Abstract
Many post‐vulvectomy vulvar reconstruction solutions, using local fasciocutaneous flaps where possible, have been proposed. We report the use of V‐Y advancement flaps from the gluteal fold in medium to large vulvar reconstructions and a simple modification we made to the technique in order to minimise wound‐related complications. Between 2006 and 2016, 30 vulvar reconstructions were performed via a total of 59 flaps, 24 of which were raised using the proposed modification to the plasty design. Short‐ and long‐term (24 months) follow‐up data were analysed, postoperative flap sensitivity was tested, and any arising complications were recorded. The mean age of patients treated was 75.3 years (51‐92 years). The mean monolateral defect dimensions were 7.5 × 4.7 × 2.8 cm. Minor complications were recorded in 23% of patients (14% of flaps). One case of ostial stenosis occurred. Micturition and ambulation recovery was rapid, and flap sensitivity was fully restored 24 months after reconstruction. Scars were well hidden by natural soft tissue folds. The outcomes in this case series confirm that the gluteal V‐Y advancement fasciocutaneous flap is a useful and simple technique for reconstructing even large vulvar defects. It has a low functional and aesthetic impact and enables rapid return to autonomy. Moreover, the simple modification to the V‐Y flap proposed, designed to reduce tension at the apical part of the wound, appears to reduce the complication rate.
Keywords: perineal reconstruction, surgical technique, surgical wound complications
1. INTRODUCTION
Vulvar carcinoma is a rare type of gynaecological tumour that affects roughly 5% of women. The most common histotype is spinocellular carcinoma, which is typically related to human papilloma virus (HPV) in young patients, but not in older patients. Although older patients are currently the predominant population, there is a growing trend for younger women to be diagnosed with different histological types of HPV‐related in situ carcinomas. Other histological types of tumours affecting the vulvar region are extramammary Paget disease, melanoma, sarcoma, basal cell carcinoma, adenocarcinoma, and verrucous carcinoma.1 Radical surgical resection and regional lymph nodes evaluation are the current cornerstones in the vulvar carcinoma work‐up; surgical removal techniques may involve local excision, modified radical vulvectomy, and radical vulvectomy, up to pelvic exenteration. The status of lymph nodes must be ascertained as this is the major determinant of survival2; in selected cases, sentinel node can be identified, and in most cases, mono‐ or bilateral inguinal lymphadenectomy must be performed. Adjuvant treatments, namely, radio‐ and/or chemotherapy, may be administered depending on the degree of infiltration, the characteristics of the tumour, and the surgical margins.
Indeed, the local relapse rate is directly correlated with the distance from the tumour to the resection margins,3 and radical vulvectomy creates a resection perimeter that can profoundly damage the perineal membrane. That being said, many gynaecologists prefer a more conservative approach, a decision supported by recent indications that exeresis radicality is reached after histological confirmation of a 1 to 2‐cm peripheral margin and a 1‐cm deep margin free of cancer.4
To minimise the disfiguration caused by radical excision and reduce the functional sequelae, which may have a significant impact because of the structures present in this anatomical region, and thereby improve patients' quality of life, numerous reconstructive techniques have been proposed.5 These include skin grafts, myocutaneous flaps, local and regional fasciocutaneous flaps, and microsurgical flaps,6, 7, 8 with local fasciocutaneous flaps being the most common because of their reliability, the ease and speed of flap raising, the suitable quality of the flap tissue, and the limited donor site morbidity and cicatricial sequelae.
Here, we provide a retrospective report of 59 V‐Y internal pudendal artery perforator advancement flap procedures, with the fasciocutaneous flap being raised from the gluteal fold, in 30 patients treated for vulvar carcinoma from 2006 to 2016. We present a simple modification to the technique that enabled us to reduce the tension on the upper part of the wound, which, together with the frequent maceration and contamination that occurs in the inguino‐perineal area,9 is one of the main causes of complications of this type of surgery.
2. MATERIALS AND METHODS
All reconstructive procedures were performed by the same reconstructive surgery team at two regional hospitals (Ospedale Santa Maria della Misericordia, Udine; Presidio Ospedaliero Sant'Antonio Abate, Tolmezzo, Udine) in Italy. All patients gave informed consent for the use of their personal information for research purposes. Patients' histories were collected, including information regarding their tumours and any neoadjuvant therapy received. Inter‐operative data regarding the dimensions of the defect and reconstruction time were recorded.
Peri‐operative care consisted of catheterisation until the wound was partially closed (1 week), as well as daily medication: 1‐week antibiotic prophylaxis with amoxicillin + clavulanic acid 1 Cp/12 hours and thromboembolism prophylaxis up to 21 days post‐surgery. Furthermore, the number of days in hospital was reported, and short‐ and long‐term data regarding complications at the surgical site, namely, infections, dehiscence, total or partial flap necrosis, and scarring problems; total flap necrosis; re‐admission to hospital; and functional complications (ostial stenosis and micturition issues after catheter removal) were considered major complications. The sensitivity of the advancement flap was tested at 6 months and 1 year via the two‐points discrimination (2PD) test, considering a value of ∼40 mm normal.10 Likewise, superficial pain was assessed using the “sharp or dull” test, and thermal sensation was assessed by applying hot and cold objects to the flap.11 After hospital discharge, patients attended outpatient follow‐up sessions at 1, 3, 6, 12, and 24 months post‐reconstruction.
2.1. Surgical technique
The technique we used to raise the V‐Y advancement flap from the gluteal fold has been widely reported in the literature and is described in detail in one of our previous papers.12, 13 In brief, before the surgery, the gluteal fold is identified and marked, as are the internal pudendal artery perforator branches at the top of the thigh, which will serve as the pedicle. Triangular flap(s) are marked after careful assessment of the loss of residual tissue after resection; the base of the flap is made up of the tissue margins of the resection site, and the apex is positioned along the gluteal fold, below the ischial tuberosity. It is important to remember that the internal pudendal artery perforator branches in the gynaecological area are concentrated medially and inferiorly to the ischial tuberosity. Flap raising is performed from medial to distal in a plane above or below the deep fascia, depending on the degree of advancement required. Flap sensitivity is ensured by the inclusion of the superficial branches of the posterior femoral cutaneous nerve, which must be identified and preserved at the gluteal fold, and the terminal branches of the pudendal nerve. A Jackson‐Pratt (JP) 10‐type suction drain is positioned, and then, the raised flap is positioned and sutured to the mucocutaneous junction at the margins of the defect using eversion sutures, taking care to avoid tension (Figure 1).
Figure 1.
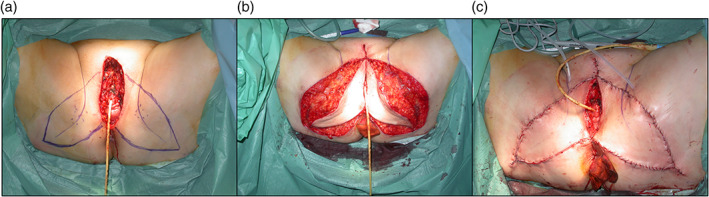
Preoperative markings of the V‐Y bilateral advancement flap along the gluteal fold. The perforators were previously identified with a pocket Doppler probe (A) and flap inset and wound closure (B, C)
Often, it is impossible to reach the apex of the defect with the cranial apex of such flaps, but the latter can be closed by suturing a few centimetres of the apical margins of the vulvectomy to each other. This enables the reduction in the tension at the apex of the flap(s), which are particularly susceptible to necrosis. After suturing, the wound will have the shape of a cross (Figure 2).
Figure 2.
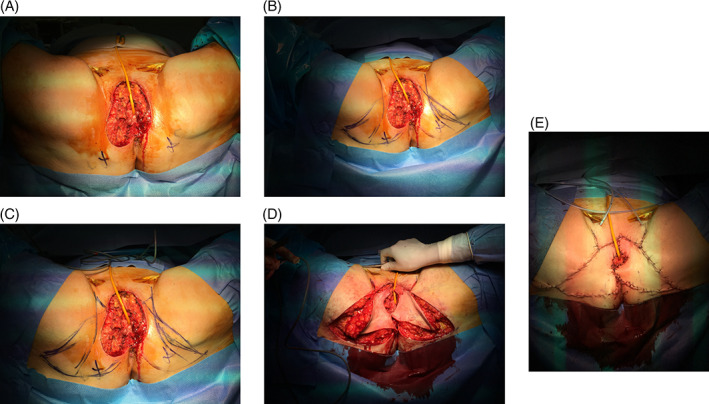
Flap inset according to the modified technique. A, Ischial tuberosity is identified; B, Classical V‐Y flap making. C, Continuing the incision along the inguinal fold and then heading it 60° towards the vulvectomy margin, the modification of the technique is obtained (C). D, Flap inset, and E, Final aspect of the wounds
We modified the pre‐surgical marking strategy, applying the “trident” plasty approach for closure of the triangular tissue defect and planning the surgical incisions for raising the cranial apex of the flap in the anterior portion of the defect—the site of the most tension (Figure 3). Indeed, this area of the flap is relatively poorly vascularised and is often thinned to better fit the mucocutaneous junctions.6
Figure 3.
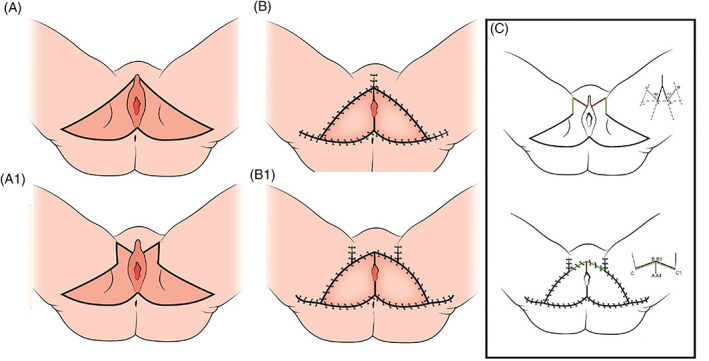
Schematic representation of the preoperative markings of classical V‐Y flap (A) and modified V‐Y flap (A1) and the aspect of the surgical wounds after closure (B, B1). In schema C, the lines configuring the trident plasty are highlighted
The superior margin of the flap, which should extend to the vulvectomy margin, was marked cranially and laterally along the inguinal fold for a few centimetres (a distance equal to or 1 cm superior to the projection of the superior apex of the vulvectomy margin on the inguinal fold). From this point, a line was traced at 60° with respect to the inguinal fold towards the vulvectomy margin. The resulting markings formed a “trident” pattern—a combination of Y‐V plasty and two Z plasties. The superior apex of the flap had a rectangular, rather than triangular, shape and a greater surface area because of the addition of tissue from the fold as illustrated in Figure 3; the modification can be schematised as a “trident” plasty, which leads to a more favourable distribution of the tension along the flap perimeter, which is elongated, and the inguinal wound, partially relaxing the median zone.
3. RESULTS
Between 2006 and 2016, 30 vulvar reconstructions were performed using the gluteal fold fasciocutaneous V‐Y flap and were based on the branches of the internal pudendal perforator artery. Of a total of 59 flaps, 24 (in 12 patients) were raised according to the modified procedure described above. The mean age of patients treated was 75.3 years (range 51‐92 years), and all patients presented with squamous cell carcinoma, staged as follows: stage IA n = 1, stage IB n = 13, stage II n = 9, stage III n = 4, and stage IVA n = 3. The patients at stages III and IVA received adjuvant radiotherapy.
The patient at stage IA underwent a wide resection plus a monolateral V‐Y flap, while the remaining 29 patients underwent radical vulvectomy plus bilateral lymphadenectomy and reconstruction with a bilateral V‐Y advancement flap from the gluteal fold. The mean dimensions of the monolateral defects were as follows: length 7.5 × 4.7 (range: 3‐12 × 1.3‐8 cm) and depth 2.8 cm (range 1‐5 cm). The mean operating time per flap was 40 minutes (range 30‐64 minutes), and the mean hospital stay was 13 days (range 10‐19 days). On the first postoperative day, all patients were mobilised in a sitting position, and ambulation was reacquired during the following week (7‐10 days).
No necrosis, wound dehiscence, or infection was observed in patients who underwent surgery according to the modified surgical approach. Among those treated using the traditional V‐Y technique, wound dehiscence was observed in four cases—one at the vaginal mucocutaneous margin and three at the inguinal wound; all the remaining wounds healed by secondary intention. Among these cases, surgical revision was necessary and was performed under local anaesthetic 5 weeks after the initial surgery in one patient, and in one of the inguinal dehiscence cases, a concomitant infection of the surgical wound was resolved by means of targeted oral antibiotic therapy. No cases of flap necrosis were observed, with the exception of three cases of local necrosis at the cranial apex of the vulvectomy, in patients treated using the unmodified technique; these required prolonged medication to achieve second‐intention healing. Overall, minor complications occurred in 14% (n = 8) of flaps and 23% (n = 7) of patients. With regard to major complications, vaginal ostial stenosis occurred in one 82‐year‐old patient who had undergone radical vulvectomy and bilateral flap reconstruction; no surgical correction was performed. Table 1 summarises the complications recorded.
Table 1.
Complications recorded
| Minor complications | No. of flaps (%) | No. of patients (%) |
|---|---|---|
| Early | ||
| Wound dehiscence | 4 (7%) | 4 (13%)a |
| Partial flap necrosis | 3 (5%) | 3 (10%) |
| Infection | 1 (2%) | 1 (3%)a |
| Late | ||
| Scarring problems | 0 (0%) | 0 (0%) |
| Permanent sensibility alterations | 0 (0%) | 0 (0%) |
| Total | 8 (14%) | 7 (23%)a |
| Major complications | No. of patients (%) | |
|---|---|---|
| Early | ||
| Total flap necrosis | 0 (0%) | |
| Rehospitalisation | 0 (0%) | |
| Late | ||
| Functional impairment | 1 (3%) | |
| Total | 1 (3%) | |
Summary of the complications recorded in our series, grouped into minor and major and early and late. Note that minor complications are expressed per flap and per patient, while major complications are expressed as per patient.
One of the cases presented with both inguinal disunion and local infection.
At 6‐month follow up, sensitivity was recovering but not completely normalised in all patients. At 1 year, six patients were deceased, one because of a cardiovascular accident and five because of the advanced stage of their disease. In the remaining patients, only one demonstrated altered sensitivity on 2PD testing (>40 mm). The remaining patients exhibited complete recovery of sensitivity according to objective testing and self‐report. At 1 year, there was no appreciable difference in sensitivity between the proximal and distal regions of the flap. Only seven patients reported a certain amount of preoperative sexual activity, but at 1 year after vulvectomy and reconstruction, nine patients reported recovery of their sexual activity. A further three patients died before the 24‐month follow up, one because of breast carcinoma and two because of advanced disease. The patient staged as IA was treated via laser ablation for a vulvar intraepithelial neoplasia (VIN) III‐type relapse 14 months after surgery. All patients reported satisfaction with surgical outcomes (Figure 4).
Figure 4.
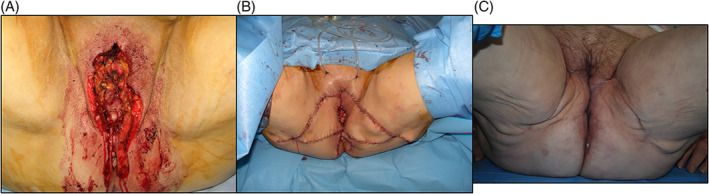
Loss of substance after vulvectomy was performed (A). intraoperative final aspect after repair with bilateral V‐Y advancement flap (B). C, Result 18 months postop.: the scars are well hidden in the physiological cutaneous folds
4. DISCUSSION
The choice of reconstructive method after vulvectomy depends on various factors, some of which are linked to the size and type of defect or the individual characteristics of the patient, while others are associated with the flap. Local fasciocutaneous flaps are generally the first choice of many surgeons, except in cases of widespread tissue destruction, for example, in the dissection of several lymph nodes or pelvic exenteration, when muscle flaps are preferred because of their greater mass.14 Indeed, the perineal area, buttocks, and medial portion of the thigh are supplied by a rich system of perforator vessels, mainly branches of the internal pudendal and deep femoral arteries but also the inferior gluteal artery, upon which to base local fasciocutaneous flaps (perforator or not) of various shapes and sizes.15, 16 Owing to the cicatricial outcomes at the donor site, flaps from the gluteal and perineal regions are generally preferred to those from the medial portion of the thigh. Indeed, the infragluteal region is, in the majority of cases, considered a good site for donor tissue. Furthermore, there are no lymphatic vessels—through which vulvar cancer can spread—in the gluteal fold, making this tissue suitable from an oncological, as well as surgical, perspective.17
Among the flaps raised from the gluteal fold, the lotus flap,18 V‐Y perforator flap,16 gluteal fold island flap,19 profunda artery perforator (PAP) flap,20, 21 and fasciocutaneous infragluteal (FCI) flap22 have been used successfully in vulvar and perineal reconstruction, and various different protocols have been proposed. However, gluteal V‐Y advancement fasciocutaneous flaps are preferred by many authors for small‐ to medium‐sized mono‐ or bilateral defects or larger defects that do not extend anteriorly.23 These flaps are based on the internal pudendal perforator artery and are relatively quick and easy to raise as dissection of the vascular pedicle is not required. This technique also minimises the risk of damage to branches of the pudendal nerve—as demonstrated by the excellent sensitivity outcomes in our sample. That being said, it should be noted that we cannot provide data regarding preoperative sensitivity, and only one patient underwent monolateral surgery, and therefore, no meaningful control data are available. Moreover, although the 2PD test is a good indicator of tactile sensation recovery, it has several limitations, particularly those linked to age‐related changes in sensitivity and inter‐individual variability, which can be substantial.24
Another advantage of gluteal V‐Y advancement fasciocutaneous flaps is that they can be raised bilaterally and are therefore applicable in cases of hemivulvectomy and total vulvectomy. They are also reliable in post‐radiotherapy cases as they are based on a complex of blood vessels rather than a single perforator artery. Partially because of this rich blood supply, no cases of full‐flap necrosis have been reported in other studies or ours, in which apical necrosis occurred in only 5% of patients.
In our experience, this flap can be used successfully to cover defects with a surface area of up to 80 cm2. Indeed, the complications encountered in our cases, like those reported in the literature, were minor, being linked mainly to wound healing and apical flap necrosis.12, 13 Furthermore, the flap modification described above—grounded on the basic principles of plastic surgery and involving a small variation in how the cranial apex of the flap is raised—enables redistribution of the tension along the inguinal fold, away from the demolition area, and reduces the superior and medial traction exerted by the vulvectomy margins on the apex of the flap. Although we have only applied this procedure in 12 patients—a total of 24 flaps—the complication rate in our series was very low, with only minor complications being observed.
A further advantage of the technique is aesthetic—the donor site scars are hidden by the gluteal fold, which remains well defined. Moreover, the scar along the superior half of the inguinal fold introduced by the modification is easily covered by underwear. Furthermore, the colour, thickness, and texture of the flap were well matched with the demolition area, and the pliability of the tissue promoted good adaptation of the mucocutaneous margin. Indeed, no additional surgery was required for scar correction or flap debulking, which may be necessary in cases of perforator or island flaps, especially in patients with obesity.25
The patients in our series were relatively elderly (mean 75 years) with an advanced stage of the disease—factors that are reflected in the high mortality rate at follow up (nine patients, 30%). This also made it difficult to conduct an analysis of sexual activity and sexual dysfunction (FSD) before and after surgery using the standard tools (Female Sexual Function Index (FSFI), Sexual Quality of Life‐ Female questionnaire (SQoL‐F)).26, 27 However, seven patients did report a certain level of sexual activity in the preoperative period, which had been recovered 1 year later, and two patients reported recovery of sexual activity despite having had none before the operation.
The outcomes in this case series confirm that the gluteal V‐Y advancement fasciocutaneous flap is a useful and simple technique for reconstructing even large vulvar defects. It has low functional and aesthetic impact and enables a rapid return to autonomy, which is particularly beneficial in older patients. Moreover, the simple modification to the V‐Y flap design proposed above appears to reduce the rate of complications associated with the surgical wound.
Conflict of interest
The authors have no conflicts of interest to report.
Fin A, Rampino Cordaro E, Guarneri GF, Revesz S, Vanin M, Parodi PC. Experience with gluteal V‐Y fasciocutaneous advancement flaps in vulvar reconstruction after oncological resection and a modification to the marking: Playing with tension lines. Int Wound J. 2019;16:96–102. 10.1111/iwj.12997
REFERENCES
- 1. Koh WJ, Greer BE, Abu‐Rustum NR, et al. Vulvar cancer, version 1.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2017;15(1):92‐120. [DOI] [PubMed] [Google Scholar]
- 2. Stehman FB, Look KY. Carcinoma of the vulva. Obstet Gynecol. 2006;107:719‐733. [DOI] [PubMed] [Google Scholar]
- 3.Vulvar Cancer Treatment (PDQ®)–Health Professional Version. Bethesda, MD: 2015. http://www.cancer.gov/types/vulvar/hp/vulvar‐treatment‐pdq#section/_1. Accessed August 3, 2015.
- 4. Heaps JM, Fu YS, Montz FJ, Hacker NF, Berek JS. Surgical‐pathologic variables predictive of local recurrence in squamous cell carcinoma of the vulva. Gynecol Oncol. 1990;38:309‐314. [DOI] [PubMed] [Google Scholar]
- 5. Weikel W, Hofmann M, Steiner E, Knapstein PG, Koelbl H. Reconstructive surgery following resection of primary vulvar cancers. Gynecol Oncol. 2005;99(1):92‐100. [DOI] [PubMed] [Google Scholar]
- 6. Negosanti L, Sgarzani R, Fabbri E, et al. Vulvar reconstruction by perforator flaps: algorithm for flap choice based on the topography of the defect. Int J Gynecol Cancer. 2015;25(7):1322‐1327. [DOI] [PubMed] [Google Scholar]
- 7. Di Donato V, Bracchi C, Cigna E, et al. Vulvo‐vaginal reconstruction after radical excision for treatment of vulvar cancer: evaluation of feasibility and morbidity of different surgical techniques. Surg Oncol. 2017;26(4):511‐521. [DOI] [PubMed] [Google Scholar]
- 8. Zhang W, Zeng A, Yang J, et al. Outcome of vulvar reconstruction by anterolateral thigh flap in patients with advanced and recurrent vulvar malignancy. J Surg Oncol. 2015;111(8):985‐991. [DOI] [PubMed] [Google Scholar]
- 9. Guarner F, Malagelada JR. Gut flora in health and disease. Lancet. 2003;361:512‐519. [DOI] [PubMed] [Google Scholar]
- 10. Mancini F, Bauleo A, Cole J, et al. Whole‐body mapping of spatial acuity for pain and touch. Ann Neurol. 2014;75(6):917‐924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Walker HK, Hall WD, Hurst JW. Clinical Methods: The History, Physical, and Laboratory Examinations. Chapter 67. 3rd ed. Boston: Butterworths; 1990. [PubMed] [Google Scholar]
- 12. Lazzaro L, Guarneri GF, Rampino Cordaro E, et al. Vulvar reconstruction using a "V‐Y" fascio‐cutaneous gluteal flap: a valid reconstructive alternative in post‐oncological loss of substance. Arch Gynecol Obstet. 2010. Nov;282(5):521‐527. [DOI] [PubMed] [Google Scholar]
- 13. Lee PK1, Choi MS, Ahn ST, Oh DY, Rhie JW, Han KT. Gluteal fold V‐Y advancement flap for vulvar and vaginal reconstruction: a new flap. Plast Reconstr Surg. 2006;118(2):401‐406. [DOI] [PubMed] [Google Scholar]
- 14. Gentileschi S, Servillo M, Garganese G, et al. Surgical therapy of vulvar cancer: how to choose the correct reconstruction? J Gynecol Oncol. 2016;27(6):e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Huang JJ, Chang NJ, Chou HH, et al. Pedicle perforator flaps for vulvar reconstruction—new generation of less invasive vulvar reconstruction with favorable results. Gynecol Oncol. 2015;137(1):66‐72. [DOI] [PubMed] [Google Scholar]
- 16. Chen YC, Scaglioni MF, Kuo YR. Profunda artery perforator based V‐Y rotation advancement flap for total vulvectomy defect reconstruction—a case report and literature review. Microsurgery. 2015;35(8):668‐671. [DOI] [PubMed] [Google Scholar]
- 17. McClean JM. Anatomy and physiology of the vulval area. In: Ridley CM, ed. The Vulva. Edinburgh: Churchill Livingstone; 1988. [Google Scholar]
- 18. Argenta PA, Lindsay R, Aldridge RB, Siddiqui N, Burton K, Telfer JR. Vulvar reconstruction using the "lotus petal" fascio‐cutaneous flap. Gynecol Oncol. 2013;131(3):726‐729. [DOI] [PubMed] [Google Scholar]
- 19. Moschella F, Cordova A. Innervated island flaps in morphofunctional vulvar reconstruction. Plast Reconstr Surg. 2000;105(5):1649‐1657. [DOI] [PubMed] [Google Scholar]
- 20. Ito R, Huang JJ, Wu JC, Lin MC, Cheng MH. The versatility of profunda femoral artery perforator flap for oncological reconstruction after cancer resection—clinical cases and review of literature. J Surg Oncol. 2016;114(2):193‐201. [DOI] [PubMed] [Google Scholar]
- 21. Chang TN, Lee CH, Lai CH, et al. Profunda artery perforator flap for isolated vulvar defect reconstruction after oncological resection. J Surg Oncol. 2016;113(7):828‐834. [DOI] [PubMed] [Google Scholar]
- 22. Windhofer C, Papp C, Staudach A, Michlits W. Local fasciocutaneous infragluteal (FCI) flap for vulvar and vaginal reconstruction: a new technique in cancer surgery. Int J Gynecol Cancer. 2012;22(1):132‐138. [DOI] [PubMed] [Google Scholar]
- 23. Kuokkanen H, Mikkola A, Nyberg RH, Vuento MH, Kaartinen I, Kuoppala T. Reconstruction of the vulva with sensate gluteal fold flaps. Scand J Surg. 2013;102(1):32‐35. [DOI] [PubMed] [Google Scholar]
- 24. Shimokata H, Kuzuya F. Two‐point discrimination test of the skin as an index of sensory aging. Gerontology. 1995;41(5):267‐272. [DOI] [PubMed] [Google Scholar]
- 25. Ragoowansi R, Yii N, Niranjan N. Immediate vulvar and vaginal reconstruction using the gluteal‐fold flap: long‐term results. Br J Plast Surg. 2004. Jul;57(5):406‐410. [DOI] [PubMed] [Google Scholar]
- 26. Vital M, de Visme S, Hanf M, Philippe HJ, Winer N, Wylomanski S. Using the Female Sexual Function Index (FSFI) to evaluate sexual function in women with genital mutilation undergoing surgical reconstruction: a pilot prospective study. Eur J Obstet Gynecol Reprod Biol. 2016. Jul;202:71‐74. [DOI] [PubMed] [Google Scholar]
- 27. Symonds T, Boolell M, Quirk F. Development of a questionnaire on sexual quality of life in women. J Sex Marital Ther. 2005;31:385‐397. [DOI] [PubMed] [Google Scholar]


