Abstract
Chronic, nonhealing diabetic foot ulcers (DFU) are increasing in prevalence and are often unresponsive to conventional therapy. Adipose tissue, containing adipose‐derived stem cells, and platelet rich plasma (PRP) are regenerative therapies rich in growth factors which may provide a solution to chronic wound healing. This study aimed to assess the feasibility of conducting a definitive randomised controlled trial (RCT) to investigate the efficacy of these therapies for the treatment of DFU. This was a single centre, feasibility, three‐arm, parallel group RCT. Eligible DFU patients were randomised on a 1:1:1 basis to three intervention arms: control (podiatry); fat grafting; fat grafting with PRP. The intervention was delivered once and patients were followed‐up for 12 weeks. The primary objective was to assess measures of trial feasibility. Clinical outcomes and health‐related quality of life (HRQoL) were also evaluated. Three hundred and thirty four patients were screened and 32 patients (9.6%) were deemed eligible with 18 enrolled in the trial (6 per arm) over 17 months. All participants completed the trial with no withdrawals or crossover. Participant engagement was high with most HRQoL questionnaires returned and only 4.8% follow‐up appointments missed. There were five adverse events (AEs) related to the trial with no serious AEs. Five (28%) of the wounds healed. There was no difference between any of the groups in terms of clinical outcomes. This feasibility study demonstrated that a multi‐centre RCT is safe and feasible with excellent patient engagement. We have highlighted crucial information regarding methodology and recruitment, which will guide future trial design. Registration number: NCT03085550 clinicaltrials.gov. Registered 01/03/2017.
Keywords: diabetic foot ulcers, fat grafting, platelet rich plasma, wound healing
1. INTRODUCTION
The prevalence of diabetes mellitus is increasing, with an estimated 4 million people with diabetes in the UK and 422 million worldwide. 1 Chronic wounds, a common sequelae of diabetes, cost the National Health Service (NHS) over £5billion per year. 2 Approximately, 6% of the 4 million people in the UK with diabetes will develop diabetic foot ulcers (DFUs) and 85% of all amputations are preceded by an ulcer. 3 Complications or co‐morbid conditions in DFU such as infection, foot deformities and vascular insufficiency can be managed effectively with a multi‐disciplinary approach. However, there remains a significant number of DFU patients who suffer with chronic nonhealing wounds. Podiatry care for these patients can be time‐consuming, costly, and have inconsistent efficacy. Conventional reconstructive options, such as grafts and flaps, have limited efficacy in DFU and infection rates are high. Therefore, there is a need to explore new therapies, which may enhance healing in a cost‐effective manner.
Fat grafting is popular in reconstructive surgery due to the ease of tissue harvest, favourable safety profile, and abundance of autologous tissue available. It has been used effectively for contouring procedures for several years. 4 However, interest regarding the regenerative potential of fat has increased in recent years. This may, in part, be due to fat containing adipose‐derived stem cells (ADSCs), which unlike bone‐derived stem cells, are easily obtained and can be grafted directly without the need for culture or expansion. 5 Fat tissue is also rich in growth factors, and cytokines, which encourage healing through cell migration, reduced inflammation, and angiogenesis. 6 Also fat tissue, unlike purified ADSCs, is not subject to therapeutic and legislative restrictions. 7 Several studies have shown fat to have regenerative effects in reducing scar tissue 8 and in treating scleroderma, 9 burns, 10 and osteoarthritis. 11 There is also some evidence to suggest a benefit of fat grafting in the healing of chronic and acute wounds. However, a systematic review by the authors (in press) of the 10 case series 10 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 in the literature that have evaluated fat grafting for wound healing has shown that the standard of evidence is low due to most studies presenting only observational data with low numbers, wide methodological heterogeneity, and inadequate reporting of technical detail. The proportion of wounds achieving complete healing in these 10 studies was 58.4% and the average time to healing was 15 weeks. A limitation of fat grafting in the context of wound healing is that a significant majority of grafted fat will not survive after implantation, with a reported resorption rate of up to 80% 21 in the months following surgery, due to inadequate vascularisation, re‐perfusion injury, and anoikis. 22 Well‐vascularised fat grafts have improved retention rates in experimental conditions. 23 However, diabetic adipose tissue is more prone to poor vascularisation, as well as having fewer ADSCs, which secrete fewer growth factors and are less angiogenic. 24
Platelet‐rich plasma (PRP), an autologous blood product rich in growth factors and cytokines, may assist in fat vascularisation. PRP alone has been shown to have a regenerative effect on wounds, 25 burns, 26 alopecia, 27 osteoarthritis, 28 and nerves. 29 It may also have antimicrobial properties, which may enhance wound healing by limiting biofilm growth and infection. 30 PRP may act as a fibrin scaffold for ADSCs, preventing anoikis, retaining them at the graft site for longer, reducing cell death, and increasing the secretion of growth factors. 31 , 32 , 33 Several animal studies have shown improved fat graft retention in contouring procedures when PRP is co‐transplanted, with an enhancement in fat vascularity and reduction in fat necrosis. 34 , 35 , 36 These findings have also been reproduced in human studies. 37 , 38 A single animal study has investigated PRP‐fat co‐transplantation in a porcine wound healing model, and the authors identified an improvement in wound vascularity and cosmesis but no overall benefit to re‐epithelialisation. 39 Three human studies 40 , 41 , 42 have investigated PRP‐fat therapy in wound healing, (one poorly reported randomised trial and two case series), and however the quality of evidence was low, with many key aspects of methodology and outcome measure not reported. We conducted a systematic review of these studies and found 67% of wounds achieved complete healing and, where reported, average time to healing was 7.5 weeks. 43
As demonstrated, the evidence for both fat grafting and PRP‐fat grafting in wound healing is extremely limited, with a lack of level one evidence. This is further compounded by the wide variation in the methodology of preparing fat and PRP in the literature, where the reporting of procedural information is often inconsistent and inadequate. 44 These issues make clinical decision‐making regarding whether fat grafting or fat/PRP may be beneficial in wound healing very difficult. However, given that both these treatments are widely available, cheap and easy to deliver, and given that fat grafting is already widely used (often in contexts without an evidence base) high‐level evidence is needed to evaluate their efficacy and cost effectiveness. DFU patients represent a large pool of patients who may potentially benefit from these treatments; however, recruitment to DFU trials can be difficult. 45 Therefore, the aim of this study was to assess the methodology for, and the feasibility of, conducting a definitive randomised controlled trial (RCT) for fat grafting and fat/PRP grafting in DFU. The data generated from this trial would, if feasibility and safety was demonstrated, be used to power a larger multi‐centre study.
2. METHODS
2.1. Study design and participants
This study was a, randomised, single‐site, three‐armed, and parallel group feasibility trial. Patients undergoing treatment for DFU from the podiatry service at the Royal Free Hospital were pre‐screened against the eligibility criteria by the investigating team. Patients who were deemed to be not responding to treatment and therefore not on a healing trajectory were considered for enrolment. All DFUs that were showing evidence of healing with conventional therapy were not considered although this was not explicitly part of the eligibility criteria. Eligibility criteria are summarised in Table 1.
TABLE 1.
Trial inclusion and exclusion criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
| Age 18 to 90 at time of consent | Wound with active infection |
| Diabetic foot ulcer measuring more than 0.5 × 0.5 cm and less than 10 × 10 cm | Underlying severe vascular insufficiency (ankle branchial pressure index <0.3) |
| Wound clean with a healthy granulating bed and minimal adherent slough | Uncontrolled diabetes mellitus (HbA1C >90 mmol/mol) |
| Patient is able to provide their own consent and is willing to attend weekly followup visits | The presence of significant comorbidity rendering them unsuitable for ulcer healing trial: End stage renal disease (eGFR<15) or on renal replacement therapy, liver failure, auto‐immune disease, immunosuppression |
| Unfit for surgery (ASA Classification >3) |
Briefly, the trial recruited male or female adults (aged 18‐90 years) with at least one DFU, ulcer area >25 mm2 and <10 000 mm2, ankle branchial pressure index (ABPI) ≥0.7 taken within the previous 3 months or, if the ABPI was incompressible, other forms of clinical assessment to exclude severe peripheral arterial disease (e.g. peripheral pulse examination, vascular scans such as duplex ultrasound or angiography, and clinical judgement). After low initial recruitment numbers, the ABPI criteria were altered to ≥0.3 as detailed in the results section. All patients with vascular insufficiency were under the care of a vascular surgeon and if revascularisation was planned, they were excluded from consideration for the trial. All enrolled patients with previous wound infections were off antibiotics, with clean wounds as assessed by the treating surgeon or infectious diseases physician, any patient with suspicion of osteomyelitis was cleared by appropriate imaging (X‐ray or magnetic resonance imaging) prior to enrolment. For patients with more than one ulcer, the largest ulcer was chosen as the reference ulcer for the purposes of the trial. Potentially eligible participants were provided with an information sheet to consider for at least 24 hours before being approached by the research team for written informed consent. A full eligibility check was conducted for patients who gave consent and an eligibility case report form (CRF) was completed by an investigating doctor. This trial was sponsored by University College London (UCL), and had approval from the Health Research Authority (HRA), the National Research Ethics Service Committee London‐Chelsea (project ID: LO/16/0231) and from the National Health Service Research & Development Department, Royal Free Hospital. The trial has been registered at clinicaltrials.gov (ID NCT03085550).
2.2. Randomisation, allocation concealment, and blinding
Once consented, patients were randomly assigned on a 1:1:1 basis to either: control (podiatry standard of care); fat grafting; fat grafting with PRP. A random allocation sequence was generated independently of the research team by a medical statistician at UCL. The allocation sequence was sealed in identical opaque numerically ordered envelopes and given to the enrolling researcher upon receipt of patient consent. Owing to the nature of the interventions, it was not possible to blind participants or healthcare professionals to the intervention; however, the assessor for the primary clinical outcome measure was blinded. Following randomisation, we aimed to deliver interventions to all participants within 7 days.
2.3. Intervention group: Fat grafting
Patients randomised to this intervention arm underwent a single episode of fat grafting on Day 0 in the operating theatre. The aim was for all cases to be conducted under local anaesthesia, general anaesthesia would only be offered in exceptional circumstances after consultation with a consultant plastic surgeon and consultant anaesthetist. Baseline wound measurements and 3D volumetric pictures were taken of the wound prior to fat grafting but after debridement, and these were added to the CRF as the Day 0 measurements.
Fat grafting was performed as per current standard practice in our unit using the Coleman technique. 4 After administration of anaesthesia, the wound was initially debrided of all nonviable tissue and hyper‐keratotic areas and the wound edges were sharply debrided using a scalpel. Wounds were then covered with saline soaked gauze while fat was harvested. Fat was harvested from the abdomen in all cases. Prior to harvest, the abdominal fat was infiltrated with a tumescence solution (mix of 500 mL saline, 30 mL 0.5% bupivacaine, 1 unit of 1:10 000 adrenaline) as per the standard tumescent technique of liposuction. 46 A tumescent technique with bupivacaine was used as the use of this anaesthetic has been shown to maintain adipocyte viability. 47 Fat was then harvested using a 3.5 mm harvesting cannula attached to a manual syringe and then centrifuged at 3000 rpm for 3 minutes. This fat preparation process has also been shown to enhance fat graft survival. 48 Water and oil layers were discarded and the isolated lipoaspirate was transferred to a syringe attached to a sharp Y‐shaped infiltration cannula. An incision was made at least 1 cm from the wound edge, and fat was infiltrated using a threading technique to allow fat to fall into natural tissue planes. Minimal pressure was used and fat was infiltrated at a rate of 0.5 mL/s with care taken to avoid overfilling and blanching of the wound edges. Approximately 2 mL of lipoaspirate per square centimetre wound size was infiltrated. Following the procedure, the wound was dressed with Silflex silicone dressing (Advancis Medical, Nottinghamshire, UK) or Kaltostat alginate dressing (Convatec, Deeside, UK) if there was wound bleeding and an appropriate outer dressing as deemed necessary by the treating clinician.
2.4. Intervention group: Fat grafting with PRP
For patients randomised to this group the baseline wound measurements, initial debridement and fat grafting was identical to the fat group and patients underwent a single treatment on Day 0 in an operating theatre.
PRP was prepared using the Food and Drug Administration‐approved and CE‐marked Angel PRP processing device (Arthrex, Naples, Florida). Whole blood (52 mL) was obtained via peripheral venipuncture and was immediately combined with 8 mL of adenosine citrate dextrose acid (ACD‐A) in a 60 mL syringe. PRP with a haematocrit of 8% was produced using the Angel system. The system utilises a 3‐sensor ultraviolet flow cytometry method that senses cell specific wavelengths of light for platelets, red blood cells and platelet poor plasma, allowing their separation into sterile compartments. PRP was then combined with processed lipoaspirate in a 1:4 ratio and infiltrated as above. PRP was not activated with adjunctive agents prior to infiltration.
Following intervention, each patient was followed‐up weekly in the podiatry clinic and received standard wound care as decided by the treating podiatrist.
2.5. Control group: Podiatry care
Control participants received standard podiatry wound care on a weekly basis. This comprised:
review of recent haematology, biochemistry, microbiology, and radiology.
debridement of the base of the ulcer and any peri‐wound callus.
ulcer measurement as per protocol.
ulcer photography as per protocol.
redressing the ulcer with an appropriate dressing (as selected by the podiatrist).
offloading the ulcer with a device appropriate to the patient and ulcer site.
-
Where clinical signs of infection were present:
prescribed antibiotics
tissue taken for microbiology
radiology requested if osteomyelitis suspected
blood tests
The dressing selected for each participant was in line with local guidance and the podiatrist's clinical judgement, which is usual practice within the clinic and in line with NICE Guidance NG19. 49 A range of dressings types were used across the group and were selected on ulcer presentation, as would normally occur outside trial conditions. Participation in the trial did not alter dressings selected.
Offloading devices were selected by considering patient mobility, patient preference, and ulcer site. Offloading was not standardised within the trial, as this would have excluded some participants such as those with mobility issues, or where it was not required e.g. dorsal foot ulceration.
2.6. Baseline data and follow‐up
At the baseline visit, the following data were collected from each participant: demographics, wound size, and characteristics; 3D volumetric wound photographs using an Eykona wound management system camera (The QT company, Espoo, Finland); pressure ulcer scale for healing (PUSH) score, a validated wound assessment tool shown in Table 2 50 ; and a diabetic foot ulcer scale (DFS) validated quality of life questionnaire. 51 Day 0 was defined as the day of intervention or first podiatry appointment following randomisation. After randomisation, participants were followed up for 12 weeks, with weekly visits from weeks 1 to 6 and a final visit at week 12, or until 100% wound healing was achieved. At each visit, a doctor or podiatrist completed follow‐up CRFs to record wound measurements and characteristics, PUSH score, adverse events (AEs), the dressings used and their cost. 3D volumetric photographs were also taken at each visit and were stored in the patient's digital photo diary; an independent blinded analysis of the photo diary was carried out at the end of the trial. Between weeks 6 and 12 patients had routine follow‐up visits as deemed necessary by the podiatry team; if the wound was found to be healed within this time the investigating team were alerted and that appointment was deemed to be the end of the trial. At the end of trial visit (either 12 weeks or 100% healing) the CRF paperwork was completed, a final 3D photograph was taken and the patient completed a post‐trial DFS questionnaire.
TABLE 2.
Pressure ulcer scale for healing score wound assessment tool
| Length × Width (in cm2) | 0 | 1 | 2 | 3 | 4 | 5 | Sub‐score |
| 0 | <0.3 | 0.3‐0.6 | 0.7‐1.0 | 1.1‐2.0 | 2.1‐3.0 | ||
| 6 | 7 | 8 | 9 | 10 | |||
| 3.1‐4.0 | 4.1‐8.0 | 8.1‐12.0 | 12.1‐24.0 | >24.0 | |||
| Exudate amount | 0 | 1 | 2 | 3 | Sub‐score | ||
| None | Light | Moderate | Heavy | ||||
| Tissue type | 0 | 1 | 2 | 3 | 4 | Sub‐score | |
| Closed | Epithelial tissue | Granulation tissue | Slough | Necrotic tissue | |||
| Total score |
2.7. Feasibility
As the primary aim of this study was to assess trial feasibility, the primary outcome measures for feasibility of the trial were: eligibility rates (number meeting eligibility criteria versus the total number screened); reasons for ineligibility; enrolment rates (number randomised versus number eligible); reasons for nonenrolment in those eligible, withdrawal rates, and reasons; compliance with follow‐up schedule and reasons for noncompliance; ease of delivery of interventions including time to treatment following randomisation and number of local versus general anaesthetics. The trial protocol stated that each patient should have wound measurements, PUSH score, CRF, and 3D photography at each trial visit. Podiatry staff were provided training to record these assessments and the accuracy and completeness of each clinical outcome measure was evaluated for feasibility. The feasibility of using 3D photographs as the primary outcome measure was also assessed by comparing accuracy of volumetric measurements using the Eykona software to the manual measurements of wound size taken at each clinic visit.
2.8. Clinical outcomes
2.8.1. Wound size and healing
The primary clinical outcome in a definitive trial would be change in wound volume (mm3) over 12 weeks as measured by the 3D volumetric photographic wound management system. Secondary outcome measures would be: number of wounds achieving complete healing; time in days to complete healing; change in wound area (mm2) over 12 weeks as manually measured by a ruler at each visit; change in PUSH score over 12 weeks. Complete wound healing was defined as 100% re‐epithelialisation as judged by the treating podiatrist and clinician with no need for further dressing therapy. Hundred percentage wound healing would be confirmed by the blinded assessor on 3D analysis.
2.8.2. Adverse events
Data were collected on all AEs throughout the trial. All AEs were recorded in the trial log and these were reported to the sponsor within a week of occurrence if they were deemed to be “unexpected”. All serious AEs that were unexpected and related to the research procedures were reported to the sponsor in accordance with UCL guidelines.
2.8.3. Cost implications
The cost of the dressings used at each follow‐up visit was recorded and this was used as an indicator for the cost implications of the interventions. The feasibility of using this as a representation of cost of the interventions was also assessed.
2.8.4. Health‐related quality of life
All participants were asked to complete a validated health‐related quality of life (HRQoL) questionnaire that is specific to DFU patients, the DFS, before intervention and after the completion of the trial. The DFS has been validated in cross‐sectional and longitudinal studies and can discriminate between patients with healed ulcers and those with active wounds. The DFS examines the effect of DFU on 11 domains of patient's lives: leisure, physical health, daily activities, emotions, noncompliance with therapy, family, friends, positive attitude, treatment of DFU, satisfaction with medical care, and financial. A score out of 100 is assigned to each domain and data generated were used to evaluate the effect of the interventions on HRQoL. Compliance with completing the questionnaire was used as a measure of feasibility.
2.8.5. Sample size
As this was a feasibility trial, and the primary objective was to determine measures of feasibility rather than to detect a treatment effect, a formal power calculation was not conducted. The study aimed to randomise approximately 30 patients (10 per treatment arm) in order to assess eligibility, participation, and withdrawal of participants. The target was to recruit and complete the follow‐up of all 30 patients within 18 months from opening of the trial.
2.9. Statistical analysis
Analyses were conducted using the principles of intention to treat. As this was a feasibility study then clinical outcomes are reported descriptively with accompanying statistical analysis with the understanding that the study wasn't expected to be powered to show a treatment effect. Statistical analysis was performed to assess suitability of the data for the tests. Change in wound volume, size, PUSH score, and DFS score over time were analysed using an analysis of covariance (ANCOVA) for independent samples and repeated‐measures ANOVA for within‐group analysis. Quality of life data were analysed using a paired samples t‐test for within‐group analysis and comparison of group means was done using an independent sample t‐test or Mann Whitney U test for nonparametric data. Time to healing was summarised using Kaplan‐Meier curves and investigated using a stratified log‐rank test. A Bland‐Altman plot 52 was used to assess agreement of the measurement methods for wound size (3D photographs versus manual method) and a one‐sample t test was used to evaluate the difference between measurements.
3. RESULTS
The trial opened for recruitment on 8th February 2018 at the Royal Free Hospital, London and ran for 17 months, closing to recruitment on 2nd July 2019. Participant follow‐up was completed on 28th October 2019 after 21 months. The trial closed slightly beyond the target of 18 months for completion of follow‐up to allow further recruitment. Consent for enrolment was obtained from 18 patients with no exclusions before randomisation. Six participants were randomised to each of the three arms and all participants completed the full trial follow‐up period with no withdrawals. A summary diagram of participant flow through the trial is illustrated in Figure 1.
FIGURE 1.

CONSORT diagram depicting the flow of the participants through the trial
Only two patients were randomised in the first 3 months of the trial, and the most common excluding criteria as identified by the research team was ABPI ≥0.7. A substantial amendment was therefore submitted to the HRA on 11th May 2018 to alter these criteria to ABPI ≥0.3. This amendment was approved by the HRA on 25th June 2018 and 16 patients were recruited in the subsequent 12 months. The patient list was re‐screened following this amendment to ensure capture of any patient who was previously excluded who then met the inclusion criteria.
3.1. Baseline characteristics
Baseline data for the three groups are summarised in Table 3. The average age of participants was 57.6 years (range 35‐78), there were 15 male and 3 female recruits. Seventeen patients were taking oral hypoglycaemic agents and 11 were taking insulin; the mean HbA1C was 60 mmol/mol (range 31.1‐88). All of the patients had peripheral neuropathy as defined by loss of protective sensation and all had previously been on antibiotic therapy of ≥4 weeks for either wound infection or osteomyelitis. Only four patients had never smoked. Average duration of the wound was 49 weeks (range 8‐132), mean wound volume was 1489 mm3 (range 730‐11 120) and mean wound surface area was 360 mm2 (range 25‐1750). The offloading regimen chosen for each participant is summarised in Table 4.
TABLE 3.
A summary of participant demographics
| Total | Control | Fat grafting | Fat grafting + platelet rich plasma | |
|---|---|---|---|---|
| Age (years), mean (range) | 57.6 (35‐78) | 55.2 (41‐69) | 60.2 (45‐78) | 57.5 (35‐71) |
| Sex (M:F) | 15:3 | 4:2 | 6:0 | 5:1 |
| Body mass index (kg/m2), mean (range) | 29.3 (20.1‐42.9) | 30.7 (24‐42.9) | 26.9 (20.1‐30.3) | 28.9 (24‐34.3) |
| Insulin | 11 | 3 | 5 | 3 |
| Oral hypogylcaemics | 14 | 6 | 5 | 3 |
| HbA1C (mmol/mol), mean (range) | 60 (31.1‐88) | 53.9 (31.1‐74) | 68.6 (55.2‐88) | 57.6 (35.5‐82.5) |
| Smoking | ||||
| Never | 4 | 2 | 2 | 0 |
| Ex‐smoker | 11 | 2 | 4 | 5 |
| Current | 3 | 2 | 0 | 1 |
| Peripheral vascular disease | 6 | 2 | 2 | 2 |
| Previous vascular intervention | 5 | 1 | 2 | 2 |
| Previous amputation | 11 | 1 | 4 | 2 |
| Charcot foot deformity | 3 | 2 | 1 | 0 |
| Previous long term antibiotic therapy | 18 | 6 | 6 | 6 |
| Size of ulcer | ||||
| Volume (mm3) | ||||
| Mean (SD) | 1489 (2655) | 3105 (4123) | 816 (1115) | 546 (986) |
| Median (range) | 2300 (73‐11 120) | 1996 (172‐11 120) |
389 (111‐3000) |
156 (73‐2556) |
| Area (mm2) | ||||
| Mean (SD) | 360 (400) | 640 (590) | 310 (130) | 130 (140) |
| Median (range) | 290 (25‐1750) | 500 (120‐1750) | 340 (110‐490) | 65 (25‐340) |
| Duration of ulcer (weeks) | 47 (8‐132) | 49 (20‐132) | 41 (18‐88) | 54 (8‐104) |
| Wound location | ||||
| Plantar | 10 | 3 | 3 | 4 |
| Toe | 3 | 2 | 1 | 0 |
| Heel | 4 | 0 | 2 | 2 |
| Dorsum | 1 | 1 | 0 | 0 |
TABLE 4.
Distribution of offloading
| Offloading regimen | Control | Fat | Fat/platelet rich plasma |
|---|---|---|---|
| Darco + Peggassist | 1 | 2 | 2 |
| Aircast boot | 3 | 0 | 1 |
| Removable cast | 2 | 1 | 0 |
| Own shoe | 0 | 1 | 1 |
| Total contact casting (TCC) | 0 | 1 | 1 |
| Backslab | 0 | 0 | 1 |
| Surgical shoe | 0 | 1 | 0 |
3.2. Feasibility outcomes
3.2.1. Eligibility and participation
A total of 334 DFU patients were screened for trial eligibility with 32 patients (9.6%) being identified as potential recruits. Reasons for ineligibility are summarised in Figure 1. Of the 32 eligible patients 18 (56%) consented to participate in the trial. Reasons for nonparticipation in the 14 patients who refused were: unwilling to commit to follow‐up (8 patients); refusal to undergo intervention if randomised (2 patients); wound too small by time of review (2 patients); transport issues to attend (1 patient); and concerns regarding patient's capacity to consent to trial (1 patient).
3.2.2. Success of trial protocol
All of the 18 randomised participants completed the full trial period of 12 weeks or until 100% healing of their wound, giving a trial withdrawal rate of 0%. All patients remained within their randomised intervention arm and received the intervention as per protocol with no methodological deviations. A summary of the procedural details is summarised in Table 5. There were six missed follow‐up appointments out of a total of 126 (4.8%), with all patients attending on Weeks 1, 2, 4, and 12 (or final appointment after fully healed). The reasons for these missed appointments were: participant's holiday (4 cases), transport issues (2 cases). Aside from the six missed appointments, there were 10 further missed 3D photographs (16 missed in total, 12.7%) either due to a technical glitch when uploading to the software (five cases) or researcher error (five cases) where the reviewing podiatrist was not trained in using the 3D camera and research members were not available at the time to assist. Manual wound measurement, CRF and PUSH score were successfully recorded at 100% of attended follow‐up appointments.
TABLE 5.
A summary of procedural data for each intervention
| Total | Fat grafting | Fat grafting + PRP | |
|---|---|---|---|
| Volume of fat harvested (mL), mean (range) | 19.5 (8‐30) | 17.5 (8‐30) | 21.5 (10‐30) |
| Volume of fat or fat/PRP infiltrated (mL), mean (range) | 2.8 (2‐4.5) | 2.9 (2‐4.5) | 2.6 (2.4) |
| Volume of tumescent fluid used (mL), mean (range) | 43 (15‐150) | 57 (30‐150) |
29 (15‐50) |
| Volume of PRP harvested (mL), mean (range) | — | — | 3.2 (3‐4) |
| Local: general anaesthetic | 11:1 | 6:0 | 5:1 |
| Length of procedure (minutes), mean (range) | 36 (19‐62) | 38 (19‐62) | 35 (25‐50) |
Abbreviation: PRP, platelet rich plasma.
For participants randomised to intervention arms the average time to intervention was 21 days (range 8‐48). The protocol aimed to treat all patients within 7 days and this was achieved in zero participants. The primary reason (in 14 cases, 77.7%) was a lack of available theatre space in a large tertiary department where trauma and cancer procedures were prioritised. Three cases of delay were because of the patient's availability and one was due to the Christmas period reducing both clinical and participant availability with the decision taken to commence the trial in the New Year. Eleven of the 12 patients underwent their procedure under local anaesthetic. One patient received a general anaesthetic due to severe needle phobia. All patients received the interventions as day case procedures with no participants requiring hospital admission.
3.3. Clinical outcomes
3.3.1. Wound size
The primary clinical outcome measure was change in wound volume (mm3) over time and the percentage changes in mean wound volume for each group is illustrated in Figure 2. Because the number of 3D photos taken at Week 6 was inadequate for comparison this week's data have been excluded. The mean wound volume changes from Week 0 to Week 12 for each group were: Control—3105 to 2216 mm3; Fat grafting—816 to 815 mm3; Fat/PRP grafting—545 to 587 mm3. Wound area (mm2) was also measured manually at each follow‐up visit and the percentage change in mean and median wound area for each group is illustrated in Figure 3. The mean wound area changes from Week 0 to Week 12 for each group were: Control 640 to 340 mm2; Fat grafting 310 to 140 mm2; Fat/PRP grafting 130 to 90 mm2. A repeated measures ANOVA did not demonstrate a significant difference in the change in wound volume or area within any of the groups over time. There was also no significant difference in change in wound volume or area over 12 weeks between any of the groups. There was a significant difference in the Week 0 volume and size of the control group compared with the fat/PRP group (P < .05) indicating that the groups were not fairly matched on wound size despite randomisation.
FIGURE 2.

Percentage change in mean wound volume (mm3) over 12 weeks
FIGURE 3.

Percentage change in mean wound area (mm2)
The Bland‐Altman plots (Figure 4) indicated that measurement of wound volume in mm3 using 3D photographs and digital analysis estimated a higher wound volume compared with manual measurement with a ruler (length × width × depth). The average difference in these measurements was 407 mm3 (95% confidence intervals [CIs] −2734 and 3505 mm3). The plot indicated that the majority of cases fell within the 95% CI and that smaller wounds were more likely to be measured similarly with larger wounds showing the greatest difference. The difference between the two methods of measurement was significant (P < .05).
FIGURE 4.

Bland‐Altman plots illustrating the difference between volumetric measurements (mm3) taken by 3D photographs and manual ruler measurement. The red lines indicate the 95% limits of agreement and the blue line represents the mean of the difference. Each dot represents a wound measurement
3.3.2. Wound healing
Five wounds (28%) out of 18 achieved 100% healing during the trial. Two patients healed from the Fat/PRP group (at 73 and 80 days); two patients from the Fat group (at 78 and 80 days); and one patient healed from the control group at 64 days (Figure 5). There was no significant difference in time to healing on log‐rank test (P > .05).
FIGURE 5.

Kaplan‐Meier curve showing time from commencement of trial to 100% wound healing
3.3.3. PUSH score
The change in mean PUSH score over time is illustrated in Figure 6. The mean PUSH score changes from week 0 to week 12 for each group were: Control −3; Fat grafting −4; Fat/PRP grafting −2. This change over time was significant for all three groups (P < .05) indicating a reduction in severity of the wounds in all groups. There was no significant difference between any of the groups for change in PUSH score over 12 weeks (P > .05). There was no difference in mean PUSH score at Day 0 between any of the groups (P > .05).
FIGURE 6.
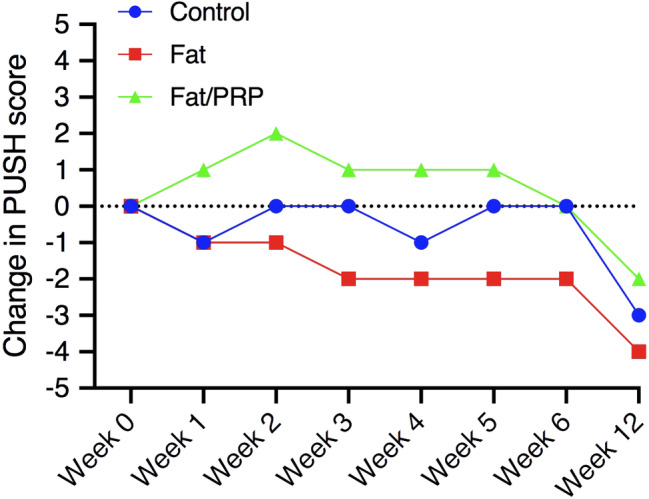
Mean change in pressure ulcer scale for healing score over 12 weeks
3.3.4. Cost of dressings
The change in the average cost of dressings per group is illustrated in Figure 7. The average percentage change in dressing cost at week 12 compared with week 0 for each group was: control −9.4%; fat grafting −6.3%; fat/PRP grafting −49%. Both the fat/PRP and control groups saw an increase in the cost of dressings over weeks 0 to 6 before showing a decrease at week 12, whereas the fat group remained relatively stable. The average total spend per patient on dressings was Control £15.01; Fat grafting £8.09; Fat/PRP £13.45. There was no significant difference in the cost of dressings within or between any of the groups over time (P > .05).
FIGURE 7.

The change in average cost of dressings in (mean £ ± SEM) over 12 weeks
3.3.5. Adverse events
All procedures were carried out as day cases with no intra‐operative or immediate post‐operative complications. There were three (17%) instances of infection in the target ulcers, which all resolved with courses of oral antibiotics. One patient suffered an allergic reaction to the antibiotics prescribed and this was reported as an AE. One patient developed a superficial fluid collection along the track created by the fat grafting cannula, which required drainage in clinic. There were four serious AEs all relating to admission to hospital for pre‐existing conditions (2 for infection to a nontrial ulcer requiring debridement; 1 for chest sepsis; 1 for heart failure), none of these were related to the target wound. Four patients developed separate DFUs during the trial period and one patient developed a necrotic toe.
3.3.6. Health‐related quality of life
All 18 participants returned the pre‐trial questionnaire and 15 (83%) returned the post‐trial questionnaire with one participant from each group not returning. Pre‐trial there were several areas where the control group was found to have significantly lower (P < .05) HRQoL scores compared with the Fat group (daily activities, emotions, compliance, friends, treatment, and financial) and the Fat/PRP group (emotions, compliance, and financial). Post‐trial the majority of these significant lower scores remained in the control group, with only the differences in financial domains (both groups) and emotions (Fat/PRP) no longer being significant. Post‐trial there were also noted to be further significantly lower scores (P < .05) in the control group compared with the Fat group (leisure, family) and Fat/PRP group (treatment); however, interestingly this is despite the control group scoring significantly higher than the Fat group in satisfaction and positive attitude. We also compared the post‐trial results between healed and nonhealed patients, and found those that healed had higher scores in every domain except positive attitude and satisfaction with significantly higher scores in physical health, daily activities and financial (P < .05). There were no significant changes in HRQoL scores within groups from pre to post‐trial. Both the control and Fat/PRP groups were found to have an increase in HRQoL score in 6 domains and a decrease in 5, whereas the Fat group had an increase in 10 domains and a decrease in only one domain (which was a decrease of a single point). The HRQoL scores of each group and healed and nonhealed participants are summarised in Figure 8.
FIGURE 8.

A summary of the mean health related quality of life scores from the diabetic foot ulcer scale questionnaire
4. DISCUSSION
Our feasibility study has provided valuable information on the feasibility of conducting a large‐scale RCT for fat grafting ± PRP in DFUs and our findings will be important in directing the design of future clinical trials. An RCT investigating these two treatment options for wound healing has not previously been conducted in any wound context.
Eighteen patients were recruited to the trial at a single site over 17 months, with follow‐up complete by 21 months. This recruitment number was 60% of the target sample size of 30. The demographic of patients recruited was generalisable to the wider DFU patient population. Fifteen patients were type 2 diabetics and the majority of participants were insulin‐treated, they had an average age in their late 50s and the majority were ex‐smokers with a raised body mass index and at least one other significant comorbidity. More men (83%) were recruited than women. There were a number of patient‐specific barriers to recruitment; in particular, a large number of patients were excluded at initial screening due to kidney failure, vascular disease, wound dimensions, transport/follow‐up issues, and chronic infection. A further 14 (44%) eligible patients refused enrolment primarily due to issues with follow‐up with only two patients stating the interventions themselves as the reason for not consenting. Once the protocol was altered to allow enrolment of patients with vascular disease, the recruitment rate improved to 16 recruits in 12 months. Based on this recruitment rate, a single site could hope to recruit 32 patients over a 24 month period. However, it must be noted that this study benefitted from access to a well‐established and efficiently run diabetic foot service with excellent engagement in the trial by the podiatry service and the associated community team. Patient recruitment was taken from an existing patient population who were already receiving routine care on‐site and there was no resistance to trial recruitment from any element of the DFU multidisciplinary team. A larger study would benefit from using sites with similar DFU services, which not only assists in recruitment but also in follow‐up of participants.
Despite the low recruitment rate in this study, the trial protocol was successfully implemented for all randomised participants with all patients completing the full follow‐up period within their originally randomised treatment arm with no crossovers or withdrawals. This illustrates the high‐level of patient engagement with the study, which is further emphasised by the high rate of attendance at follow‐up appointments with only 4.8% being missed. The 100% retention rate would need to be accounted for by future researchers when undertaking a sample size calculation for a larger study.
The successful implementation of the trial protocol was also illustrated by all participants receiving their intervention as a day case procedure with no hospital admissions and with 11 out of 12 undergoing the procedure under a local anaesthetic. The safety of the interventions was also demonstrated by the low number of AEs (n = 5) that were directly related to the intervention or the target ulcer. This is particularly encouraging given the participants are a high‐risk population with significant co‐morbidities who developed further DFU during the course of the trial.
A failure of the study protocol was identified by a difference in average wound volumes and areas at the start of the trial between groups, with these being significantly larger in the control group than in the fat/PRP group. This may be due to the small numbers of recruits and in a larger study that was adequately powered this difference should not be present. However, a future study could consider stratification of the randomisation schedule by ulcer size to ensure similar sizes in each group.
A further failure in the implementation of the protocol was that there was a delay to intervention following randomisation in all cases with no patients receiving treatment within the target of 7 days (average 21 days, range 8‐48 days). The seven‐day target was optimistic in the current healthcare climate with significant pressures being placed on acute hospitals to manage a high volume of patients and in the majority of cases, the delay to intervention was due to a lack of theatre availability. This is a limitation of the protocol (and of the interventions themselves) as they must be conducted in an operating theatre, or minor operating room, to ensure sterility. This delay to intervention has the potential to result in participants becoming ineligible due to changes in wound size, infection etc. which may affect study numbers. However, in this trial, none of the enrolled patients became ineligible during the delay to intervention and this is perhaps due to the intractable nature of the ulcers in our population. Any future study should continue to aim for a short time to intervention to prevent dropout, with 21 days a more pragmatic target.
A further consideration in a future larger trial would be the heterogeneity of patients with DFU and whether subgroup analysis would be an aim of the study. The successful increase in recruitment following the relaxation of the criteria for vascular disease (from ABPI ≥0.7 to ≥0.3) was beneficial for recruitment, and probably more representative of the overall clinical population, but in a larger study this may make comparability of data more difficult due to the heterogenous population.
As the wounds being treated in this trial were chronic (average duration was 47 weeks) and resistant to routine podiatry care, a change in wound volume (mm3) was chosen as the primary clinical outcome measure. This was used because the goal of the interventions was to improve wound size with the secondary aim of shortening the time to wound healing. Also as the number of wounds expected to heal was low (actual n = 5), the change in wound volume would provide more data to use as a comparison between groups in a small study population. Despite the low healing rate (28%), a larger study may want to consider time to healing or proportion of wounds healed as a more clinically tangible primary outcome measure. Furthermore, the use of change in wound volume as the primary outcome measure relies on the collection of sufficient data. In this study, 10 3D volumetric pictures were not available for analysis despite the patient attending the follow‐up appointment for that week. This was due either to a technical error in uploading to the analysis software or due to researcher unavailability to assist an untrained podiatrist. These errors meant that week 6 could not be included in the final analysis due to insufficient data. A future trial would have to ensure adequate training for all participating clinical staff and consider the use of backup drives to prevent loss of data. There was also a significant discrepancy in estimated wound volume between 3D photo digital analysis and manual ruler measurement with 3D measurement overestimating the wound volume. Anecdotally, this may be due to the manual measurement of wound depth using a probe, which was unlikely to accurately measure the maximum depth of the wound, whereas the measurement of depth can be easily estimated on specialist software. The 3D analysis software used in this trial was simple to operate and could be mastered with minimal training. The 3D photographs were also successfully blinded from the assessor in this study and the blinded assessor identified the five healed wounds from 3D photographs only. Therefore, if wound volume was to be a clinical outcome measure in future studies then digital analysis of volume would be preferable to manual measurement.
Our study has demonstrated that collection of primary and secondary outcome data to compare these interventions was straightforward and feasible with 100% of CRF measures being recorded at follow‐up appointments. However, the limited study population does not allow us to draw any meaningful conclusions regarding treatment effect, and this was not a primary aim of the study. There were no significant differences in change in wound volume, wound area, time to healing, PUSH score, and cost of dressings between the groups. A multi‐centre RCT is required to fully assess the efficacy of these treatments in DFU patients.
As previously discussed, studies in the literature that have investigated both fat grafting and fat with PRP for wound healing have been poorly reported with wide variation in methodology. Several of these studies utilised multiple treatment episodes and some included adjunctive modalities to enhance the healing effect, such as negative pressure therapy, meaning the conclusions that could be drawn on the specific effect of fat or fat/PRP were difficult to determine. Furthermore, several studies utilised ADSCs rather than fat grafts, which is less applicable to clinical practice given the restrictions on the clinical use of stem cells. The aim of a larger study based on our protocol would be to identify the effect of a single episode of these therapies on wound healing. The follow‐up period would need to be considered in a larger trial as only five wounds (28%) healed within the 12 weeks. Our review of the 10 published case series evaluating fat therapy for wound healing found an average time to healing of 15 weeks. No accurate conclusions can be drawn from the three published articles, which have evaluated fat/PRP as only two of these published time to healing as an outcome measure. Two multi‐centre trials evaluating hard‐to‐heal DFUs found 34% 53 and 48% 54 healing at 20 weeks. Therefore, limiting a trial to the short follow‐up period used in this trial is likely to see lower healing rates and is unlikely to provide definitive conclusions; a larger study should consider a longer follow‐up period of 20 weeks or above.
Return of HRQoL questionnaires by participants was excellent, with 100% and 83% returning the pre‐trial and post‐trial questionnaires, respectively, illustrating again the high level of patient engagement in the study. Unsurprisingly, our findings illustrated that patients with healed ulcers scored better than those who did not heal. We also found that patients in both intervention groups tended to score higher HRQoL scores than the control group following the trial, which was despite the control arm patient being more satisfied with their treatment. These findings should be interpreted carefully given the low participant numbers and do not allow any firm conclusions to be drawn. However, the use of a HRQoL measure within this study has been successfully demonstrated and any future researchers should retain this outcome measure in a larger trial. However, although the DFS is specific to DFU and therefore very suitable for measuring quality of life as an indicator of the success of the interventions, it should be mentioned that because the DFS questionnaire is sub‐divided into 11 domains it would not provide a single HRQoL value which would be necessary to perform a quality adjusted life years calculation, which would provide information on the health economic benefits of the interventions. In the case of the DFS, assumptions would have to be made in order to aggregate the HRQoL values from each subdomain to a single value. If calculation of quality‐adjusted life years was an aim of a larger trial then the EQ‐5D‐3L may be more suitable as this has been used extensively in diabetes in the literature. 55 Cost of dressings was evaluated in this trial to attempt to quantify the cost of treatment; however, this is unlikely to be accurate as significant costs arise from community care, hospital and societal costs, and the EQ‐5D‐3L would allow this full cost utility analysis.
4.1. Limitations
The debridement undertaken in the intervention arm by the surgical team is potentially more aggressive than that carried out on patients in the control arm by the podiatry team without anaesthesia. This is likely to alter the wound biology to a more acute wound, which in healing terms would be beneficial to the patient. However, given that this is not the case in the control arm this more aggressive debridement may be a confounder which provides inherent bias towards the intervention arms and which was not mitigated for in the trial design. Future studies should consider whether a less aggressive debridement would help to prevent bias but at the potential cost of poorer healing outcomes.
A potential limitation of the study protocol was that the control arm of the study, and the follow‐up regimen for the intervention arms, was not standardised to a specific dressing therapy and the judgement as to what dressing or type of off‐loading the patient required was left to the treating podiatrist. This decision was taken because this treatment regimen was more representative of the actual clinical care these patients would receive, and standardisation to a particular dressing would create a false environment and may be detrimental to the wound as the incorrect dressing may have been used for certain wound scenarios. However, it does increase the heterogeneity of the patient population. A further RCT would be recommended to allow usual care to remain the remit of the DFU multidisciplinary team but could consider methods of standardisation such as drawing up specific treatment algorithms based on wound data generated from the follow‐up appointments.
A further limitation of the study protocol was the lack of a PRP specific arm, which may have allowed a more accurate comparison of both the effect of PRP and fat on the wounds. The PRP arm could have acted as a control arm, along with fat grafting alone, in a noninferiority method to allow more patients to enter intervention arms. However, this would not have allowed comparison with the current standard of care and would have examined unproven, experimental procedures against each other would have been less useful in guiding clinical practice. A sham surgery arm, where a fat grafting cannula would be used around the wound without any injection of fat or fat/PRP, could also have been a useful comparator to assess whether local tissue disruption and the subsequent inflammatory response may be the reason why healing improved. However, this would have created a multiple‐arm study which, given the low numbers of patients expected to be recruited, would not have been possible. A larger RCT would have to consider whether these intervention arms would be a useful addition to the study.
A further limitation was that this study did not blind the participants or researchers to the interventions due to the nature of the procedures. However, if the infrastructure at the site had allowed for it, then researchers assessing patients at follow‐up appointments could have been blinded when filling in the CRF. This trial did not have the manpower to do this as the follow‐up appointments were often conducted with a member of the surgical team present; however, this would be recommended in a larger trial if possible.
5. CONCLUSION
In conclusion, this feasibility trial has identified that an RCT investigating fat grafting and fat grafting with PRP in DFU patients is feasible and safe. We have identified key barriers to recruitment and have highlighted recommendations for a larger multicentre study in trial design, outcome measurement, data collection, and protocol implementation. This trial does not allow recommendations on the clinical effectiveness of these treatments and a larger RCT is required to assess their efficacy.
Abbreviations
- ABPI
ankle brachial pressure index
- ACD‐A
adenosine citrate dextrose acid
- ADSC
adipose derived stem cell
- AE
adverse event
- ANOVA
analysis of variance
- ANCOVA
analysis of co‐variance
- BMI
body mass index
- CRF
case report form
- DFS
diabetic foot ulcer scale
- DFU
diabetic foot ulcer
- FDA
Food and Drug Administration
- HRA
Health Research Authority
- HRQoL
health related quality of life
- HbA1C
glycated haemoglobin
- NHS
National Health Service
- OM
osteomyelitis
- PRP
platelet rich plasma
- PUSH
pressure ulcer scale for healing
- RCT
randomised controlled trial
- SAE
significant adverse event
- UCL
University College London
- UK
United Kingdom
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
AUTHORS' CONTRIBUTIONS
O. Smith made a substantial contribution to the concept and design of the trial, the acquisition and interpretation of the data and was the major contributor to writing the manuscript. R. Leigh made a substantial contribution to the concept and design of the trial and the acquisition of the data. M. Kanapathy made a substantial contribution to the concept and design of the trial and the acquisition and interpretation of the data and made a contribution to writing the manuscript. P. Macneal was the blinded assessor and provided a contribution to the design of the trial. G. Jell made a substantial contribution to the design of the study and the interpretation of the data. N. Hachach‐Haram and H. Mann made substantial contributions to the concept and design of the trial. A. Mosahebi was the Chief Investigator and led on the concept, design and implementation of the trial and the interpretation of the data. All authors read and approved the final manuscript.
ACKNOWLEDGEMENTS
The authors would like to that all the participants who took part in the trial. We would also like to thank the research team, research administrators, and especially the Royal Free Hospital podiatry service who helped screen and recruit patients, collected data and supported the trial. This trial was part funded by a grant from the British Association of Plastic, Reconstructive and Aesthetic Surgeons. The lead author (OS) was supported by the National Institute for Health Research (NIHR) as an academic clinical fellow. The PRP consumables for the trial were donated by Biotherapy Services Ltd (Windsor, UK). Academic Clinical Fellow (ACF) programme.
Smith OJ, Leigh R, Kanapathy M, et al. Fat grafting and platelet‐rich plasma for the treatment of diabetic foot ulcers: A feasibility‐randomised controlled trial. Int Wound J. 2020;17:1578–1594. 10.1111/iwj.13433
Funding information BAPRAS; National Institute for Health Research; British Association of Plastic, Reconstructive and Aesthetic Surgeons
REFERENCES
- 1. Global report on diabetes 2016 . Geneva: World Health Organisation; 2016. https://apps.who.int/iris/bitstream/handle/10665/204871/9789241565257_eng.pdf;jsessionid=813CCBD2E0DB86E3FD8AD6939C4B31E2?sequence=1. Accessed January 12, 2020.
- 2. Phillips CJ, Humphreys I, Fletcher J, Harding K, Chamberlain G, Macey S. Estimating the costs associated with the management of patients with chronic wounds using linked routine data. Int Wound J. 2016;13:1193‐1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhang P, Lu J, Jing Y, Tang S, Zhu D, Bi Y. Global epidemiology of diabetic foot ulceration: a systematic review and meta‐analysis. Ann Med. 2017;49(2):106‐116. [DOI] [PubMed] [Google Scholar]
- 4. Coleman SR. Structural fat grafting: more than a permanent filler. Plast Reconstr Surg. 2006;118(Suppl):108S‐120S. [DOI] [PubMed] [Google Scholar]
- 5. Naderi N, Combellack EJ, Griffin M, et al. The regenerative role of adipose‐derived stem cells (ADSC) in plastic and reconstructive surgery. Int Wound J. 2017;14(1):112‐124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Planat‐Benard V, Silvestre JS, Cousin B, et al. Plasticity of human adipose lineage cells toward endothelial cells: physiological and therapeutic perspectives. Circulation. 2004;109:656‐663. [DOI] [PubMed] [Google Scholar]
- 7. Arshad Z, Halioua‐Haubold CL, Roberts M, et al. Adipose‐derived stem cells in aesthetic surgery: a mixed methods evaluation of the current clinical trial, intellectual property, and regulatory landscape. Aesthet Surg J. 2018;38(2):199‐210. [DOI] [PubMed] [Google Scholar]
- 8. Fredman R, Katz AJ, Hultman CS. Fat grafting for burn, traumatic and surgical scars. Clin Plast Surg. 2017;44(4):781‐791. [DOI] [PubMed] [Google Scholar]
- 9. Griffin MF, Almadori A, Butler PE. Use of lipotransfer in scleroderma. Aesthet Surg J. 2017;37:S33‐S37. [DOI] [PubMed] [Google Scholar]
- 10. Piccolo NS, Piccolo MS, Piccolo MTS. Fat grafting for treatment of burns, burn scars, and other difficult wounds. Clin Plastic Surg Elsevier. 2015;42(2):263‐283. [DOI] [PubMed] [Google Scholar]
- 11. Herold C, Rennekampff HO, Groddeck R, Allert S. Autologous fat transfer for thumb carpometacarpal joint osteoarthritis: a prospective study. Plast Reconstr Surg. 2017;140(2):327‐335. [DOI] [PubMed] [Google Scholar]
- 12. Marangi GF, Pallara T, Cagli B, et al. Treatment of early‐stage pressure ulcers by using autologous adipose tissue grafts. Plast Surg Int. 2014;2014:1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Caviglia H, Landro ME, Gallo E, Douglas Price AL, Galatro G, Neme D. Is it possible to use autologous adipose graft for wound repair in patients with coagulation disorders? Haemophilia. 2016;22(2):298‐302. [DOI] [PubMed] [Google Scholar]
- 14. Kim JH, Park SH, Lee BH, Jeong HS, Yang HJ, Suh IS. Early intervention with highly condensed adipose‐derived stem cells for complicated wounds following filler injections. Aesthet Plast Surg. 2016;40(3):428‐434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bene M, Pozzi M, Rovati L, Mazzola I, Erba G, Bonomi S. Autologous fat grafting for scleroderma‐induced digital ulcers. An effective technique in patients with systemic sclerosis. Handchir Mikrochir Plast Chir. 2014;46(4):242‐247. [DOI] [PubMed] [Google Scholar]
- 16. Del Papa N, Di LG, Sambataro D, et al. Regional implantation of autologous adipose tissue‐derived cells induces a prompt healing of long‐lasting indolent digital ulcers in patients with systemic sclerosis. Cell Transplant. 2015;24:2297‐2305. [DOI] [PubMed] [Google Scholar]
- 17. Marino G, Moraci M, Armenia E, et al. Therapy with autologous adipose‐derived regenerative cells for the care of chronic ulcer of lower limbs in patients with peripheral arterial disease. J Surg Res. 2013;185(1):36‐44. [DOI] [PubMed] [Google Scholar]
- 18. Carstens MH, Gómez A, Cortés R, et al. Non‐reconstructable peripheral vascular disease of the lower extremity in ten patients treated with adipose‐derived stromal vascular fraction cells. Stem Cell Res. 2017;18:14‐21. [DOI] [PubMed] [Google Scholar]
- 19. Stasch T, Hoehne J, Huynh T, De Baerdemaeker R, Grandel S, Herold C. Debridement and autologous lipotransfer for chronic ulceration of the diabetic foot and lower limb improves wound healing. Plast Reconstr Surg. 2015;136(6):1357‐1366. [DOI] [PubMed] [Google Scholar]
- 20. Chopinaud M, Labbé D, Creveuil C, et al. Autologous adipose tissue graft to treat hypertensive leg ulcer: a pilot study. Dermatology. 2017;233(2–3):234‐241. [DOI] [PubMed] [Google Scholar]
- 21. Delay E, Garson S, Tousson G, Sinna R. Fat injection to the breast: technique, results, and indications based on 880 procedures over 10 years. Aesthet Surg J. 2009;29:360‐376. [DOI] [PubMed] [Google Scholar]
- 22. Sommer B, Sattler G. Current concepts of fat graft survival: histology of aspirated adipose tissue and review of the literature. Dermatol Surg. 2000;26:1159‐1166. [PubMed] [Google Scholar]
- 23. Baran CN, Celebioğlu S, Sensz O, et al. The behavior of fat grafts in recipient areas with enhanced vascularity. Plast Reconstr Surg. 2002;109:1646‐1651. 1652. [DOI] [PubMed] [Google Scholar]
- 24. Cianfarani F, Toietta G, Di Rocco G, et al. Diabetes impairs adipose tissue‐derived stem cell function and efficiency in promoting wound healing. Wound Repair Regen. 2013;21:545‐553. [DOI] [PubMed] [Google Scholar]
- 25. Sommeling CE, Heyneman A, Hoeksema H, Verbelen J, Stillaert FB, Monstrey S. The use of platelet‐rich plasma in plastic surgery: a systematic review. J Plast Reconstr Aesthet Surg. 2013;66:301‐311. [DOI] [PubMed] [Google Scholar]
- 26. Venter NG, Marques RG, Santos JS, et al. Use of platelet rich plasma in deep second and third degree burns. Burns. 2016;42(4):807‐814. [DOI] [PubMed] [Google Scholar]
- 27. Cervantes J, Perper M, Wong LL, et al. Effectiveness of platelet rich plasma for androgenetic alopecia: a review of the literature. Skin Appendage Disord. 2018;4(1):1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ye Y, Zhou X, Mao S, Zhang J, Lin B. Platelet rich plasma versus hyaluronic acid in patients with hip osteoarthritis: a meta‐analysis of randomized controlled trials. Int J Surg. 2018;53:279‐287. [DOI] [PubMed] [Google Scholar]
- 29. Ikumi A, Hara Y, Yoshioka T, Kanamori A, Yamazaki M. Effect of local administration of platelet‐rich plasma (PRP) on peripheral nerve regeneration: an experimental study in the rabbit model. Microsurgery. 2018;38(3):300‐309. [DOI] [PubMed] [Google Scholar]
- 30. D'asta F, Halstead F, Harrison P, Zecchi Orlandini S, Moiemen N, Lord J. The contribution of leucocytes to the antimicrobial activity of platelet‐rich plasma preparations: a systematic review. Platelets. 2018;29(1):9‐20. [DOI] [PubMed] [Google Scholar]
- 31. Kang YH, Jeon SH, Park JY, et al. Platelet‐rich fibrin is a bioscaffold and reservoir of growth factors for tissue regeneration. Tissue Eng Part A. 2011;17:349‐359. [DOI] [PubMed] [Google Scholar]
- 32. Siegel KR, Clevenger TN, Clegg DO, Proctor DA, Proctor CS. Adipose stem cells incorporated in fibrin clot modulate expression of growth factors. Art Ther. 2018;34(2):581‐591. [DOI] [PubMed] [Google Scholar]
- 33. Sivan U, Jayakumar K, Krishnan LK. Constitution of fibrin‐based niche for in vitro differentiation of adipose‐derived mesenchymal stem cells to keratinocytes. Biores Open Access. 2014;3(6):339‐347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Li F, Guo W, Li K, et al. Improved fat graft survival by different volume fractions of platelet‐rich plasma and adipose‐derived stem cells. Aesthet Surg J. 2015;35(3):319‐333. [DOI] [PubMed] [Google Scholar]
- 35. Nakamura S, Ishihara M, Takikawa M, et al. Platelet‐rich plasma (PRP) promotes survival of fat‐grafts in rats. Ann Plast Surg. 2010;65:101‐106. [DOI] [PubMed] [Google Scholar]
- 36. Oh DS, Cheon YW, Jeon YR, Lew DH. Activated platelet‐rich plasma improves fat graft survival in nude mice: a pilot study. Dermatol Surg. 2011;37:619‐625. [DOI] [PubMed] [Google Scholar]
- 37. Jin R, Zhang L, Zhang YG. Does platelet rich plasma enhance the survival of grafted fat? An update review. Int J Clin Exp Med. 2013;6(4):252‐258. [PMC free article] [PubMed] [Google Scholar]
- 38. Kim DY, Ji YH, Kim DW, Dhong ES, Yoon ES. Effects of platelet‐rich plasma, adipose‐derived stem cells, and stromal vascular fraction on the survival of human transplanted adipose tissue. J Korean Med Sci. 2014;29:S193‐S200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Blanton MW, Hadad I, Johnstone BH, et al. Adipose stromal cells and platelet‐rich plasma therapies synergistically increase revascularization during wound healing. Plast Reconstr Surg. 2009;123(Suppl:56S‐64S. [DOI] [PubMed] [Google Scholar]
- 40. Cervelli V, Gentile P, Grimaldi M. Regenerative surgery: use of fat grafting combined with platelet‐rich plasma for chronic lower‐extremity ulcers. Aesthetic Plast Surg. 2009;33(3):340‐345. [DOI] [PubMed] [Google Scholar]
- 41. Cervelli V, De Angelis B, Lucarini L, et al. Tissue regeneration in loss of substance on the lower limbs through use of platelet‐rich plasma, stem cells from adipose tissue, and hyaluronic acid. Adv Skin Wound Care. 2010;23(6):262‐272. [DOI] [PubMed] [Google Scholar]
- 42. Raposio E, Bertozzi N, Bonomini S, et al. Adipose derived stem cell added to platelet rich plasma for chronic skin ulcer therapy. Wounds. 2016;28(4):126‐131. [PubMed] [Google Scholar]
- 43. Smith OJ, Kanapathy M, Khajuria A, et al. Systematic review of the efficacy of fat grafting and platelet rich plasma for wound healing. Int Wound J. 2018;15(4):519‐526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Luck J, Smith OJ, Mosahebi A. A systematic review of autologous platelet rich plasma and fat graft preparation methods. Plast Recontr Surg Glob Open. 2017;5(12):e1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jeffcoate WJ, Vileikyte L, Boyko EJ, Armstrong DG, Boulton AJM. Current challenges and opportunities in the prevention and management of diabetic foot ulcers. Diabetes Care. 2018;41(4):645‐652. [DOI] [PubMed] [Google Scholar]
- 46. Klein JA. The tumescent technique. Anesthesia and modified liposuction technique. Dermatol Clin. 1990;8(3):425‐437. [PubMed] [Google Scholar]
- 47. Keck M, Zeyda M, Gollinger K, et al. Local anesthetics have a major impact on viability of preadipocytes and their differentiation into adipocytes. Plast Reconstr Surg. 2010;126:1500‐1505. [DOI] [PubMed] [Google Scholar]
- 48. Strong AL, Cederna PS, Rubin JP, Coleman SR, Levi B. The current state of fat grafting: a review of harvesting, processing, and injection techniques. Plast Reconstr Surg. 2015;136(4):897‐912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. NICE Guidelines diabetic foot problems: prevention and management. https://www.nice.org.uk/guidance/ng19. Accessed April 17 2020
- 50. de Gouveia Santos VL, Sellmer D, Massulo MM. Inter rater reliability of pressure ulcer scale for healing (PUSH) in patients with chronic leg ulcers. Rev Lat Am Enfermagem. 2007;15(3):391‐396. [DOI] [PubMed] [Google Scholar]
- 51. Abetz L, Sutton M, Brady L, McNulty P, Gagnon DD. The diabetic foot ulcer scale (DFS): a quality of life instrument for use in clinical trials. Pract Diab. 2002;19(6):167‐175. [Google Scholar]
- 52. Bland MJ, Altman D. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307‐310. [PubMed] [Google Scholar]
- 53. Game F, Jeffcoate W, Tarnow L, et al. LeucoPatch system for the management of hard‐to‐heal diabetic foot ulcers in the UK, Denmark, and Sweden: an observer‐masked, randomised controlled trial. Lancet Diabetes Endocrinol. 2018;6(11):870‐878. [DOI] [PubMed] [Google Scholar]
- 54. Edmonds M, Lazaro‐Martinez JL, Alfayate‐Garcia JM, et al. Sucrose octasulfate dressing versus control dressing in patients with neuroischaemic diabetic foot ulcers (explorer): an international, multicentre, double‐blind, randomised, controlled trial. Lancet Diabetes Endocrinol. 2018;6(3):186‐196. [DOI] [PubMed] [Google Scholar]
- 55. Janssen MF, Lubetkin EI, Sekhobo JP, Pickard AS. The use of the EQ‐5D preference‐based health status measure in adults with type 2 diabetes mellitus. Diabet Med. 2011;28(4):395‐413. [DOI] [PubMed] [Google Scholar]


