Abstract
Wide local excision offers a potential cure for severe axillary hidradenitis suppurativa. However, the gold standard for reconstruction has yet to be defined. Here, we describe our rotation advancement flap technique, which allows for one‐stage closure of large axillary defects, with minimal functional morbidity to the axilla. We performed a retrospective review of all patients who underwent one‐stage surgical management for axillary hidradenitis suppurativa at a single‐centre tertiary care hospital from 2009 to 2018. We identified 34 patients, with a total of 53 operative sites. The majority were female (85%) with a mean age of 31 years and body mass index 35 kg/m2. The median defect size was 84 cm2 and the majority were treated using the rotation advancement flap technique (86%). A quarter of operative sites experienced minor complications with only one requiring re‐operation. At a median follow‐up of 32 months, two (4%) sites showed decreased range of motion. However, all patients had achieved remission without any further recurrence of disease. We describe a one‐stage rotation advancement flap technique for management of moderate to severe hidradenitis suppurativa that achieves a high local cure rate, minimal functional morbidity, and acceptable wound complication rates.
Keywords: axilla, flap, hidradenitis suppurativa, one stage, surgery
1. INTRODUCTION
Hidradenitis suppurativa (HS) is a chronic, debilitating disease that affects the intertriginous areas of the body, with the axilla being the most commonly involved site.1, 2 The presentation varies from mild, asymptomatic to painful, draining sinus tracts and tends to affect women more often than men.3, 4, 5 The aetiology appears to be multifactorial with risk factors, including diabetes, smoking, and African American race.3, 4, 5, 6, 7 Although management with antibiotics and incision and drainage are often used for mild disease, these efforts only temporarily improve the disease and are not curative. Any efforts short of complete excision of the diseased areas of skin will invariably lead to recurrence. The current standard of care for treatment of severe stage disease is wide local excision, which yields the lowest recurrence rate.8, 9, 10, 11, 12
Wide local excision often leads to large defects that are difficult to close primarily.13 This is a particular concern for the axilla where function and range of motion are prioritised. However, there is no consensus on the optimal surgical technique for management of severe axillary HS.14 Numerous options exist for surgical reconstruction, such as healing by second intention, split‐thickness skin grafts (STSGs), and the use of local tissue flaps, but these methods are associated with increased complications, such as decreased range of motion, poor scarring, and prolonged recovery.15, 16, 17 Moreover, STSG and flap reconstruction have increased operative times and may lead to an increased number of surgical procedures.18
The objective of this study was to describe our one‐stage rotation advancement flap technique for management of moderate to severe axillary HS following wide local excision. In addition, we will evaluate clinical outcomes, including complications, recurrences, and shoulder mobility.
2. METHODS
2.1. Data collection
We reviewed all cases of local tissue rearrangement for axillary HS performed at a single tertiary university hospital from 2009 to2018. Institutional review board approval was obtained prior to any data collection. Patient data were retrieved from prospectively maintained computerised databases and medical records. Details were collected on patient demographics, extent and severity of HS, intra‐operative details, and postoperative course, including complications within 90 days, unplanned readmission within 90 days, shoulder mobility, and recurrence of disease. HS severity was classified using the Hurley staging system.19 Operative time was defined as time from first incision until application of wound dressings. Bilateral cases were excluded from the calculation of the mean operative duration. Recurrence was defined as a return of rash or painful nodules within an operative site. Data were tabulated and analysed descriptively using Microsoft Excel (Microsoft 2016, Redmond, Washington). Means and SD are reported for data that follows a normal distribution whereas non‐parametric data is described using medians and the interquartile range (IQR).
2.2. Operative technique
Incisional markings are completed preoperatively while the patient is awake. The diseased areas of HS are carefully marked and include all areas of active and previous disease as evidenced by scarred tissue, sinus tracts, and abscesses. The procedure is performed under general anaesthesia, with the patient placed supine on the operating table. All pressure points are padded and the axilla and arm are prepped and draped in a sterile fashion. There are two surgical phases: wide local excision and axillary reconstruction. During the first phase, the preoperatively marked areas are confirmed to ensure removal of all areas of diseased skin. The entirety of disease is excised down to the subcutaneous fat, 1 cm thick underneath the skin. The diseased sites are then copiously irrigated with clorpactin or bacitracin containing solution and careful haemostasis is assured with electrocautery.
During the second phase, the defects are measured, and adjacent tissue rearrangement is performed (Figure 1). In order to offset tension in the upper arm, a back‐cut is performed to create a skin and subcutaneous tissue flap. The incision is extended in a curvilinear manner taking advantage of the common redundancy typically present in tissue of the upper/lateral back area. If the patient has soft tissue redundancy in upper arm or chest, the flap can be recruited from this excess tissue instead (Figure 2). The flap is then elevated and advanced into the defect to allow for primary closure. Once haemostasis is achieved, wounds are closed in multiple layers to obliterate dead space. The rotation‐advancement flaps are secured in place with 2‐0 Vicryl tacking sutures, then 3‐0 Vicryl deep dermal sutures to anchor the skin flaps in place, followed typically by 3‐0 Vicryl interrupted sutures along the incision line. Lastly, iodoform packing gauze is placed in between the incision line to absorb additional moisture (removed the next day), followed by gauze fluffs, abdominal pads, Kerlix around the extremity, and Ace wraps as compressive dressings. Alternatively, for select patients, a negative pressure wound therapy device is applied in lieu of the dressings mentioned above (n = 3).
Figure 1.
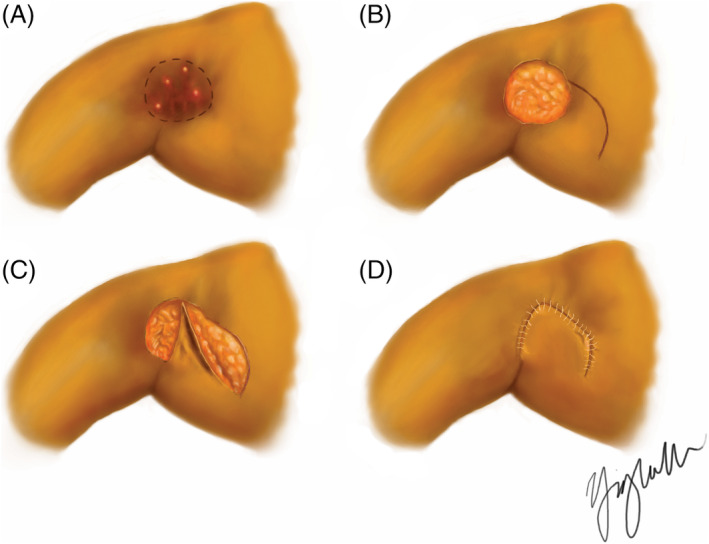
Rotation advancement flap closure technique. A, Preoperative illustration of right axilla with preoperative markings outlining area of disease for resection. B, Following excision of affected area. C, Flap rotation and advancement. D, Flap inset and closure with 3‐0 Vicryl interrupted sutures
Figure 2.
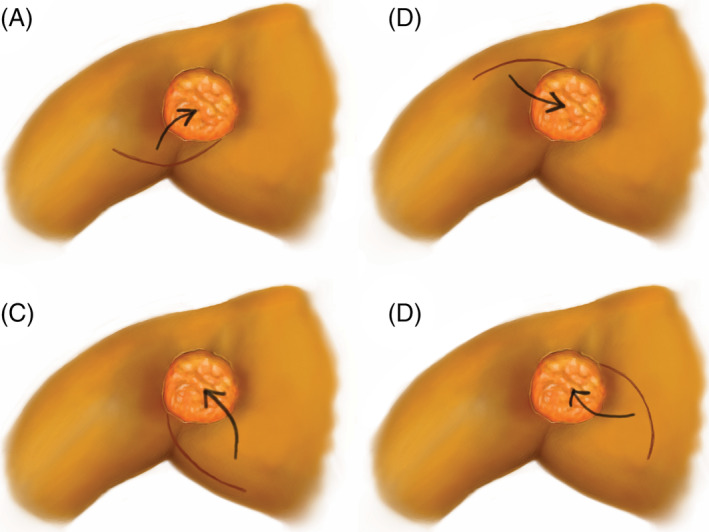
Alternative configurations of the rotation advancement flap. Design based on where there is skin and soft tissue redundancy. Arrow shows arc of rotation
2.3. Postoperative management
All procedures are performed on an outpatient basis and barring any complications during recovery in the post‐anaesthesia care unit, patients are discharged on the same day. Patients are instructed to keep the operative site dry for the first 48 hours. Showering is acceptable after 48 hours, but patients are instructed not to scrub the operative site. Upon discharge, patients are advised to apply bacitracin ointment to the surgical site twice daily for the first week. For the first 6 weeks postoperatively, patients are instructed to restrict arm movement to below the height of the shoulder and not to perform any heavy lifting (defined as weight greater than a gallon of milk).
3. RESULTS
Thirty‐four patients met our inclusion criteria. The cohort was predominantly female (85%) and African American (85%) with a mean age of 31 ± 9 years and body mass index 34.7 ± 8.6 kg/m2. Two‐thirds of patients had severe axillary HS defined as Hurley grade III, whereas the remainder had moderate disease burden (Hurley grade II). Additional patient demographics are reported in Table 1.
Table 1.
Demographic characteristics of 34 local tissue rearrangement patients
| Variables | Patients (n = 34) |
|---|---|
| Sex | |
| Female | 29 (85%) |
| Male | 5 (15%) |
| Mean age at first operation (yr) | 30.7 ± 9.0 |
| Mean body mass index (kg/m2) | 34.7 ± 8.6 |
| Race | |
| African American | 29 (85%) |
| White | 3 (9%) |
| Hispanic | 1 (3%) |
| Unknown | 1 (3%) |
| Diabetes mellitus | 7 (21%) |
| Tobacco use | |
| Never smoker | 16 (47%) |
| Previous smoker | 8 (24%) |
| Current smoker | 10 (29%) |
| Hurley grade | |
| II | 12 (35%) |
| III | 22 (65%) |
There were a total of 53 operative sites (Table 2). The most common type of tissue rearrangement procedure performed was our one‐stage rotation advancement flap technique (86%) (Figure 3). The median size of excised disease was 84 cm2 (IQR: 69‐120). The operations lasted for a mean of 46 ± 16 minutes with a median hospital stay of 0 days (IQR: 0‐0).
Table 2.
Summary of 53 cases of local tissue arrangement
| Variables | Operative sites (n = 53) |
|---|---|
| Median size of axillary defect (cm2) | 84 (interquartile range: 69‐120) |
| Type of procedure | |
| Rotation advancement flap | 46 (86%) |
| Primary sutured closure | 4 (8%) |
| Z‐plasty | 1 (2%) |
| Multiple closure typesa | 2 (4%) |
V‐Y advancement flap and Z‐plasty performed together.
Figure 3.
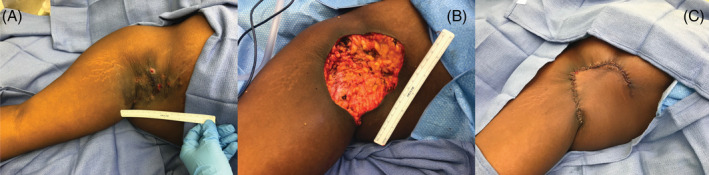
Case example of a patient treated with a rotation advancement flap. A, Severe axillary HS (Hurley grade III) in a 19‐year‐old African American female, body mass index 37.5 kg/m2. B, Wide local excision of diseased area 180 cm2. C, Immediate postoperative photograph showing wound closure
Following surgical excision, one‐quarter of operative sites experienced complications within the first 90 days postoperatively (n = 13), giving a complication rate of 25% (Table 3). All operative sites with a complication had dehiscence (n = 13). Two sites also had decreased shoulder range of motion, and one site had a surgical site infection. Dehiscence and surgical site infection were managed medically (ie, frequent dressing changes or oral antibiotics). There was a single readmission within 90 days due to wound dehiscence, which was managed with re‐operation (Table 4).
Table 3.
Postoperative complications within 90 days
| Complication | Number of operative sites (n = 53) |
|---|---|
| Dehiscence | 13 (25%) |
| Surgical site infection | 1 (2%) |
| Decreased shoulder range of motion | 2 (4%) |
Table 4.
Management for complications
| Management for complication | Number of complications |
|---|---|
| Medical management | 12 (80%) |
| Negative pressure wound therapy | 2 (13%) |
| Re‐operation | 1 (7%) |
There were five recurrences in 53 (10%) operative sites with a median disease‐free interval of 5 months (IQR: 4‐8). Three of these recurrences were treated with surgical intervention while two were managed conservatively. At a median follow‐up of 32 months (IQR: 10‐41), all patients treated with local tissue rearrangement for axillary HS have been in remission without any further evidence of recurrence.
4. DISCUSSION
In this study, we found a low incidence of complications, minimal functional morbidity, short operative times, and a high local cure rate. This not only demonstrates that the one‐stage rotation advancement flap technique is an effective method for the management of moderate to severe axillary HS, but also reinforces the widely held notion that surgery offers the best hope for curative therapy.
Our overall complication rate was 26%, but the majority were minor in nature and managed medically with the exception of one operative site which required re‐operation. This is consistent with previous studies that report complication rates of 14% to 65%.20, 21, 22, 23 A challenge of wide local excision of large axillary defects is preservation of shoulder mobility. Healing by secondary intention often leaves a retractile scar band and has the disadvantage of prolonged recovery time.24 In contrast, STSG requires immobilisation of the arm during the recovery period and can result in significant donor site morbidity.15 In our study, only 4% of operative sites showed evidence of decreased shoulder range of motion, which is lower than rates achieved through healing by secondary intention.25 This may be attributed to the curved scar that results from closure using our technique, which minimises the risk of linear scar contracture.
In addition to the low complication rate and low shoulder morbidity, the rotation advancement technique yielded a shorter mean operative time (46 ± 16 minutes) compared with that of other studies (76‐210 minutes), therefore reducing the overall time under general anaesthesia.18, 26 Moreover, this technique allows for single‐staged rotation advancement flap closure, which results in fewer total operations.
One of the most challenging aspects of axillary HS for both patients and surgeons is the recurrent nature of the disease. While recurrence rates after surgical excision reported in the literature range from 6% to 47%,10, 21 the recurrence rate in our study was low (10%). In our cohort, the median disease‐free interval for sites where a recurrence occurred was 5 months, which is similar to that of other studies.8, 27, 28, 29 Although sufficient resection of diseased skin is the most important factor in preventing recurrence,11, 30 more recent reports have suggested that the number of affected regions, location of hidradenitis, and type of surgical reconstruction may also play a role.10, 21, 29 Numerous methods for closure of axillary defects after wide local excision include primary complex closure, healing by secondary intention, STSG, and local flap rearrangement.20, 23, 31, 32, 33, 34 However, the types of reconstruction that appear to offer the best hope of remission are local tissue rearrangement and STSG.10, 21 Our rotation advancement flap technique falls within the subset of local tissue rearrangement, which may explain our low recurrence rates.
The main limitations of this study are its single‐centre retrospective nature and lack of a control arm to compare results. Furthermore, due to inconsistencies in patient records, we were unable to use an objective measure, such as the Disabilities of the Arm, Shoulder, and Hand questionnaire or the Dermatology Life Quality Index, to obtain a quantitative measure of shoulder mobility after axillary reconstruction. However, our study is strengthened by the longer follow‐up duration and patient demographics, which were consistent with the HS population characteristics described in the literature.3, 4, 5, 6, 7, 35
5. CONCLUSION
Axillary HS is a difficult disease to manage due to its recurrent nature and the need to preserve shoulder mobility, which can limit excision size. Our one‐stage rotation advancement flap is a promising closure technique, which yields a high local cure rate, minimal functional morbidity, short operative times, and acceptable wound complication rates.
CONFLICT OF INTEREST
None declared.
Wu Y, Ngaage LM, Ge S, Rada EM, Silverman RP, Rasko YM. Reconstruction for axillary hidradenitis suppurativa using one‐stage local tissue rearrangement: A retrospective analysis of 53 cases. Int Wound J. 2020;17:701–707. 10.1111/iwj.13319
REFERENCES
- 1. Mustafa EB, Ali SD, Kurtz LH. Hidradenitis suppurativa: review of the literature and management of the axillary lesion. J Natl Med Assoc. 1980;72(3):237‐243. [PMC free article] [PubMed] [Google Scholar]
- 2. Micheletti RG. Natural history, presentation, and diagnosis of hidradenitis suppurativa. Semin Cutan Med Surg. 2014;33(3 suppl):S51‐S53. [DOI] [PubMed] [Google Scholar]
- 3. Garg A, Kirby JS, Lavian J, Lin G, Strunk A. Sex‐ and age‐adjusted population analysis of prevalence estimates for hidradenitis suppurativa in the United States. JAMA Dermatol. 2017;153(8):760‐764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vazquez BG, Alikhan A, Weaver AL, Wetter DA, Davis MD. Incidence of hidradenitis suppurativa and associated factors: a population‐based study of Olmsted County, Minnesota. J Invest Dermatol. 2013;133(1):97‐103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vlassova N, Kuhn D, Okoye GA. Hidradenitis suppurativa disproportionately affects African Americans: a single‐center retrospective analysis. Acta Derm Venereol. 2015;95(8):990‐991. [DOI] [PubMed] [Google Scholar]
- 6. Revuz JE, Canoui‐poitrine F, Wolkenstein P, et al. Prevalence and factors associated with hidradenitis suppurativa: results from two case‐control studies. J Am Acad Dermatol. 2008;59(4):596‐601. [DOI] [PubMed] [Google Scholar]
- 7. Miller IM, Ellervik C, Vinding GR, et al. Association of metabolic syndrome and hidradenitis suppurativa. JAMA Dermatol. 2014;150(12):1273‐1280. [DOI] [PubMed] [Google Scholar]
- 8. Ritz JP, Runkel N, Haier J, Buhr HJ. Extent of surgery and recurrence rate of hidradenitis suppurativa. Int J Colorectal Dis. 1998;13:164‐168. [DOI] [PubMed] [Google Scholar]
- 9. Büyükaşik O, Hasdemir AO, Kahramansoy N, et al. Surgical approach to extensive hidradenitis suppurativa. Dermatol Surg. 2011;37(6):835‐842. [DOI] [PubMed] [Google Scholar]
- 10. Mehdizadeh A, Hazen PG, Bechara FG, et al. Recurrence of hidradenitis suppurativa after surgical management: a systematic review and meta‐analysis. J Am Acad Dermatol. 2015;73(5 suppl 1):S70‐S77. [DOI] [PubMed] [Google Scholar]
- 11. Soldin MG, Tulley P, Kaplan H, et al. Chronic axillary hidradenitis—the efficacy of wide excision and flap coverage. Br J Plast Surg. 2000;53(5):434‐436. [DOI] [PubMed] [Google Scholar]
- 12. Ge S, Ngaage LM, Orbay H, Silverman RP, Rasko YM, Rada EM. Surgical management of pediatric hidradenitis suppurativa: a case series and review of the literature. Ann Plast Surg. 2020. 10.1097/SAP.0000000000002195. [DOI] [PubMed] [Google Scholar]
- 13. Chuang CJ, Lee CH, Chen TM, et al. Use of a versatile transpositional flap in the surgical treatment of axillary hidradenitis suppurativa. J Formos Med Assoc. 2004;103(8):644‐647. [PubMed] [Google Scholar]
- 14. Rambhatla PV, Lim HW, Hamzavi I. A systematic review of treatments for hidradenitis suppurativa. Arch Dermatol. 2012;148(4):439‐446. [DOI] [PubMed] [Google Scholar]
- 15. Calibre C, Bouhanna A, Salmin JP, Bodin F, Benaïssa‐Beck M, Bruant‐Rodier C. [Axillary hidradenitis suppurativa: a single‐stage surgical treatment]. Ann Chir Plast Esthet. 2013;58(6):670‐675. [DOI] [PubMed] [Google Scholar]
- 16. Yamashita Y, Hashimoto I, Matsuo S, Abe Y, Ishida S, Nakanishi H. Two‐stage surgery for hidradenitis suppurativa: staged artificial dermis and skin grafting. Dermatol Surg. 2014;40(2):110‐115. [DOI] [PubMed] [Google Scholar]
- 17. Hynes PJ, Earley MJ, Lawlor D. Split‐thickness skin grafts and negative‐pressure dressings in the treatment of axillary hidradenitis suppurativa. Br J Plast Surg. 2002;55(6):507‐509. [DOI] [PubMed] [Google Scholar]
- 18. Ruan QZ, Chen AD, Singhal D, Lee BT, Fukudome EY. Surgical management of hidradenitis suppurativa: procedural trends and risk factors. J Surg Res. 2018;229:200‐207. [DOI] [PubMed] [Google Scholar]
- 19. Hurley HJ. Axillary hyperhidrosis, apocrine bromhidrosis, hidradenitis suppurativa, and familial benign pemphigus: surgical approach. In: Roenigk RK, Roenigk HH, eds. Dermatologic Surgery. New York, NY: Marcel Dekker; 1996:623‐645. [Google Scholar]
- 20. Ching DL, Mughal M, Papas A, Soldin M. Axillary reconstruction for hidradenitis suppurativa with an inner‐arm transposition flap creating a brachioplasty effect. Arch Plast Surg. 2017;44(3):228‐233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mendes RRDS, Zatz RF, Modolin MLA, et al. Radical resection and local coverage of hidradenitis suppurativa—acne inversa: analysis of results. Rev Col Bras Cir. 2018;45(3):e1719. [DOI] [PubMed] [Google Scholar]
- 22. Elgohary H, Nawar AM, Zidan A, Shoulah AA, Younes MT, Hamed AM. Outcome of pedicled thoracodorsal artery perforator flap in the surgical treatment of stage II and III hidradenitis suppurativa of axilla. Ann Plast Surg. 2018;81(6):688‐693. [DOI] [PubMed] [Google Scholar]
- 23. Schmidt M, Dunst‐huemer KM, Lazzeri D, et al. The versatility of the islanded posterior arm flap for regional reconstruction around the axilla. J Plast Reconstr Aesthet Surg. 2015;68(7):953‐959. [DOI] [PubMed] [Google Scholar]
- 24. van Rappard DC, Mooij JE, Mekkes JR. Mild to moderate hidradenitis suppurativa treated with local excision and primary closure. J Eur Acad Dermatol Venereol. 2012;26(7):898‐902. [DOI] [PubMed] [Google Scholar]
- 25. Humphries LS, Kueberuwa E, Beederman M, Gottlieb LJ. Wide excision and healing by secondary intent for the surgical treatment of hidradenitis suppurativa: a single‐center experience. J Plast Reconstr Aesthet Surg. 2016;69(4):554‐566. [DOI] [PubMed] [Google Scholar]
- 26. Nail‐barthelemy R, Stroumza N, Qassemyar Q, et al. Evaluation of the mobility of the shoulder and quality of life after perforator flaps for recalcitrant axillary hidradenitis. Ann Chir Plast Esthet. 2019;64(1):68‐77. [DOI] [PubMed] [Google Scholar]
- 27. Deckers IE, Dahi Y, van der Zee HH, Prens EP. Hidradenitis suppurativa treated with wide excision and second intention healing: a meaningful local cure rate after 253 procedures. J Eur Acad Dermatol Venereol. 2018;32:459‐462. [DOI] [PubMed] [Google Scholar]
- 28. DeFazio MV, Economides JM, King KS, et al. Outcomes after combined radical resection and targeted biologic therapy for the management of recalcitrant hidradenitis suppurativa. Ann Plast Surg. 2016;77:217‐222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ngaage LM, Wu Y, Ge S, et al. Factors influencing the local cure rate of hidradenitis suppurativa following wide local excision. Int Wound J. 2020;17:117‐123. 10.1111/iwj.13241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tanaka A, Hatoko M, Tada H, Kuwahara M, Mashiba K, Yurugi S. Experience with surgical treatment of hidradenitis suppurativa. Ann Plast Surg. 2001;47(6):636‐642. [DOI] [PubMed] [Google Scholar]
- 31. Alharbi M, Perignon D, Assaf N, Qassemyar Q, Elsamad Y, Sinna R. Application of the inner arm perforator flap in the management of axillary hidradenitis suppurativa. Ann Chir Plast Esthet. 2014;59(1):29‐34. [DOI] [PubMed] [Google Scholar]
- 32. Egemen O, Özkaya Ö, Bingöl D, et al. Islanded perforator flaps in the reconstruction of hidradenitis suppurativa defects. J Reconstr Microsurg. 2013;29(5):297‐302. [DOI] [PubMed] [Google Scholar]
- 33. Altmann S, Fansa H, Schneider W. Axillary hidradenitis suppurativa: a further option for surgical treatment. J Cutan Med Surg. 2004;8(1):6‐10. [DOI] [PubMed] [Google Scholar]
- 34. Ortiz CL, Castillo VL, Pilarte FS, et al. Experience using the thoracodorsal artery perforator flap in axillary hidradentitis suppurativa cases. Aesthetic Plast Surg. 2010;34(6):785‐792. [DOI] [PubMed] [Google Scholar]
- 35. Sartorius K, Emtestam L, Jemec GB, et al. Objective scoring of hidradenitis suppurativa reflecting the role of tobacco smoking and obesity. Br J Dermatol. 2009;161(4):831‐839. [DOI] [PubMed] [Google Scholar]


