatory and other harmful biomarkers. Despite the limitations inherent to a small cohort of patients and the lack of a concurrent control group receiving the conventional therapy without EGF treatment, this seems to be the first clinical demonstration to our knowledge, describing the putative effects of EGF on the systemic endovascular inflammation in diabetic subjects. This assumption may broaden the spectrum of EGF‐associated pharmacological bounties and would confirm basic science studies.19, 20
Our group had previously demonstrated that EGF intralesional infiltrations for 3–4 weeks (9–12 infiltrations) associated with the standard of care, beyond promoting granulation tissue over 75% of the wounded area, and contributed to restore the circulating levels of several redox biology parameters up to values close to those of non‐ulcerated diabetic patients and non‐diabetic subjects.11 In this opportunity, using a similar clinical investigation protocol and under a 3‐week treatment timeframe, we investigated the impact of EGF intervention on four major fields including1: ordinary/routine blood chemistry,2 acute reactants and pro‐inflammatory mediators,3 oxidative and nitrosilation stress, and4 the AGE/RAGE axis.
The current standard of care21 supported with the EGF intervention under the in‐hospital regimen showed not to significantly impact on the haemochemical parameters studied. Of note is, however, that EGF accounted for a significant reduction at T1 of erythrocyte sedimentation rate and IL‐6 although with no modifications of albumin levels. Albumin circulating levels would be expected to marginally increase given the attenuation of the acute‐phase reactant.22 The fact that EGF somewhat opposed to acute‐phase reactants is supported by the fact that 3 weeks after treatment, CRP median values were arithmetically reduced to 45%. A hypothetical interpretation of these clinical findings incites to speculate that the systemic exposure to EGF contributes to attenuate the hepatic‐reactive phenotype.23 Previous studies document the modulating effect of circulating EGF on hepatic‐derived acute phase proteins.24 Other in vitro studies illustrate the effect of EGF on modulating acute‐phase proteins on hepatic cells.25, 26
Although with an impartial effect on ICAM‐1 and the inflammasome effector IL‐1β, the observation that EGF decreased IL‐6 associated with a significant reduction of both sFAS and MIP1‐a circulating levels is at least of pharmacological connotation. IL‐6 plays a definitive pathogenic role in T2‐DM onset, progression, and complications as has been demonstrated.27 sFAS has been associated with insulin resistance, onset of T2‐DM, as to the ensued endovascular inflammation, diabetes‐related apoptosis, and multiorgan complications.28 The chemokine MIP1‐a is one of the inflammatory cytokines induced by high glucose levels,29 and pathogenically involved in prediabetes and diabetic chronic inflammation.30 Collectively, our findings suggest that systemic exposure to EGF may be modulating these three major well‐renowned biomarkers of T2‐DM and its ensued complications pathophysiology. This fact reinforces the need for more comprehensive clinical studies. Simply said, dampening IL‐6 circulating levels could contribute to restore metabolic homeostasis in diabetes‐afflicted patients, as to reinstate tissues' pro‐anabolic programmes in the course of the healing process.31
By its side, the analyses of anti‐inflammatory cytokines do not show a clear‐cut distribution, although with a clear trend to the induction of IL‐13, IL‐10, and IL‐5 in patients with higher pro‐inflammatory cytokine levels. Again, further studies including a broader sampling window will be necessary.
Diabetes is a multifactorial toxic entity in which glucotoxicity is associated with increased reactive oxygen species levels, disruption of the balance between pro‐degradative an pro‐synthetic forces, as to the AGE/RAGE noxious impact, thus leading to cellular proliferative and survival reserves demise.32 In line with this, our group had previously demonstrated that ulcerated diabetic subjects behave as a particular diseased group, as they exhibit an exacerbation of the oxidative stress arm as compared with non‐ulcerated diabetics.11 Here, we confirm that nine EGF infiltrations over 3 weeks were sufficient to reduce the circulating levels of oxidative reactants although with no effect on the circulating antioxidant defence reserves. The high level of oxidative stress induced by hyperglycaemia and other diabetes‐derived derangements has the potential to foster premature organismal senescence32 and therefore to disrupt the physiological healing response.33
Hyperglycaemia along with other metabolic changes appears proximal to nitric oxide (NO) production and utilisation impairments by an endothelial dysfunction.34 The fact that EGF treatment significantly reduced the nitrite levels, as the main NO‐derived oxidation product35 at T1 is of paramount clinical relevance. Converging types of evidence indicate that nitrosilative stress is strongly associated with a myriad of diabetes‐related complications, organs' dysfunctions, and ultimately to patients outcome.36, 37 Cutaneous wound repair is also dramatically influenced by NO metabolism38 where an exquisite fine‐tuning is required at both organismal and in the wound milieu levels for a proper healing trajectory.39
AGE are a heterogeneous group of molecules that upon their interaction with the cell‐bound RAGE results in generation of oxygen radicals, pro‐inflammatory cytokines, and cell adhesion molecules overexpression, altogether largely involved in diabetic multiorgan complications pathophysiology.40 Here, we show that although EGF did not contribute to reduce the burden of AGE circulating levels, pentosidine appeared as the unique responder among the constellation of AGE. Pentosidine is not only a glycation but an oxidation‐derived product (glycoxidation product), which may theoretically correlate with the detected attenuation of the oxidative reactants by EGF. Previous reports associate pentosidine circulating levels with diabetic chronic complications, hence pentosidine has been invoked as a bona fide predictor for diabetic complications.41 An enlightening finding related to the AGE/RAGE is the significant elevation of plasma levels of endogenous soluble RAGE, which acts as a “decoy receptor” that inhibits the AGE/RAGE signalling axis, thus preventing the amplification of cytotoxic episodes. This observation prompts further studies for confirmation given its potential therapeutic significance.
A cascade of serendipitous events led to the discovery of salivary EGF about 60 years ago.42 EGF production is impaired by diabetes and it has been invoked as a major factor for wound chronification.43 Experimentally induced diabetes damages the submandibular salivary glands physiology, accounting for a concomitant reduction of EGF output.44 Furthermore, reduced levels of salivary EGF in diabetic patients contribute to the development of oral and systemic complications of diabetes.45 This theoretical background encouraged us to examine the possible impact of EGF infiltration on its salivary levels. Ultimately, there was no variation on the morning salivary content of EGF after completing the nine infiltration sessions when wounds had healed to 75%. Additional studies are required to shed light on the potential involvement of EGF endocrine and paracrine profiles in diabetic‐ulcerated individuals.
An integral vision of these findings advice that behind the healing success achieved upon EGF infiltration, with a resulting reduction of amputation risks and long‐term low recurrence rates,10, 16 there is an extensive profile of pharmacodynamic effects, given by the systemic restoration of multiple and converging molecular events that facilitate the resumption of a physiologic healing trajectory. Infiltrated EGF, in addition to assistance in the amendment of soluble imbalanced mediators, is theoretically endowed with the ability to counteract the diabetic‐associated premature senescence.46 The actual validation of this notion could provide the bases for novel therapeutic horizons to deter diabetic complications.
ACKNOWLEDGEMENTS
The authors hereby acknowledge the support provided by the Director of the National Institute of Angiology and Vascular Surgery, in Havana, Cuba, for his logistic assistance.
García‐Ojalvo A, Berlanga Acosta J, Figueroa‐Martínez A, et al. Systemic translation of locally infiltrated epidermal growth factor in diabetic lower extremity wounds. Int Wound J. 2019;16:1294–1303. 10.1111/iwj.13189
REFERENCES
- 1. Fang P, Shi M, Zhu Y, Bo P, Zhang Z. Type 2 diabetes mellitus as a disorder of galanin resistance. Exp Gerontol. 2016;73:72‐77. [DOI] [PubMed] [Google Scholar]
- 2. Kolb H, Martin S. Environmental/lifestyle factors in the pathogenesis and prevention of type 2 diabetes. BMC Med. 2017;15(1):131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Piaggesi A, Apelqvist J. The Diabetic Foot Syndrome. Pisa, Lund: Karger; 2018. [Google Scholar]
- 4. Martin P, Nunan R. Cellular and molecular mechanisms of repair in acute and chronic wound healing. Br J Dermatol. 2015;173(2):370‐378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Williams FN, Herndon DN. Metabolic and endocrine considerations after burn injury. Clin Plast Surg. 2017;44(3):541‐553. [DOI] [PubMed] [Google Scholar]
- 6. Han G, Ceilley R. Chronic wound healing: a review of current management and treatments. Adv Ther. 2017;34(3):599‐610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yazdanpanah L, Nasiri M, Adarvishi S. Literature review on the management of diabetic foot ulcer. World J Diabetes. 2015;6(1):37‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Frykberg RG, Banks J. Challenges in the treatment of chronic wounds. Adv Wound Care (New Rochelle). 2015;4(9):560‐582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Berlanga‐Acosta J, Fernandez‐Montequin J, Valdes‐Perez C, et al. Diabetic foot ulcers and epidermal growth factor: revisiting the local delivery route for a successful outcome. Biomed Res Int. 2017;2017:2923759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lopez‐Saura PA, Yera Alos IB, Valenzuela‐Silva C, et al. Medical practice confirms clinical trial results of the use of Intralesional human recombinant epidermal growth factor in advanced diabetic foot ulcers. Adv Pharmacoepidem Drug Safety. 2013;2(2):128. [Google Scholar]
- 11. Ojalvo AG, Acosta JB, Mari YM, et al. Healing enhancement of diabetic wounds by locally infiltrated epidermal growth factor is associated with systemic oxidative stress reduction. Int Wound J. 2017;14(1):214‐225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wagner FW. The dysvascular foot: a system for diagnosis and treatment. Foot Ankle. 1981;2(2):64‐122. [DOI] [PubMed] [Google Scholar]
- 13. Armstrong DG, Lavery LA, Nixon BP, Boulton AJ. It's not what you put on, but what you take off: techniques for debriding and off‐loading the diabetic foot wound. Clin Infect Dis. 2004;39(Suppl 2):S92‐S99. [DOI] [PubMed] [Google Scholar]
- 14. Martinez A, Lopez LD, Perez R, et al. Uso del Factor de Crecimiento Epidérmico humano recombinante en crema de sulfadiacina de plata en el tratamiento de pacientes quemados. Biotecnología Aplicada. 1994;11(3):209‐212. [Google Scholar]
- 15. Cinza AM, Quintana M, Lombardero J, et al. A batch process for production of human epidermal growth factor in yeast. Product characterization. Biotecnología Aplicada. 1991;8(2):166‐173. [Google Scholar]
- 16. Berlanga J, Fernandez JI, Lopez E, et al. Heberprot‐P: a novel product for treating advanced diabetic foot ulcer. MEDICC Rev. 2013;15(1):11‐15. [DOI] [PubMed] [Google Scholar]
- 17. Schultz GS, Sibbald RG, Falanga V, et al. Wound bed preparation: a systematic approach to wound management. Wound Repair Regen. 2003;11(Suppl 1):S1‐S28. [DOI] [PubMed] [Google Scholar]
- 18. Valenzuela‐Silva CM, Tuero‐Iglesias AD, Garcia‐Iglesias E, et al. Granulation response and partial wound closure predict healing in clinical trials on advanced diabetes foot ulcers treated with recombinant human epidermal growth factor. Diabetes Care. 2013;36(2):210‐215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Berlanga‐Acosta J, Gavilondo‐Cowley J, Lopez‐Saura P, et al. Epidermal growth factor in clinical practice ‐ a review of its biological actions, clinical indications and safety implications. Int Wound J. 2009;6(5):331‐346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Circolo A, Pierce GF, Katz Y, Strunk RC. Antiinflammatory effects of polypeptide growth factors. Platelet‐derived growth factor, epidermal growth factor, and fibroblast growth factor inhibit the cytokine‐induced expression of the alternative complement pathway activator factor B in human fibroblasts. J Biol Chem. 1990;265(9):5066‐5071. [PubMed] [Google Scholar]
- 21. American DA. 10. Microvascular complications and foot care: standards of medical Care in Diabetes‐2018. Diabetes Care. 2018;41(Suppl 1):S105‐S118. [DOI] [PubMed] [Google Scholar]
- 22. Chang DC, Xu X, Ferrante AW Jr, Krakoff J. Reduced plasma albumin predicts type 2 diabetes and is associated with greater adipose tissue macrophage content and activation. Diabetol Metab Syndr. 2019;11:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Roncero C, Fabregat I, Benito M. Regulation of gene expression by interleukin‐6 in fetal rat hepatocyte primary cultures: role of epidermal growth factor and dexamethasone. Hepatology. 1995;22(6):1769‐1775. [PubMed] [Google Scholar]
- 24. Trautwein C, Boker K, Manns MP. Hepatocyte and immune system: acute phase reaction as a contribution to early defence mechanisms. Gut. 1994;35(9):1163‐1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Koj A, Rokita H, Kordula T, Kurdowska A, Travis J. Role of cytokines and growth factors in the induced synthesis of proteinase inhibitors belonging to acute phase proteins. Biomed Biochim Acta. 1991;50(4–6):421‐425. [PubMed] [Google Scholar]
- 26. Mackiewicz A, Speroff T, Ganapathi MK, Kushner I. Effects of cytokine combinations on acute phase protein production in two human hepatoma cell lines. J Immunol. 1991;146(9):3032‐3037. [PubMed] [Google Scholar]
- 27. Pickup JC. Inflammation and activated innate immunity in the pathogenesis of type 2 diabetes. Diabetes Care. 2004;27(3):813‐823. [DOI] [PubMed] [Google Scholar]
- 28. Margaryan S, Witkowicz A, Arakelyan A, Partyka A, Karabon L, Manukyan G. sFasL‐mediated induction of neutrophil activation in patients with type 2 diabetes mellitus. PLoS One. 2018;13(7):e0201087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li MF, Zhang R, Li TT, et al. High glucose increases the expression of inflammatory cytokine genes in macrophages through H3K9 Methyltransferase mechanism. J Interferon Cytokine Res. 2016;36(1):48‐61. [DOI] [PubMed] [Google Scholar]
- 30. Meshkani R, Vakili S. Tissue resident macrophages: key players in the pathogenesis of type 2 diabetes and its complications. Clin Chim Acta. 2016;462:77‐89. [DOI] [PubMed] [Google Scholar]
- 31. Ridker PM. From C‐reactive protein to Interleukin‐6 to interleukin‐1: moving upstream to identify novel targets for atheroprotection. Circ Res. 2016;118(1):145‐156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Palmer AK, Tchkonia T, LeBrasseur NK, Chini EN, Xu M, Kirkland JL. Cellular senescence in type 2 diabetes: a therapeutic opportunity. Diabetes. 2015;64(7):2289‐2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Berlanga‐Acosta J, Schultz GS, Lopez‐Mola E, Guillen‐Nieto G, Garcia‐Siverio M, Herrera‐Martinez L. Glucose toxic effects on granulation tissue productive cells: the diabetics' impaired healing. Biomed Res Int. 2013;2013:256043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Adela R, Nethi SK, Bagul PK, et al. Hyperglycaemia enhances nitric oxide production in diabetes: a study from south Indian patients. PLoS One. 2015;10(4):e0125270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Allen JD, Miller EM, Schwark E, Robbins JL, Duscha BD, Annex BH. Plasma nitrite response and arterial reactivity differentiate vascular health and performance. Nitric Oxide. 2009;20(4):231‐237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Assmann TS, Brondani LA, Boucas AP, et al. Nitric oxide levels in patients with diabetes mellitus: a systematic review and meta‐analysis. Nitric Oxide. 2016;61:1‐9. [DOI] [PubMed] [Google Scholar]
- 37. Mishra S, Mishra BB. Study of lipid peroxidation, nitric oxide end product, and trace element status in type 2 diabetes mellitus with and without complications. Int J Appl Basic Med Res. 2017;7(2):88‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shi HP, Most D, Efron DT, Witte MB, Barbul A. Supplemental L‐arginine enhances wound healing in diabetic rats. Wound Repair Regen. 2003;11(3):198‐203. [DOI] [PubMed] [Google Scholar]
- 39. Andre‐Levigne D, Modarressi A, Pepper MS, Pittet‐Cuenod B. Reactive oxygen species and NOX enzymes are emerging as key players in cutaneous wound repair. Int J Mol Sci. 2017;18(10):2‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Prasad K, Tiwari S. Therapeutic interventions for advanced Glycation‐end products and its receptor‐ mediated cardiovascular disease. Curr Pharm des. 2017;23(6):937‐943. [DOI] [PubMed] [Google Scholar]
- 41. Haddad M, Knani I, Bouzidi H, Berriche O, Hammami M, Kerkeni M. Plasma levels of pentosidine, carboxymethyl‐lysine, soluble receptor for advanced glycation end products, and metabolic syndrome: the metformin effect. Dis Markers. 2016;2016:6248264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cohen S. Origins of growth factors: NGF and EGF. Ann N Y Acad Sci. 2004;1038:98‐102. [DOI] [PubMed] [Google Scholar]
- 43. Berlanga‐Acosta J, Mendoza‐Mari Y, Garcia‐Ojalvo A, Acosta‐Buxado JA, Fernandez‐Mayola M, Guillen‐Nieto G. Epidermal growth factor (EGF) intralesional infiltrations: from the bench to the diabetic ulcers cells. Integr Mol Med. 2019;6(1):1‐7. [Google Scholar]
- 44. El Sadik A, Mohamed E, El Zainy A. Postnatal changes in the development of rat submandibular glands in offspring of diabetic mothers: biochemical, histological and ultrastructural study. PLoS One. 2018;13(10):e0205372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Oxford GE, Tayari L, Barfoot MD, Peck AB, Tanaka Y, Humphreys‐Beher MG. Salivary EGF levels reduced in diabetic patients. J Diabetes Complications. 2000;14(3):140‐145. [DOI] [PubMed] [Google Scholar]
- 46. Berlanga‐Acosta J, Mendoza‐Mari Y, Garcia‐Ojalvo A, Fernandez‐Mayola M, Guillen‐Nieto G. Epidermal growth factor therapy impact on scar tissue resilience of diabetic lower limbs ulcers‐an enlightening hypothesis. J Diabetes Metab. 2018;9(7):798. [Google Scholar]


