Abstract
Prodromal signs of a non‐healing wound after revascularisation, which might be strictly linked with impending failure of vascular reconstructions, are associated with an inflammatory response mediated by several circulating adhesion molecules, extracellular endopeptidases, and cytokines. The aim of our study was to investigate the role of selected plasma biomarkers in the prediction of both wound healing and failure of infrapopliteal vein graft or percutaneous trans‐luminal angioplasty (PTA) with selective stent positioning of the superficial femoral artery (SFA) in a population affected with critical limb ischaemia. A total of 68 patients who underwent either surgical or endovascular revascularisation of the inferior limb with autologous saphenous vein infrapopliteal bypass or PTA and selective stenting of the SFA were enrolled in our study. Patients were divided into two groups according to treatment: 41 patients were included in Group 1 (open surgery) and 27 in Group 2 (endovascular procedure). Plasma and blood samples were collected on the morning of surgery and every 6 months thereafter for up to 2 years of follow‐up or until an occlusion occurred of either the vein bypass graft or the vessel treated endovascularly. Fifteen age‐matched healthy male volunteers were considered a reference for biological parameters. Vascular cell adhesion molecule 1 [VCAM‐1]/CD106, inter‐cellular adhesion molecule‐1 [ICAM‐1]/CD54), interleukin‐1 (IL‐1), interleukin‐6 (IL‐6), tumour necrosis factor alpha (TNF‐α), and metalloproteinases (MMP)‐2 and ‐9 plasma levels were measured with enzyme‐linked immunosorbent assay (ELISA) kits. The mean observed time to heal of 54 wounds was 13 ± 4 months, with no statistically significant differences among the groups. The healing failure of the remaining wounds was strictly related to an unsuccessful open (n = 12) or endovascular (n = 8) treatment. The 2‐year primary patency rate was 65% (SE = .09) in Group 1 and 52% (SE = .1) in Group 2. When compared with mean concentration values of Group 1, VCAM‐1 and ICAM‐1 were always significantly higher during follow‐up in patients of Group 2 (P < .05). Furthermore, in the same group, IL‐6 and tumour necrosis factor alpha (TNF‐α) were found to be significantly higher at 6‐ and 12‐month (P < .05) when compared with surgically treated patients. Cox regression analysis showed that elevated plasma levels of VCAM‐1, ICAM‐1, IL‐6, and TNF‐α during follow up were strongly related to impaired wound healing and/or revascularisation failure (P < .05). Elevated plasma levels of inflammatory markers VCAM‐1, ICAM‐1, IL‐6, and TNF‐α may be related to the failure of wound healing and revascularisation procedures. Interestingly, we have observed that endovascular treatments cause a higher level of these inflammation biomarkers when compared with a vein graft, although wound‐healing and patency and limb salvage rates are not influenced.
Keywords: wound healing, peripheral vessels, cytokines, circulating adhesion molecules, extracellular endopeptidases
1. INTRODUCTION
Open or endovascular reconstructive vascular surgery may fail in patients with critical limb ischaemia (CLI), and non‐healing wounds are the prodromal signs of impending failure.1 Evidence recently showed that revascularisation options for CLI are strictly associated with the release of numerous inflammatory cytokines,2, 3 as well as the wound‐healing process, after foot amputation2; however, the specific role of the various molecular actors is still undetermined. It is well known that inflammation contributes importantly to the initiation and progression of the graft failing processes, which may impair open or endovascular graft patency. The recruitment, adhesion, and subsequent trans‐endothelial migration of leukocytes are necessary for the start and maintenance of the inflammatory process.4 Inflammatory cytokines such as vascular cell adhesion molecule 1 (VCAM‐1) and inter‐cellular adhesion molecule‐1 (ICAM‐1), interleukin‐1 (IL‐1), interleukin‐6 (IL‐6), and tumour necrosis factor‐α (TNF‐α) have been shown, beyond the development of atherosclerotic plaques, to have specific roles in neointima formation in arterial segments involved by restenosis, which may be ascribed as the ultimate cause of reconstructive vascular failure.5, 6 Furthermore, the process of restenosis can be induced by the synthesis and dysregulation of several metalloproteinases (MMPs),7 a family of zinc‐dependent endopeptidases with proteolytic activity against a wide range of extracellular proteins. MMPs‐2 and ‐9, which are involved in vascular remodelling because of their primary affinity for collagen type IV and laminin,8 are the key components of the basement membrane separating the intimal and medial layers of the vessel wall.9 MMPs expression can be induced by cytokines, growth factors, stress, or inflammation. Cytokines such as IL‐6 and TNF‐α have been shown to up‐regulate MMPs in a number of cell types, including fibroblasts, endothelial cells, and vascular smooth muscle cells (VSMC).10 A correlation between high plasma levels of MMPs and advanced stages of CLI with poor outcomes in term of limb loss, non‐healing ulcers, and fatal complications was recently demonstrated.11, 12 Elevated plasma levels of these cytokines may prove beneficial as markers of endovascular or peripheral artery bypass graft failures. At present, clinical studies regarding the serological marker of graft failure and consequent non‐healing wound are limited, and only few inflammatory circulating factors were effectively investigated. A clear role of these molecules is still under debate.
The purpose of the present study was the identification of valuable plasma biomarkers that are able to monitor the fate of revascularisation procedures, the release differences between open or endovascular surgical options, and their impact on the wound‐healing process.
2. MATERIALS AND METHODS
2.1. Study design
Between January 2010 and December 2015, 68 patients of both genders affected with Rutherford Grade III Category 5 to 6 CLI13 and a moderate to high risk of limb amputation (clinical stages 3‐4 according to Wound Ischaemia foot Infection—WIfI—classification),14 who underwent an infrapopliteal vein graft or PTA with selective stent positioning of the SFA following the current ESVS guidelines,15 were enrolled in the present study. This research complies with the principles laid down in the Declaration of Helsinki and was approved by the Institutional Research Committee. Written informed consent for the treatment and the analysis of data for scientific purpose was obtained from all patients. This report is in accordance with the STROBE statement checklist of reporting on cohort studies.16
A total of 41 patients who had a long occlusion of the superficial femoral and popliteal arteries with one or two tibial vessel run‐offs revascularised by infrapopliteal reversed autogenous vein graft were included (Group 1). Of the patients, 27 with a stenosis greater than 80% or an occlusion of at least 8 cm in length involving the femoro‐popliteal tract with one‐, two‐, or three‐vessel run‐offs showing at least 50% of vessel patency underwent an endovascular procedure (Group 2). Fifteen age‐matched, male, healthy volunteers (mean age, 69 ± 10 years; median age, 65 years; age range, 52‐84 years), who were non‐smokers, without atherosclerotic lesions (excluded by carotid and aortic ultrasonography, and ankle brachial index measurements), and with normal glycaemic profile and LDL‐cholesterol values, served as a reference for biological parameters.
Exclusion criteria were the presence of chronic venous insufficiency; arterial aneurysms; insulin‐dependent or ‐independent diabetes; acute ischaemia; connective tissue disorders including rheumatoid arthritis; and use of medications impairing wound healing (ie, cytotoxic antineoplastic, immunosuppressive agents, and corticosteroids), blood disorders (clotting factors deficiencies and prothrombotic state because of cancer), and the presence in the endovascular group of orificial or total atherosclerotic occlusion of SFA and concomitant lesions in the distal popliteal and proximal or distal tibial arteries.
2.2. Collection of baseline data
Hospital charts were retrospectively reviewed to collect demographic data, including age, gender, risk factors, and associated diseases. Traditional cardiovascular risk factors were registered in an electronic database and were defined as follows: hypertension (diastolic blood pressure, ≥85 mm Hg), renal disease (blood urea nitrogen, >7.1 mmol/L; creatinine level, >266 μmol/L; creatinine clearance, <50 mL/min), pulmonary disease (PO2, <60 mm Hg; PCO2, >50 mm Hg; pulmonary function tests, <80% of predicted; tested with arterial blood gas test and spirometry), total cholesterol level ≥5 mmol/L, low‐density lipoprotein level ≥4 mmol/L, high‐density lipoprotein level ≤1 mmol/L, non‐fasting triglyceride level ≥1.7 mmol/L, and obesity (body mass index [BMI] – kg/m2 >20% of ideal). In addition, data on active smoking and on anamnestic cardiac disease (prior myocardial infarction, stable or unstable angina, or ST segment alteration on electrocardiogram) were collected and included in the database. Information obtained from diagnostic studies consisting of B‐mode ultrasonography and colour imaging and computed tomography angiography (CTA), inflow and run‐off status, surgical procedural details, minor and major complications, length of hospitalisation, and early‐ and mid‐term outcomes were recorded.
2.3. Operative procedures and clinical follow up
Surgical risk was determined according to the standard (ASA Physical Status Classification System) proposed by the American Society of Anesthesiologists,17 and all patients belonged to classes II to IV. CTA images were used to plan the surgical treatment.
A reversed autogenous vein graft was performed using standard techniques under loco‐regional anaesthesia. The autogenous conduit always included the great saphenous vein. Inflow site included the common femoral artery; distal anastomoses were performed on the tibial vessels. All reconstructions were tunnelled in an anatomical position. At the end of the procedure, the vein graft was studied using angiography to identify flow disturbances or intrinsic vein disease. These were handled with anastomotic revision or removal of the diseased vein segment with another vein portion replacement. Preferentially, all bypass grafts were performed to revascularise the ischaemic angiosome as previously described.18
The endovascular procedures were performed in an operating room with a mobile C‐arm (General Electric Healthcare OEC 9800 Plus, Salt Lake City, Utah) connected with the workstation and were performed under local anaesthesia and conscious sedation. Procedures were most frequently performed from the contralateral groin using 6 or 7 Fr sheaths. Intravenous heparin was routinely administered (100 I.U./kg). Lesions were crossed with 0.035 in. hydrophilic wires supported by 4 Fr angled glide catheters and were treated with PTA and selective stent positioning. The balloon was chosen with the same diameter as the adjacent normal‐appearing artery. Self‐expanding, uncovered nitinol stents with the same diameter (from 6 to 8 mm) of the normal‐appearing artery were selectively deployed, according to the manufacturers' recommendations, preferentially at the Hunter canal level. Angioplasty was considered to be successful if residual stenosis was less than 20% and antegrade flow was established.
All patients, 3 days after revascularisation according to clinical presentation and transcutaneous oxygen tension measurements, had partial toe or toe amputations; metatarso‐phalangeal disarticulation; or transmetatarsal, Lisfranc, or Chopart amputations. As described previously,19 transmetatarsal, Lisfranc, and Chopard amputation wounds were left open, and no attempts of direct closure were made because it was impossible to safely use the plantar flap to cover the wound. A broad‐spectrum short course (2‐4 days) of intravenous antibiotic therapy was started. Negative pressure wound therapy (NPWT) with vacuum‐assisted closure (VAC therapy system) (KCI Medical S.r.l. via Meucci, 1 Assago, MI, 20090) was used to accelerate wound healing and to prevent infection during in‐hospital stay and was continued on a domiciliary basis. When the NPWT was discontinued, the wounds were medicated until complete healing with standard, comprehensive care, including wound off‐loading, local wound debridement, and wound moisture balance with appropriate dressings.
Postoperative medical therapies consisted of aspirin 100 mg/day and atorvastatin 10 or 20 mg/day. All patients were followed up with physical examination, ankle brachial index (ABI) measurement, and B‐mode ultrasonography and colour imaging every 4 months. Suspected graft or PTA with selective stent positioning occlusion was confirmed by means of B‐mode ultrasonography and colour imaging and CTA.
2.4. Wound healing
As previously described,18 wound healing was assessed through direct ulcer tracing onto a clear plastic sheet and subsequent computerised planimetry. Healing was calculated by subtracting the final ulcer area (FUA), expressed in centimetres, from the initial ulcer area (IUA), expressed in centimetres, and then by dividing the number of follow up months to obtain the total area healed per month (cm2/month).
2.5. Definition of endpoints
The primary endpoint of the study was vein graft or endovascular failure and wound healing defined as complete epithelialisation without adverse complications during follow up.
2.6. Laboratory measurements
Plasma and blood samples were collected on the morning of surgery and every 6 months thereafter for up to 2 years of follow up or until the healing of the wound and vein graft or PTA with selective stent positioning failure by a direct venipuncture either on an antecubital vein of the arm or on a superficial vein as proximal as possible to the site of necrosis and, during follow up, to the amputation site. The samples were collected into tubes containing K2‐EDTA (Terumo Europe NV, Leuven, Belgium) or sodium citrate (BD‐Plymouth, PL67BP, UK) and immediately transported to our laboratory under controlled conditions of temperature and humidity. After centrifugation at 3000 rpm for 10 minutes of the sampled blood, the plasma levels of human adhesion molecules (VCAM‐1/CD106 and ICAM‐1/CD54) were measured by Quantikine enzyme‐linked immunosorbent assay (ELISA) kits (R&D Systems, Inc., Minneapolis, Minnesota). The plasma levels of IL‐1 and IL‐6 were measured with human IL‐1 and IL‐6 Quantikine ELISA kits (R&D Systems, Inc.). The concentration of plasma TNF‐α was determined by human TNF‐α Quantikine ELISA kits (R&D Systems). Measurements of MMP‐2 and ‐9 were taken using Quantikine ELISA kits (R&D Systems). The mean intra‐ and inter‐assay coefficients of variation for MMP‐2 estimation were <6% and <10% for all cases, respectively. The mean intra‐ and inter‐assay coefficients of variation for MMP‐9 estimation were <3% and <8% for all cases, respectively. All plasma‐level inflammatory markers determinations were performed according to the manufacturer's instructions.
2.7. Statistical analysis
Data were analysed with a computer software programme (SPSS Ver. 25.0.0.1; SPSS Chicago, Illinois for OS X El Capitan ver. 10.11.6, Apple Inc. 1983‐2017 Cupertino, California). All results are expressed as the mean ± standard deviation. Continuous variables were analysed with either the Mann‐Whitney U test or Kruskal‐Wallis H test (one‐way ANOVA), followed by the Bonferroni post‐hoc test, calculated by dividing the P value (.05) by the number of paired comparisons made. Categorical variables were analysed using the χ² test or Fisher's exact test. Survival, primary patency, and limb salvage rates were assessed using the Kaplan‐Meier method. Patency and limb salvage rates were calculated based on the number of limbs at risk and survival rates of patients. Cox regression analysis was applied to assess the influence of demographics, risk factors, associated diseases, and biochemical markers in wound healing and graft patency. Variables that significantly differed at a level of significance <.05 (vessel run‐off, VCAM‐1, ICAM‐1, IL‐6, TNF‐α) were entered into the model, whose goodness of fit was assessed by the Hosmer‐Lemeshow test. Differences with an α‐level of <.05 were considered statistically significant.
3. RESULTS
3.1. Patients overview
Table 1 describes the demographics and preoperative clinical characteristics of our population. Overall, 33 (48%) patients were considered to be at high surgical risk, and 35 (52%) had a moderate or low operative risk. No adverse effects related to systemic heparinisation administration, required during open or endovascular procedures, were recorded.
Table 1.
Patients' demographics and preoperative characteristics of patients affected with PAD
| Group 1 (n = 41) | Group 2 (n = 27) | Significance | |
|---|---|---|---|
| Gender (male/female) | 34/7 | 22/5 | .878 |
| Age (year ± SD) | 72 ± 8 | 74 ± 7 | .137 |
| Active tobacco use | 26 (63) | 17 (63) | .970 |
| Cardiac disease | 14 (34) | 6 (22) | .291 |
| Hypertension | 24 (58) | 13 (48) | .400 |
| Renal disease | 4 (10) | ‐ | .094 |
| Pulmonary disease | 3 (7) | 3 (11) | .589 |
| Total cholesterol (mmol/L ± SD) | 5.4 ± .8 | 5.2 ± .7 | .887 |
| Low‐density lipoprotein (mmol/L ± SD) | 4.2 ± .6 | 4.3 ± .8 | .621 |
| High‐density lipoprotein (mmol/L ± SD) | .7 ± 1.7 | .8 ± 1.8 | .553 |
| Non‐fasting triglyceride (mmol/L ± SD) | 3.6 ± .8 | 3.6 ± .7 | .702 |
| Obesity | 2 (5) | 3 (11) | .335 |
| Preoperative ABI (mm Hg ± SD) | 0.36 ± 0.1 | 0.35 ± 0.1 | .823 |
| ASA II | 19 (46) | 16 (59) | .550 |
| ASA III | 19 (46) | 9 (33) | .550 |
| ASA IV | 3 (8) | 2 (8) | .550 |
| Vessel run‐off (1/2/3) | 30/11/0 | 12/13/2 | .026 |
| Partial toe amputation | 3 (7) | 8 (30) | .074 |
| Toe amputation | 6 (15) | 4 (15) | .074 |
| Metatarso‐phalangeal disarticulation | 7 (17) | 7 (26) | .074 |
| Transmetatarsal amputation | 13 (32) | 5 (18) | .074 |
| Lisfranc amputation | 8 (19) | 3 (11) | .074 |
| Chopart amputation | 4 (10) | 0 (0) | .074 |
Percentage is reported between brackets.
Abbreviations: ABI, Ankle Brachial Index; ASA, American Society of Anesthesiologists; PAD, peripheral arterial disease.
One (42%‐62%), two (24%‐35%), or three (2%‐3%) vessels were the run‐off status of our population, but patients in Group 1 had a significantly worse run‐off status (P = .026). In Group 1, a reversed autologous saphenous vein graft with proximal anastomosis on the common femoral artery and distal anastomosis on the anterior tibial artery was constructed in 11 (27%) cases, on the posterior tibial artery in 19 (46%) cases, and on the peroneal artery in 11 (27%) cases. Twenty‐four (58%) bypasses were constructed on the angiosome‐related artery. After intraoperative angiography, an anastomotic revision was required in one case and the removal of a diseased vein segment with another vein portion in one case.
Thromboendoarterectomies of the femoral bifurcation were never associated with the infrapopliteal vein graft.
The mean length of the stenotic lesions in group 2 was 28 ± 5 cm (median 29; range min 25‐max 38 cm), and we selectively used a nitinol stent in 12 (44%) cases. The stent was always deployed at the Hunter canal level because control biplanar angiography demonstrated residual stenosis. No statistical differences were recorded among the two groups regarding the level of postoperative amputations (P = NS), and the description of the type of amputation is reported in Table 1. The overall length of stay was 10 ± 3 days (median 10 days; range min 4‐max 19 days). Group 1 had a length of stay that was significantly longer when compared with group 2 (P < .01).
3.2. Early outcomes
There were no early technical failures, and no major complications were recorded. In Group 1, we recorded four minor complications (two lymphorrheas at the level of the groin and two superficial wound infections), which spontaneously resolved (P = NS), whereas in Group 2, we noticed one hematoma, which did not require further treatment.
3.3. Long‐term outcomes
Overall, there were four (6%) ongoing wound infections, and 2 (3%) wounds required repeated tissue debridement, both complications requiring specific antibiotic therapy and a domiciliary VAC therapy. No osteomyelitis was recorded. No differences between the groups were observed (P = NS). Complete follow‐up information was available for all patients, and no patients were lost for reasons other than death. Overall mean follow up duration was 20 ± 9 months (range min 7‐max 41 months; median 18 months). No differences were recorded among the two groups (P = NS).
During follow up, nine (13%) patients died (seven myocardial infarctions and two strokes) at 8, 9, 10, 15, 16, 17, 19, 21, and 27 months. Overall 2‐year survival rate was 81% (SE = .058). No differences were recorded among the two groups (P = NS). Two‐year primary patency rate was 65% (SE = .09) for Group 1 and 52% (SE = .1) for Group 2. We observed 12 vein graft occlusions at 8, 8, 9, 10, 14, 14, 14, 15, 18, 19, 20, and 21 months and eight SFA PTA with selective stent positioning thromboses at 7, 9, 9, 10, 13, 13, 16, 25, 26, and 27 months (P = NS). Five vein grafts and four SFA PTA with selective stent positioning thromboses required a major amputation (five above‐knee and four below‐knee) because of non‐healing wounds (P = NS). The limb salvage rate at 2 years was 82% (SE = .08) and 58% (SE = .2), respectively, for Groups 1 and 2 (P = NS).
3.4. Wound healing
After the various types of amputations, mean wound diameter was 19 ± 7 cm2 (range min 12‐max 32 cm2; median 14 cm2). No statistical difference was observed between groups (P = .839). A total of 54 wounds (37 in Group 1 and 25 in Group 2) healed in 13 ± 4 months (range min. 6‐max. 18 months; median 10 months). The remaining 14 wounds failed to heal because of the failure of the reconstruction (eight wounds in Group 1 and six in Group 2). In four (10%) patients of Group 1 and in two (7%) patients of Group 2, the ulcers healed but reopened at the failure of the vascular reconstruction. Table 2 describes the healing process between the groups. No statistical differences were observed between the groups at 6, 12, 18, and 24 months.
Table 2.
Wound‐healing process
| Group 1 (n = 41) | Group 2 (n = 27) | Significance | |
|---|---|---|---|
| 6‐month wounds at risk | 41 | 27 | |
| Wound‐healing process | 1.6 ± .3 cm2/months | 1.7 ± .4 cm2/months | .789 |
| 12‐month wounds at risk | 37 | 19 | |
| Wound‐healing process | 1.7 ± .4 cm2/months | 1.5 ± .7 cm2/months | .567 |
| 18‐month wounds at risk | 32 | 16 | |
| Wound‐healing process | 1.6 ± .8 cm2/months | 2.0 ± 1.2 cm2/months | .424 |
| 24‐month wounds at risk | 29 | 13 | |
| Wound‐healing process | 1.4 ± 1.1 cm2/months | 1.5 ± 1.1 cm2/months | .572 |
3.5. Modification of inflammatory markers during the process of wound and vascular healing
There were no statistical differences in the plasma level of VCAM‐1, ICAM‐1, MMP‐2, MMP‐9, IL‐1, IL‐6, and TNF‐α between the blood samples collected from an antecubital vein of the arm and those from a superficial vein as proximal as possible to the necrosis or amputation site (P = NS).
Preoperative plasma levels of VCAM‐1, ICAM‐1, MMP‐2, MMP‐9, IL‐1, IL‐6, and TNF‐α were significantly higher in Groups 1 and 2 when compared with the control (P = .0001) (Figure 1A‐G), but no differences were recorded between the two groups (P = NS). At 6, 12, 18, and 24 months, we observed a significant lower release of VCAM‐1 and ICAM‐1 in Group 1 when compared with Group 2 (6 months: P = .003 and P = .011;12 months: P = .05 and P = .045;18 months: P = .042 and P = .04; 24 months: P = .006 and P = .01, respectively) (Figure 1A‐G). Furthermore, at 6 and 12 months, we observed a significant lower release of IL‐6 and TNF‐α in Group 1 when compared with Group 2 (6 months: P = .022 and P = .002, 12 months: P = .04 and P = .011, respectively) (Figure 1F and G).
Figure 1.
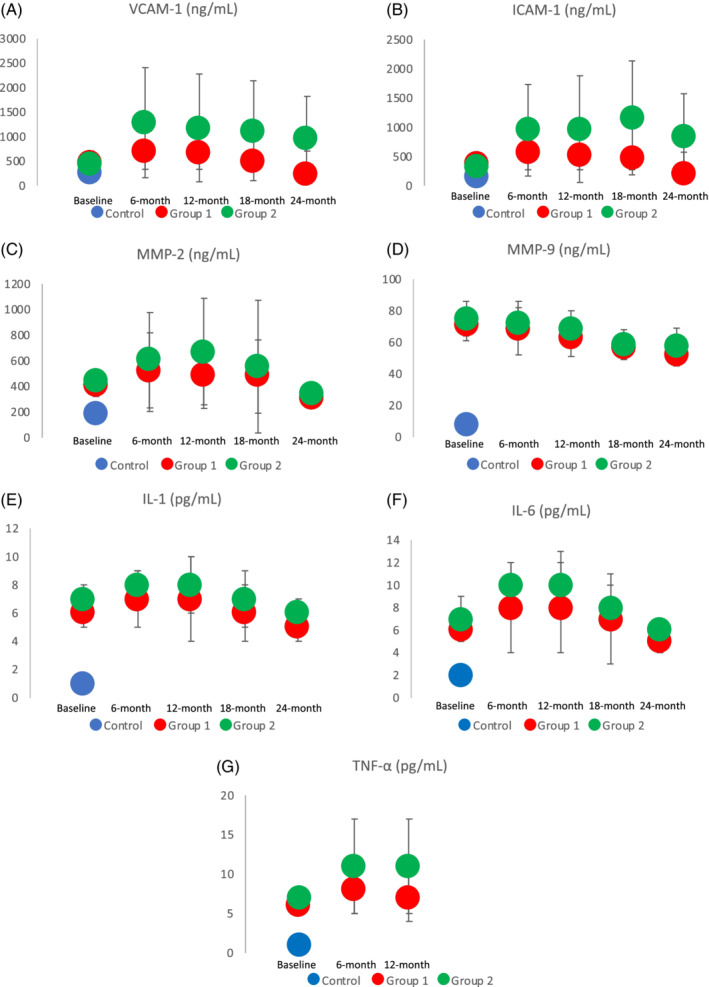
A‐G, Plasma‐level modification of vascular cell adhesion molecule 1 (VCAM‐1), inter‐cellular adhesion molecule‐1 (ICAM‐1), MMP‐2, MMP‐9, interleukin‐1 (IL‐1), interleukin‐1 (IL‐6), and tumour necrosis factor alpha (TNF‐α) between the two groups at 6, 12, 18, and 24 months are depicted. Significances are marked with an asterisk
We also compared the release of these biological markers between patent and occluded vein or endovascular reconstructions in the two groups of patients. We found that, at 12 and 18 months, the release of VCAM‐1 (P = .043 and P = .046, respectively), ICAM‐1 (P = .023 and P = .039, respectively), IL‐6 (P = .035 and P = .011, respectively), and TNF‐α (P = .04 and P = .036, respectively) was significantly lower in Group 1 when compared with Group 2 (Figure 2).
Figure 2.
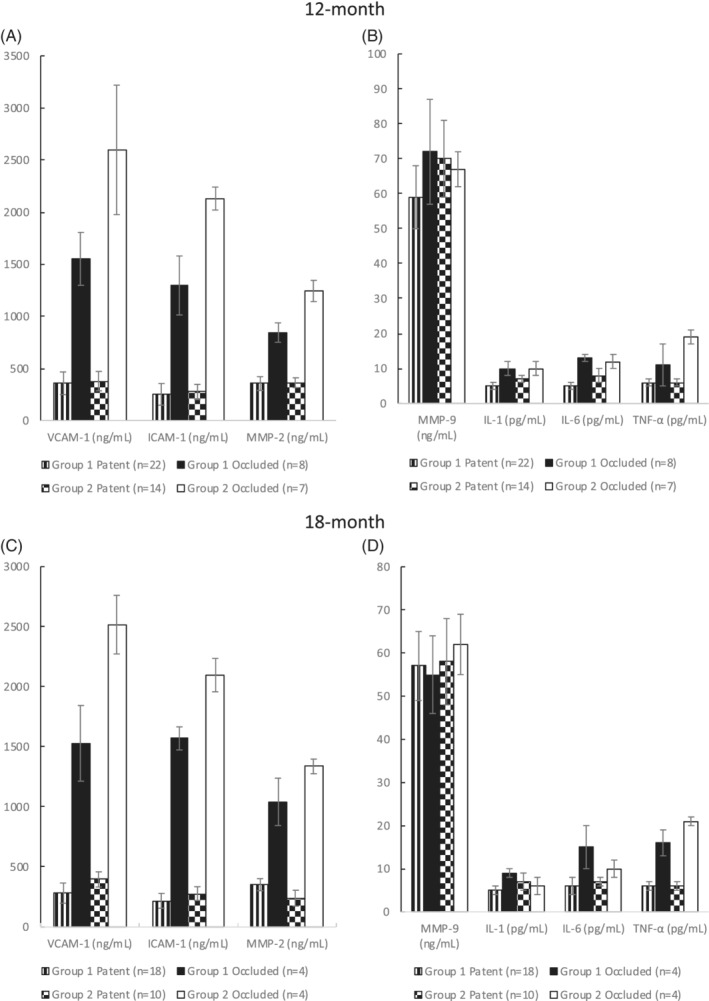
Modifications in vascular cell adhesion molecule 1 (VCAM‐1), inter‐cellular adhesion molecule‐1 (ICAM‐1), MMP‐2, MMP‐9, interleukin‐1 (IL‐1), interleukin‐1 (IL‐6), and tumour necrosis factor alpha (TNF‐α) plasma release between patent and occluded vascular reconstructions at 12 and 18 months are reported. The number of grafts at risk at each interval are given between brackets
Cox regression analysis was conducted to predict factors influencing wound healing and patency rates of vein grafts and PTA with selective stent positioning, after the inclusion into the model of variables, which had resulted statistically significant at univariate analysis (ie, vessel run‐off, VCAM‐1, ICAM‐1, IL‐6 and TNF‐α). Analysis showed that VCAM‐1, ICAM‐1, IL‐6, and TNF‐α were able to predict the fate of the wound healing and vascular reconstruction (Table 3).
Table 3.
Cox regression analysis to determine predictors of wound healing and patency rates after vein graft or PTA
| Odds ratio | 95% CI | P | |
|---|---|---|---|
| Vessel run‐off | .522 | 135‐2.017 | .346 |
| VCAM‐1 | 1.003 | 1.002‐1.006 | .038 |
| ICAM‐1 | 1.002 | .997‐.1.004 | .049 |
| IL‐6 | 1.855 | 1.297‐2.652 | .001 |
| TNF‐α | .679 | .512‐.902 | .007 |
Model fit after Hosmer‐Lemeshow test df = 8, P = .998.
Abbreviations: CI, confidence Interval; ICAM‐1, inter‐cellular adhesion molecule‐1, IL‐6, interleukin‐6, TNF‐α, tumor necrosis factor alpha; VCAM‐1, vascular cell adhesion molecule 1.
4. DISCUSSION
In our study, we highlighted that the release of numerous and different soluble inflammatory mediators is the physiopathological central moment of the wound‐healing process after foot amputation and also after open or endovascular procedures related to restenosis; however, the exact sequence, association, and real clinical importance of their rise are still uncertain. The markers included in the present analysis were chosen in order to assess their predictive value in wound healing and vascular reconstructive failures.
Recent evidence focused on the role of specific inflammatory markers in different stages of arterial disease, and a linear correlation between plasma MMP‐9 levels and the severity of ischaemia11 has been demonstrated. Our population had a baseline‐altered inflammatory markers profile because of their clinical status, and all patients had an advanced stage of atherosclerotic peripheral arterial disease.
We also observed that, regardless the type of revascularisation, higher plasma‐circulating levels of these biochemical markers were strictly associated with an increased risk of failure of wound healing and revasculative procedures and limb loss. A role for the inflammation markers in clinical risk prediction among a homogeneous cohort of patients exclusively undergoing peripheral reconstruction for CLI has been already demonstrated previously.2
VCAM‐1 expression is associated with an endothelial‐leukocyte20 interaction, a consequence of the endothelial damage that was probably created during the PTA with selective stent positioning of the SFA. We hypothesized that the endothelial damage was greater after PTA with selective stent positioning than after vein graft implantation and continued, even if this observation is difficult to explain, during the postoperative period. Similarly, ICAM‐1, which is poorly detected in a normal endothelium but is expressed by a damaged endothelium, triggering the leukocyte adhesion stage and its trans‐endothelial migration,21, 22, 23 had a significantly higher plasma level in patients undergoing endovascular procedures. Our findings are compatible with the role of these molecules in promoting the beginning and maintenance of the restenosis process. Furthermore, the increase of VCAM‐1 and ICAM‐1 was also able to predict the impending failure of the reconstructions regardless the type.
The activation of leukocytes is mediated by IL‐1, IL‐6, and TNF‐α and permits the contact between leukocytes and the adhesion molecules on the endothelium.12, 24 Furthermore, the activated macrophages release IL‐1, IL‐6, and TNF‐α, contributing to the maintenance of the inflammatory state created by each revascularisation procedure. In macrophage depletion studies, a reduction in inflammatory cytokines, as well as neointima formation, has been observed previously.25, 26, 27
In our study, the postoperative plasma level of IL‐6 and TNF‐α were significantly higher for the first year in patients who underwent PTA with selective stent positioning of the SFA when compared with those who underwent vein revascularisation procedures. However, IL‐6 and TNF‐α were positively related to impending reconstructive surgery failure.
MMPs mainly govern the turnover of the basement membrane and concur on neointima formation.28, 29 In particular, MMP‐2 and MMP‐9 cause matrix degradation, apoptosis of smooth muscle cells, and promotion of inflammatory cell infiltration.28, 29 In our study, MMP‐2 and ‐9 plasma concentration were similar among the groups.
Multivariate analysis has shown a strong relationship between the elevated plasma levels of VCAM‐1, ICAM‐1, IL‐6, and TNF‐α and the prediction of revascularisation procedure and wound‐healing failure.
During follow up, the plasma levels of these inflammatory markers were serially reassessed to eliminate the risk that the advanced clinical presentation of our population might have an influence in their release. After successful revascularisation, the release of inflammatory markers significantly decreases, thus reflecting the influence either of the reconstruction or other factors, such as NPWT. Although this was not an endpoint of our study, the efficacy of NPWT is still undefined in this subset of patients, and the literature yields variable results30, 31; however, it may exert a beneficial effect on the healing process.32, 33 We left all mid‐foot amputations open, and healing by second intention was attempted. Primary closure by the plantar flap should be tried when a rich arterial network, arising from the plantar artery, is present; in addition, closure without skin tension must be achievable for a primary closure attempt,20 and in our series, it was impossible to obtain because of the advanced arterial disease. It is also important to consider that the risk of ongoing wound infection, the need for tissue debridement, or osteomyelitis, although theoretically similar between the two groups of patients, might have a concurrent role in the plasma level elevation of these markers, thus mitigating their specificity.34, 35
We believe that the introduction of this panel of inflammatory marker in the clinical assessment of inferior limb‐revascularised patients could be cost‐effective. Whether the activation of the inflammatory system is just a marker of poor postoperative outcome or if it instead plays a direct role in vascular disease remains to be established. Our findings also raise the important question of whether a more aggressive anti‐inflammatory therapy associated with peripheral arterial reconstruction may improve the postoperative outcome of patients with CLI.36, 37 We prescribed the systematic use of aspirin and statin, but patency and life expectancy were apparently not influenced, and this was not the aim of the present study.
We are aware that our study has some limitations, first of which is the small sample size. Second, the measurements were performed at 6‐month intervals to reduce the costs of biological kits and follow up. Therefore, we could not exclude the variability of plasma levels during the time course, particularly in the proximity of graft failures. Third, a wound bed can be hypoxic even when the reconstructions were patent because of local pathologic changes in the vascular bed, peri‐wound fibrosis, and oedema, which increases the distance between capillaries, simply because, in the phase of healing, peripheral collaterals will dilate in an attempt to supply the wound with the required nutrients, yet the base of the wound is lacking, particularly in oxygen. This in turn might also alter the release of the circulating biomarkers analysed in our study. Fourth, although symptoms at presentation were similar, the arterial occlusions of patients in Groups 1 and 2 were different as the vein graft was always planned to be femoro‐crural, and the haemodynamically significant lesions treated with PTA and selective stent positioning were restricted to the SFA.
In conclusion, elevated plasma levels of selected circulating biomarkers are strongly related to healing wound and graft patency, suggesting their use as a marker of postoperative graft failure in patients treated with an inferior limb revascularisation procedure. Furthermore, the endovascular procedures determine a higher plasma level of the inflammation markers during the follow up. This increase is, at least theoretically, explained by the fact that PTA with selective stent positioning of the SFA causes an endothelial damage or dysfunction greater than a vein graft.
Sapienza P, Mingoli A, Borrelli V, et al. Different inflammatory cytokines release after open and endovascular reconstructions influences wound healing. Int Wound J. 2019;16:1034–1044. 10.1111/iwj.13154
REFERENCES
- 1. Thompson JR, Henke PK. Contemporary management of critical limb ischemia. Adv Surg. 2018;52(1):257‐274. [DOI] [PubMed] [Google Scholar]
- 2. Sapienza P, Mingoli A, Borrelli V, et al. Inflammatory biomarkers, vascular procedures of lower limbs, and wound healing. Int Wound J. 2019. 10.1111/iwj.13086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Radak D, Djukic N, Tanaskovic S, Obradovic M, Cenic‐Milosevic D, Isenovic ER. Should we be concerned about the inflammatory response to endovascular procedures? Curr Vasc Pharmacol. 2017;15(3):230‐237. [DOI] [PubMed] [Google Scholar]
- 4. Cucina A, Borrelli V, Randone B, Coluccia P, Sapienza P, Cavallaro A. Vascular endothelial growth factor increases the migration and proliferation of smooth muscle cells through the mediation of growth factors released by endothelial cells. J Surg Res. 2003;109:16‐23. [DOI] [PubMed] [Google Scholar]
- 5. Friedl R, Li J, Schumacher B, et al. Intimal hyperplasia and expression of transforming growth factor‐β1 in saphenous veins and internal mammary arteries before coronary artery surgery. Ann Thorac Surg. 2004;78:1312‐1318. [DOI] [PubMed] [Google Scholar]
- 6. Wild SH, Byrne CD, Tzoulaki I, et al. Metabolic syndrome, haemostatic and inflammatory markers, cerebrovascular and peripheral arterial disease: the Edinburgh artery study. Atherosclerosis. 2009;203:604‐609. [DOI] [PubMed] [Google Scholar]
- 7. Borrelli V, di Marzo L, Sapienza P, Colasanti M, Moroni E, Cavallaro A. Role of platelet‐derived growth factor and transforming growth factor beta1 the in the regulation of metalloproteinase expressions. Surgery. 2006;140:454‐463. [DOI] [PubMed] [Google Scholar]
- 8. Serra R, Grande R, Montemurro R, et al. The role of matrix metalloproteinases and neutrophil gelatinase‐associated lipocalin in central and peripheral arterial aneurysms. Surgery. 2015b;157:155‐162. [DOI] [PubMed] [Google Scholar]
- 9. Jacob MP. Extracellular matrix remodeling and matrix metalloproteinases in the vascular wall during aging and in pathological conditions. Biomed Pharmacother. 2003;57:195‐202. [DOI] [PubMed] [Google Scholar]
- 10. Sapienza P, di Marzo L, Borrelli V, et al. Basic fibroblast growth factor mediates carotid plaque instability through metalloproteinases‐2 and ‐9 expression. Eur J Vasc Endovasc Surg. 2004;28:89‐97. [DOI] [PubMed] [Google Scholar]
- 11. De Caridi G, Massara M, Spinelli F, et al. Matrix metalloproteinases and risk stratification in patients undergoing surgical revascularisation for critical limb ischaemia. Int Wound J. 2016b;13:493‐499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Serra R, Ielapi N, Barbetta A, Andreucci M, de Franciscis S. Novel biomarkers for cardiovascular risk. Biomark Med. 2018;12(9):1015‐1024. [DOI] [PubMed] [Google Scholar]
- 13. Dayal R, Kent C. Standardized Reporting Practices. Rutherford Vascular Surgery. 6th ed. Philadelphia, PA: Elsevier Saunders Inc; 2005:41‐52. [Google Scholar]
- 14. Mills JL, Conte MS, Armstrong DG, et al. The Society for Vascular Surgery Lower Extremity Threatened Limb Classification System: risk stratification based on wound, ischemia, and foot infection (WIfI). J Vasc Sur. 2014;59:220‐234. [DOI] [PubMed] [Google Scholar]
- 15. Authors/Task Force Members , Aboyans V, Ricco JB, et al. Editor's choice—2017 ESC guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European Society for Vascular Surgery (ESVS). Eur J Vasc Endovasc Surg. 2018;55:305‐368. [DOI] [PubMed] [Google Scholar]
- 16. von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg. 2014;12:1495‐1499. [DOI] [PubMed] [Google Scholar]
- 17. ASA Physical Status Classification System. Last Approved by ASA House of Delegates on 2014. American Society of Anesthesiologists; 2014. http://www.asahq.org/~/media/sites/asahq/files/public/resources/standards-guidelines/asa-physical-status-classification-system.pdf. [Google Scholar]
- 18. Sapienza P, Venturini L, Grande R, et al. Is the endovascular treatment of mild iliac stenoses worthwhile to improve wound healing in patients undergoing femorotibial bypass? Ann Vasc Surg. 2018;47:162‐169. [DOI] [PubMed] [Google Scholar]
- 19. Ammendola M, Sacco R, Butrico L, Sammarco G, de Franciscis S, Serra R. The care of transmetatarsal amputation in diabetic foot gangrene. Int Wound J. 2017;14(1):9‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Leeuwenberg JF, Smeets EF, Neefjes JJ, et al. E‐selectin and intercellular adhesion molecule‐1 are released by activated human endothelial cells in vitro. Immunology. 1992;77:543‐549. [PMC free article] [PubMed] [Google Scholar]
- 21. Witkowska A, Borawska M. Soluble intercellular adhesion molecule‐1 (sICAM‐1): an overview. Eur Cytokine Netw. 2004;15:91‐98. [PubMed] [Google Scholar]
- 22. Klouche M, May AE, Hemmes M, et al. Enzymatically modified, nonoxidized LDL induces selective adhesion and transmigration of monocytes and T‐lymphocytes through human endothelial cell monolayers. Arterioscler Thromb Vasc Biol. 1999;19:784‐793. [DOI] [PubMed] [Google Scholar]
- 23. Tishko VV, Sokolov AA, Belshkih AN, Ivanov AM, Meshkova ME, Skorinova TS. Impact of double filtration plasmapheresis on adhesion molecules levels in patients with stable coronary heart disease after coronary stenting. Atheroscler Suppl. 2017;30:92‐98. [DOI] [PubMed] [Google Scholar]
- 24. Sapienza P, di Marzo L, Cucina A, et al. The effect of locally administered anti‐growth factor antibodies on neointimal hyperplasia formation in expanded polytetrafluoroethylene grafts. Ann Vasc Surg. 2009;23:398‐409. [DOI] [PubMed] [Google Scholar]
- 25. Hoch JR, Stark VK, van Rooijen N, Kim JL, Nutt MP, Warner TF. Macrophage depletion alters vein graft intimal hyperplasia. Surgery. 1999;126:428‐437. [PubMed] [Google Scholar]
- 26. Wolff RA, Tomas JJ, Hullett DA, Stark VE, van Rooijen N, Hoch JR. Macrophage depletion reduces monocyte chemotactic protein‐1 and transforming growth factor‐beta1 in healing rat vein grafts. J Vasc Surg. 2004;39:878‐888. [DOI] [PubMed] [Google Scholar]
- 27. Sterpetti AV, Lepidi S, Borrelli V, et al. Growth factors and experimental arterial grafts. J Vasc Surg. 2016;64:1444‐1449. [DOI] [PubMed] [Google Scholar]
- 28. Sapienza P, di Marzo L, Borrelli V, et al. Metalloproteinases and their inhibitors are markers of plaque instability. Surgery. 2005;137:355‐363. [DOI] [PubMed] [Google Scholar]
- 29. Sapienza P, Borrelli V, di Marzo L, Cavallaro A. MMP and TIMP alterations in asymptomatic and symptomatic severe recurrent carotid artery stenosis. Eur J Vasc Endovasc Surg. 2009;37:525‐530. [DOI] [PubMed] [Google Scholar]
- 30. De Caridi G, Massara M, Greco M, et al. VAC therapy to promote wound healing after surgical revascularisation for critical lower limb ischaemia. Int Wound J. 2016a;13:336‐342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ubbink DT, Westerbos SJ, Nelson EA, Vermeulen H. A systematic review of topical negative pressure therapy for acute and chronic wounds. Br J Surg. 2008;95:685‐692. [DOI] [PubMed] [Google Scholar]
- 32. Lo Torto F, Ruggiero M, Parisi P, Borab Z, Sergi M, Carlesimo B. The effectiveness of negative pressure therapy on infected wounds: preliminary results. Int Wound J. 2017;14:909‐914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. De Caridi G, Serra R, Massara M, et al. VAC therapy for the treatment of complex wounds after cardio‐thoracic surgery. Int Wound J. 2016c;13:759‐762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Serra R, Grande R, Butrico L, et al. Chronic wound infections: the role of Pseudomonas aeruginosa and Staphylococcous aureus . Expert Rev Anti Infect Ther. 2015;13:605‐613. [DOI] [PubMed] [Google Scholar]
- 35. Flores‐Herrera H, García‐López G, Díaz NF, et al. An experimental mixed bacterial infection induced differential secretion of proinflammatory cytokines (IL‐1‐ß, TNF‐α) and proMMP‐9 in human fetal membranes. Placenta. 2012;33:271‐277. [DOI] [PubMed] [Google Scholar]
- 36. Sapienza P, Borrelli V, Sterpetti AV, Dinicola S, Tartaglia E, di Marzo L. Dose‐dependent effect of rosuvastatin in the regulation of metalloproteinase expression. Ann Vasc Surg. 2011;25:823‐829. [DOI] [PubMed] [Google Scholar]
- 37. Sapienza P, Borrelli V, Sterpetti AV, et al. Statins reduce levels of metalloproteinases in patients with carotid occlusive disease. Int Angiol. 2014;33:530‐539. [PubMed] [Google Scholar]


