Abstract
This study evaluates the current clinical evidence of Negative Pressure Wound Therapy with Instillation and dwell time (NPWTi‐d) to establish its clinical application and efficacy. MEDLINE, EMBASE, and CENTRAL databases were searched from 1946 to July 2019 for studies reporting clinical outcomes on wounds treated with NPWTi‐d. The primary outcome was proportion of wounds with complete healing. The secondary outcomes were mean time for healing, NPWTi‐d settings, cost, length of stay, and adverse events. Thirteen articles were included with a total of 624 wounds in 542 patients involving wounds of various aetiology. The pooled proportion of wound that achieved complete healing was 93.65% (95%CI: 84.02‐99.04). Normal saline was the most commonly used instillation solution with the mean dwell time of 14.23 minutes (95%CI: 10.88‐17.59) and instillation cycle every 4.17 ± 2.32 hourly. The mean therapy duration was 10.69 days (95%CI: 10.46‐10.91) with daily cost of $194.80. The mean hospital stay was 18.1 days (95%CI: 17.20‐19.00). There were no severe adverse effects reported. NPWTi‐d is an adjuntive therapy to aid complete healing of the vast majority of wounds. However, the current data are limited by the lack of level 1 evidence.
Keywords: negative pressure wound therapy with instillation, NPWTi‐d, Veraflo
1. INTRODUCTION
Negative pressure wound therapy (NPWT) is a recognised, widely applied adjunct in the management of complex wounds since 1997. 1 , 2 An important evolution of the NPWT, first introduced in 2004, involves instillation of a topical solution with a period of dwell which is followed by its removal via negative pressure cycles, termed NPWT with instillation and dwell time (NPWTi‐d). 3 , 4 This technology promotes wound healing and its cyclical cleaning reduces the bacterial bioburden. 5 , 6
The NPWTi‐d is currently used in a varied clinical setting, and several studies report of its superiority as an adjunct of managing a heterogenous variety of wounds. NPWTi‐d has been suggested to be a more effective management for trunk and extremity wounds, in terms of time taken to complete wound healing, compared with NPWT alone and standard wet‐to‐moist dressing changes. 7 , 8 , 9 , 10 Further, NPWTi‐d has been proposed as a treatment in complex wounds where currently used adjuncts are ineffective, such as in the management of invasive osteomyelitis of the proximal femur and complex spine wounds. 11 , 12 Significantly, in the recent Medtech Innovation Briefing (MIB) developed by the National Institute for Health and Care Excellence (NICE), the NPWTi was advised to have a role in replacing normal NPWT in standard care in people with open infected wounds or chronic wounds that do not respond to standard care. 13
Although there are several reports on the successful management of infected wounds with NPWTi‐d, the evidence on the efficacy of this technology for wound healing has yet to be outlined. The reports of wounds treated with this technology have been heterogeneous in nature, across the fields of plastic, general, orthopaedic, and spinal surgery, with no consensus on what type of wounds would benefit most from this treatment. As a result, the clinical indication and outcomes of using this technology are variable, and its use in clinical practice is guided largely by expert preference rather than evidence‐based recommendations. A recent international consensus guideline (2019) on the general framework of NPWTi‐d use was developed by a consensus panel composed of experts on this topic which updated the earlier consensus guideline published in 2013, however, this evidence is limited as it is an expert opinion and the current available evidence has yet to be synthesised in a systematic manner. 4 , 14
This systematic review aims to synthesise the current evidence on the current clinical use NPWTi‐d and to establish the efficacy of this technology by measuring the outcome of wounds treated with NPWTi‐d. The current clinical usage of NPWTi‐d will also be summarised.
2. METHODS
The protocol for this systematic review was registered with PROSPERO international prospective registration of systematic reviews (registration number: CRD42019140918). This systematic review was conducted and reported according to the Meta‐Analysis of Observational Studies in Epidemiology (MOOSE) guidelines. 15
2.1. Search strategies
A systematic review of the literature was performed using the MEDLINE (OvidSP), EMBASE (OvidSP) and Cochrane databases from 1946 to July 2019 to identify studies of relevance to this review. The search strategy included a combination of text words and Medical Subject Headings (MeSH) terms. No language or publication restrictions were applied.
A sample search strategy for MEDLINE (OvidSP) is shown and similar strategy was adapted for other databases:
1) [“negative pressure wound therapy”] AND [“instillation” OR “irritation” OR “dwell”] OR [“veraflo”] OR [“NPWTi‐d”]
2.2. Inclusion criteria
All primary studies involving adult patients (>18 years old) with wounds of any size and aetiology treated by NPWTi‐d were included.
2.3. Exclusion criteria
The exclusion criteria were case series of less than 10 cases, studies describing NPWTi‐d only without clinical outcome data, review articles, non‐English language articles, laboratory studies, and studies involving animal models. Abstracts and conference proceedings without full text were not included because of the difficulty in evaluating incomplete information. Ongoing trials without complete data were not included.
2.4. Outcome measures
The primary outcome measure was the proportion of wound with complete closure. Wound closure is defined as 100% re‐epithelialization or closure without further need for wound dressing. 12
The secondary outcome measures were the mean time for wound closure, proportion of wounds with failed treatment, mean dwell time, mean dwell frequency, mean instillation time, type of instillation solution, mean number of dressing changes, mean therapy duration, difference in cost, length of stay, and adverse events.
2.5. Study selection and data management
Study selection was conducted in a two‐stage process. The titles and abstracts were initially screened by two reviewers (NK and AM) for potential eligibility, after excluding duplicate records. Next, studies identified as relevant underwent full‐text review by both reviewers. Any discrepancies between the reviewers were resolved by discussion or referring to a third reviewer (MK). The data from all full‐text articles accepted for the final analysis were independently retrieved by NK and AM using a standardised data extraction form. Any discrepancies between the reviewers were resolved by discussion or referring to MK. The search results, including abstracts, full‐text articles, and record of reviewer's decisions, including reasons for exclusion, were recorded in Endnote X8 (Clarivate Analytics). Extracted data are summarised in Table 1. Data were extracted from the studies as presented or were calculated (e.g. mean age and mean wound size).
TABLE 1.
Overview of the included studies
| Citation | Blome‐Eberwein et al 19 | Brinkert et al 20 | Chowdhry et al 21 | Daeschlein et al 22 | Gabriel et al 7 | Gabriel et al 8 | Garcia‐Ruano et al 10 |
|---|---|---|---|---|---|---|---|
| Year | 2018 | 2013 | 2019 | 2016 | 2014 | 2008 | 2016 |
| Country | USA | France | USA | Germany, Switzerland, UK | USA | USA | Spain |
| Study type | Single centre retrospective case series | Multicentre prospective case series | Retrospective cohort study | Prospective case series | Retrospective case series | Prospective case series | Retrospective case series |
| Number of patients | 21 | 131 | 15 | 9 | 48 | 15 | 11 |
| Male | 76.2% | 58.1% | 53.3% | 33.3% | 50.0% | N/R | 63.6% |
| Mean age (year) | 48.7 | 59.2 | 70.5 (6.8) | 74 ± 10 | 42.7 | 57.13 ± 11.64 | 64.8 ± 15.2 |
| Number of wounds | 21 (15 Burns, 6 Necrotising Fasciitis.) | 131 | 15 | 33 | 48 | 15 | 11 |
| Mean wound duration (week) | N/R | N/R | 7.9 (3.1) | N/R | N/R | N/R | N/R |
| NPWTi‐d settings | |||||||
| Dwell time (minutes) |
Burns: 7.73 ± 2.22 Necrotising Fasciitis: 11.0 ± 4.69 |
10 | 20 | 20 | 1‐60 seconds | 30‐45 seconds | 5 |
| Dwell Frequency | N/R | 6‐hourly | 2‐hourly | 1‐hourly | N/R | N/R | 3‐5 hourly |
| NPTW‐I Time (hours) | 4‐6 | 4‐12 | N/R | N/R | 1‐2 | 2 | N/R |
| Instillation solution |
Mafenide solution (n = 14) ¼ Dankins Solution (n = 6), Normal Saline (n = 1) |
Normal Saline | 1/8th Strength Dankins Solution | 0.02% Polyhexanide (SERAG‐Wiessner, Naila, Germany) |
Saline or polyhexanide (Prontosan, B. Braun Medical Inc, Bethlehem, Pennsylvania) |
Silver nitrate |
Hypertonic Saline ‐ 30‐50 mL depending on wound size (Apiroserum, Hypertonic NaCl 2%) |
| Number of dressing changes | Every 2‐3 days | Every 3 days | Every 3 days | N/R | Every 2‐3 days | N/R | Every 3 days |
| Therapy duration (days) | N/R | 12 | 5.4 ± 2.1 | N/R | 4.1 | 9.87 ± 4.31 days | 19.73 ± 9.5 days |
| Time to wound closure | 10.0 ± 6.83 days | N/R | 7.9 ± 2.3 | N/R | 4.1 days | 13.20 ± 6.75 days | N/R |
| Method of wound closure |
Split‐thickness skin graft (n = 13) Graft and flap (n = 3) Secondary intention (n = 1) Primary intention and graft (n = 1) Amputation (n = 3) |
Skin graft 74 (57·76%)Flap 22 (17·33%)Primary suture 32 (24·83%) |
Pectoralis Major flap reconstruction | I/R | N/R |
Primary Intention (n = 2) Secondary intention (n = 4) Skin graft (n = 5) Local flap (n = 4) |
Simple closure (n = 5) Debridement and closure (n = 1) |
| Number of wounds with complete closure | 18 | 128 | 15 | 33 | N/R | 15 | 6 |
| Number of wounds that failed treatment | 3 | 3 | 0 | 0 | N/R | 0 | 5 |
| Complication |
Average pain using NPWT‐i 2.95 ± 2.40 out of 10. |
Death unrelated to therapy (n = 2). Recurrence of chronic osteomyelitis (n = 1) | N/R | N/R | N/R | N/R |
Recurrence of hernia(n = 3) New wound dehiscence (n = 1) |
| Length of follow up | 32.0 ± 26.1 days | 4‐12 months | 90 days | 3 months | 8.1 days | 14.67 ± 9.18 days | 66 days |
| Citation | Jain et al 11 | Kim et al 16 | Kim et al 9 | Ludolph et al 23 | Milcheski et al 24 | Omar et al 5 |
|---|---|---|---|---|---|---|
| Year | 2018 | 2015 | 2014 | 2018 | 2017 | 2016 |
| Country | USA | USA | USA | Germany | Brazil | Germany |
| Study type | Retrospective case series | Prospective, Randomised, Effectiveness study | Retrospective, Historical, Cohort, Controlled study | Prospective case series | Prospective case series | Prospective series and matched retrospective control |
| Number of patients | 10 |
83 Normal Saline n = 42 0.1% Polyhexadine +0.1% Betaine n = 41 |
68 | 111 | 10 | 10 |
| Male | 9 | 61 | 42 | 66 | 4 | 6 |
| Mean age (year) | 40 | 59.45 |
6 minute dwell group1 = 63 ± 16 20‐minute dwell group2 = 55 ± 17 |
58.6 | 40.7 | 49 |
| Number of wounds | 11 | 100 | 68 | 111 | 10 | 10 |
| Mean wound duration (week) | N/R | N/R | N/R | N/R | N/R | N/R |
| NPWTi‐d settings | ||||||
| Dwell time (minutes) | 10 | 20 | 6, 20 | 20 | 20 | 15 |
| Dwell frequency | N/R | N/R | N/R | 2 hourly | 2 hourly | 4 hourly |
| NPTW‐I time (hours) | 3.5 | 2 | 3.5 hours for group 1, 2 hours for group 2 | 2 | 2 | 4 |
| Instillation solution |
Normal Saline = 9 ¼ Dankins Solution = 2 |
Normal Saline = 42 0.1% Polyhexadine +0.1% Betaine = 41 |
Polyhexamethylene biguanide (0.1%) + betaine (0.1%) (Prontosan ©) |
0.4 mg/1 mL Polihexanide | Normal Saline | Normal Saline |
| Number of dressing changes | N/R | N/R | N/R | 3 times a week | N/R | N/R |
| Therapy duration (days) |
Mean = 10 Median = 7 |
Mean = 12.95 |
11.9 ± 7.8 days for group 1 11.4 ± 5.1 days for group 2 |
11.5 days (SD 3.9 days) range of 7 up to 31 days |
11.4 days | Range = 1‐7 days |
| Time to wound closure | 4.5 days |
NSS = 5.6 days 0.1% PH + 0.1% B = 7.5 days |
7.8 ± 5.2 days (group 1) 7.5 ± 3.1 days (group 2) | N/R | 6.3 days | Median = 9 |
| Method of wound closure | Delayed partial or primary closure | Primary closure, local or free flap coverage, application of a xenograft or a Split thickness Skin Graft, or debridement | N/R | Local/Free Flaps, Skin Grafts, Secondary Wound Closure | Skin Grafting/Surgical Flaps | SSG or Secondary closure |
| Number of wounds with complete closure | 6 | 62 |
24 (group 1) 14 (group 2) |
N/R | 2.4 | 10 |
| Number of wounds that failed treatment | 0 | N/R | N/R | N/R | 0 | 0 |
| Complication | N/R | N/R | N/R | N/R | 0 | N/R |
| Length of follow up |
Mean = 12.11 1 lost to f/u |
30 days | 30 days | N/R |
Mean = 6 Range = 3‐9 |
N/R |
Abbreviations: I/R, incomplete reporting; N/R, not reported.
2.6. Assessment of risk of bias of included studies
A formal risk of bias assessment was not performed as the included studies were mostly small case series.
2.7. Data analysis and synthesis
The main outcome measures of the included studies were the pooled estimate of the proportions of wounds with complete closure, proportion of wounds with failed treatment, and the mean wound healing time with the corresponding 95% confidence intervals (CI).
A meta‐analysis of proportion was performed for the proportion of wound closure. A meta‐analysis of summary was performed for the mean time for wound closure, mean dwell time, mean frequency of instillation cycle, mean therapy duration, and length of stay. Narrative synthesis was performed to summarise the different NPWTi‐d settings used, cost evaluation, length of hospital stay, and adverse events. The outcomes were analysed using Stats‐Direct Statistical software (StatsDirect statistical software, version 2·8·0; StatsDirect, Altrincham, UK).
3. RESULTS
3.1. Literature search results
We found 165 articles in the MEDLINE database search, 141 articles in the EMBASE database search, and 17 articles in the CENTRAL database search. References from these three searches were combined, and after removing the duplicates, 227 articles were available for title and abstract reviewing. Of these, 191 articles did not meet the inclusion criteria and were excluded. Following full‐text review of the remaining 36 articles, 24 articles were excluded as the inclusion criteria were not met. A total of 13 articles were included and formed the basis of this systematic review (Figure 1). Cross‐checking of the reference list revealed that no article was missed by the initial search. Details of the included studies were summarised in Table 1.
FIGURE 1.
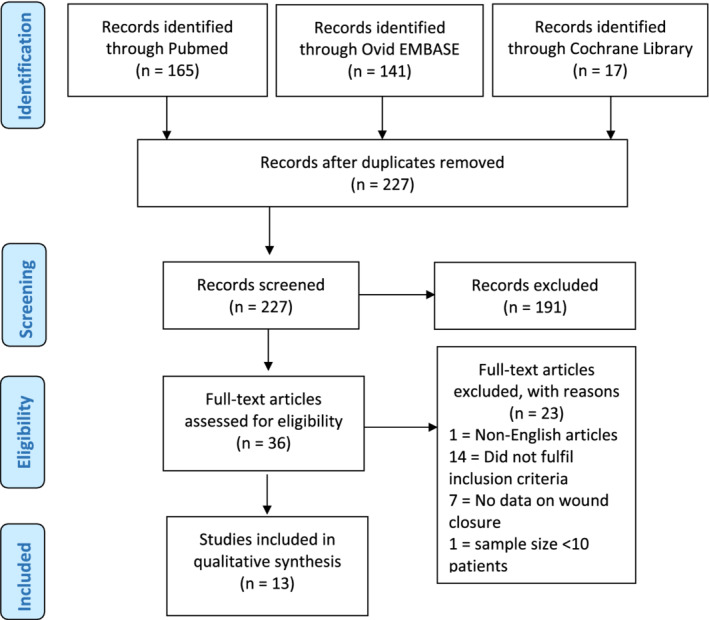
The PRISMA (preferred reporting items for systematic reviews and meta‐analyses) flow diagram
3.2. Clinical application of NPWTi‐d
A total of 624 wounds in 542 patients were treated with NPWTi‐d. The mean age of the patients was 56.67 ± 1.06 years.
NPWTi‐d was used on wounds of varying aetiologies and was reported in 521 wounds. These included surgical wounds (n = 186), trauma (n = 112), pressure ulcers (n = 73), neuropathic (n = 56), infection (n = 28), diabetic ulcers (n = 20), necrotizing fasciitis (n = 19), burns (n = 15), venous (n = 10), and vasculitis (n = 2).
3.3. Wound closure outcome
Wounds treated with NPWTi‐d achieved complete closure via several different techniques, with the commonest being split‐thickness skin graft (n = 126) followed by local/free flap (n = 99), primary closure (n = 45), and secondary intention (n = 32). Data on the method of wound closure and number of wounds were reported in 8 studies.
The pooled proportion of wounds that achieved complete closure was 93.65% (95% confidence interval: 84.02‐99.04) (Figure 2). Data on the number of wounds with complete closure were reported in 11 studies, involving 333.4 wounds. Meanwhile, the pooled proportion of wounds that failed treatment was 6.35% (95% confidence interval: 0.96‐15.98). Data on the number of wounds that failed treatment were reported in nine studies, involving 11 wounds.
FIGURE 2.
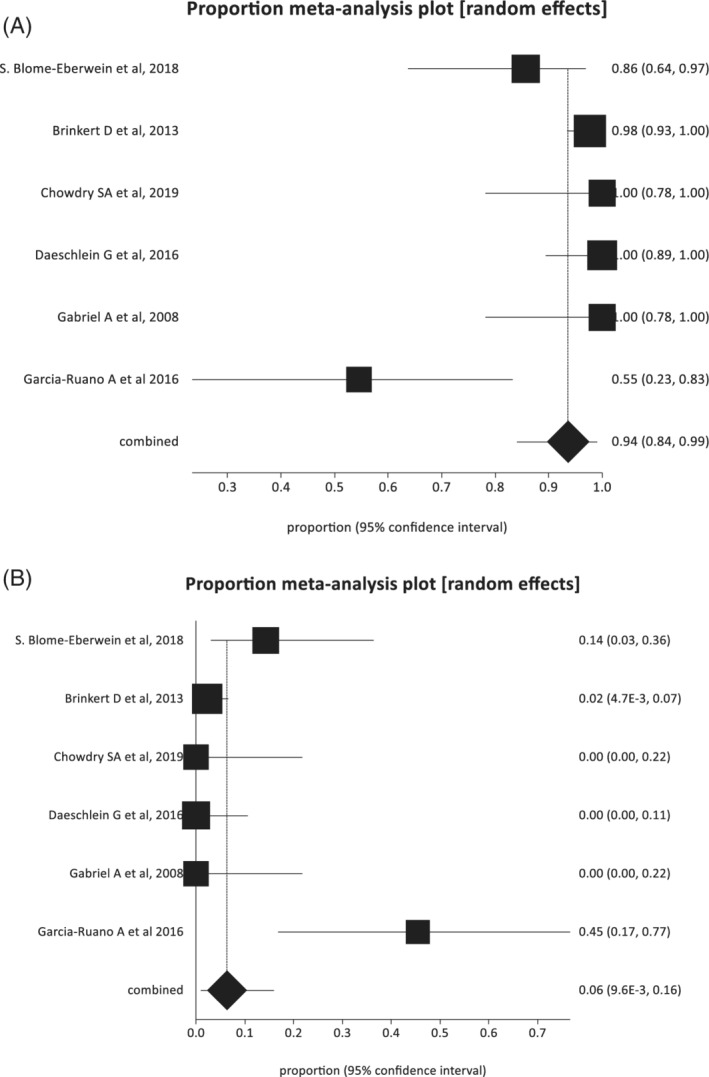
A, Pooled estimate of the proportion of wounds that achieved complete closure (random‐effects plot). B, Pooled estimate of the proportion of wounds that failed treatment (random‐effects plot). Proportions are shown with 95% confidence intervals
The pooled mean time for wound closure for all wound was 8.49 days (95% confidence interval: 5.09‐11.90) (Figure 3). The mean time for wound closure was reported in 9 out of the 13 included studies.
FIGURE 3.
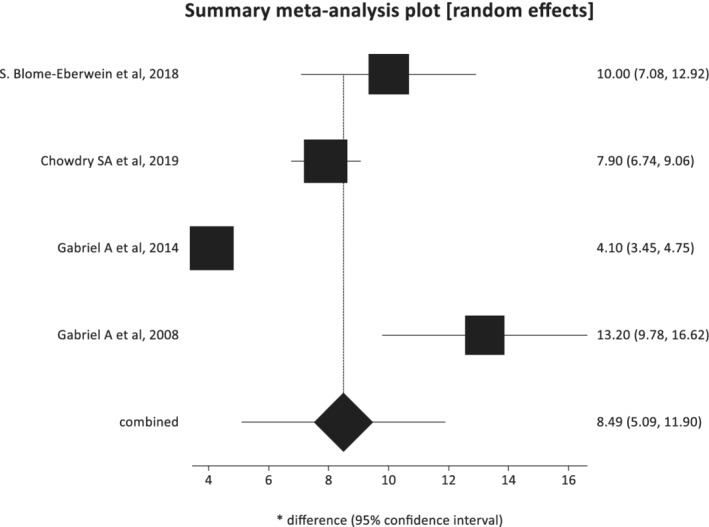
Pooled estimate of the mean time for wound closure (random‐effects plot) with 95% confidence intervals
3.4. NPWTi‐d settings
Most of the included studies reported data on the NPWTi‐d settings and instillation solution used. The pool mean dwell time was 14.23 minutes (95% confidence interval: 10.88 to 17.59) with a mean frequency of instillation cycle of every 4.17 ± 2.32 hourly.
With regards to the type of instillation solution, Normal Saline Solution (NSS) was the commonest used (7 out 12 studies). Other solutions used included these antimicrobials: 0.1% polyhexadine plus 0.1% betaine solution, mafenide solution, 1/4 and 1/8 Dakins solution, 0.02% polyhexadine and silver nitrate. In a randomised controlled trial comparing NSS and 0.1% polyhexadine plus 0.1% betaine solution in 100 patients, the NSS group was found to require less time to complete wound closure (5.6 days versus 7.5 days, P = .04) as well as no significant differences in length of hospital stay, proportion of wounds with complete closure and number of subsequent debridements required. 16
The mean frequency of NPWTi‐d dressing changes, as reported in four studies, was every 2.75 ± 0.43 days. The mean therapy duration, reported in 11 studies, was 10.69 days (95% confidence interval: 10.46‐10.91).
3.5. Cost of NPWTi‐d
Only one study discussed the cost of NPWTi‐d, which was compared to the cost of NPWT. 6 In this retrospective study, Gabriel et al used a theoretical economic model, which incorporated the costs of the therapy unit, cannisters used, and dressings for both treatment modalities. The daily cost of NPWTi‐d was reported to be $194.80, while the daily cost of NPWT was $106.08. However, throughout the length of treatment, NPWTi‐d was reported to be more cost effective by $1418 because of the reduced duration of hospital stay in the NPWTi‐d groups (NWPTi‐d:8.1 day versus NWPT:27.4 days). Further, there was an estimated savings of $8143 by using NPWTi‐d because of the fewer surgical debridements needed in this group (mean of two debridements in the NPWTi‐d group versus 4.4 debridements in the NPWT group).
3.6. Length of stay
The length of hospital stay varied greatly according to the aetiology of the wound. The pooled mean length of hospital stay in 184 patients with wounds in the torso and limbs was 18.1 days (95% confidence interval: 17.2‐19 days). However, the length of hospital stay in patients with burns and necrotising fasciitis was reported to be much longer, with an average length of hospital stay of 30.7 ± 29.2 days and 35.2 ± 17.8 days, respectively. 13 One study reported that patients with abdominal wound dehiscence with mesh exposure to have a longer length of stay with a median of 66 days. 9
3.7. Adverse events
Out of 12 studies reviewed, adverse events and complications were reported in only 2 papers. 9 , 13 Pain was one of the adverse events reported in a retrospective study involving 21 patients, which recorded mild pain with mean of 2.95 ± 2.40 in a 10‐point Likert scale (3.20 ± 2.18 for burns patients and 2.33 ± 3.01 for necrotizing fasciitis patients). 13 In another study, which evaluated the use of NPWTi‐d in 11 patients with abdominal wound dehiscence with mesh exposure, a new wound dehiscence was reported in 1 patient. 9
4. DISCUSSION
The aim of this systematic review was to evaluate the efficacy of using negative pressure wound therapy with instillation for wound healing by synthesising the current available evidence on NPWTi‐d. We found 13 articles, and no randomised controlled trials comparing NPWTi‐d against NPWT or other dressings. We found one article which was a randomised effectiveness study comparing outcomes for different types of instillation solutions in NPWTi‐d but no comparison was made with other mode of treatment. A majority of the current evidence involves retrospective analyses and prospective case series.
We found that complete healing was achieved in 93.65% (95% confidence interval: 84.02‐99.04) of the wounds, with a mean time to complete healing of 8.49 days (95% confidence interval: 5.09‐11.90). The failure rate of wounds to achieve complete healing was 6.35% (95% confidence interval: 0.96‐15.98). However, the quality of these results is limited, as the studies employed NPWTi‐d on wounds of various aetiology and sizes along with different wound closure techniques, often collectively analysed in the same study. Nevertheless, this finding demonstrates the ability of this technology to improve wound healing in a broad range of wounds with several indications. Furthermore, a direct comparison between NPWTi‐d and NPWT or standard moist wound care therapy was not performed in a prospective randomised controlled trial to establish the difference in outcome. Similar to the included studies, the recent international consensus on NPWTi‐d agreed on its use for a broad range of wound aetiology, however, highlighted that NPWTi‐d does not replace debridement or appropriate surgical care but can be used as adjunct for debridement and wound bed preparation. 4 Importantly, the panel highlighted a range of wounds that is contra‐indicated for NPWTi, which includes wounds with presence of exposed, unprotected organs and vessels, wounds with presence of undrained abscess, over split‐thickness skin grafts, over dermal substitutes, and in acutely ischaemic wounds. 4
In terms of NPWTi‐d settings, the pool mean dwell time was 14.23 minutes (95% confidence interval: 10.88‐17.59) with a mean frequency of instillation cycle of every 4.17 ± 2.32 hourly. Kim et al compared a dwell time of 6 to 20 minutes in 68 wounds of varying aetiologies, and there were no statistically significant differences in hospital stay, time to complete wound healing, percentage of failed treatments, and number of subsequent debridements required. 9 , 16 The international consensus recommended instillation solution dwell time of 10 minutes to allow adequate time to cleanse the wound. 4 In addition, the panel recommended considering shorter dwell times in wounds that are challenging to seal and longer dwell times in wounds with areas of fibrinous tissue. 4
The choice of the instillation solution in the studies was varied with no clear evidence supporting the choice of solutions used. Normal Saline solution was the most commonly used irrigation solution, with reported similar wound outcomes when compared to the antiseptic 0.1% polyhexadine plus 0.1% betaine solution in a randomised controlled trial. 16 The recent international consensus recommends the use of either of the following five solutions for installation: normal saline, hypochlorous acid solution, sodium hypochlorite solution (dilute Dakin's solution 0.125% or quarter strength), acetic acid solution (0.25%‐1.0%), and polyhexamethylene biguanide (0.1%) + betaine (0.1%). 4 , 13 However, due to lack of comparative studies comparing the outcomes of these solutions against normal saline, the highest level of current evidence, supported by the single randomised controlled trial, would recommend the use of normal saline as the instillation solution.
NPWTi‐d adds a new dimension to the standard NPWT in that there is a combined action of negative pressure therapy with intermittent instillation of solution into the wound bed, resulting in extended wound cleansing as a component of the wound therapy. 17 This results in the dilution and removal of wound exudate and infectious materials, maintains a clean wound environment, and so the cellular metabolic resources are utilised in the healing pathway (for example, cell proliferation, and matrix deposition) instead of excessive inflammatory and immune reaction. 18 The superiority of this technology over NPTW in promoting wound healing via increased granulation was initially demonstrated in porcine animal models by Lessing et al18. NPTWi was reported to result in 44% more granulation tissue and decreased wound perimeter and surface area compared with different settings of NPWT (continuous, intermittent, and dynamic) at day 7 of treatment. 18 These data suggest that the increased granulation tissue response with NPTWi‐d is due to the extended wound cleansing after debridement rather than the intermittent nature of negative pressure therapy alone.
The evidence in this study is limited by the lack of high‐quality level 1 evidence. The existing studies were mostly small retrospective case series, which are often at high risk of bias. Formal bias assessment was not performed because of this reason. There were no prospective, randomised control studies with direct comparison of NPWTi‐d to NPWT or standard moist dressing changes, which limits our comparison of this new therapy to currently used adjuncts. Besides that, our search criteria excluded case series of lesser than 10 cases which may have eliminated report on the use of this technology in our clinical settings. Further, the proportions of wounds with complete healing were reported collectively for all wound sizes and aetiologies, rather than performing a subgroup analysis, because of incomplete reporting of the included studies. Moreover, despite wounds of various aetiologies were included in the studies, more than half of the study population involved surgical and traumatic wounds, potentially limiting the generalisability of the results. Similarly, the different types of instillation solutions in NPWTi‐d were assumed to produce similar wound outcomes for the purposes of this review, however, this is potentially not the case and further studies need to evaluate the exact effect of various therapeutics. Lastly, the data on NPTWi‐d cost analysis included in this review was based on reports from a single study; hence, despite giving an idea on the average cost, it may not be representative to all settings.
5. CONCLUSION
In conclusion, NPWTi‐d offers a high proportion of wounds with complete healing and has versatility to improve wound healing in a broad range of wounds. However, our conclusions are limited by the lack of high‐quality evidence. Randomised controlled trials evaluating the efficacy of NPWTi‐d against NPWT or standard dressings is required to outline the wound closure outcome, NPWTi‐d settings and its cost effectiveness.
CONFLICT OF INTEREST
IY has received financial reimbursement from 3 M for speaking engagements. Other authors have no conflict of interest to declare.
Kanapathy M, Mantelakis A, Khan N, Younis I, Mosahebi A. Clinical application and efficacy of negative pressure wound therapy with instillation and dwell time (NPWTi‐d): A systematic review and meta‐analysis. Int Wound J. 2020;17:1948–1959. 10.1111/iwj.13487
Contributor Information
Angelos Mantelakis, Email: aggelosmantelakis@yahoo.com, Email: ibbyyounis@hotmail.com.
Ibby Younis, Email: ibbyyounis@hotmail.com.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available in PubMed (References 1‐24).
REFERENCES
- 1. Aycart MA, Eble DJ, Ross KM, Orgill DP. Mechanisms of action of instillation and dwell negative pressure wound therapy with case reports of clinical applications. Cureus. 2018;10(9):e3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Argenta LC, Morykwas MJ. Vacuum‐assisted closure: a new method for wound control and treatment: clinical experience. Ann Plast Surg. 1997;38(6):563‐577. [PubMed] [Google Scholar]
- 3. Broder K, Li A. The use of V.a.C. instill in the wounded pediatric population. Wounds. 2010;22(4):E13‐E16. [Google Scholar]
- 4. Kim PJ, Attinger CE, Constantine T, et al. Negative pressure wound therapy with instillation: international consensus guidelines update. Int Wound J. 2020;17(1):174‐186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Omar M, Gathen M, Liodakis E, et al. A comparative study of negative pressure wound therapy with and without instillation of saline on wound healing. J Wound Care. 2016;25(8):475‐478. [DOI] [PubMed] [Google Scholar]
- 6. Jerome D. Advances in negative pressure wound therapy: the VAC instill. J Wound Ostomy Continence Nurs. 2007;34(2):191‐194. [DOI] [PubMed] [Google Scholar]
- 7. Gabriel A, Kahn K, Karmy‐Jones R. Use of negative pressure wound therapy with automated, volumetric instillation for the treatment of extremity and trunk wounds: clinical outcomes and potential cost‐effectiveness. Eplasty [Electron Resource]. 2014;14:e41. [PMC free article] [PubMed] [Google Scholar]
- 8. Gabriel A, Shores J, Heinrich C, et al. Negative pressure wound therapy with instillation: a pilot study describing a new method for treating infected wounds. Int Wound J. 2008;5(3):399‐413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kim PJ, Attinger CE, Steinberg JS, et al. The impact of negative‐pressure wound therapy with instillation compared with standard negative‐pressure wound therapy: a retrospective, historical, cohort, controlled study. Plast Reconstr Surg. 2014;133(3):709‐716. [DOI] [PubMed] [Google Scholar]
- 10. Garcia‐Ruano A, Deleyto E, Garcia‐Fernandez S. VAC‐instillation therapy in abdominal mesh exposure: a novel indication. J Surg Res. 2016;206(2):292‐297. [DOI] [PubMed] [Google Scholar]
- 11. Jain N, Horn CB, Andrade EG, Punch L. Combination of Girdlestone Pseudoarthroplasty and negative pressure wound therapy with instillation and dwell in the treatment of invasive osteomyelitis of the proximal femur. Cureus. 2018;10(11):e3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. West JM, Jordan SW, Mendel E, Khan SN, Chandawarkar RY, Valerio IL. Instillation negative pressure wound therapy: an effective tool for complex spine wounds. Adv Wound Care (New Rochelle). 2018;7(10):333‐338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. NICE . The V.A.C. Veraflo Therapy system for infected wounds. Medtech innovation briefing [MIB189]. 2019. https://www.nice.org.uk/advice/mib189.
- 14. Kim PJ, Attinger CE, Steinberg JS, et al. Negative‐pressure wound therapy with instillation: international consensus guidelines. Plast Reconstr Surg. 2013;132(6):1569‐1579. [DOI] [PubMed] [Google Scholar]
- 15. Stroup DF, Berlin JA, Morton SC, et al. Meta‐analysis of observational studies in epidemiology: a proposal for reporting. Meta‐analysis of observational studies in epidemiology (MOOSE) group. JAMA. 2000;283(15):2008‐2012. [DOI] [PubMed] [Google Scholar]
- 16. Kim PJ, Attinger CE, Oliver N, et al. Comparison of outcomes for Normal saline and an antiseptic solution for negative‐pressure wound therapy with instillation. Plast Reconstruct Surg. 2015;136(5):657e‐664e. [DOI] [PubMed] [Google Scholar]
- 17. Lessing MC, James RB, Ingram SC. Comparison of the effects of different negative pressure wound therapy modes‐continuous, noncontinuous, and with instillation‐on porcine excisional wounds. Eplasty. 2013;13:e51. [PMC free article] [PubMed] [Google Scholar]
- 18. Lessing C, Slack P, Hong KZ, Kilpadi D, McNulty A. Negative pressure wound therapy with controlled saline instillation (NPWTi): dressing properties and granulation response in vivo. Wounds: A Compend Clin Res Pract. 2011;23(10):309‐319. [PubMed] [Google Scholar]
- 19. Blome‐Eberwein S, Lozano D, Amani H. Utility of negative pressure wound therapy with instillation in a burn centre. Burns Open. 2018;2(4):208‐212. [Google Scholar]
- 20. Brinkert D, Ali M, Naud M, Maire N, Trial C, Teot L. Negative pressure wound therapy with saline instillation: 131 patient case series. Int Wound J. 2013;10(Suppl 1):56‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chowdhry SA, Wilhelmi BJ. Comparing negative pressure wound therapy with instillation and conventional dressings for sternal wound reconstructions. Plast Reconstruct Surg Global Open. 2019;7(1):e2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Daeschlein G, Napp M, Lutze S, et al. Comparison of the effect of negative pressure wound therapy with and without installation of polyhexanide on the bacterial kinetic in chronic wounds. Wound Med. 2016;13:5‐11. [Google Scholar]
- 23. Ludolph I, Fried FW, Kneppe K, Arkudas A, Schmitz M, Horch RE. Negative pressure wound treatment with computer‐controlled irrigation/instillation decreases bacterial load in contaminated wounds and facilitates wound closure. Int Wound J. 2018;15(6):978‐984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Milcheski DA, Portocarrero ML, Alvarez DM, Mazuca L, Monteiro AAJ, Gemperli R. Initial experience with negative‐pressure wound therapy with instillation in complex wounds. Revista Do Colegio Brasileiro de Cirurgioes. 2017;44(4):348‐353. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are openly available in PubMed (References 1‐24).


