Abstract
There are many chemicals that can cause burns. Although they are generally acidic and basic in nature, there are more than one million known chemical compounds, of which 300 have been declared highly hazardous chemical substances by the National Fire Protection Society. Chemical burns account for approximately 10.7% of all burn injuries and 30% of deaths because of burns. Chemicals can be classified as acid, alkali, organic, and inorganic compounds. Acids act by denaturing and coagulating proteins. Alkaline burns cause deeper burns than acid burns.
Keywords: chemical burns injury, injury mechanism, rare chemical agents, treatment strategies
1. INTRODUCTION
The skin acts as a barrier that protects the body from heat and fluid loss. The elimination of this barrier leads to many pathophysiological changes and this leads to infection. The heat and fluid balance of the body is disturbed. Burn is associated with acute injury caused by exposure of skin and/or subcutaneous tissues to heat, cold, electricity, radiation, or chemical agents.1, 2 Although damage occurs in skin and subcutaneous tissues, it is a complex trauma that determines the prognosis by the pathophysiology of the whole organism because of the reasons such as the depth of the burn, surface area, the agent causing the burn, area of the burn on body, additional diseases and the infection, and metabolic conditions that may occur during the follow‐up period.3
There are many chemicals that can cause burns. Although they are generally acidic and basic in nature, there are more than one million known chemical compounds, of which 300 have been declared by the National Fire Protection Society as highly hazardous chemical substances.4 Chemical burns account for about 10.7% of all burn injuries and 30% of deaths because of burns.5
Chemicals can often be classified as acid, alkali, organic, and inorganic compounds. Acids act by denaturing and coagulating proteins. Alkaline burns cause deeper burns than acid burns.6 Alkaline compounds saponification on the surface epithelium of the skin and laxity causes necrosis.7 Organic solutions cause injury by dissolving the lipid membrane, leading to disruption of physiological processes. Inorganic solutions cause injury through denaturation mechanisms.6 The type and pathophysiology of some rare chemical burns are summarised in Table 1.
Table 1.
Rare chemical agents and injury mechanism
| Chemical Substance | Chemical Agent | Mechanism Of Action | Pathological Process | Primary Treatment |
|---|---|---|---|---|
| Laundry | ||||
| Cleaners |
• Ammonia • Sodium hypochlorite |
Alkaline, Corrossive Effect | Potent alkali causing oxidization and liquefactive necrosis | Very Large Rotaring Water |
| Bleach | • Sodium hypochlorite | As previous | ||
| Pool cleaner | • Sodium hypochlorite | As previous | ||
| Kitchen | ||||
| Oven cleaners | •Sodium (or potassium) hydroxide | Alkaline Corrossive Effect | Potent alkali causing oxidation and production of heat (exothermic) | Very Large Rotaring Water |
| • Hydrofluoric acid | Acidic Corrosive Effect, Skin Burn |
As previous and metabolic hypocalcemia, hyperkalemia and myocardial arrhythmia |
Duration High Flow Water Decontamination |
|
| Bathroom | ||||
| Toilet cleaner |
• Precursors of sulohuric acid • Hypochlorite • Hydrochloric acid |
Alkaline and Acidic Corrosive Effect Lipid Barier Disruption and Skin Burn |
Potent acids and alkalis as previous |
Duration High Flow Water Decontamination |
| Drain cleaner |
• Sulphuric acid • Sodium hydroxide |
Alkaline, Corrossive Lipid Barier Disruption |
Potent acids and alkalis as previous | Very Large Rotaring Water |
| Other | ||||
| Cement | •Quicklime | full‐thickness skin burn | full thickness thermal burn |
Duration High Flow Water Decontamination |
| Super Glue |
•Cyanoacrylate (and Methyl‐, Ethyl‐, İsobutyl‐, İsohexyl‐ And Octyl‐CA |
Exothermic Reaction By Contact With Cotton and İrritant |
causing thermal burn 2nd degree burn( in epidermis and dermiş) |
Irrigated Under Cold Water |
| Drugs(Expigment and IL‐33 solution) |
•Hidrokinon •Dichloroacetic Acid •Monochloroacetic Acid •Trichloroacetic Acid |
Acidic Corrosive Effect | Potent acid causing coagulative necrosis | Irrigated Under Large Rotaring Water |
| Hot Bitum | •Tar |
Lipid Barier Disruption |
full thickness thermal burn | Cloths Impregnated With Olive Oil Or Remove The Bitumen After Cooling Or Irrigation With Abundant Warm Water |
| Nail polish remover | Acetone: dimethyl ketone; dimethyl ketal; ‐ketopropane | Skin barrier disruption, disruption, dries and irritates the skin. also narcosis effect, skin inflammation | with suppressed NGF expression correlating with a reduction in intraepidermal nerve density |
High Flow Water Decontamination |
| White Vinegar |
•Potassium Hydrate •Aldehyde •4‐5% Acetic Acid •Alcohol etc. |
superficial skin burn | Potent acid causing coagulative necrosis |
High Flow Water Decontamination |
| Airbag Gas |
•Various hydrocarbons (nitrogen, carbon monoxide, carbon dioxide, ammonia •Sodium hydroxide |
Alkaline, Corrossive Lipid Barier Disruption |
Potent acids and alkalis as previous | Very Large Rotary Water |
| Liquid Oxygen | •Oxygen | Localized Cold İnjury And Cellular Destruction‐ Severe Skin İrritation, And Frostbite | İschemia and necrosis as a result of hyptermia, Cryogenic Characteristic, Comes İn Contact With Skin İt Causes Numbness, | Contaminated Skin Must Be Promptly Washed Using Soap Or Mild Detergent And Water. |
The aim of this study is to discuss the types of damage caused by the rare cases of chemical burns and the primary treatment strategies performed in our burn unit.
2. SPECIAL ISSUES FOR RARE COMPONENTS
2.1. Cement
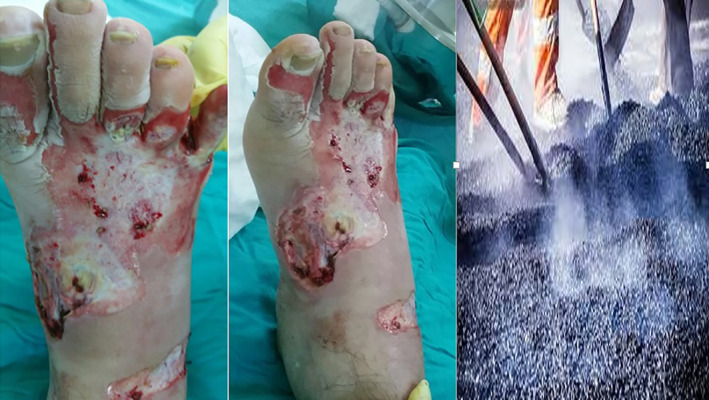
Cement is widely used in the world and cause burns outside the industrial environment. The dominant compound is alkaline calcium hydroxide formed when calcium oxide is exposed to water. This is the compound accused of chemical burns. The onset of symptoms is typically insidious and may cause full‐thickness burns. The dry material which is loose in its treatment is purified from the skin, then the contaminated areas are thoroughly washed and applied with antibacterial ointment. Wounds are observed regularly because of possible debridement and grafts8 (Figure 1).
Figure 1.
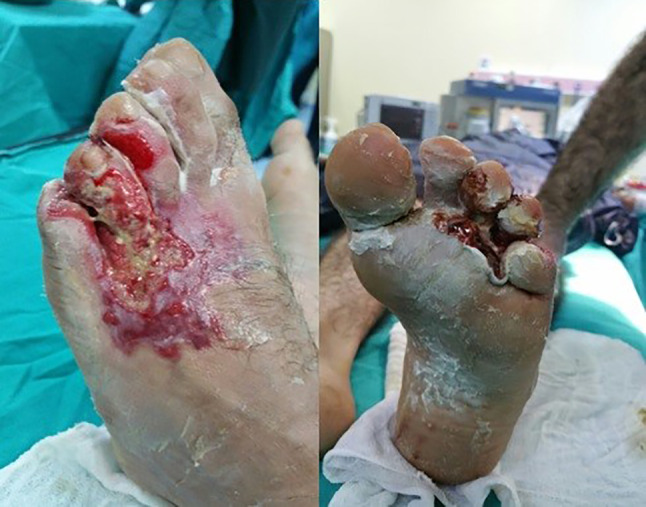
Burns on the foot because of a tear in the sole of the shoe. Burn and blisters on the foot the morning after the injury (cement image)
2.2. Super Glue (Cyanoacrylate)
Cyanoacrylate (CA) was first described in 1949 and used as an adhesive. Homologues of CA glue, including methyl‐, ethyl‐, isobutyl‐, isohexyl‐, and octyl‐CA, have been used in different industrial areas. In order to create a less toxic product in the 1960s, the formulation was changed to butyl CA for possible medical use. There is an increasing use in houses. It now has widespread use for all purposes. However, frequent application of CA is locally adhesive. CA burns are unusual chemical burns. When it contacts with cotton, a serious exothermic reaction will occur and result in thermal burns.9, 10, 11, 12, 13 Single skin contact with CA is generally safe, but repeated contact may cause dermatitis,14 irritant paronychia, or allergic onycholysis.15 There are four patients, among which two of them were children, reported with CA burns.11, 12 The cyanacrylate cutaneous burn of one of the children reported was caused by nail adhesive (Figure 2).
Figure 2.
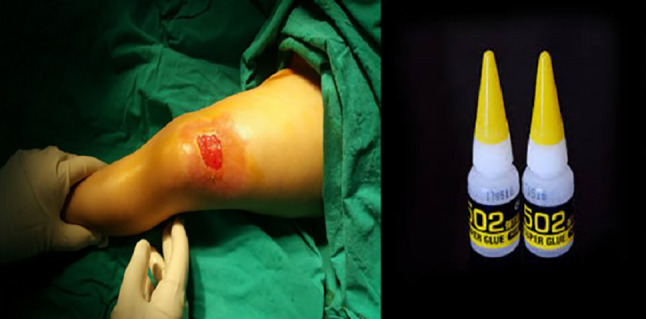
Burn to the foot after the adhesive agent was spilt on the socks of a child
2.3. Drugs (Expigment 30 Gr Cream: 4% hydroquinone ORVA dermatologic products)
Expigment is a drug in which the active ingredient is hydroquinone, with concentrations of 2% and 4%, and higher concentrations are prohibited. It is a drug that provides the skin to be opened in dark colour spots because of excessive increase of pigment in the skin or tissue. Expigment cream (2% hydroquinone density) is 20 mg in 1 g; the 4% of Expigment cream is 40 mg in 1 g. If the medication touches your mouth, nose, and eye, it should be washed with plenty of water. During treatment, it is necessary not to use the drug with hard soaps, hard shampoos, hair dye, hair removal, and alcoholic skin products that may cause skin irritation. Side effects, such as mild burning, stinging, and redness, may be seen. Hydroquinone‐induced occlusion and colloid milia development have been reported for skin whitening.16 No hydroionone burns were reported in the literature (Figure 3).
Figure 3.
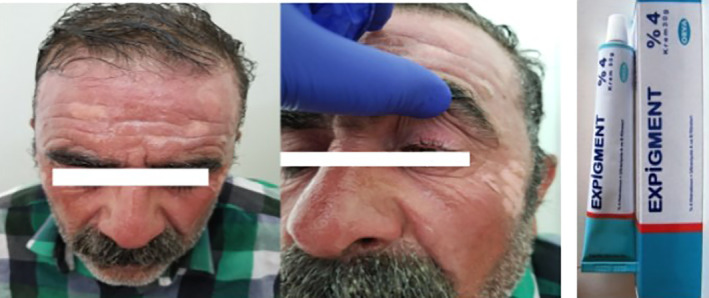
A first‐degree burn on the face because the cream was left on the face overnight in an effort to obtain more effective treatment
2.4. Sink opener (sodium hydroxide) (lye and alkaline drain cleaners)
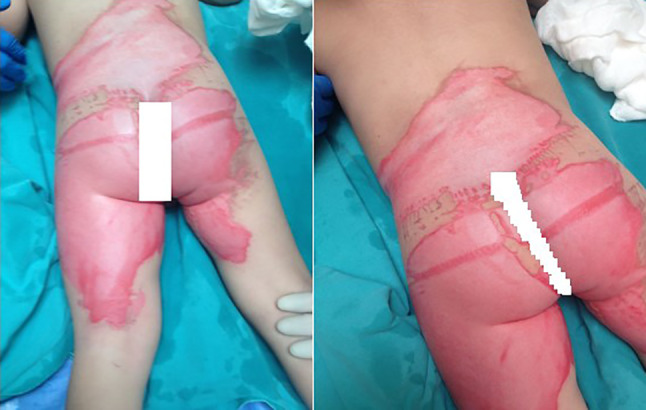
Alkali or dry sodium hydroxide is a strong alkaline compound, and when combined with water, causes a strong exothermic reaction. Aqueous solutions are generally marketed as sewage (channel) drainage and as oven cleaners in the kitchen. Alkali substances penetrate deeply into the tissue and cause progressive damage until it is removed or diluted. They can cause full‐thickness skin loss and saponification of subcutaneous lipids.17 A large amount of water was required to return the skin to a neutral pH.18, 19 Mafenide acetate dressing has been recommended because of the antibacterial properties and inert by‐products inside20 (Figure 4).
Figure 4.
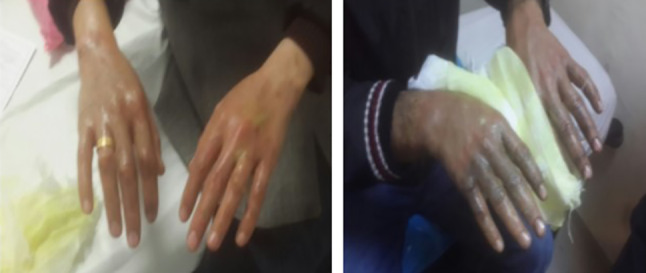
Hand burns after contact with domestic drain cleaner
2.5. Sink opener (hydrofloric acid)
Hydrofluoric acid is present in various products such as rust suppressors with concentrations differing between 6% and 12%. It can also be stored as hydrofluoride (HF) and is used in industrial glass and microchip engraving with concentrations exceeding 70%. Hydrofluoric acid is particularly dangerous because of its double action mechanism. In addition to the acidic corrosive effect, fluoride ions penetrate deeply into the tissues and react with calcium and magnesium to cause multiple local and systemic effects. HF activity is proportional to the size of the damage caused. This is because of the shifts of potassium in intracellular compartment, which leads to ongoing nerve depolarization.21 Other local effects include slow healing wounds and osteolysis. Systemic hypocalcemia and resulting hyperkalemia may cause myocardial arrhythmia. Hypocalcemia may develop even with 1% total body surface area exposure in concentrated HF22 (Figure 5).
Figure 5.
A burn‐out of the acidic contents of domestic drain cleaner
2.6. Hot tar (bitumen)
The most preferred coating material in the construction of the superstructures of the roads is hot asphalt. It is preferred that the hot asphalt properties are more suitable to the superstructures of the roads than other coating materials. It is hot long lasting flooring material. Its application is more practical than other coating materials. It is produced with a mixture of grega (broken or unbroken materials in various sizes with artificial or both types of dense mineral materials) dried at a temperature of 145°C‐160°C and heated with a heated aggregate viscous liquidised bitumen (tar) by heating to about the same temperature (tar), a mixture of high boiling hydrocarbons, asphalt paving, a solid maroon‐coloured mixture used in waterproofing roofs and papers, and so on). As a result of foot contact in the road workers often creates a second degree burn (Figure 6).
Figure 6.
A burn on the foot of a patient and the substance that caused it
2.7. Acetone (Nail Polish Remover)
Acetone is naturally found in plants and animals, such as poultry and fish.23 It is the only methyl ketone detected in animal tissues. Acetone is used as a solvent for resins, lacquers, oils, oils, waxes, rubber cements, plastics, cotton, cellulose acetate, and acetylene. It is used in the production of flax, acetic anhydride, methyl methacrylate, diacetone alcohol, methyl isobutyl ketone, isophorone, chloroform, iodoform, and vitamin C. It is also used in paint and varnish industries; in rubber, plastic, dyeing, celluloid, photographic, and explosive industries; and in the production of artificial silk and leather. It can be found in products such as acetone, solvents, cooking fuels, corn remover, drawing ink, fuel system protector, glue, nail polish remover, paint brush cleaners, paint and varnish removers, tile, film, and fishing rods.
It causes narcotic effect after inhaler poisoning, and skin contact causes skin inflamation. Factory workers are often exposed to acetone, which is used as a cleaning agent.24 Acetone is largely responsible for the remarkable potential reinforcing effect of isopropanol, which may be important when considering occupational exposure, where exposure to a variety of chemicals may alter the toxic effects of any one. Acetone burns which are detected by electron microscope cause skin damages with middle layer disorganisation, vacuolization and organelle changes, mild cutaneous edema and hyperemia, and cell damage at the stratum corneum and stratum.25 Acetone and toluene, which are used to remove the nail polish from the nail, are listed as harmful substances. These substances provide the colours of nail polishes remaining in liquid form, but when evaporating rapidly causes large amount of airborne toxins (Figure 7).
Figure 7.
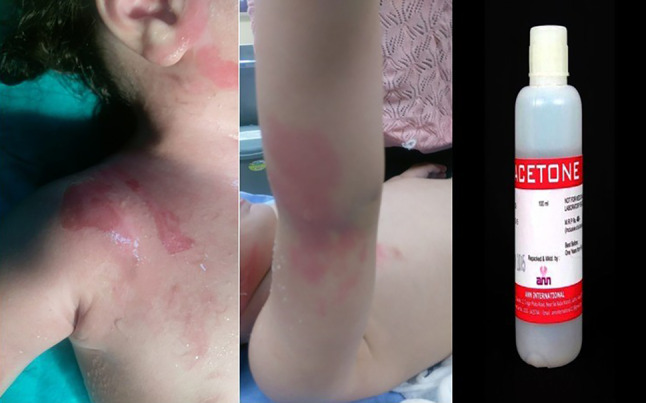
Burns on a child exposed to acetone (nail polish remover) at home and pure acetone used to remove paint and nail polish
2.8. White vinegar
The white vinegar contains the amino acid, potassium hydrate, aldehyde, propionic acid, 4%‐5% acetic acid, 1% alcohol, pectin, and fruit flavouring according to the fruit content produced. It is a sour fruit juice used as a sweetener in salads or as a protector like brine. It can cause severe burns and deaths because of the alcohol it contains and especially in children that the skin of them is thin.26 The content of 4%‐5% acetic acid in white vinegar causes skin and esophageal burns in newborn infants and children (Figure 8).
Figure 8.
A vinegar burn case caused by pure white vinegar
2.9. Airbag gas
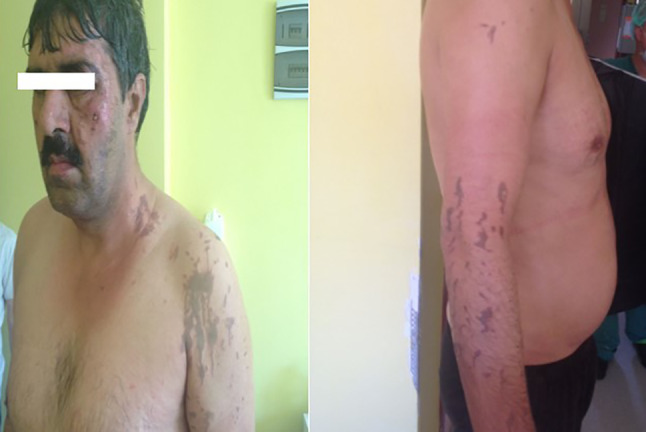
Burns of airbag gas may occur in the form of chemical and thermal burns because of contact with chemical substance or friction burns because of exposure to exhaled chemicals after the airbag inflates or deflates.24 Air pads usually cause burns in the upper and lower extremities, as they drain the gas from the side and bottompart of the car.25 The contents of the airbag gas are sodium hydroxide. When deflating airbag gases, such as the nitrogen, carbon monoxide, carbon dioxide, ammoniac, various hydrocarbons, and alkaline aerosols are released from the airbags. Burns are caused by alkaline corrosives, especially sodium hydroxide.26 Sodium hydroxide is a highly corrosive substance and its solid state releases heat when it comes into contact with water. The affected skin and eye are irrigated with plenty of water to remove the agent for the treatment of sodium hydroxide burns.27 The exposed sodium hydroxide also has ocular effects. Alkaline chemical keratitis has been reported because of airbag injuries (Figure 9).
Figure 9.
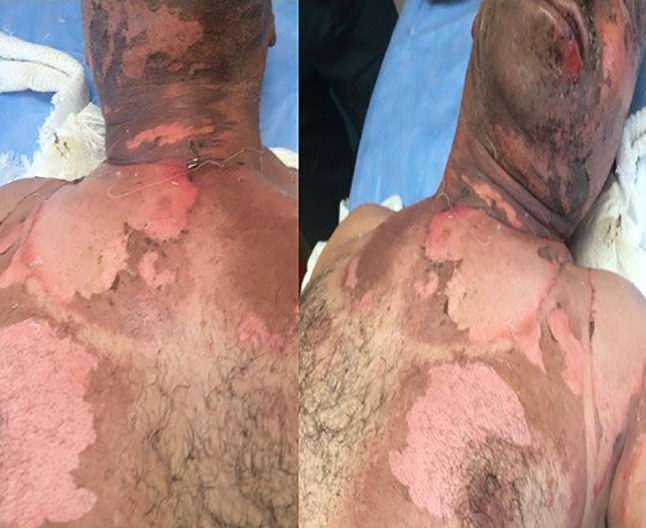
A long‐term burn‐out from an airbag which exploded after a traffic accident and a representative airbag image
2.10. Refrigerator or air conditioner refrigerant gas and liquid oxygen
The refrigerator or air conditioner refrigerant gas contains chlorofluorocarbons (CFCs). CFC is used in production of air conditioners and refrigerators sectors. The first refrigerators used the ammoniac and sülfür dioxide in early periods of refrigerator production. The CFCs were introduced to the market in the 1980s but their use with the Montreal Protocol was prohibited because of the harmful effects on the ozone layer. One of the refrigerant gas, R‐22 gas, is prohibited for serious damage to the ozone layer. This gas, which is prohibited in many countries of the world, is still being used in some countries for cheap air conditioning systems. R12 was one of the most widely used refrigerant gases up to date. As a result of the research, it has negative effects on the ozone layer and it is prohibited because of its toxic, explosive, and non‐flammable effects. R11 is a refrigerant gas with low pressure. The production of this gas was also terminated because of damage to the ozone layer. It was fireproof and odourless. R123 is used in centrifugal cooling systems. The pressure contact to the extremity regions for any reason during the repair of these gases causes 1° or 2° burns in the frostbite style28 (Figures 10 and 11).
Figure 10.

A case of refrigerator gas exacerbation
Figure 11.
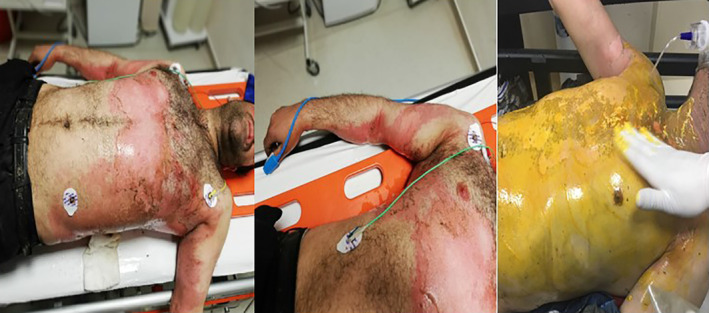
Industrial liquid leakage burns
2.11. İL‐33 10 cc solution (produced by istanbul pharmaceutical industry and trade)
İL‐33 contains 0.2 g dichloroacetic acid, 0.8 g monochloroacetic acid, 9.0 g trichloroacetic acid, and 10.0 mL deionised water. It is used to treat warts (verrus). It causes shrinkage in the wart mass with cauterising effect and also shows antiviral activity against the causative human papilloma. The drug is in the form of a burner acid and is used locally only as indicated externally. A local irritant effect can be seen when it comes into contact with normal skin around the warts. Irritation can only be controlled by applying a short period of time to the area of application location. If the skin contacts widely, it causes acid burns (Figure 12).
Figure 12.
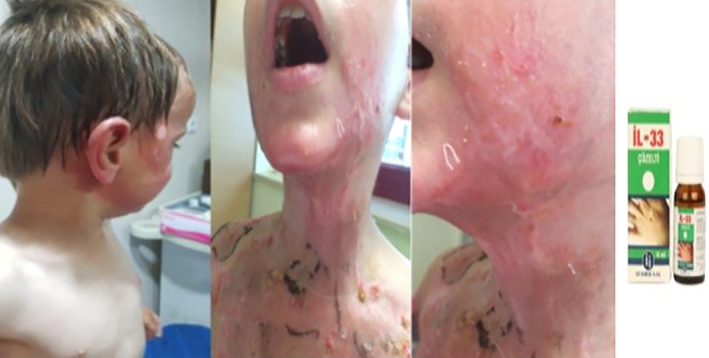
A case of acid burn caused by IL‐33 solution spilt on a child at home and the drug that caused it
The analysis of rare chemical burns seen in our clinic is summarised in Table 2.
Table 2.
Analysis of rare chemical burns seen in our clinic
| Patient Group | N | Chemical Agent | Mean Tbsa% | Degree of Burn | Location of the Event | First Intervention | Secondary Treatment at the Hospital | Outcome |
|---|---|---|---|---|---|---|---|---|
| Cement | 3 |
•Calcium Oxide •Quicklime |
4,66 | 2,3 | Workplace |
• No Intervention in Two Cases • Cold Water Application in a Case |
Alloplastic Dermis Equivalent + Collagen + Antibiotic Baktiras Cushion + Skin Graft | No Scar. Discharged with Healing |
| Super Glue | 2 | • Cyanoacrylate (Ca) | 3 | 2,3 | Home |
•No Intervention. • Application of ice in a Case |
Alloplastic Dermis Equivalent + Collagen + Antibiotic Baktiras Cushion | Previous Same |
| Airbag Gas | 2 |
•Nitrogen, Carbon Monoxide, •Carbon Dioxide, •Ammonia •Sodium Hydroxide |
7 | 2,3 | External environment |
•No Intervention • Cold Water Application in a Case |
Facial Antibiotic Jelonet Cover + Collagen + Silver Bandage | Previous Same |
| Liquid Oxygen | 2 | •Oxygen | 19 | 2,3 | Workplace |
•Flame Extinguishing in a Case • Cold Water Application in a Case |
Alloplastic Dermis Equivalent + Collagen + Antibiotic Baktiras Cushion | Previous Same |
| Drain cleaner | 2 |
•Hidrofluoric Acid •Naoh |
5 | 2 | Home |
• No Intervention. |
Alloplastic Dermis Equivalent + Collagen + Antibiotic Baktiras Cushion | Previous Same |
| Nail Polish Remover | 2 | •Acetone: Dimethyl Ketone; Dimethyl Ketal; ‐Ketopropane | 12,5 | 2 | Home | • Cold water | Alloplastic Dermis Equivalent + Antibiotic Baktiras Cover | Previous Same |
| Expigment 4% | 1 | •Hidroquinone | 10 | 2 | Home | •No Intervention | Antibiotic Baktiras Cover | Previous Same |
| Hot Bitumen | 1 | •Tar | 9 | 2 | Workplace | • Cold water | Antibiotic Baktiras Cover | Previous Same |
| Il‐33 Solution | 1 | •Dichloracetic acid •Monochloracetic acid •Trichloracetic acid | 4 | 2 | Home | •Normal Su | Alloplastic Dermis Equivalent + Antibiotic Baktiras Cover | Previous Same |
| White Vinegar | 1 |
•Potassium Hydrate •Aldehyde •4‐5%Acetic Acid •Alcohol Etc. |
10 | 2 | Home | •No Intervention | Antibiotic Baktiras Cover | Previous Same |
| Refrigerator or Air Conditioning Refrigerant Gas | 1 | •R11,R1,R22 Gas | 3 | 2 | Workplace | •No Intervention | Alloplastic Dermis Equivalent + Antibiotic Baktiras Cover | Previous Same |
3. CONCLUSION
As a result, although chemical burns vary according to their types, they generally cause deep burns. Although they appear to affect superficially initially, they may cause a larger scar and bad appearance in their final form. In the early period, the removal of the substance and neutralisation with water is the most important step in the treatment. In addition, the metabolic and systemic effects that may develop with absorption should be considered. There are problems of successful treatment of such burns, such as lack of first aid support until hospital admission, deficiencies in patient follow‐up, and lack of multidisciplinary approach. In our study, we tried to emphasise the importance and treatment of the first intervention which we need to do in these cases, in spite of the common chemical burns and in rare cases, we may encounter some types of chemical burns. In such rare burns, it is of great importance to know the nature of the chemical and its systemic and local effects.
CONFLICT OF INTEREST
There are no conflicts of interests related to this work.
Akelma H, Karahan ZA. Rare chemical burns: Review of the Literature. Int Wound J. 2019;16:1330–1338. 10.1111/iwj.13193
Hakan Akelma and Zeki A. Karahan contributed equally to the development of this study.
REFERENCES
- 1. Yin S. Chemical and common burns in children. Clin Pediatr. 2017;56:8S‐12S. 10.1177/0009922817706975. [DOI] [PubMed] [Google Scholar]
- 2. Diler B, Dalgic N, Karadag CA, Dokucu AI. Epidemiology and infections in a pediatric burn unit: experience of three years. Çocuk Enfeksiyon Dergisi/J Pediatr Infect. 2012;6:40‐45. 10.5152/ced.2012.10. [DOI] [Google Scholar]
- 3. Zor F, Ersöz N, Külahçı Y, Kapı E, Bozkurt M. Birinci basamak yanık tedavisinde altın standartlar Gold standards for primary care of burn management. Dicle Tıp Dergisi. 2009;36:219‐225. [Google Scholar]
- 4. Barillo DJ, Cancio LC, Goodwin CW. Treatment of white phosphorus and other chemical burn injuries at one burn center over a 51‐year period. Burns. 2004;30:448‐452. 10.1016/j.burns.2004.01.032. [DOI] [PubMed] [Google Scholar]
- 5. Maghsoudi H, Gabraely N. Epidemiology and outcome of 121 cases of chemical burn in East Azarbaijan province, Iran. Injury. 2008;39:1042‐1046. 10.1016/j.injury.2008.03.019. [DOI] [PubMed] [Google Scholar]
- 6. Richards A, Dafydd H. Key Notes on Plastic Surgery. Chichester, UK: John Wiley & Sons, Ltd; 2014. 10.1002/9781118757017. [DOI] [Google Scholar]
- 7. Kozawa S, Kakizaki E, Muraoka E, Koketsu H, Setoyama M, Yukawa N. An autopsy case of chemical burns by hydrochloric acid. Legal Med. 2009;11:S535‐S537. 10.1016/j.legalmed.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 8. Palao R, Monge I, Ruiz M, Barret JP. Chemical burns: pathophysiology and treatment. Burns. 2010;36:295‐304. 10.1016/j.burns.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 9. Tang CL, Larkin G, Kumiponjera D, Rao GS. Vanity burns: an unusual case of chemical burn caused by nail glue. Burns. 2006;32:776‐777. 10.1016/j.burns.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 10. Jamnadas‐Khoda B, Khan MAA, Thomas GPL, Ghosh SJ. Histoacryl glue: a burning issue. Burns. 2011;37:e1‐e3. 10.1016/j.burns.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 11. Bélanger RE, Marcotte M‐E, Bégin F. Burns and beauty nails. Paediatr Child Health. 2013;18:125‐126. 10.1093/pch/18.3.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Clarke TFE. Cyanoacrylate glue burn in a child—lessons to be learned. J Plas Reconstructive Aesthetic Surg. 2011;64:e170‐e173. 10.1016/j.bjps.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 13. Akelma H, Kılıc ET, Kaçar CK, et al. Accidental full thickness burns by super glue. Ann Med Health Sci Res. 2017;7:70‐71. [Google Scholar]
- 14. Shelley ED. Chronic dermatitis simulating small‐plaque parapsoriasis due to cyanoacrylate adhesive used on fingernails. JAMA. 1984;252:2455‐2456. 10.1001/jama.1984.03350170057021. [DOI] [PubMed] [Google Scholar]
- 15. Kanerva L, Estlander T. Allergic onycholysis and paronychia caused by cyanoacrylate nail glue, but not by photobonded methacrylate nails. Eur J Dermatol. 2000;10:223‐225. [PubMed] [Google Scholar]
- 16. Coleman WP. Handbook of cosmetic science and technology—3rd edition. Dermatol Surg. 2010;36:382. 10.1111/j.1524-4725.2009.01444.x. [DOI] [Google Scholar]
- 17. Aydogan C, Luay O, Borman H, Zeyneloglu P, Haberal M. Early excision and grafting in a case of a strong alkali agent damaged arm. Burns. 2009;35:S24. 10.1016/j.burns.2009.06.095. [DOI] [Google Scholar]
- 18. Dünser MW, Öhlbauer M, Rieder J, et al. Critical care management of major hydrofluoric acid burns: a case report, review of the literature, and recommendations for therapy. Burns. 2004;30:391‐398. 10.1016/j.burns.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 19. Dalamaga M, Karmaniolas K, Nikolaidou A, Papadavid E. Hypocalcemia, Hypomagnesemia, and hypokalemia following hydrofluoric acid chemical injury. J Burn Care Res. 2008;29:541‐543. 10.1097/BCR.0b013e3181711152. [DOI] [PubMed] [Google Scholar]
- 20.Opdyke DLJ. Methyl ethyl ketone. Monographs on Fragrance Raw Materials. 1973;11:1011‐1081. 10.1016/B978-0-08-023775-6.50404-5. [DOI] [PubMed] [Google Scholar]
- 21. Kjellberg A, Wigaeus E, Engstrom J, Astrand I, Ljungquist E. Long term effects of exposure to styrene in a polyester plant. Arbete Och Halsa. 1979;18:25. [Google Scholar]
- 22. Lupulescu AP, Birmingham DJ, Pinkus H. An electron microscopic study of human epidermis after acetone and kerosene administration. J Invest Dermatol. 1973;60:33‐45. 10.1111/1523-1747.ep13069780. [DOI] [PubMed] [Google Scholar]
- 23. Brayer C, Micheau P, Bony C, et al. Brûlure néonatale accidentelle à l'isopropanol. Archives de Pédiatrie. 2004;11:932‐935. 10.1016/j.arcped.2004.04.023. [DOI] [PubMed] [Google Scholar]
- 24. Suhr M, Kreusch T. Burn injuries resulting from (accidental) airbag inflation. J Cranio‐Maxillofacial Surg. 2004;32:35‐37. 10.1016/j.jcms.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 25. Ulrich D, Noah E‐M, Fuchs P, Pallua N. Burn injuries caused by air bag deployment. Burns. 2001;27:196‐199. 10.1016/S0305-4179(00)00091-7. [DOI] [PubMed] [Google Scholar]
- 26. Eroglu M, Mutluoglu M, Uzun G, Ay H. Caustic skin burn caused by sodium hydroxide. Case Reports. 2012;2012:bcr2012007103. 10.1136/bcr-2012-007103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Alquraini TA, Aggour MA, Zamzam AM. Airbag induced facial and bilateral ocular injuries in a 14‐year‐old child. Saudi J Ophthalmol. 2011;25:421‐425. 10.1016/j.sjopt.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Heggers JP, McCauley RL, Phillips LG, Robson MC. Cold‐Induced Injury: Frostbite. Total Burn Care. Vol 408. London: WB Saunders; 1996:14. [Google Scholar]


