Abstract
Deep sternal wound infection (DSWI) is a severe complication in patients after open heart surgery (OHS). But there is a lack of appropriate imaging tool to detect the infection sites, which may lead to incomplete debridement. The present study aims to investigate the value of 18F‐fluorodeoxyglucose positron emission tomography/computed tomography (18F‐FDG PET/CT) in comparison with CT scan in diagnosing and localising DSWI. A total of 102 patients with DSWI after OHS were retrospectively collected from January 2012 to December 2017 in our hospital. All the patients had surgical debridements for DSWI with pretreatment imaging of either 18F‐FDG PET/CT or CT scan. The sensitivity, specificity, and accuracy of localising infection sites were compared between PET/CT and CT groups, with surgical, microbiological, and histopathological findings as the gold standard. The length of hospital stays and the rate of recurrence were also compared. Ten patients in the PET/CT group had a follow‐up PET/CT scan after debridement, and the correlations between the changes of PET/CT findings and surgical outcomes were analysed. 18F‐FDG PET/CT is more accurate than CT in diagnosing and localising DSWI after OHS, which leads to a more successful surgical debridement with a lower rate of recurrence and a shorter length of hospital stay. In addition, follow‐up PET/CT after debridement could evaluate the treatment effect.
Keywords: PET/CT, deep sternal wound infection, debridement, 18F‐FDG
1. INTRODUCTION
Deep sternal wound infection (DSWI) is a severe complication after open heart surgery (OHS). DSWI often attacks bone and mediastinum, which is also named as mediastinitis. 1 Though the morbidity of DSWI is rare from 1.1% to 2.4%, it is associated with a longer hospital stay, worse survival, and higher medical cost.2, 3, 4 After OHS, patients with DSWI have twice the mortality rate compared with those without DSWI. 5
Diagnosis of DSWI can be made according to the Centre of Disease Control criteria: (a) organisms cultured from mediastinal tissue or fluid obtained during a surgical operation or needle aspiration. (b) Evidence of mediastinitis was observed during a surgical operation or histopathological examination. (c) At least one of the following signs or symptoms with no other recognised causes, such as fever (>38°C), chest pain, or sternum instability, and at least one of the following: (a) purulent discharge from mediastinal area; (b) organisms cultured from blood, or discharge from mediastinal area; (c) mediastinal widening on X‐rays. 6 Chronic DSWI that occurs in months to years after OHS is hard to be diagnosed and cured. 7 Patients with chronic DSWI usually present chronic fistulas, subcutaneous abscess, osteomyelitis, rib infection, costal chondritis, and infection spreading into mediastinum sometimes. 8
When DSWI is diagnosed, a surgical treatment is needed as the first‐line treatment. However, the optimal surgical method is hard to choose. Different methods of debridements are based on the common surgical rules for infection management with different institutional experiences. Generally, treatment methods include the following: (a) complete debridement to clean up all the infections after reopening the wound; (b) reconstruction of sternum using metal wires, titanium plates, and muscular flaps to make it stable; (c) negative‐pressure wound therapy (NPWT) after immediate or secondary closure; (d) antibiotics that are sensitive to bacteria.5, 8, 9, 10 Even though new methods such as NPWT have shown promising results with faster healing of the sternal defect and decreased recurrence and mortality, recurrent DSWI still often occurs making complete debridement a real challenge. 11
Identification of infection sites and assessment of sternum stability are the two key points for a successful treatment of DSWI. 5 A computed tomography (CT) scan with 3D reconstruction of the sternum is often used before surgical treatment to evaluate of the sternum stability. However, CT has a low accuracy in localising the infection sites. Magnetic resonance imaging (MRI) is widely used for diagnosing infection in bone and soft tissue. It is sensitive but may include large areas of nonspecific signal changes.12, 13 Moreover, patient with metal wires or titanium plate on sternum after OHS cannot accept MRI. The debridement is performed based on clinical manifestations and findings during surgical exploration. Frequently, atypical symptoms make the decision of appropriate surgical debridement difficult. In some cases, recurrent infections are localised at the costal cartilage with the same pathogen as earlier, suggesting incomplete debridement during the initial surgery.11, 14 So far, there is no appropriate imaging examination for diagnosing and localising the sites of DSWI.
18F‐fluorodeoxyglucose positron emission tomography/computed tomography (18F‐FDG PET/CT), as a functional imaging procedure, is mainly used in the diagnosis and treatment evaluation of tumour. Recently, FDG PET/CT is also used in the field of infection such as fever of unknown origin, infectious endocarditis, osteomyelitis, spondylodiscitis, infected prosthesis, and so on.15, 16 The inflammatory cells including neutrophil and macrophage in infection sites have an enhanced glucose metabolism. The increase of glycolysis in inflammatory cells makes infection sites as “hot spots” in FDG PET/CT imaging.15, 16 In this study, we evaluate the role of FDG PET/CT in surgical treatment of patients with DSWI after OHS.
2. METHODS
2.1. Patients and data collection
A retrospective analysis was performed of prospectively collected data of 108 patients with suspected DSWI who underwent CT scan and PET/CT scan with 3D reconstruction of sternum and ribs before surgical debridement in our hospital from January 2012 to December 2017. There were six patients who were excluded because of missing clinical data. A total of 56 cases who underwent PET/CT scan were collected as experimental group, and 46 cases who underwent CT scan were collected as control group. Ten patients forming the PET/CT group received follow‐up PET/CT scans after debridement. Patients were followed up 1 year after surgical treatment and their data of recurrence were collected.
Data regarding patients' demographics, laboratory findings, blood and wound culture, imaging characteristics, methods of surgical treatment, the length of hospital stay, and outcomes were recorded based on chart review. This retrospective study was approved by our hospital IRB.
2.2. Imaging procedures
Imaging scan was performed for all patients after last surgery (OHS or surgical debridement) more than 1 month in order to avoid the false‐positive results of postoperative changes.
18F‐FDG PET/CT imaging studies were performed on Discovery VCT unit (GE Medical Systems, Waukesha) or on uMI S‐96R unit (United Imaging, Shanghai, China) with a 64/16‐MDCT scanner. All patients fasted at least 6 hours before PET/CT scan. For diabetic patients using insulin, the insulin was discontinued for at least 6 hours, and a serum blood glucose level was verified to be below 10 mmol/L before examination. All patients received FDG intravenously as 4.4 MBq/kg and then rested quietly around 60 minutes. A thoracic PET was acquired from apex to basis of the lung in 3D imaging mode (2 minutes per bed). Helical CT scan was matched with the PET scan's field and slice thickness with a tube voltage of 120 to 140KV and a tube current of 200 mA. All PET/CT scans were displayed on an uWS‐MI R001 workstation (United Imaging, Shanghai, China). 3D reconstruction of the sternum and ribs was performed.
CT scans were obtained with the helical technique using 16‐detector (Somatom Definition AS; Siemens, Forchheim, Germany) scanners. The field of scan was performed from apex to base of the lung. The scan parameters were 120 kV and 170 to 200 mA, and mediastinal, lung, and bone window images were evaluated on the monitors. 3D reconstruction of the sternum and ribs was performed.
2.3. Imaging analysis
Two nuclear medicine physicians and two radiologists evaluated the PET/CT and CT images independently. The image interpretation is focused on identifying the locations of infection sites in DSWI patients.
Positive findings of PET were defined as increased focal or diffused FDG uptake involving the sternum, costal cartilage, or mediastinum, compared with that of surrounding normal bone or soft tissue. Mild increase of uptake should be distinguished from postoperative change, which is general homogeneous. Positive findings of CT were defined as follows: (a) wide dehiscence and shifting between two halves of sternum; (b) the signs of osteomyelitis such as cortical lysis, bone resorption, and periosteal reaction in the sternum, ribs, and costal cartilages; (c) large air of fluid collections, obvious oedema of soft tissue in mediastina; (d) abscess, fistulas, or skin ulcers in pre‐sternal tissue. Small gaps of sternum, little gas or fluid collections, and mild oedema should be distinguished with postoperative change.17, 18
The imaging findings were summarised in three compartments as sternum, ribs/costal cartilages, and mediastinum based on the location of infection sites. The imaging diagnosis was correlated with surgical findings, pathologic diagnosis, bacterial cultures, and follow‐up.
2.4. Surgical treatment
Surgical debridements and exploration were performed based on the CT or PET/CT scan and surgical findings. During debridement, all previous sternal wires and foreign matters were removed. Necrotic soft tissues and all infected sternum, ribs, and costal cartilages were resected until healthy tissue was visible. Then, mediastinum was inspected for signs of infected pacemaker leads, infected vascular grafts and necrotic tissues, and cleaned if any. The sternal regions were reconstructed with pectoralis major muscle flaps. The instable sterna that were hardly rewired were reconstructed with titanium plate. The surgery was completed by one stage or stages based on the general conditions of patients and range of infections. All the patients received NPWT intravenous antibiotics that were sensitive to pathogens after the surgical debridement.
2.5. Statistical analysis
Categorical variables were presented as number and percent. Continuous variables were presented as means and SD. Significant differences were assessed with either chi‐square test or Fisher's exact test for categorical variables and independent samples. Student's t test was applied for continuous variables. The P value of <.05 was considered statistically significant. All statistical analyses were performed by using IBM SPSS version 22.0.
3. RESULTS
3.1. Demographics of the patients
As shown in Table 1, no statistically significant differences were found in the basic characteristics of the patients between CT and PET/CT group in terms of gender, age, body mass index (BMI), smoking, hypertension, diabetes, chronic obstructive pulmonary disease (COPD), left ventricular ejection fraction (LVEF), and renal disease. The primary OHS included coronary artery bypass grafting (CABG) in 56 patients (28 in the CT group and 28 in the PET/CT group), repair or replacement of valves in 32 patients (12 in the CT group and 20 in the PET/CT group), CABG together with valve replacement in 2 patients (2 in PET/CT group), thoracic aortic dissection in 10 patients (4 in CT group and 6 in PET/CT group), and tetralogy of Fallot in 2 patients (2 in CT group) (P = .506). Forty‐four patients (43.1%) had histories of prior debridement (18 in the CT group and 26 in the PET/CT group, P = .548). The time interval from the OHS to the DSWI surgical debridement was 1 month to 13 years (median of 6 months) (8.6 months in the CT group and 17.3 months in the PET/CT group, P = .258). There were no statistically significant differences in the types of OHS, histories of debridement, and the months between OHS and DSWI surgery in two groups.
TABLE 1.
Demography and clinical characteristics of patients
| CT group (n = 46) | PET/CT group (n = 56) | P | |
|---|---|---|---|
| Sex, Male | 28 (60.9%) | 38 (67.9%) | .462 |
| Age, y | 63.2 ± 9.2 | 57.2 ± 12.7 | .056 |
| BMI | 23.9 ± 2.4 | 24.3 ± 3.9 | .678 |
| Smoking | 10 (21.7%) | 20 (35.7%) | .123 |
| Hypertension | 22 (47.8%) | 32 (57.1%) | .348 |
| Diabetes | 18 (39.1%) | 28 (39.3%) | .987 |
| COPD | 8 (17.4%) | 8 (14.3%) | .668 |
| LVEF | 58.0 ± 11.3 | 59.8 ± 9.8 | .558 |
| Renal disease | 4 (8.7%) | 8 (14.3%) | .383 |
| Type of OHS | .506 | ||
| CABG | 28 (60.9%) | 28 (50.0%) | |
| Valve surgery | 12 (26.1%) | 20 (35.7%) | |
| CABG with valve surgery | 0 (0.0%) | 2 (3.5%) | |
| Thoracic aortic dissection | 4 (8.6%) | 6 (10.7%) | |
| Tetralogy of Fallot | 2 (4.3%) | 0 (0.0%) | |
| History of debridement (>1) | 18 (39.1%) | 26 (46.4%) | .548 |
| Months from OHS to DSWI surgical debridement | 8.6 ± 16.9 | 17.3 ± 33.3 | .258 |
Note: Data are presented as n (%) and mean ± SD.
Abbreviations: BMI, body mass index; CABG, coronary artery bypass grafting; COPD, chronic obstructive pulmonary disease; DSWI, deep sternal wound infection; LVEF, left ventricular ejection fraction; OHS, open‐heart surgery.
3.2. Imaging findings and diagnosis of DSWI
As shown in Table 2, FDG PET/CT showed a higher sensitivity, specificity, and accuracy in localising infection sites than CT in the patients who underwent DSWI. The infection sites were divided into three sub‐groups as sternum, ribs/costal cartilages, and mediastinum by its location. PET/CT had a significantly higher accuracy than CT in the diagnosis of sternal infections (100.0% vs 87.0%, P = .007). And PET/CT could show the exact range of infection in manubrium sterni, manubrium, or xiphoid process (Figure 1). In ribs/costal cartilages, the sensitivity of PET/CT was significantly higher than CT (78.6% vs 16.2%, P = .001). It should be noted that the location of increased uptake was not completely matched because of breathing artefact, presenting which was in the surrounding soft tissue, or which was just involving part of the infected ribs/costal cartilages (Figure 1). In mediastinum, the sensitivity of PET/CT was higher than CT (100.0% vs 16.7%, P = .001), but the specificity is lower (61.5% vs 16.2%, P = .001).
TABLE 2.
Diagnostic performance of CT and PET/CT
| Infection sites | CT group (%) | PET/CT group (%) | P | |
|---|---|---|---|---|
| Sternum | Sensitivity | 90.5 | 100.0 | .370 |
| Specificity | 50.0 | 100.0 | .429 | |
| Accuracy | 87.0 | 100.0 | .007 | |
| Ribs/costal cartilages | Sensitivity | 16.2 | 78.6 | .001 |
| Specificity | 98.2 | 98.6 | .580 | |
| Accuracy | 88.8 | 97.2 | .001 | |
| Mediastinum | Sensitivity | 16.7 | 100.0 | .001 |
| Specificity | 94.1 | 61.5% | .002 | |
| Accuracy | 73.9 | 82.1% | .315 |
Note: The bold values means P Abbreviations: PET/CT, positron emission tomography/computed tomography.
FIGURE 1.

A, A 62‐year‐old male had infections in sternum and ribs/costal cartilages who accepted valve surgery 5 years ago. 18F‐FDG PET/CT showed increased FDG uptake at the infection sites. The maximum standardized uptake value (SUVmax) is 11.5. B, A 58‐year‐old male presented with fistulas and wound separation 5 months after CABG. 18F‐FDG PET/CT showed infection sites in the sternum and left costal cartilage with increased FDG uptake (SUVmax = 14.1). 3D reconstruction of the sternum and ribs/costal cartilages was performed. CABG, coronary artery bypass grafting; 18F‐FDG PET/CT, 18F‐fluorodeoxyglucose positron emission tomography/computed tomography
3.3. Surgical debridement procedures and outcomes
Table 3 shows that 70 patients with DSWI accepted surgical debridements by one stage and 32 patients by stages. Among these people, 52 patients had reconstructions of sternum with titanium plate and 50 without titanium plate. The patients in CT group had a longer hospital length of stay than PET/CT group (28.8 ± 20.9 vs 17.9 ± 7.7 days, P < .001). The rate of recurrent DSWI in PET/CT group is lower than CT group significantly (21.4% vs 41.3%, P = .003).
TABLE 3.
Outcome data of surgical debridement
| CT group | PET/CT group | P | |
|---|---|---|---|
| Type of surgical debridement | |||
| One‐stage surgery | 30 (65.2%) | 40 (71.4%) | .501 |
| Surgery by stages | 16 (34.7%) | 16 (28.6%) | |
| Type of reconstruction of sternal | |||
| With titanium plate | 12 (52.2%) | 14 (50.0%) | .827 |
| Without titanium plate | 11 (47.8%) | 14 (50.0%) | |
| Recurrent DSWI | 19 (41.3%) | 12 (21.4%) | .03 |
| Hospital length of stay (days) | 28.8 ± 20.9 | 17.9 ± 7.7 | <.001 |
Note: The bold values means P Abbreviations: DSWI, deep sternal wound infection; PET/CT, positron emission tomography/computed tomography.
3.4. Follow‐up PET/CT could evaluate the treatment effect of debridement
After debridement, 10 patients had a second FDG PET/CT scan to evaluate the treatment effect. In 6 patients, FDG uptake in the original infection sites was increased or expanded in the second PET/CT imaging compared with the first scan. One patient whose original infections became decreased and reduced was found to have new uptake sites in costal cartilages (Figure 2). All of these 7 patients were confirmed to be recurrent DSWI. Another 2 patients had no recurrence during follow‐up, and the uptake of original infection sites was decreased with reduced range or disappeared in the second PET/CT imaging (Figure 3). The last one patient presented with wound separation and discharge about 1 month after the surgical debridement of infected sternum. A second PET/CT scan was performed and showed that the original infected range was reduced and the increased uptake was just localised in the chest wall. The patient was diagnosed with recurrent superficial sternal wound infection and had debridements for chest wall only. The patient avoided unnecessary surgical treatment and had no recurrence in follow‐up (for more details, please refer to Table 4).
FIGURE 2.
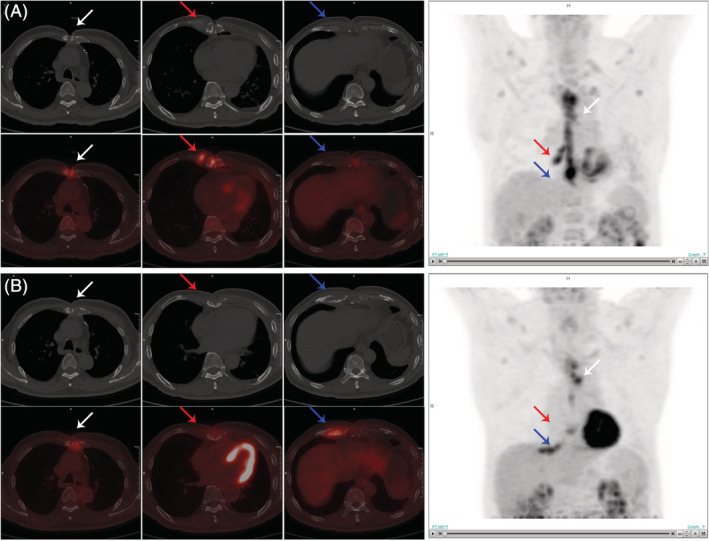
A 72‐year‐old male with DSWI accepted surgical debridement 6 months after CABG. A, The 18F‐FDG PET/CT 1 week before debridement showed increased uptake in sternum (SUVmax = 7.9) and right fifth costal cartilage (SUVmax = 7.7). B, The follow‐up PET/CT 5 months after debridement showed decreased uptake in sternum (SUVmax = 3.6) and right fifth costal cartilage (SUVmax = 1.6), and new increased uptake at left second (SUVmax = 6.4) and right sixth costal cartilages (SUVmax = 6.2). The patient has recurrent DSWI and resurgery. CABG, coronary artery bypass grafting; DSWI, deep sternal wound infection; 18F‐FDG PET/CT, 18F‐fluorodeoxyglucose positron emission tomography/computed tomography
FIGURE 3.
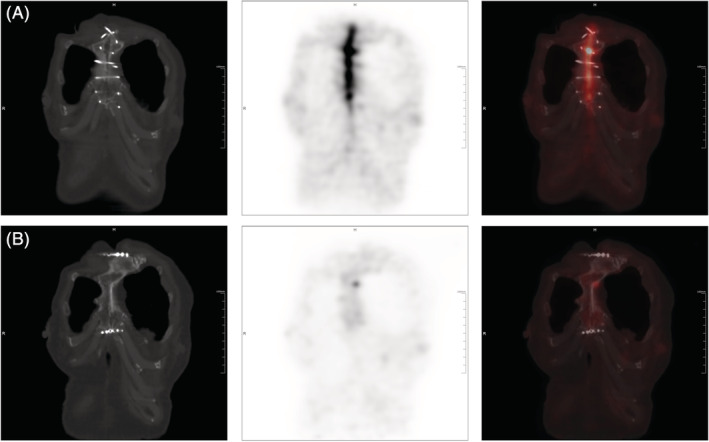
A, A 72‐year‐old female with DSWI accepted surgical debridement 2 months after aortic valve replacement. The 18F‐FDG PET/CT 1 week before debridement showed increased uptake in sternum (SUVmax = 12.6). B, The follow‐up PET/CT 1 year after debridement showed decreased uptake in original infection site (SUVmax = 1.9). The patient has no recurrence. DSWI, deep sternal wound infection; 18F‐FDG PET/CT, 18F‐fluorodeoxyglucose positron emission tomography/computed tomography
TABLE 4.
Follow up 18F‐FDG PET/CT image findings
| Infection sites with increased uptake in the first PET/CT scan | Changes of infection sites after treatment in the second PET/CT scan | Follow‐up and treatment | |
|---|---|---|---|
| 1 | Sternum | Uptake decreased, range reduced | No recurrence |
| 2 | Sternum | Uptake decreased | No recurrence |
| Right fifth to sixth and left fourth to sixth costal cartilage | Uptake decreased, range reduced | ||
| 3 | Sternum | Uptake decreased, range expanded | Recurrence, no surgery, died due to upper gastrointestinal bleeding |
| Superior and anterior mediastinum | Uptake decreased | ||
| 4 | Sternum | Uptake increased | Recurrence, resurgery |
| Right pericardium | Uptake decreased | ||
| 5 | Sternum | Uptake decreased | Recurrence, resurgery |
| Anterior and middle mediastinum | Uptake increased | ||
| 6 | Sternum | Range reduced | SSWI, cured by debridements for chest wall only |
| 7 | Sternum | Uptake decreased, range reduced | Recurrence, resurgery |
| Subxyphoid | Uptake increased | ||
| Right pericardium | Uptake increased | ||
| 8 | Sternum | Uptake increased, range expanded | Recurrence, resurgery |
| Right second costa | Uptake increased | ||
| Superior and anterior mediastinum | Uptake increased | ||
| 9 | Sternum | Uptake decreased, range reduced | Recurrence, resurgery |
| Superior mediastinum | Uptake increased, range expanded | ||
| 10 | Sternum | Uptake decreased, range reduced | Recurrence, resurgery |
| Right fifth costal cartilage | Uptake decreased, range reduced | ||
| New increased uptake at left second and right sixth costal cartilage | |||
| Subxiphoid | Uptake decreased, range reduced |
Abbreviations: 18F‐FDG PET/CT, 18F‐fluorodeoxyglucose positron emission tomography/computed tomography; SSWI, superficial sternal wound infection.
4. DISCUSSION
DSWI is a serious complication after OHS, often associated with incomplete debridement and high recurrence, in the absence of accurate presurgical evaluation of the infection sites. In our study, FDG PET/CT shows a high accuracy in diagnosing and localising infection sites of DSWI compared with CT, which could help to make plans of debridement, leading to a short postsurgical hospital stay and lower rate of recurrence. Follow‐up PET/CT could potentially evaluate the effect of surgical debridement.
FDG PET/CT has shown a unique value in diagnosis and treatment effect evaluation of infection, including osteomyelitis. It has been reported that PET/CT has a sensitivity and a specificity of 92.3% and 92.0%, respectively, in the diagnosis of chronic osteomyelitis. 19 For diabetic foot osteomyelitis, PET/CT has a sensitivity and a specificity of 74% and 91%, respectively,which is effective in differential diagnosis of osteomyelitis and Charcot neuroarthropathy. 13 PET/CT could also better localise active spinal infections than MRI in delineating the surgical field for successful minimally invasive surgery. 20 In addition, PET/CT could be used in the evaluation of antibiotic treatment response in spondylodiscitis. 21 Even though there is different anatomical position, DSWI has many similarities with these infectious diseases. A previous case series report has suggested that PET/CT could potentially localise the site of infections, guide surgical treatment, and evaluate treatment effect for DSWI. 22 Our results confirmed the findings with a larger sample number and showed that PET/CT is superior to CT scan.
CT with 3D reconstruction is usually used to evaluate the sternum stability of DSWI patients, but not for infection. In the current study, we used PET/CT with 3D reconstruction instead of CT before debridement, which shows that PET/CT is more accurate than CT in localising DSWI after OHS. CT could hardly find the infections in ribs/costal cartilages with a low sensitivity as 16.2%. For infection in mediastinum, the typical sign of CT is air of fluid collection in mediastina, 18 which is hard to distinguish infection from changes after OHS in real clinical settings. The sensitivity of CT in mediastinum group was 16.7%. However, FDG PET/CT could localise the infection sites by showing increased uptake of FDG, especially in ribs/costal cartilages and mediastinum. Moreover, PET/CT could show the exact range of infection in sternum and surrounding tissue, which helps to avoid unnecessary exploration and surgical injury. PET/CT scan provides a “map” of infections to guide a successful debridement, resulting in a shorter length of hospital stay and lower rate of recurrence than CT.
In addition, we have found that PET/CT could evaluate the treatment effect of surgical debridement for DSWI patients. In a follow‐up PET/CT, persistent increased FDG uptake in the original infection sites, with an expanded range, or new uptake is indicative of recurrent DSWI. On the other side, decreased uptake and reduced range demonstrate that the treatment is effective.
In summary, our data show that 18F‐FDG PET/CT is superior to CT in localising the infection sites and evaluating the range of infections in patients with DSWI after OHS, particularly in the regions of ribs/costal cartilages and mediastinum. Compared with CT, PET/CT could successfully guide surgical debridement with a reduced rate of recurrent DSWI and a shorter hospital stay. In addition, follow‐up PET/CT could potentially evaluate the treatment effect of surgical debridement.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
ACKNOWLEDGEMENTS
This study was commissioned and funded by Shanghai Municipal Key Clinical Specialty (shslczdzk03401).
Liu S, Zhang J, Yin H, Pang L, Wu B, Shi H. The value of 18F‐FDG PET/CT in diagnosing and localising deep sternal wound infection to guide surgical debridement. Int Wound J. 2020;17:1019–1027. 10.1111/iwj.13368
Funding information Shanghai Municipal Key Clinical Specialty, Grant/Award Number: shslczdzk03401
REFERENCES
- 1. Lazar HL, Salm TV, Engelman R, Orgill D, Gordon S. Prevention and management of sternal wound infections. J Thorac Cardiovasc Surg. 2016;152(4):962‐972. [DOI] [PubMed] [Google Scholar]
- 2. Baillot R, Cloutier D, Montalin L, et al. Impact of deep sternal wound infection management with vacuum‐assisted closure therapy followed by sternal osteosynthesis: a 15‐year review of 23499 sternotomies. Eur J Cardiothorac Surg. 2010;37(4):880‐887. [DOI] [PubMed] [Google Scholar]
- 3. Filsoufi F, Castillo JG, Rahmanian PB, et al. Epidemiology of deep sternal wound infection in cardiac surgery. J Cardiothorac Vasc Anesth. 2009;23(4):488‐494. [DOI] [PubMed] [Google Scholar]
- 4. Juhl AA, Hody S, Videbaek TS, Damsgaard TE, Nielsen PH. Deep sternal wound infection after open‐heart surgery: a 13‐year single institution analysis. Ann Thorac Cardiovasc Surg. 2017;23(2):76‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rupprecht L, Schmid C. Deep sternal wound complications: an overview of old and new therapeutic options. Open J Cardiovasc Surg. 2013;6:9‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care‐associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36(5):309‐332. [DOI] [PubMed] [Google Scholar]
- 7. Pairolero PC, Arnold PG. Management of recalcitrant median sternotomy wounds. J Thorac Cardiovasc Surg. 1984;88(3):357‐364. [PubMed] [Google Scholar]
- 8. Gudbjartsson T, Jeppsson A, Sjogren J, et al. Sternal wound infections following open heart surgery—a review. Scand Cardiovasc J. 2016;50(5–6):341‐348. [DOI] [PubMed] [Google Scholar]
- 9. Kaye AE, Kaye AJ, Pahk B, McKenna ML, Low DW. Sternal wound reconstruction. Ann Plast Surg. 2010;64(5):658–666. [DOI] [PubMed] [Google Scholar]
- 10. Fleck T, Fleck M. Negative pressure wound therapy for the treatment of sternal wound infections after cardiac surgery. Int Wound J. 2014;11(3):240‐245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gaudreau G, Costache V, Houde C, et al. Recurrent sternal infection following treatment with negative pressure wound therapy and titanium transverse plate fixation. Eur J Cardiothorac Surg. 2010;37(4):888‐892. [DOI] [PubMed] [Google Scholar]
- 12. Fuster D, Tomas X, Mayoral M, et al. Prospective comparison of whole‐body (18)F‐FDG PET/CT and MRI of the spine in the diagnosis of haematogenous spondylodiscitis. Eur J Nucl Med Mol Imaging. 2015;42(2):264‐271. [DOI] [PubMed] [Google Scholar]
- 13. Treglia G, Sadeghi R, Annunziata S, et al. Diagnostic performance of fluorine‐18‐fluorodeoxyglucose positron emission tomography for the diagnosis of osteomyelitis related to diabetic foot: a systematic review and a meta‐analysis. Foot (Edinburgh, Scotland). 2013;23(4):140‐148. [DOI] [PubMed] [Google Scholar]
- 14. Phan TQ, Depner C, Theodorou P, et al. Failure of secondary wound closure after sternal wound infection following failed initial operative treatment: causes and treatment. Ann Plast Surg. 2013;70(2):216‐221. [DOI] [PubMed] [Google Scholar]
- 15. Revest M, Patrat‐Delon S, Devillers A, Tattevin P, Michelet C. Contribution of 18fluoro‐deoxyglucose PET/CT for the diagnosis of infectious diseases. Med mal Infect. 2014;44(6):251‐260. [DOI] [PubMed] [Google Scholar]
- 16. Vaidyanathan S, Patel CN, Scarsbrook AF, Chowdhury FU. FDG PET/CT in infection and inflammation—current and emerging clinical applications. Clin Radiol. 2015;70(7):787‐800. [DOI] [PubMed] [Google Scholar]
- 17. Keidar Z, Militianu D, Melamed E, Bar‐Shalom R, Israel O. The diabetic foot: initial experience with 18F‐FDG PET/CT. J Nucl Med. 2005;46(3):444‐449. [PubMed] [Google Scholar]
- 18. Goodman LR, Kay HR, Teplick SK, Mundth ED. Complications of median sternotomy: computed tomographic evaluation. AJR Am J Roentgenol. 1983;141(2):225‐230. [DOI] [PubMed] [Google Scholar]
- 19. Wang GL, Zhao K, Liu ZF, Dong MJ, Yang SY. A meta‐analysis of fluorodeoxyglucose‐positron emission tomography versus scintigraphy in the evaluation of suspected osteomyelitis. Nucl Med Commun. 2011;32(12):1134‐1142. [DOI] [PubMed] [Google Scholar]
- 20. Nakahara M, Ito M, Hattori N, et al. 18F‐FDG‐PET/CT better localizes active spinal infection than MRI for successful minimally invasive surgery. Acta Radiol. 2015;56(7):829‐836. [DOI] [PubMed] [Google Scholar]
- 21. Riccio SA, Chu AK, Rabin HR, Kloiber R. Fluorodeoxyglucose positron emission tomography/computed tomography interpretation criteria for assessment of antibiotic treatment response in pyogenic spine infection. Canadian Assoc Radiol J. 2015;66(2):145‐152. [DOI] [PubMed] [Google Scholar]
- 22. Read C, Branford OA, Verjee LS, Wood SH. PET‐CT imaging in patients with chronic sternal wound infections prior to reconstructive surgery: a case series. J Plast Reconstr Aesthet Surg. 2015;68(8):1132‐1137. [DOI] [PubMed] [Google Scholar]


