Abstract
Pressure injuries (PIs) have now become a common complication of the elderly patients. Some studies have observed that pressure injuries may increase mortality, but this area of evidence has not been evaluated and summarised. The aim of this study was to compare the mortality of patients with pressure injuries and those without pressure injuries. A meta‐analysis of observational studies was performed. PubMed, Cochrane Library, Embase, and Web of Science were searched up to April 2019. Studies about mortality among the elderly patients with and without pressure injuries were included. Methodological quality was assessed by the Newcastle‐Ottawa Scale (NOS). The fixed effect or random effect model was determined by the test of heterogeneity. The subgroup analysis was performed based on the pressure injuries stages, the region, and the type of study design. The meta‐regression analysis was performed to investigate the relationship between the mortality and patients' enrolled year, average age, the incidence of pressure injuries, and gender ratio. The sensitivity analysis was used to explore the impact of an individual study by excluding one at a time. The hazard ratio (HR) and 95% confidence intervals (CIs) in terms of the comparison of two groups were extracted for meta‐analysis. A survival curve between two groups by individual patient‐level was drew. Eight studies with 5523 elderly patients were included in the analysis. Follow‐up periods for the included studies ranged from about 0.5 to 3 years. The elderly patients who complicated with pressure injuries had a higher risk of death. The pooled HR was 1.78 (95% CI 1.46‐2.16). A funnel plot showed no publication bias. Further subgroup analysis showed that HR values for the patient stage 3 to 4 pressure injuries (HR:2.41; 95% CI:1.08‐5.37) were higher than stage 1‐4 and 2‐4 pressure injuries (HR: 1.66; 95% CI: 1.35‐2.05; HR: 1.74; 95% CI: 1.16‐2.60). The meta‐regression analysis found that patients' enrolled year, average age, the incidence of pressure injuries, and gender ratio were not the sources of heterogeneity. Sensitivity analyses showed that the outcomes of the study did not change after removing the Onder's article. The survival curve at the individual patient‐level also indicated that patients complicated with pressure injuries significantly increased the risk of death (HR: 1.958; 95% CI: 1.79‐2.14) in elderly patients. Our meta‐analysis indicated that patients complicated with pressure injuries are estimated to have a two times higher risk on mortality compared with patients without pressure injuries during the 3 years follow‐up period. Particular attention should be given to the elderly patients who are at higher risk for mortality.
Keywords: meta‐analysis, mortality, older patients, pressure injuries
1. INTRODUCTION
Pressure injuries (PIs) are common complications in some elderly patients, especially in hip fractured elderly and other patients who have difficulty turning over in bed.1, 2 Aljezawi et al's study showed that the prevalence of PIs among hospitalised patients with cancer was 15.5% and heel PIs were the most frequent (64.7%).3 The National Pressure Ulcer Advisory Panel (NPUAP) performed PI testing on 5947 elderly patients in five European countries. They found that the prevalence of PIs was 8.3% in Italy, 12.5% in Portugal, and 21% to 22.9% in Belgium, Sweden, and the United Kingdom.4 Seventy percentage of PIs occurred in people over 70 years of age.5 According to the studies of several European and American countries, the prevalence of hospital PIs ranged from 6% to 25%.4, 6 With the advent of population aging and the increase of life expectancy, PIs are the main problem faced by elderly people in all medical institutions.7 There is an article showing that in elderly patients with PIs hospitalised in intensive care units, the length of hospital stay and the time to use non‐invasive mechanical ventilation were increased.8 There has been a lot of research on the effect of PIs on elderly patients. Research by McGinnis et al showed that 75% of elderly patients with PIs have pain.9 PIs can also reduce the quality of life and increase medical expenses.10 Annual medical expenses for the treatment of PIs amounted to $362 million to $3.3 billion.11 The costs for PI treatment was increased12 and the treatments of PIs were long‐term clinical challenges.13
PIs may cause a more serious hazard, which may increase the risk of death. Patients with PIs have a poor prognosis. There are studies highlighted a number of risk factors for PIs that may be present, such as pain and sedation from medication, which leads to tissue ischaemia from pressure, lower blood pressure, and so on.14, 15 The guideline of United Kingdom indicated that all patients were at risk of developing PIs.16 At the same time, elderly patients complicated with PIs have a worse prognosis which can accelerate their death. The mortality in PIs group was more than 50% when serious infectious complications occur.17 Some small sample size studies have shown that elderly patients with PIs suffer from an increased risk of mortality.18 Vanderwee et al4 reported that the odds ratio (OR) for mortality in hospitalised elderly patients with PIs was 2.81. Berlowitz et al showed a significant increase in mortality from patients with PIs (RR = 1.9).19 In view of the recent studies on the mortality of elderly patients with PIs and non‐PIs, there was a relationship between the mortality of the elderly patients and PIs. However, few studies have investigated the impact of mortality in PI elderly patients and some studies have reported conflicting results.20, 21 , 22 Although there seems to be consensus that PIs do accelerate the mortality, the impact of PIs on the mortality at the population level is less clear. What is more, the existing research sample size was relatively small. As far as we know, none of the meta‐analyses has reported relevant content. Whether they will increase the mortality remains controversial. The clinical importance and significance of these associations are poorly understood.
It was of great significance to verify whether PIs can increase patient mortality. To assess the correlation between PIs and mortality, we conducted the meta‐analysis. We proposed the following objectives: (a) identify the association between PIs and mortality and (b) explore the specific effect size of PIs to accelerate the death of patients.
2. METHODS
2.1. Search strategy
We conducted a systematic review using the method suggested in the PRISMA statement.23 Four databases (PubMed, Cochrane Library, Embase, and Web of science) were searched up to April 2019. The medical terminology (MESH) terms and free text words were used. The search included the following MeSH terms: pressure ulcer, Kaplan‐Meier estimate, survival, and mortality. All MeSH terms were converted to synonyms that they were appropriate for each database. First, it is filtered by title. If the title fulfilled the inclusion criteria, the summary was filtered. Other potentially relevant studies were identified by cross‐referencing in eligible studies. The search details for each database were described in the Appendix S1.
2.2. Study selection
This systematic review was limited to published human‐based studies, focusing only on the comparison of mortality between the PI group and the non‐PI group. There were no language restrictions. The inclusion and exclusion criteria were as follows:
Inclusion criteria are as follows: (a) observational studies: cohort, case control, and cross‐sectional study; (b) PI diagnostic criteria according to NPUAP24 and there is no limit to the stage of PIs; (c) hospitalised elderly with a follow‐up period of more than 3 months; (d) all studies compared mortality in elderly patients with PIs and without PIs; and (e) the primary outcome was the mortality of PI and non‐PI group; to be eligible, the study must include at least two groups including PIs group and non‐PIs group. Study reported the sample size, mean, standard deviation, HR, or Kaplan‐Meier estimate.
Exclusion criteria are as follows: (a) we excluded the literature review, edited the letter, case reports, and animal studies. We also excluded studies that did not compare PIs with non‐PIs; (b) controlled experimental studies aimed at interventions to reduce mortality; (c) studies with follow‐up times shorter than 6 months, studies did not show corresponding outcomes. We used the electronic search to get the original research article.
2.3. Data extraction
Two screening authors extracted the following information from each included study: first author; publication year and country; study size; and the type of study (including cohort studies and case‐control studies; outcomes; HR; and follow‐up period). However, not all studies included the data on all of the information listed previously. The third reviewer would be consulted to resolve the disagreements between the two screening authors. We extracted the status of each patient to analyse the HR. The data of the survival curve were extracted through the Engauge digitizer. We calculated HR according to the excel program file provided by Tierney et al.25 HR values are recorded from included articles, and if not provided, estimated from published actuarial survival curves using spreadsheets designed for this purpose (http://www.biomedcentral.com/content/supplementary/1745-6215-8-16-S1.xls). For example, we calculated the time to death for all elderly patients during the follow‐up period due to PIs and we also extracted the deleted data.
2.4. Quality assessment
Two screening authors independently extracted relevant data according to the eligibility criteria. They discussed the results and reached a consensus. The third reviewer would be consulted to resolve the disagreements between the two reviewers. The Newcastle‐Ottawa Scale (NOS) is an effective scale for non‐randomised studies in meta‐analysis to assess the quality of included studies.26 According to this model, each study can score up to nine points and consist of three fields: four points for the study group selection, two points for the comparison between groups, and three points for the ascertainment outcome or exposure. Low‐, medium‐, and high‐quality academic scores are 0‐3, 4‐6, and 7‐9, respectively.
The HR and confidence intervals (CI) for mortality were derived from the studies for which they are reported. The baseline characteristics included the enrolled year, country, study type, participants sample size, mean age, gender ratio, PI grade, PI incidence, follow‐up period, mortality during follow‐up, and HR (95%CI).
We will add more information if necessary, especially when plotting the survival curves. We extracted measurements from the PI group and the non‐PI group, such as HR. We extracted the survival time and survival status of each patient from the survival curves of the studies selected for inclusion.
2.5. Data extraction and synthesis
The main outcome of this meta‐analysis was mortality in the follow‐up. We incorporated the pooled HR. Statistical heterogeneity of each study was assessed by χ 2 test and I2 statistics. For the I2 statistic, >50% means moderate heterogeneity, and > 75% means highly heterogeneous.27 For survival outcomes, we calculated HR and 95% CI. The random effect model was used to calculate the pooled HR, if there was the heterogeneity (I2 > 50%, P > .1).28
A funnel plot was used to assess publication bias. A symmetric funnel‐shape distribution of studies indicates no significant bias. An asymmetric funnel indicates the existence of the publication bias.29
The subgroup analysis was performed to investigate the possible sources of heterogeneity according to the stage of PIs, different regions, and the type of study design. For the stage of PIs, one group had the patients with PI stage 1‐4 and another group had patients without stage 1.
Meta‐regression analyses of patients' enrolled year, average age of the people being included, the PI incidence, and gender ratio of the included studies were conducted. The size of the symbol in the meta‐regression reflected the weight of each study included in the analysis; the larger the symbol represented the study with more precise impact. The random effects model was used to calculate the pooled HR if I2 > 50%.
The sensitivity analysis was used to investigate the stability. One study was excluded once at a time of this systematic review to detect if the results were severely distorted by a specific study.
Finally, survival curves were plotted for all included references. The data from the survival curves were extracted and calculated for each patient. We drew the survival curve with individual patient‐level data and calculated the HR with 95% CI between two groups.
The data were pooled and analysed using STATASE 11 and spss21.
3. RESULTS
Fifty‐five eligible observational studies were identified. Figure 1 showed the flow of studies through the screening details. Of the 55 full‐text articles assessed for eligibility, 47 were excluded for a variety of reasons as detailed: lack of data (HR or survival curve); follow‐up time less than half a year; incorrect comparisons; and case reports. We included eight cohort and case‐control studies with a total of 5523 participants unanimously agreed by two reviewers. Three studies explored hospitalised patients, two studies explored intensive care unit patients, and one study explored elderly patients with hip fractures. One study explored cancer patients and patients with advanced no‐cancer disorders, and one study explored pressure and non‐pressure injuries.
Figure 1.
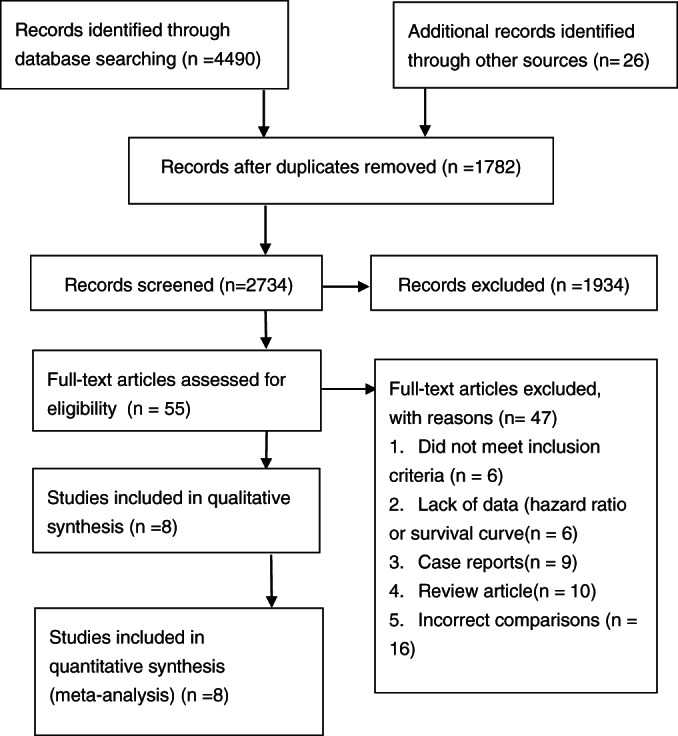
Article selection. The search strategy yielded a total of eight articles for the meta‐analysis
3.1. Quality of studies study sample
All studies used a comparison group and obtained a total NOS grading score. Assessment methods and duration of follow‐up were extracted and presented in Table 1. Four articles scored eight points,30, 31, 32, 33 and two of them were deducted by one point because of the non‐response rate. The other two were deducted one point because of insufficient follow‐up time. Two articles34, 35 scored seven points, losing two points because the follow‐up time was insufficient and did not state what they used a blind method. One article36 scored 6 points, because it was incomparable and the follow‐up time was less than 5 years. One article37 scored 5 points due to incomparability and because followed up for less than 5 years and did not report whether blinding was used. Four articles in the included literature provided HR, and the remaining five provided only survival curves.
Table 1.
Quality assessment using the Newcastle‐Ottawa Scale
| Study | Selection | Comparability | Outcome/exposure |
|---|---|---|---|
| Fernández‐Jiménez and coworkers, 201634 | **** | ** | * |
| Onder and coworkers, 200737 | **** | — | * |
| Jaul et al, 201630 | **** | ** | ** |
| Kim et al, 201831 | **** | ** | ** |
| Magny et al, 201735 | **** | ** | * |
| Maida et al, 201032 | **** | ** | ** |
| Manzano et al, 201433 | **** | ** | ** |
| Calderon‐Margalit and coworkers, 201536 | **** | — | ** |
Table 2 summarised the characteristics of these eight studies.30, 31, 32, 33, 34, 35, 36, 37 These studies included a total of 5523 people, one of which was followed up to death or to hospital discharge33; two studies were retrospective30, 31 and six studies were prospective32, 33, 34, 35, 36, 37; five studies were from Europe,30, 33, 34, 35, 37 two were from Asia,31, 36 and one was from North America.32
Table 2.
Characteristics of the included studies
| Study | Enrolled year | County | Study type | Participants | Sample size | Mean age (years) | Gendera (relative ratio) | PI grade | PIs incidence (%) | Follow‐up period (y) | Mortality during follow‐up | HR (95% CI) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PIs group | No‐PIs group | ||||||||||||
| Kim et al, 201831 | 2012‐2014 | Korea | RCC | Underwent major surgery | 129 | 61.8 ± 10.8 | 104/25 (4.16:1) | 2‐4 | 33.3 | 2 | 15/43b | 17/86b | 4.54 (1.38‐14.9)b |
| Magny et al, 201735 | 2009 | France | PC | Elderly patients with hip fracture | 567 | 86.5 ± 7 | 134/433 (0.31:1) | 1‐4 | 11.8 | 0.5 | 19/67 | 50/500 | 1.64 (1.16‐2.32)b |
| Fernández‐Jiménez and coworkers, 201634 | 2010‐2013 | Spain | PC | Elderly patients in internal medicine | 699 | 74.6 | 333/366 (0.91:1) | 1‐4 | 14.3 | 3 | 83/100b | 250/599b | 1.53 (1.14‐2.06) |
| Jaul et al, 201630 | 2008‐2012 | Denmark | RCC | Elderly patients with advanced dementia and comorbidities | 99 | 79 ± 11 | 48/51 (0.94:1) | 3‐4 | 72.7 | 3.5 | 56/72 | 18/27b | 3.86 (1.82‐8.20) |
| Calderon‐Margalit and coworkers, 201536 | 2008‐2011 | Israel | PC | Elderly patients in hospital | 174 | 77.4 ± 13.2 | 87/87 (1:1) | 3‐4 | 61.5 | 1.25 | 80/107 | 38/67 | 1.69 (1.1‐2.62)b |
| Manzano et al, 201433 | 2006 | Spain | PC | Elderly patients in ICU | 563 | 64.5 ± 18.5 | 365/198 (1.84:1) | 2‐4 | 19.5 | 2 | 66/110 | 208/453 | 1.28 (1.0‐1.65) |
| Maida et al, 201032 | 2005 | Canada | PC | Cancer elderly patients and elderly patients with advanced noncancer disorders | 189 | 81.7 ± 10.7 | 76/113 (0.67:1) | 1‐4 | 69.8 | 1 | 93/132b | 48/57b | 2.42 (1.34‐4.38) |
| Onder and coworkers, 200737 | 1998‐1999 | Italy | PC | The older person | 3103 | 78.5 ± 9.5 | 1262/1841 (0.69:1) | 2‐4 | 18.0 | 1 | 160/558 | 368/2545 | 1.90 (1.60‐2.26)b |
Male/female.
Data was extracted from the survival curves.
Abbreviations: PC, prospective cohort; RCC, retrospective case‐control.
3.2. The association between PIs and mortality in elderly patients
3.2.1. The pooled HR of meta‐analysis
Follow‐up periods for the included studies ranged from half a year to 3.5 years. Two studies followed up to death or to hospital discharge. Four articles reported the HR, one reported the adjusted HR, and the remaining four provided only survival curves. We pooled data from the four studies irrespectively and calculated the HR. The results showed that the elderly patients with PIs had a higher risk of mortality than the non‐PI group (pooled HR: 1.78; 95% CI: 1.46‐2.16). Figure 2A showed the pooled HR and 95% CI for the elderly patients with and without PIs in eight studies. The publication bias analysis showed that there was no obvious asymmetry in the funnel plot and no strong evidence of publication bias (Figure 2B).
Figure 2.
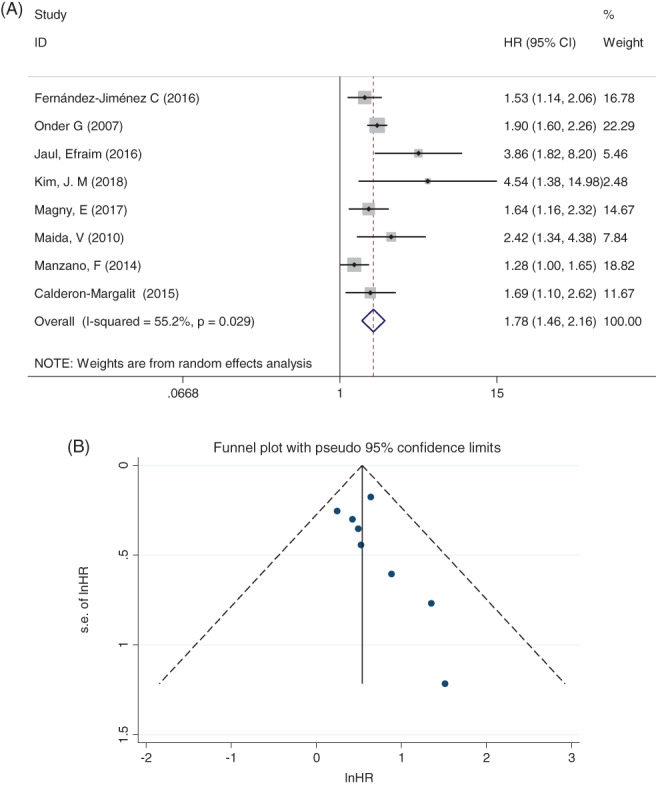
A, Pooled estimate on the risk of mortality. B, Funnel plot with 95% confidence limits. Meta‐analysis after removing one influential article
3.2.2. The subgroup analysis
The subgroup analysis was performed according to PI stage, different regions, and the type of study design. Figure 3A showed that the HR values for the patient stage 3‐4 PIs (HR:2.41; 95% CI: 1.08‐5.37) were higher than stage 1 to 4 PIs and stage 2 to 4 PIs (HR: 1.66; 95% CI: 1.35‐2.05; HR: 1.74; 95% CI: 1.16‐2.60).
Figure 3.
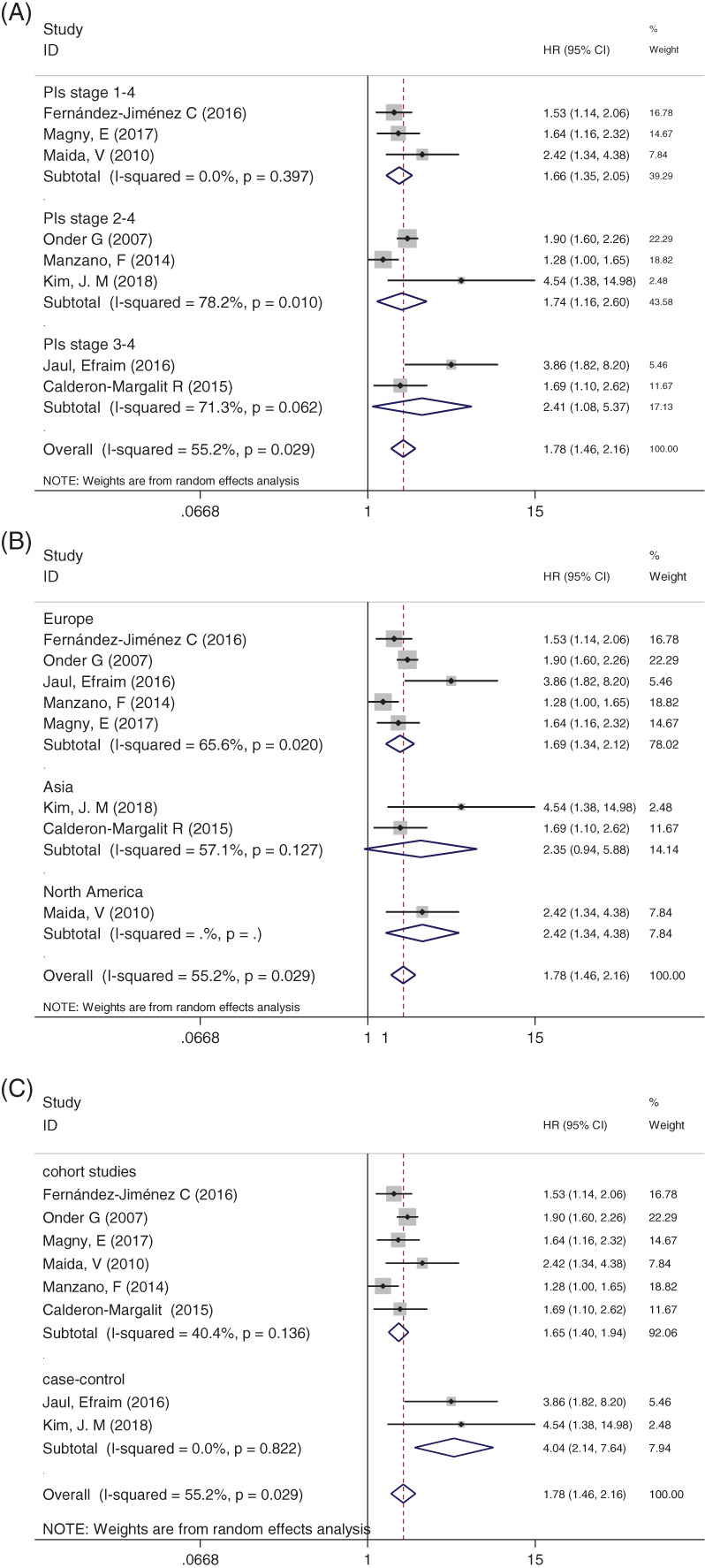
A, Subgroup of comparison between groups stratified by stage 1‐4 PIs, stage 2‐4 PIs, and 3‐4 PIs. B, Subgroup with different regions. C Subgroup with different types of study design
Figure 3B showed the HR comparison between groups stratified by regions by using the random‐effects model. The result indicated that the PI group had a higher mortality than non‐PI group between Europe (HR: 1.69; 95% CI: 1.34‐2.12) and North America (HR: 2.42; 95% CI: 1.34‐4.38). There was no difference between genders in Asia group (HR: 2.35; 95% CI: 0.94‐5.58; P = 0.127).
Figure 3C showed the mortality comparison between groups stratified by study design. The HR between prospective and retrospective studies was 1.65 (95% CI: 1.40‐1.94) and 1.78 (95% CI: 1.46‐2.16), respectively.
3.2.3. The meta‐regression analyses
The meta‐regression analysis of eight studies between the PI group and non‐PI group was performed to investigate the relationship between patients' enrolled year, average age, the incidence of PIs, and gender ratio. Figure 4A‐C showed that the heterogeneity did not come from patients' enrolled year (P = .692, CI: −1.74‐0.25), the patient's age (P = 0.46, CI: −0.14‐0.07), and the PIs incidence (P = 0.17, CI: −0.1‐0.04). Figure 4D showed the gender ratio of each study (P = 0.323, CI: −54.6‐1.4).
Figure 4.
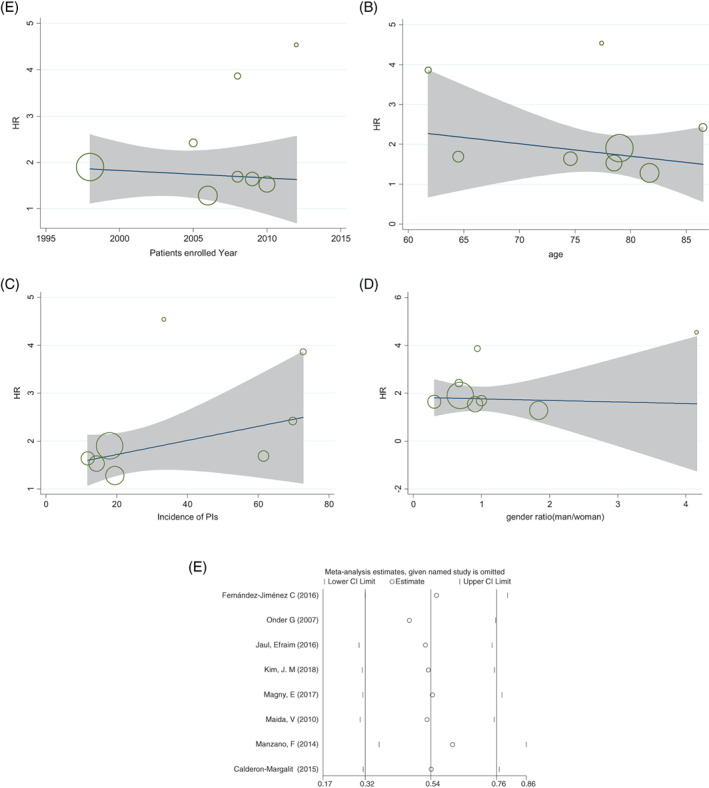
Meta‐regression on mortality in the PIs group and non‐PIs group. x = patients' enrolled year, average age, the incidence of PIs, and gender ratio, y = HR and circle diameters showed the weight of each study based on the random effect model. A, Meta‐regression analysis of the median of patients' enrolled year. B, Meta‐regression analysis of patients' average age. C, Meta‐regression analysis of the incidence of PIs of included studies. D, Meta‐regression analysis of male and female gender ratio of included studies. E, Sensitivity analysis with each study excluded
3.2.4. The sensitivity analyses
Sensitivity analyses based on the removal of a study were showed in Figure 4E. The result showed that the outcomes of the study did not change after removing the Onder's article.
3.3. Survival curves between two groups according to individual patient‐level data
Figure 5 showed the survival curve of confluence from individual patient‐level in eight studies. The survival curve at the individual patient‐level also indicated that concurrent PIs significantly increased the risk of death in elderly patients during follow‐up (HR: 1.958; 95% CI: 1.79‐2.14).
Figure 5.
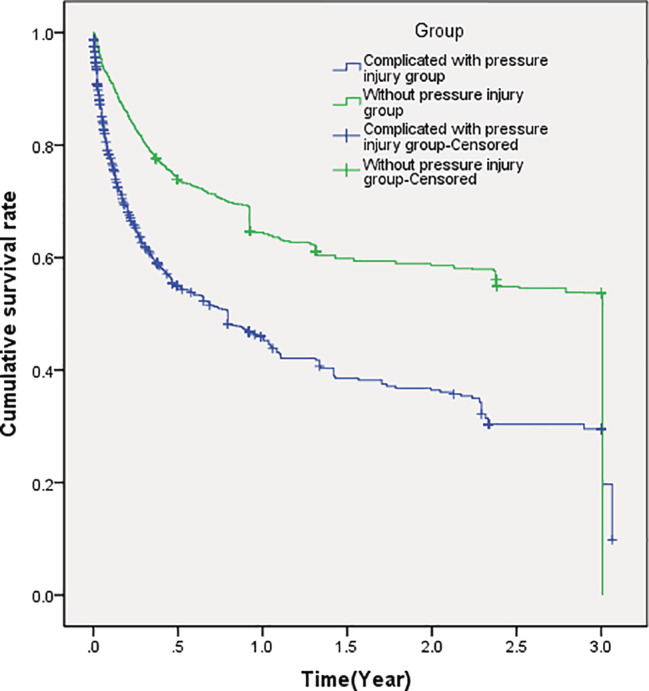
Cumulative survival rate of all elderly patients
4. DISCUSSION
Our results showed that elderly patients with concurrent PIs can accelerate mortality of the elderly patients (HR: 1.78; 95% CI: 1.46‐2.16). Our finding confirmed the previous researches on the relationship between PIs and mortality.38, 39, 40, 41, 42 There are few reports that have reported the mortality of PIs. Ueda et al showed that 23 elderly patients with a mean age of 51 years and a long follow‐up of 6 years had a mortality rate of 22%.43 Leijon et al showed that elderly patients with PIs had a 3.6‐fold increased risk of death within 21 months compared with elderly patients without PIs. The 6‐month mortality rate after hip fracture was reported to be 13.4%.2 This conclusion was drawn when the age was limited. In fact, we concluded that regardless of age, the appearance of PIs can increase the risk of death. However, the articles by Thomas DR et al. putted forward a different opinion; they believed that PIs were not identified as the direct cause of mortality.44 The development of in‐hospital PIs was associated with an increased risk of mortality at 1 year (59.5% vs 38.2%, P = .02). After adjusting for other mortality predictors, PI development was not independently associated with a reduction in survival. Therefore, they concluded that PIs that occur during acute hospitalisation are not associated with reduced 1‐year survival in high‐risk elderly.
The causes and mechanisms of PIs to accelerate death are not yet clear. The positive correlation between MMP activity and inflammatory response in elderly patients with PIs is one of the reasons for the increased mortality of elderly patients with high serum MMPs, but the association between mortality of elderly patients with serum activity of MPP‐2 and MPP‐9 has not yet been determined.2, 45 Berlowitz et al said that the PIs were the sign of an underlying disease, leading to an increased risk of complications and thus increased death.19 In addition, PIs may cause death due to complications such as osteomyelitis or sepsis.46 Studies have shown that the incidence of PIs is high in recent years. Among elderly patients with cardiac surgery, hip fracture surgery, and surgical intensive care unit, the incidence of PIs was 0.18 (95% CI 0.14‐0.22, I2 = 62.8%), 0.22 (95% CI 0.20‐0.24, I2 = 98.4%), (95% CI 0.09‐0.13, I2 = 98.5%).47 With the increase of the incidence of PIs, the mortality of elderly patients also increased. Perioperative corticosteroids administration,48 immobility, hemodynamic instability, decreased sensory, and increased risk of circulatory disorders due to organ failure can increase the risk of PIs.49 The parts of the body that has PIs are easily colonised by bacteria. Secondary bacteremia or sepsis may complicate PIs, which may be related to increased mortality.50 Stress, shear, friction, malnutrition, and lack of physical activity are contributors to the pathogenesis of PIs.51 Mustoe et al hypothesized the pathogenesis of PIs: they believed that the pathogenesis of PIs was a combination of factors and their common effects overwhelm the healing of PIs.52 Reperfusion injury has been suggested by some authors as a major determinant of PIs development. In both rat and mouse models, repeated ischemia‐reperfusion injury was more destructive to tissue than long‐term ischemia alone.53, 54 These mechanisms of PIs development may have a detrimental effect on the patient's original disease. Sun et al results showed that LRRC19 was a factor in reperfusion injury‐induced tissue damage by promoting NFkB‐dependent inflammatory responses.55
Our results showed that the HR value for the studies containing stage 1 PIs (HR: 1.66; 95%, P = .397) was smaller than those without stage 1 PIs (HR: 1.90; 95%, P = .08). This suggested that stage 1 PIs may have no effect on mortality. We suspected that there was no difference in the statistical significance which may be due to the small sample size of stage 1 PIs. However, there have been study showing support for our guess. The HACI in the Federal Register described earlier‐stage (Stage I and II) PIs no longer generate extra payment.56 The data showed that the mortality rate of elderly patients with PIs at the point of 0.5 year was 46%, and that of elderly patients in the non‐PIs group was 23%, which was the same as the mortality in the study by Michocki et al. According to the report, they found that the 6‐month mortality for hospitalised elderly patients with PIs was 77%, compared with 18% for those without PIs.57 The data showed that the PIs were associated with mortality and the longer the follow‐up period, the closer the relationship between PIs and mortality. Our meta‐regression analysis showed that mortality was independent of patients' enrolled year, average age, the incidence of PIs, and gender ratio. In all regression analyses, the P value of incidence of PIs was closest to .05 with value of .17. We suspected that the incidence of PIs may be a source of heterogeneity. However, further studies are needed.
To further determine the relationship between concomitant PIs and mortality, we extracted the data from patient‐level. In critically ill elderly patients, it has not been determined whether the increase in PI‐related mortality is due to complications of PIs or its underlying disease or other comorbidities. In addition, Khor et al have shown that according to the cox proportional hazard analysis, the mortality of elderly patients with stage 4 PIs was significantly higher than that of stage 2 PIs, but the difference is not statistically significant.58 The studies we included did not specify the elderly patient's disease and the stage of PIs. In our future research, we can explore whether the relationship between PIs and mortality is related to the type of disease or stage of PIs.
Of course, our research has some limitations.
First, there is still some heterogeneity in our article. Inadequate details of the method and insufficient follow‐up time may lead to clinical heterogeneity. Although PIs are associated with an increase in mortality, factors such as the type of disease, age, and length of hospitalisation may complicate the analysis.
In addition, although our result is consistent with the previous studies that concurrent PIs can accelerate the death of elderly patients, extracting data through software may still lead to the deviations from the original data. Such deviations indicate that we should use the patient's raw data for analysis in the future.
What is more, due to the lack of relevant research, the small sample size and the low quality of documents included in this study, the conclusions may be offset. And some studies did not implemented blinding.
5. CONCLUSION
Our study showed that patients living with PIs are estimated to have a two times higher risk on mortality compared with patients living without PIs. We recommend that particular attention should be given to the elderly patients with concurrent PIs, and effective methods of preventing PIs should be taken to alleviate the suffering of elderly patients. However, this conclusion needs to be confirmed by more high‐quality and multi‐faceted researches and patient‐level data.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
Supporting information
Appendix S1. The specific search terms for each database
ACKNOWLEDGEMENTS
The authors would like to thank Yan Zhuang and Hong‐Wu Shen for their revision of the language in the manuscript. They would also like to thank for the support by the Key Project of Medical Management (MS12017016‐3) from Nantong City Science and Technology Bureau.
Song Y‐P, Shen H‐W, Cai J‐Y, Zha M‐L, Chen H‐L. The relationship between pressure injury complication and mortality risk of older patients in follow‐up: A systematic review and meta‐analysis. Int Wound J. 2019;16:1533–1544. 10.1111/iwj.13243
Funding information Key Project of Medical Management from Nantong City Science and Technology Bureau, Grant/Award Number: MS12017016‐3
REFERENCES
- 1. Feuchtinger J, Halfens RJ, Dassen T. Pressure ulcer risk factors in cardiac surgery: a review of the research literature. Heart Lung. 2005;34(6):375‐385. [DOI] [PubMed] [Google Scholar]
- 2. Lindholm C, Sterner E, Romanelli M, et al. Hip fracture and pressure ulcers ‐ the pan‐European pressure ulcer study ‐ intrinsic and extrinsic risk factors. Int Wound J. 2008;5(2):315‐328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Aljezawi M, Tubaishat A. Pressure injuries among hospitalized patients with cancer: prevalence and use of preventive interventions. J Wound Ostomy Continence Nurs. 2018;45(3):227‐232. [DOI] [PubMed] [Google Scholar]
- 4. Vanderwee K, Clark M, Dealey C, Gunningberg L, Defloor T. Pressure ulcer prevalence in Europe: a pilot study. J Eval Clin Pract. 2007;13(2):227‐235. [DOI] [PubMed] [Google Scholar]
- 5. Bansal C, Scott R, Stewart D, Cockerell CJ. Decubitus ulcers: a review of the literature. Int J Dermatol. 2005;44(10):805‐810. [DOI] [PubMed] [Google Scholar]
- 6. Lyder CH, Wang Y, Metersky M, et al. Hospital‐acquired pressure ulcers: results from the national Medicare patient safety monitoring system study. J Am Geriatr Soc. 2012;60(9):1603‐1608. [DOI] [PubMed] [Google Scholar]
- 7. Garcia AD, Thomas DR. Assessment and management of chronic pressure ulcers in the elderly. Med Clin North Am. 2006;90(5):925‐944. [DOI] [PubMed] [Google Scholar]
- 8. García‐Molina P, Balaguer‐López E, García‐Fernández FP, Ferrera‐Fernández MLÁ, Blasco JM, Verdú J. Pressure ulcers' incidence, preventive measures, and risk factors in neonatal intensive care and intermediate care units. Int Wound J. 2018;15(4):571‐579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McGinnis E, Briggs M, Collinson M, et al. Pressure ulcer related pain in community populations: a prevalence survey. BMC Nurs. 2014;13:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gorecki C, Brown JM, Nelson EA, et al. Impact of pressure ulcers on quality of life in older patients: a systematic review. J Am Geriatr Soc. 2009;57(7):1175‐1183. [DOI] [PubMed] [Google Scholar]
- 11. Van Den Bos J, Rustagi K, Gray T, Halford M, Ziemkiewicz E, Shreve J. The $17.1 billion problem: the annual cost of measurable medical errors. Health Affairs. 2011;30(4):596‐603. [DOI] [PubMed] [Google Scholar]
- 12. Demarré L, Van Lancker A, Van Hecke A, et al. The cost of prevention and treatment of pressure ulcers: A systematic review. Int J Nurs Stud. 2015;52(11):1754‐1774. [DOI] [PubMed] [Google Scholar]
- 13. Venturi ML, Attinger CE, Mesbahi AN, Hess CL, Graw KS. Mechanisms and clinical applications of the vacuum‐assisted closure (VAC) device: a review. Am J Clin Dermatol. 2005;6(3):185‐194. [DOI] [PubMed] [Google Scholar]
- 14. Schols JM, Heyman H, Meijer EP. Nutritional support in the treatment and prevention of pressure ulcers: an overview of studies with an arginine enriched oral nutritional supplement. J Tissue Viability. 2009;18(3):72‐79. [DOI] [PubMed] [Google Scholar]
- 15. Langemo D. General principles and approaches to wound prevention and care at end of life: an overview. Ostomy Wound Manage. 2012;58(5):24‐26, 28, 30 passim. [PubMed] [Google Scholar]
- 16. Stansby G, Avital L, Jones K, Marsden G, Group GD . Prevention and management of pressure ulcers in primary and secondary care: summary of NICE guidance. BMJ. 2014;348(apr23 1):g2592‐g2592. [DOI] [PubMed] [Google Scholar]
- 17. Braga IA, Pirett CC, Ribas RM, Gontijo Filho PP, Diogo FA. Bacterial colonization of pressure ulcers: assessment of risk for bloodstream infection and impact on patient outcomes. J Hosp Infect. 2013;83(4):314‐320. [DOI] [PubMed] [Google Scholar]
- 18. Brem H, Maggi J, Nierman D, et al. High cost of stage IV pressure ulcers. Am J Surg. 2010;200(4):473‐477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Berlowitz DR, Wilking SV. The short‐term outcome of pressure sores. J Am Geriatr Soc. 1990;38(7):748‐752. [DOI] [PubMed] [Google Scholar]
- 20. Zhan C, Miller MR. Excess length of stay, charges, and mortality attributable to medical injuries during hospitalization. JAMA. 2003;290(14):1868‐1874. [DOI] [PubMed] [Google Scholar]
- 21. Bo M, Massaia M, Raspo S, et al. Predictive factors of in‐hospital mortality in older patients admitted to a medical intensive care unit. J Am Geriatr Soc. 2003;51(4):529‐533. [DOI] [PubMed] [Google Scholar]
- 22. Freeman C, Todd C, Camilleri‐Ferrante C, et al. Quality improvement for patients with hip fracture: experience from a multi‐site audit. Qual Saf Health Care. 2002;11(3):239‐245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. Epidemiol Biostat Public Health. 2009;339. [DOI] [PubMed] [Google Scholar]
- 24. NPUAP . Prevention and treatment of pressure ulcers: clinical practice guideline; 2016. http://www.internationalguideline.com/guideline. Accessed April 3, 2019.
- 25. Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time‐to‐event data into meta‐analysis. Trials. 2007;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stang A. Critical evaluation of the Newcastle‐Ottawa scale for the assessment of the quality of nonrandomized studies in meta‐analyses. Eur J Epidemiol. 2010;25(9):603‐605. [DOI] [PubMed] [Google Scholar]
- 27. Higgins JPT, Thompson SG, Deeks JJ, Altman D. Measuring inconsistency in meta‐analyses. Br Med J. 2003;327:7414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions: Cochrane Book Series. A John Wiley & Sons, Ltd;2008. [Google Scholar]
- 29. Duval S, Tweedie R. Trim and fill: a simple funnel‐plot‐based method of testing and adjusting for publication bias in meta‐analysis. Biometrics. 2000;56(2):455‐463. [DOI] [PubMed] [Google Scholar]
- 30. Jaul E, Meiron O, Menczel J. The effect of pressure ulcers on the survival in patients with advanced dementia and comorbidities. Exp Aging Res. 2016;42(4):382‐389. [DOI] [PubMed] [Google Scholar]
- 31. Kim JM, Lee H, Ha T, Na S. Perioperative factors associated with pressure ulcer development after major surgery. Korean J Anesthesiol. 2018;71(1):48‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Maida V, Ennis M, Kuziemsky C, Corban J. Wounds and survival in noncancer patients. J Palliat Med. 2010;13(4):453‐459. [DOI] [PubMed] [Google Scholar]
- 33. Manzano F, Perez‐Perez AM, Martinez‐Ruiz S, Garrido‐Colmenero C. Hospital‐acquired pressure ulcers and risk of hospital mortality in intensive care patients on mechanical ventilation. J Eval Clin Pract. 2014;20(4):362‐368. [DOI] [PubMed] [Google Scholar]
- 34. Díez‐Manglano J, Fernández‐Jiménez C, Lambán‐Aranda MP. Pressure ulcers in patients hospitalized in internal medicine: associated factors and mortality. Rev Clin Esp. 2016;216(9):461‐467. [DOI] [PubMed] [Google Scholar]
- 35. Magny E, Vallet H, Cohen‐Bittan J, Raux M, Meziere A. Pressure ulcers are associated with 6‐month mortality in elderly patients with hip fracture managed in orthogeriatric care pathway. Arch Osteoporos. 2017;12(1):77. [DOI] [PubMed] [Google Scholar]
- 36. Jaul E, Calderon‐Margalit R. Systemic factors and mortality in elderly patients with pressure ulcers. Int Wound J. 2015;12(3):254‐259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Landi F, Onder G, Russo A, Bernabei R. Pressure ulcer and mortality in frail elderly people living in community. Arch Gerontol Geriatr. 2007;44:217‐223. [DOI] [PubMed] [Google Scholar]
- 38. Barba R, Martínez JM, Zapatero A, Pérez A, Casasola GD. Mortality and complications in very old patients (90+) admitted to departments of internal medicine in Spain. Eur J Intern Med. 2011;22(1):49‐52. [DOI] [PubMed] [Google Scholar]
- 39. Cao Y, Krause JS, DiPiro N. Risk factors for mortality after spinal cord injury in the USA. Spinal Cord. 2013;51(5):413‐418. [DOI] [PubMed] [Google Scholar]
- 40. Russo CA, Steiner C, Spector W. Hospitalizations related to pressure ulcers among adults 18 years and older, 2006: statistical brief #642006. [PubMed]
- 41. Moore Z. US medicare data show incidence of hospital‐acquired pressure ulcers is 4.5%, and they are associated with longer hospital stay and higher risk of death. Evid Based Nurs. 2013;16(4):118‐119. [DOI] [PubMed] [Google Scholar]
- 42. Leijon S, Bergh I, Terstappen K. Pressure ulcer prevalence, use of preventive measures, and mortality risk in an acute care population: a quality improvement project. J Wound Ostomy Continence Nurs. 2013;40(5):469‐474. [DOI] [PubMed] [Google Scholar]
- 43. Ueda K, Tajima S, Sano S. Clinical experience with pressure sores in our department. J Osaka Med Coll. 1990;(49):58‐63. [Google Scholar]
- 44. Thomas DR, Goode PS, Tarquine PH, Allman RM. Hospital‐acquired pressure ulcers and risk of death. J Am Geriatr Soc. 1996;44(12):1435‐1440. [DOI] [PubMed] [Google Scholar]
- 45. Ladwig GP, Robson MC, Liu R, Kuhn MA, Muir DF, Schultz GS. Ratios of activated matrix metalloproteinase‐9 to tissue inhibitor of matrix metalloproteinase‐1 in wound fluids are inversely correlated with healing of pressure ulcers. Wound Repair Regen. 2002;10(1):26‐37. [DOI] [PubMed] [Google Scholar]
- 46. Sugarman B, Hawes S, Musher DM, Klima M, Young EJ, Pircher F. Osteomyelitis beneath pressure sores. Arch Intern Med. 1983;143(4):683‐688. [PubMed] [Google Scholar]
- 47. Chen H‐L, Chen X‐Y, Wu J. The incidence of pressure ulcers in surgical patients of the last 5 years: a systematic review. Wounds. 2012;24(9):234‐241. [PubMed] [Google Scholar]
- 48. Chen HL, Shen WQ, Xu YH, Zhang Q, Wu J. Perioperative corticosteroids administration as a risk factor for pressure ulcers in cardiovascular surgical patients: a retrospective study. Int Wound J. 2015;12(5):581‐585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Keller BP, Wille J, van Ramshorst B, van der Werken C. Pressure ulcers in intensive care patients: a review of risks and prevention. Intensive Care Med. 2002;28(10):1379‐1388. [DOI] [PubMed] [Google Scholar]
- 50. Schoonhoven L, Haalboom JR, Bousema MT, et al. Prospective cohort study of routine use of risk assessment scales for prediction of pressure ulcers. BMJ. 2002;325(7368):797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hoogendoorn I, Reenalda J, Koopman BFJM, Rietman JS, I H, J R, BFJM K, JS R . The effect of pressure and shear on tissue viability of human skin in relation to the development of pressure ulcers: a systematic review. J Tissue Viability. 2017;26(3):157‐171. [DOI] [PubMed] [Google Scholar]
- 52. Mustoe TA, O'Shaughnessy K, Kloeters O. Chronic wound pathogenesis and current treatment strategies: a unifying hypothesis. Plast Reconstr Surg. 2006;117(7 SUPPL):35S‐41S. [DOI] [PubMed] [Google Scholar]
- 53. Thompson D. A critical review of the literature on pressure ulcer aetiology. J Wound Care. 2005;14(2):87‐90. [DOI] [PubMed] [Google Scholar]
- 54. Peirce SM, Skalak TC, Rodeheaver GT. Ischemia‐reperfusion injury in chronic pressure ulcer formation: a skin model in the rat. Wound Repair Regen. 2000;8(1):68‐76. [DOI] [PubMed] [Google Scholar]
- 55. Sun J, Wang Z, Wang X. Suppression of LRRC19 promotes cutaneous wound healing in pressure ulcers in mice. Organogenesis. 2018;14(1):13‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Centers for Medicare and Medicaid Services DoHa, services H. Medicare program; changes to the hospital inpatient prospective payment system for acute care hospitals and fiscal year 2010 rates; and changes to the long‐term care hospital prospective payment system and rate years 2010 and 2009 rates. Final rules and interim final rule with comment period. Fed Regist. 2009;74(165):43753‐44236. [PubMed] [Google Scholar]
- 57. Michocki RJ, Lamy PP. The problem of pressure sores in a nursing home population: statistical data. J Am Geriatr Soc. 1976;24(7):323‐328. [DOI] [PubMed] [Google Scholar]
- 58. Khor HM, Tan J, Saedon NI, et al. Determinants of mortality among older adults with pressure ulcers. Arch Gerontol Geriatr. 2014;59(3):536‐541. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. The specific search terms for each database


