Abstract
This systematic review was designed to evaluate the overall efficacy of angiography‐guided drug‐eluting stent (DES) implantation vs intravascular ultrasound‐guided (IVUS) implantation for percutaneous coronary intervention. The electronic databases CENTRAL, PubMed, Cochrane, and EMBASE were searched for systematic reviews to investigate angiography‐guided and IVUS‐guided DES implantation. We measured the following six parameters in each patient: cardiovascular death, stent thrombosis, target lesion revascularisation (TLR), myocardial infarction (MI), major adverse cardiac events (MACEs), and all‐cause death. Twelve studies involving 6268 subjects were included, with 2984 receiving IVUS‐guided DES implantation and 3284 using angiography‐guided DES implantation. With regard to MACEs, TLR, MI, cardiovascular death, and all‐cause death, the IVUS‐guided DES implantation group had remarkably improved clinical outcomes. However, there was no significant statistical difference in stent thrombosis between the two groups. Dramatic decrease in MACEs through IVUS guidance was presented by trial sequential analysis. Remarkably improved clinical outcomes, including MACEs, cardiovascular death, all‐cause death, and TLR, were identified through IVUS‐guided DES implantation in comparison with angiography‐guided DES implantation. Nonetheless, the effect on stent thrombosis and MI required further confirmation. In this meta‐analysis, eligible randomised clinical trials were warranted to verify the findings and to determine the beneficial effect of IVUS guidance for patients.
Keywords: angiography, drug‐eluting stent, intravascular ultrasound, meta‐analysis, percutaneous coronary intervention
1. INTRODUCTION
Drug‐eluting stents (DES) have led to remarkable advances in cardiology and have significantly improved the outcome of percutaneous coronary intervention (PCI) and are superior to bare stents.1 However, the incidence of stent restenosis and in‐stent restenosis are still problems to be solved, especially in the treatment of complex lesions. Coronary angiography has remained a standard scheme to guide DES placement, but because of the projection angle and two‐dimensional plane judgement, it often leads to inaccurate evaluation of the severity of coronary stenosis, plaque structure, and stent placement.2 Intravascular ultrasound (IVUS), as an invasive detection technique, can provide more accurate information about coronary anatomy and plaque size, position, nature, and other characteristics; accurately judge the extent and range of vascular lesions; ensure that the appropriate diameter and length of stent is chosen; and optimise coronary stent placement. IVUS can also evaluate stent size, position, shape, wall level, and interlayer in stent implantation to avoid stent dilation, improper wall sticking, and incomplete coverage of lesions or excessive dilation of the stent, thus decreasing the risk of stent restenosis and thrombosis formation.3, 4, 5
Current guidelines have recommended the use of IVUS for selected patients to optimise stent implantation (Class of recommendation IIa, Level of evidence B),6, 7 but the evidence was based on data from observational studies and bare stent eras. At present, DES is widely used in clinical practice, so stronger evidence is needed to support the application of IVUS in PCI. Because of the different results of the related clinical randomised controlled trials (RCTs), it is necessary to use meta‐analysis for comprehensive evaluation to provide more effective evidence for choosing the rational guidance strategy for DES implantation.
2. MATERIALS AND METHODS
2.1. Search strategy
We performed the current meta‐analysis based on the Cochrane Handbook for Systematic Reviews of Interventions8 and Preferred Reporting Items for Systematic Reviews and Meta‐analyses guidelines.9 We conducted a systematic screening process using the CENTRAL, PubMed, EMBASE, and Cochrane Database of Systematic Reviews from their inception to January 2018 based on the following MeSH terms and free key words: “percutaneous coronary intervention,” “IVUS,” “intravascular ultrasound,” “angiography,” and “drug‐eluting stent.” All relevant publications were identified without language restrictions, in which we identified full‐text papers from reference materials for further evaluation.
2.2. Inclusion criteria
Articles that were related to the following inclusion criteria were included in this analysis: (a) patients were clinically diagnosed with complex coronary artery lesions and underwent PCI using DES; (b) trials compared angiography‐guided and IVUS‐guided implantation; (c) more than one of the following parameters were mentioned in studies: stent thrombosis, cardiovascular death, myocardial infarction (MI), major adverse cardiac events (MACEs), target lesion revascularisation (TLR), and all‐cause death; and (d) RCTs. Studies were excluded using the following exclusion criteria: (a) trials without a control group; (b) the reported data were clearly erroneous or incomplete and did not provide research outcomes; (c) observational studies or case reports; and (d) duplicated publications.
2.3. Risk‐of‐bias assessments
The risk of bias was evaluated in each mentioned study based on Cochrane handbook version 5.1.0 for Systematic Reviews by Cochrane Collaboration. Study quality was evaluated, including allocation concealment, blinding of outcome assessment, blinding of participants and personnel, incomplete outcome data, random sequence generation, selective reporting, and other biases. Each entry was then classified as “high risk,” “unclear risk,” and “low risk.”
2.4. Data selection and extraction
After the screening process, studies were then assigned to certain topic(s). Using Thomson Research Software (EndNote X4, Microsoft, Redmond, Washington), we extracted relevant data for accuracy assessment. Any unclear information should be with more details of original articles. “Excluded (reason),” “Pending,” and “Included” were included in the “notes” column. We retracted “Pending” articles from the references.
A self‐designed data extraction form was used to independently extract contents by two researchers, including lead author, year of publication, participant characteristics, clinical stage of cancer, histological type, tumour size, treatment measures, outcomes, effect indicators, and follow‐up duration. The literature screening process, data extraction, and quality evaluation process were performed separately by two reviewers. In case of disagreement, a third investigator was asked to help resolve the disagreement through discussion.
2.5. Statistical analysis
The Review Manager Software (RevMan5.3, Microsoft, Redmond, Washington) was used for statistical analysis. Risk ratios (RR) and its 95% confidence interval (CI) were utilised for binary data and effect size in the meta‐analysis. A χ 2 test was used to assess the significance of heterogeneity, and the degree of heterogeneity was then examined using the I 2 statistic. The fixed‐effects model was used if the assessment of heterogeneity was insignificant (P > 0.1, I 2 ≤ 50%). If the source of heterogeneity was uncertain, we used the random‐effects model for further analysis.
2.6. Trial sequential analysis
Trial sequential analysis (TSA) is a method for estimating sample size, which can adjust random errors and calculate the sample size through the TSA 0.9 Beta (available at http://www.ctu.dk/tsa). We estimated a diversity‐adjusted required information size, which consisted of type power = 80%, I error α = 5%, and two‐sided testing. The hypothesis was that 25% relative reduction could be achieved through IVUS guidance in the risk of MACEs, and in the angiography‐guided group, there was a 10% anticipated event rate for MACEs. A graph of the cumulative Z curve presented the major results, and the boundaries in this graph were then determined by the O'Brien‐Fleming α‐spending function for final non‐inferiority, inferiority, or superiority.
3. RESULTS
3.1. Study selection process
A total, 928 articles were identified initially. After 87 duplicates were removed, 804 irrelevant citations were further excluded based on the review of titles and abstracts. During full‐text reading of the 37 included articles, 25 articles were then excluded. Finally, a total of 12 studies10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21 published between 2010 and 2017 were assessed for eligibility in the meta‐analysis (Figure 1).
Figure 1.
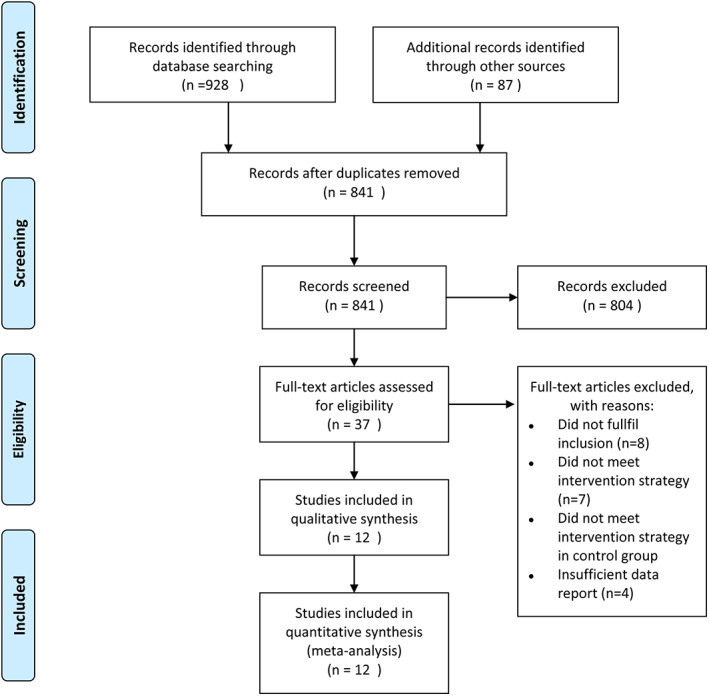
Flow diagram of study searching strategy
3.2. Quality assessment
Six10, 11, 12, 14, 15, 19 studies described random sequence generation using web‐based systems, while three studies13, 18, 21 just reported randomised trials without randomisation description; two studies17, 20 of random grouping method were assessed as a high risk of bias. Blinded methods were either used in participants or intervention providers in four trials. Most trials had comparable baseline clinical characteristics except one trial,20 which had a statistically significant difference when comparing the two groups for current smokers. Blinding of outcome assessment was independent in all studies. None of the included studies had selective reporting or incomplete reporting. Three studies10, 12, 14 were of high methodological quality, seven11, 13, 15, 16, 18, 19, 21 of moderate quality, and the remaining two studies17, 20 of low quality. Figures 2 and 3 present the summary of the quality assessment process.
Figure 2.
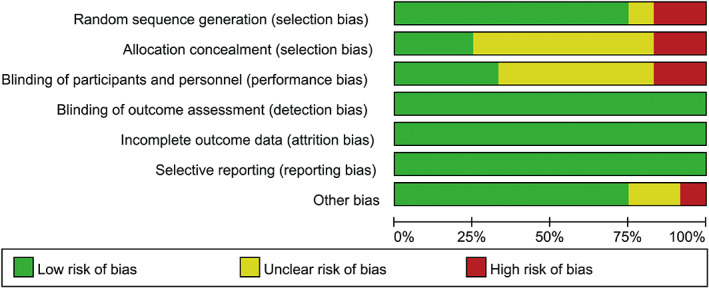
Quality assessment summary for included studies
Figure 3.
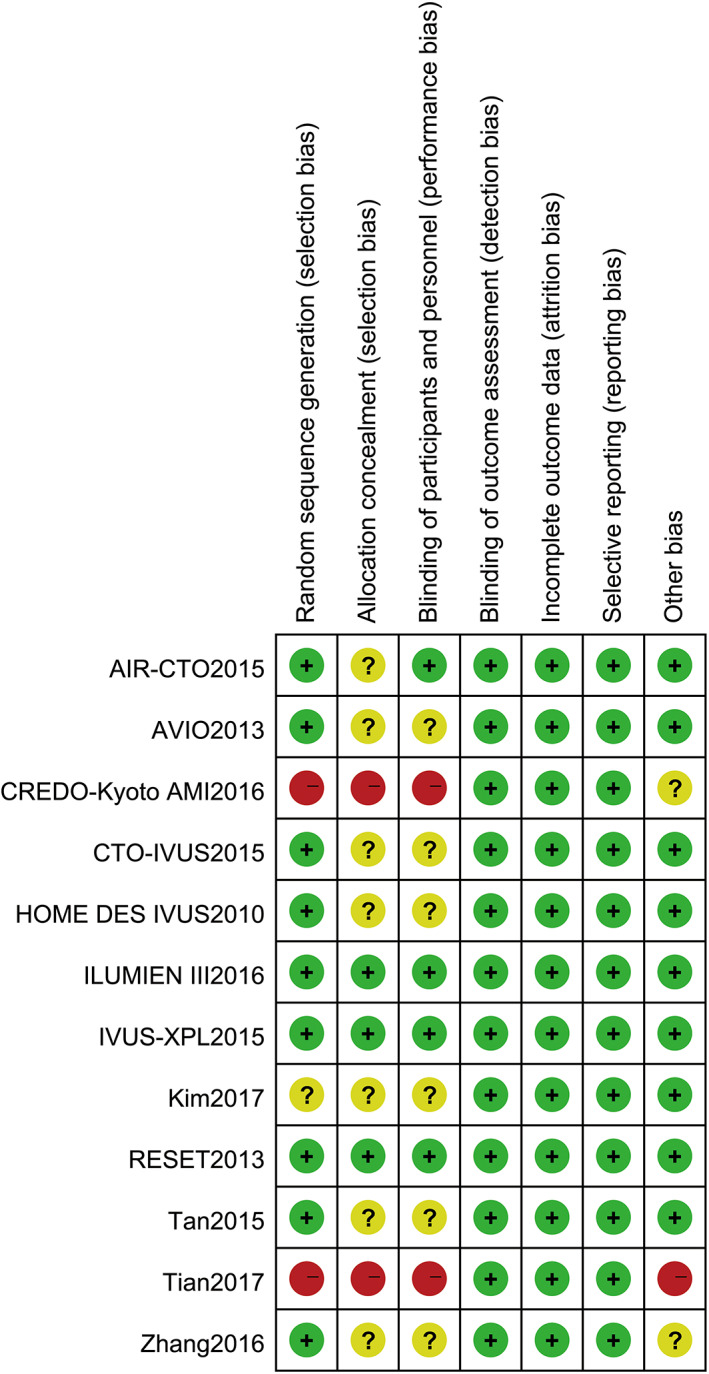
Methodological quality assessment for each included study; +, low risk of bias; −, high risk of bias; ?, unclear risk of bias
3.3. Characteristics of study selection
A total of 6268 patients were included in this meta‐analysis, including 2984 receiving IVUS‐guided DES implantation and 3284 using angiography‐guided DES implantation. Studies included patients with complex lesions, such as long lesions (implanted stent ≥28 mm in length), left main lesions, chronic total occlusion, or small vascular lesions. Among those patients, follow‐up duration varied from 30 days to 60 months, sample sizes from 84 to 1899, and mean ages from 50 to 80 years. No significant statistical difference was observed between the two groups in baseline characteristics such as diabetes, smoking status, and hypertension. Intervention strategies were similar among most of the trials. The major characteristics of the included studies are depicted in Table 1.
Table 1.
Characteristics of the included studies
| Author, y | Centre | Lesion characteristics | Procedures | Sample size/male | Patients baseline characteristics | Follow‐up | Outcome measures | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean age | Diabetes | Smoker | Hypertension | |||||||
| ILUMIEN III, 201610 | Multicentre | Complex lesions | IVUS | 146/107 | 66 (61‐72) | 55 (38%) | 19 (13%) | 113 (77%) | 12 mo | ①③④⑤ |
| Angiography | 146/107 | 67 (56‐74) | 42 (29%) | 35 (24%) | 109 (75%) | |||||
| AVIO, 201311 | Multicentre | Complex lesions | IVUS | 142/117 | 63.9 ± 10.9 | 34 (23.9%) | 49 (34.5%) | 100 (70.4%) | 24 mo | ①②③④⑤ |
| Angiography | 142/109 | 63.6 ± 11.0 | 38 (26.8%) | 44 (31.0%) | 109 (76.8%) | |||||
| IVUS‐XPL, 201512 | Multicentre | Long lesions | IVUS | 700/483 | 64 ± 9 | 250 (36%) | 155 (22%) | 454 (65%) | 12 mo | ①②③⑤ |
| Angiography | 700/481 | 64 ± 9 | 256 (37%) | 181 (26%) | 444 (63%) | |||||
| HOMEDESIVUS, 201013 | Single‐centre | Complex lesions | IVUS | 105/73 | 59.4 ± 13 | 44 (42%) | 42 (40%) | 70 (67%) | 18 mo | ①③④⑤ |
| Angiography | 105/71 | 60.2 ± 11 | 47 (45%) | 37 (35%) | 75 (71%) | |||||
| RESET, 201314 | Multicentre | Long lesions | IVUS | 269/177 | 62.8 ± 9.3 | 85(31.6%) | 58(21.6%) | 165(61.3%) | 12 mo | ①②③④⑤ |
| Angiography | 274/150 | 64.3 ± 8.7 | 82(29.9%) | 47(17.2%) | 178 (65.8%) | |||||
| CTO‐IVUS, 201515 | Multicentre | Chronic total occlusion | IVUS | 201/162 | 61.0 ± 11.1 | 70 (34.8%) | 71 (35.3%) | 126 (62.7%) | 12 mo | ①②③④⑤ |
| Angiography | 201/162 | 61.4 ± 10.1 | 68 (33.8%) | 69 (34.3%) | 128 (63.7%) | |||||
| Kim, 201716 | Single‐centre | Left main lesions | IVUS | 122/95 | 62.1 ± 10.6 | 47 (38.5%) | 36 (29.5%) | 75 (61.5%) | 30 d | ①②③④⑤ |
| Angiography | 74/53 | 64.8 ± 11.3 | 33 (44.6%) | 21 (28.4%) | 54 (73%) | |||||
| CREDO‐Kyot AMI, 201517 | Multicentre | Acute myocardial infarction | IVUS | 368 | NR | NR | NR | NR | 60 mo | ①③④⑤⑥ |
| Angiography | 237 | NR | NR | NR | NR | |||||
| Tan, 201518 | Single‐centre | Unprotected left main lesions | IVUS | 61/38 | 76.5 ± 5.0 | 21 (34.4%) | 27 (44.3%) | 25 (41.0%) | 24 mo | ①②③④ |
| Angiography | 62/43 | 75.9 ± 3.5 | 18 (29.5%) | 29 (46.8%) | 29 (46.8%) | |||||
| AIR‐CTO, 201519 | Multicentre | Chronic total occlusion | IVUS | 115/89 | NR | 35 (30%) | 45 (39%) | 86 (75%) | 12 mo | ①②③④⑤⑥ |
| Angiography | 115/80 | NR | 31 (27%) | 45 (39%) | 81 (70%) | |||||
| Tian, 201720 | Single‐centre | Unprotected left main lesions | IVUS | 713/576 | 59.6 ± 10.9 | 173 (24.3%) | 256 (35.9%) | 400 (56.1%) | 12 mo | ②③④⑤⑥ |
| Angiography | 1186/920 | 60.0 ± 10.2 | 314 (26.5%) | 316 (26.6%) | 654 (55.1%) | |||||
| Zhang, 201621 | Single‐centre | Small vascular lesions | IVUS | 42/21 | 63.2 ± 8.0 | NR | 22 (52.4%) | 27 (64.3%) | 12 mo | ①②③ |
| Angiography | 42/25 | 60.1 ± 9.4 | NR | 22 (52.4%) | 25 (59.5%) | |||||
Abbreviations: IVUS, intravascular ultrasound‐guided; NR, not report.
Outcome measures: ① major adverse cardiac events, ② cardiovascular death, ③ myocardial infarction, ④ target lesion revascularisation, ⑤ stent thrombosis, ⑥ all‐cause death.
4. OUTCOMES AND SYNTHESIS OF RESULTS
4.1. Effects of interventions for early cervical cancer
4.1.1. Major adverse cardiac events
Eleven studies10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 21 reported MACEs, including a total of 4369 patients (2098 in the angiography‐guided PCI group and 2271 in the IVUS‐guided PCI group). There was no statistical between‐study heterogeneity in the RR of studies (P = 0.26, I 2 = 19%); therefore, we used the fixed‐effects model for pooling the data. As displayed in Figure 4, the pooled estimates of effect sizes showed significant statistical difference in MACE between the two groups (RR = 0.66, 95% CI [0.56, 0.78], P < 0.0001).
Figure 4.
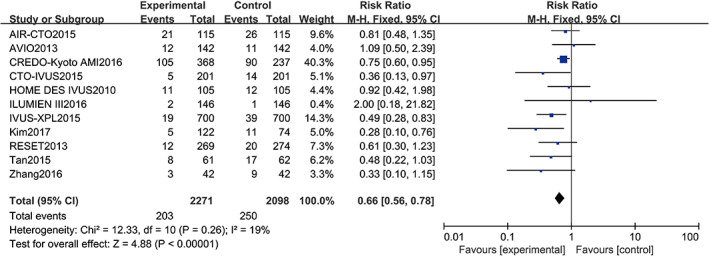
Comparison of major adverse cardiac events between intravascular ultrasound‐guided group and angiography‐guided group. CI, confidence interval
4.1.2. Cardiovascular death
Nine studies11, 12, 14, 15, 16, 18, 19, 20, 21 reported cardiovascular death, including a total of 5077 patients (2754 in the angiography‐guided PCI group and 2323 in the IVUS‐guided PCI group). There was no statistical between‐study heterogeneity in RR of studies (P = 0.79, I 2 = 0%), in which we utilised the fixed‐effects model for pooling the data. As displayed in Figure 5, the pooled estimates of effect sizes showed significant statistical difference of cardiovascular death between the two groups (RR = 0.49, 95% CI [0.29, 0.83], P = 0.007).
Figure 5.
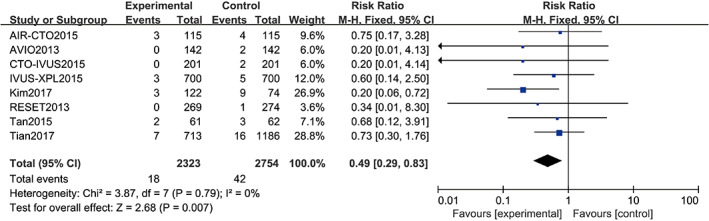
Comparison of cardiovascular death between intravascular ultrasound‐guided group and angiography‐guided group. CI, confidence interval
4.1.3. Myocardial infarction
Twelve studies10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21 reported MI including a total of 6399 patients (3415 in the angiography‐guided PCI group and 2984 in the IVUS‐guided PCI group). There was no statistical between‐study heterogeneity in the RR of studies (P = 0.61, I 2 = 0%); therefore, the fixed‐effects model was used for pooling the data. As displayed in Figure 6, the pooled estimates of effect sizes showed no significant statistical difference in progression‐free survival between the two groups (RR = 0.76, 95% CI [0.59, 0.99], P = 0.05).
Figure 6.
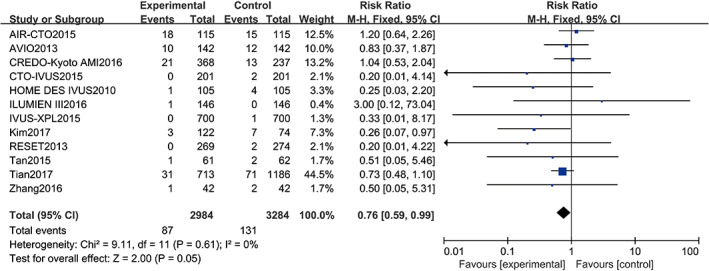
Comparison of myocardial infarction between intravascular ultrasound‐guided group and angiography‐guided group. CI, confidence interval
4.1.4. Target lesion revascularisation
Ten studies10, 11, 13, 14, 15, 16, 17, 18, 19, 20 reported TLR, including a total of 4784 patients (2542 in the angiography‐guided PCI group and 2242 in the IVUS‐guided PCI group). There was no statistical between‐study heterogeneity in RR of studies (P = 0.95, I 2 = 0%); therefore, the fixed‐effects model was used for pooling the data. As displayed in Figure 7, the pooled estimates of effect sizes showed significant statistical difference in TLR between the two groups (RR = 0.72, 95% CI [0.57, 0.91], P = 0.006).
Figure 7.
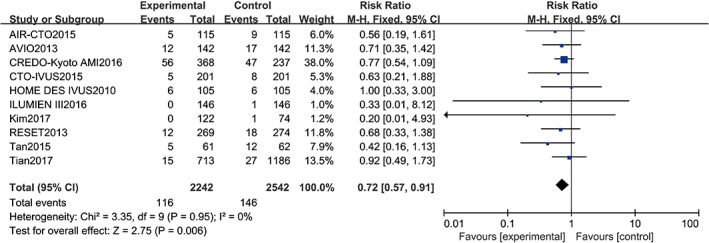
Comparison of target lesion revascularisation between intravascular ultrasound‐guided group and angiography‐guided group. CI, confidence interval
4.1.5. Stent thrombosis
Eight studies10, 11, 12, 13, 14, 15, 16, 17, 19, 20 reported stent thrombosis, including a total of 5573 patients (2960 in the angiography‐guided PCI group and 2613 in the IVUS‐guided PCI group). There was no statistical between‐study heterogeneity in the RR of studies (P = 0.61, I 2 = 0%); therefore, the fixed‐effects model was used for merging. As displayed in Figure 8, the pooled estimates of effect sizes showed no significant statistical difference in stent thrombosis between the two groups (RR = 0.82, 95% CI [0.49, 1.37], P = 0.44).
Figure 8.
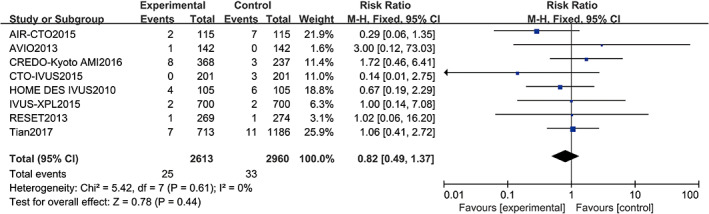
Comparison of stent thrombosis between intravascular ultrasound‐guided group and angiography‐guided group. CI, confidence interval
4.1.6. All‐cause death
Three studies17, 19, 20 reported all‐cause death, including a total of 2734 patients (2960 in the angiography‐guided PCI group and 2613 in the IVUS‐guided PCI group). There was no statistical between‐study heterogeneity in RR of studies (P = 0.81, I 2 = 0%); therefore, we used the fixed‐effects model for pooling the data. As displayed in Figure 9, the pooled estimates of effect sizes showed significant statistical difference in all‐cause death between the two groups (RR = 0.72, 95% CI [0.51, 1.01], P = 0.05).
Figure 9.
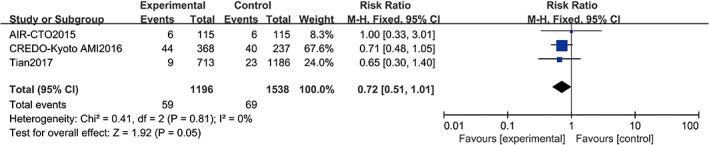
Comparison of all‐cause death between intravascular ultrasound‐guided group and angiography‐guided group. CI, confidence interval
4.1.7. Trial sequential analysis
The evaluation of MACEs through TSA indicated that the cumulative Z curve crossed the trial sequential monitoring boundaries for superiority, but there was no sufficient information on size. It further confirmed a definite decrease in MACEs through IVUS guidance, as displayed in Figure 10.
Figure 10.
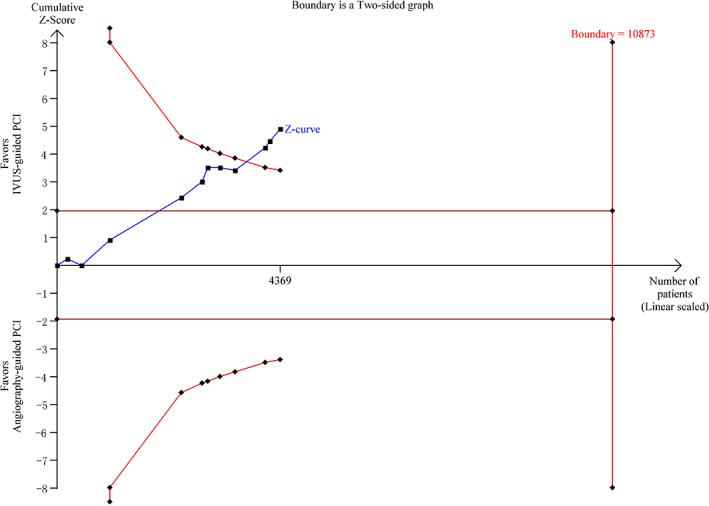
Trial sequential analyses for major adverse cardiac events. IVUS, intravascular ultrasound; PCI, percutaneous coronary intervention
5. DISCUSSION
Coronary atherosclerotic heart disease is a cardiovascular disease with a rapid increase in both morbidity and mortality, and early diagnosis and proper evaluation of the severity of coronary lesions will help develop effective treatment options. Coronary angiography has been considered a “gold standard” to evaluate the severity of coronary artery lesions and to diagnose coronary heart disease. However, with the application of IVUS, the degree of critical lesions is evaluated along with the identification of vulnerable plaque, calcification lesions, interlayer, and wall hematoma, and traditional coronary angiography significantly lags behind IVUS, especially in some complex lesions conditions, such as bifurcation lesions, left main lesion, chronic complete occlusion, small vascular lesions, stents within restenosis, and other lesions.22
Our present pooled analysis of 12 RCTs demonstrated that the IVUS‐guided group had significantly improved clinical outcomes, including MACEs, cardiovascular death, all‐cause death, MI, and TLR. However, its effect on stent thrombosis was not statistically significantly different from the angiography‐guided DES implantation group. Guagliumi et al23 showed that hypertension, diabetes, and smoking have a different effect between late stent thrombosis and very late stent thrombosis, which may be related to delayed endometrial coverage and incomplete healing. There is a remarkable difference between the IVUS‐guided and angiography‐guided group on baseline characteristics such as current smoker ratio in our study. Therefore, IVUS‐guided DES implantation is superior to coronary angiography‐guided DES implantation in terms of stent thrombosis. However, more high‐quality research should be performed to confirm the beneficial effect.
The value of IVUS‐guided DES in the treatment of coronary heart disease remains controversial. The AVIO study11 found that IVUS was superior to coronary angiography in the guidance of DES treatment, with its effect of increasing the minimum lumen diameter and reducing the incidence of stent adherent, but it cannot effectively improve the patient's clinical outcome, especially in patients with simple lesions. Yoon et al24 also pointed out that IVUS had a better effect than coronary angiography in the treatment of DES lesions; even in patients with diabetes as a high‐risk factor or acute coronary syndrome, its clinical benefit is not obvious. The findings from the CREDO‐Kyoto AMI trial17 also suggested that IVUS guidelines had no effect on reducing the incidence of TVR and TLR in first‐episode stent thrombosis‐segment elevation MI. However, most of the studies that conducted IVUS‐guided DES predicted the clinical outcomes of coronary heart disease, and the limited sample size in the above study and the potential differences between baseline and lesion characteristics of patients may affect the reliability of the results. The CREDO‐Kyoto AMI study mixed implants with a large number of bare metal stents, so the conclusions of this study cannot be fully extended to IVUS‐guided DES treatment.
A recent meta‐analysis of relevant topics showed that, in the treatment of coronary heart disease DES, IVUS‐guided DES implantation can provide a beneficial effect in clinical outcomes in comparison with angiography‐guided DES implantation.25, 26, 27, 28, 29, 30, 31 Shin et al30 reported a meta‐analysis with data on individual patient levels from 2345 randomised patients. A new generation of IVUS‐guided DES implantation can significantly reduce stent thrombosis, the risks of cardiac death, or MI for complex lesions. Three RCTs were performed particularly for left main lesions.16, 18, 20 Tan et al18 demonstrated that the IVUS‐guided group had a lower composite of non‐fatal MI, death, or TLR (13.1% vs 29.3%, P = 0.031). Tian et al20 reported MI and all‐cause death at 3 years between IVUS vs angiography guidance. IVUS guidance provided a significantly lower incidence of the composite of MI and all‐cause death (hazard ratio = 0.82, 95% CI [0.49, 1.37]; P = 0.001). Moreover, a recent pooled analysis32 has suggested that IVUS‐guided PCI is superior to angiography‐guided PCI in the left main coronary artery disease based on risk reduction in both all‐cause and cardiac death. Two RCTs15, 19 were performed particularly for chronic total occlusion. In the AIR‐CTO trial19, earlier lumen loss was observed in the IVUS‐guided group compared with the angiography‐guided group (0.28 vs 0.46 mm, P = 0.025). In the second RCT,15 the post‐procedural minimal lumen diameter was significantly larger in the IVUS‐guided group (2.64 vs 2.56 mm, P = 0.025). In patients with long lesions, IVUS‐guided implantation provided a remarkably lower rate of MACEs at 1 year compared with angiography‐guided implantation.12, 14 Therefore, it is noteworthy that IVUS guidance is beneficial for patients with complex lesions.
Several limitations of the current analysis should be discussed. First, the included studies in the pooled analysis are different in study design, patient baseline characteristics, study endpoints, and definitions. Second, the use of different types of DES in each study failed to distinguish the clinical outcomes of angiography‐guided implantation or IVUS‐guided implantation in terms of the treatment of coronary heart disease with different types of DES. Third, clinical beneficial outcomes of IVUS‐guided implantation at different baseline levels and type of lesions were not further compared. The relevant research needs to be further improved from the following aspects: larger sample size; right random allocation and allocation of hidden programs; sufficient follow‐up duration to observe the short‐term and long‐term effects; and stratified analysis of lesion characteristics, different types of DES, and patient baseline characteristics as independent factors. More comprehensive evaluation of the efficacy of IVUS‐guided implantation should be undertaken in the future.
6. CONCLUSIONS
In summary, IVUS‐guided DES implantation appears to have significantly improved clinical outcomes compared with angiography‐guided DES implantation, including MACEs, cardiovascular death, all‐cause death, TLR, and MI. However, its effect on stent thrombosis requires further confirmation. More high‐quality RCTs are warranted to verify the findings and conclusions of the current meta‐analysis and determine the IVUS‐guidance effect on different patients with lesions.
CONFLICTS OF INTEREST
The authors have no conflicts of interest to report.
Tan Y‐Y, Man X‐X, Liu L‐Y, Xu H. Comparison of clinical outcomes between intravascular ultrasound‐guided and angiography‐guided drug‐eluting stent implantation: A meta‐analysis of randomised control trials and systematic review. Int Wound J. 2019;16:649–658. 10.1111/iwj.13073
REFERENCES
- 1. Schofer J, Pinto DS, Stone GW, Ellis SG. Safety and efficacy of sirolimus‐ and paclitaxel‐eluting coronary stents. N Engl J Med. 2007;356:998. [DOI] [PubMed] [Google Scholar]
- 2. Kornowski R. The complexity of stenting in bifurcation coronary lesions. JACC Cardiovasc Interv. 2013;6:696‐697. [DOI] [PubMed] [Google Scholar]
- 3. Hibi K, Kimura K, Umemura S. Clinical utility and significance of intravascular ultrasound and optical coherence tomography in guiding percutaneous coronary interventions. Circ J. 2015;79(1):24‐33. [DOI] [PubMed] [Google Scholar]
- 4. Witzenbichler B, Maehara A, Weisz G, et al. Relationship between intravascular ultrasound guidance and clinical outcomes after drug‐eluting stents. Circulation. 2014;129:463‐470. [DOI] [PubMed] [Google Scholar]
- 5. Kadohira T, Kobayashi Y. Intravascular ultrasound‐guided drug‐eluting stent implantation. Cardiovasc Interv Ther. 2016;314:1‐11. [DOI] [PubMed] [Google Scholar]
- 6. Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention. A report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines and the society for cardiovascular angiography and interventions. J Am Coll Cardiol. 2011;58:e44‐e122. [DOI] [PubMed] [Google Scholar]
- 7. Windecker S, Alfonso F, Collet J‐P, et al. ESC/EACTS Guidelines on myocardial revascularization The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio‐Thoracic Surgery (EACTS). Eur Heart J. 2014;2014:31. [DOI] [PubMed] [Google Scholar]
- 8. Higgins J, Green SR. Cochrane Handbook for Systematic Reviews of Interventions, version 5.1.02011). Somerset, NJ: Wiley. http://training.cochrane.org/handbook. Accessed November 22, 2017. [Google Scholar]
- 9. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate healthcare interventions: explanation and elaboration. PLoS Med. 2009;6:e1‐e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ali ZA, Maehara A, Généreux P, et al. Optical coherence tomography compared with intravascular ultrasound and with angiography to guide coronary stent implantation (ILUMIEN III: OPTIMIZE PCI): a randomised controlled trial. Lancet. 2016;388:2618‐2628. [DOI] [PubMed] [Google Scholar]
- 11. Chieffo A, Latib A, Caussin C, et al. A prospective, randomized trial of intravascular‐ultrasound guided compared to angiography guided stent implantation in complex coronary lesions: the AVIO trial. Am Heart J. 2013;165:65‐72. [DOI] [PubMed] [Google Scholar]
- 12. Hong SJ, Kim BK, Shin DH, et al. Effect of intravascular ultrasound‐guided vs angiography‐guided everolimus‐eluting stent implantation: the IVUS‐XPL randomized clinical trial. JAMA. 2015;314:2155‐2163. [DOI] [PubMed] [Google Scholar]
- 13. Jakabcin J, Spacek RM, Kvasnak M, et al. Long‐term health outcome and mortality evaluation after invasive coronary treatment using drug eluting stents with or without the IVUS guidance. Randomized control trial. HOME DES IVUS. Catheter Cardiovasc Interv. 2010;75:578‐583. [DOI] [PubMed] [Google Scholar]
- 14. Kim JS, Kang TS, Mintz GS, et al. Randomized comparison of clinical outcomes between intravascular ultrasound and angiography‐guided drug‐eluting stent implantation for long coronary artery stenoses. JACC Cardiovasc Interv. 2013;6:369‐376. [DOI] [PubMed] [Google Scholar]
- 15. Kim BK, Shin DH, Hong MK, et al. Clinical impact of intravascular ultrasound‐guided chronic total occlusion intervention with Zotarolimus‐eluting versus biolimus‐eluting stent implantation: randomized study. Circ Cardiovasc Interv. 2015;8:e002592. [DOI] [PubMed] [Google Scholar]
- 16. Kim YH, Her AY, Rha SW, et al. Three‐year major clinical outcomes of angiography‐guided single stenting technique in non‐complex left Main coronary artery diseases. Int Heart J. 2017;58:704‐713. [DOI] [PubMed] [Google Scholar]
- 17. Nakatsuma K, Shiomi H, Morimoto T, et al. Intravascular ultrasound guidance vs. angiographic guidance in primary percutaneous coronary intervention for ST‐segment elevation myocardial infarction ‐ long‐term clinical outcomes from the CREDO‐Kyoto AMI registry. Circ J. 2015;80:477‐484. [DOI] [PubMed] [Google Scholar]
- 18. Tan Q, Wang Q, Liu D, Zhang S, Zhang Y, Li Y. Intravascular ultrasound‐guided unprotected left main coronary artery stenting in the elderly. Saudi Med J. 2015;36:549‐553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tian NL, Gami SK, Ye F, et al. Angiographic and clinical comparisons of intravascular ultrasound‐ versus angiography‐guided drug‐eluting stent implantation for patients with chronic total occlusion lesions: two‐year results from a randomised AIR‐CTO study. EuroIntervention. 2015;10:1409‐1417. [DOI] [PubMed] [Google Scholar]
- 20. Tian J, Guan C, Wang W, et al. Intravascular ultrasound guidance improves the long‐term prognosis in patients with unprotected left main coronary artery disease undergoing percutaneous coronary intervention. Sci Rep. 2017;7:2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang J‐Q, Shi R, Pang W, Guo Q. Application of intravascular ultrasound in stent implantation for small coronary arteries. J Clin Invasive Cardiol. 2016;3:1‐8. [Google Scholar]
- 22. Hong SJ, Jang Y, Kim BK. Clinical Evidence of Intravascular Ultrasound‐Guided Percutaneous Coronary Intervention. In: Hong MK, ed. Coronary Imaging and Physiology. Singapore: Springer; 2018. [Google Scholar]
- 23. Guagliumi G, Sirbu V, Musumeci G. Examination of the in vivo mechalisms of late drug‐eluting stent thrornbosis:findings form optical coherence tomography and intravascular ultmsound imaging. JACC Cardiovasc Interv. 2012;5:12‐20. [DOI] [PubMed] [Google Scholar]
- 24. Yoon YW, Shin S, Kim BK, et al. Usefulness of intravascular ultrasound to predict outcomes in short‐length lesions treated with drug‐eluting stents. Am J Cardiol. 2013;112:642‐646. [DOI] [PubMed] [Google Scholar]
- 25. Jang JS, Song YJ, Kang W, et al. Intravascular ultrasound‐guided implantation of drug‐eluting stents to improve outcome: a meta‐analysis. JACC Cardiovasc Interv. 2014;7:233‐243. [DOI] [PubMed] [Google Scholar]
- 26. Ahn JM, Kang SJ, Yoon SH, et al. Meta‐analysis of outcomes after intravascular ultrasound‐guided versus angiography‐guided drug‐eluting stent implantation in 26,503 patients enrolled in three randomized trials and 14 observational studies. Am J Cardiol. 2014;113:1338‐1347. [DOI] [PubMed] [Google Scholar]
- 27. Elgendy IY, Mahmoud AN, Elgendy AY, Bavry AA. Outcomes with intravascular ultrasound‐guided stent implantation: a meta‐analysis of randomized trials in the era of drug‐eluting stents. Circ Cardiovasc Interv. 2016;9:e003700. [DOI] [PubMed] [Google Scholar]
- 28. Steinvil A, Zhang YJ, Lee SY, et al. Intravascular ultrasound‐guided drug‐eluting stent implantation: an updated meta‐analysis of randomized control trials and observational studies. Int J Cardiol. 2016;216:133‐139. [DOI] [PubMed] [Google Scholar]
- 29. Cheng Q, Hong F, Cao J, Zhang G, Wang Y. Intravascular ultrasound guidance in drug‐eluting stents implantation: a meta‐analysis and trial sequential analysis of randomized controlled trials. Oncotarget. 2017;8:59387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shin DH, Hong SJ, Mintz GS, et al. Effects of intravascular ultrasound‐guided versus angiography‐guided new‐generation drug‐eluting stent implantation: meta‐analysis with individual patient‐level data from 2,345 randomized patients. JACC Cardiovasc Interv. 2016;9:2232‐2239. [DOI] [PubMed] [Google Scholar]
- 31. Ye Y, Yang M, Zhang S, Zeng Y. Percutaneous coronary intervention in left main coronary artery disease with or without intravascular ultrasound: a meta‐analysis. PLoS One. 2017;12:e0179756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. de la Hernandez T, Baz Alonso JA, Gómez Hospital JA, et al. Clinical impact of intravascular ultrasound guidance in drug‐eluting stent implantation for unprotected left main coronary disease: pooled analysis at the patient‐level of 4 registries. J Am Coll Cardiol. 2013;62:244‐254. [DOI] [PubMed] [Google Scholar]


