Abstract
Chronic diabetic foot ulcers (DFUs) are a common problem in patients with diabetes and are often difficult to treat. The application of newly developed dressing material in patients with chronic DFUs has been reported to be effective. The purpose of this study was to evaluate the usefulness of allogeneic keratinocyte treatment for chronic DFUs. We performed weekly allogeneic keratinocyte treatment for up to 12 weeks in 71 patients with intractable DFUs. We investigated healing rate, wound‐healing velocity, and time to 50% wound size reduction and analysed factors affecting ulcer healing. Fifty‐six patients (78.8%) had complete wound healing. Forty‐six patients (64.7%) showed complete healing within an average of 6.1 weeks, and 10 patients (14.1%) showed partial healing with an average 35.5% reduction vs initial size at the end of follow up. The 10 patients who showed partial healing continued to receive treatment after the 12‐week study period. The mean time to complete wound healing was 7.8 weeks. Fifteen patients (21.1%) experienced treatment failure because of infection, local necrosis, no change in ulcer size, or osteomyelitis during the follow‐up period. No adverse events were observed. Allogeneic keratinocyte treatment is effective for chronic, difficult‐to‐treat DFUs.
Keywords: diabetic foot ulcer, keratinocytes, treatment outcome, wound healing
1. INTRODUCTION
In general, patients with diabetes are less likely to recover from injuries compared with people without diabetes. In diabetic feet, there are many concerns to overcome, including microvascular disease, peripheral neuropathy, decreased immunity, concomitant infection, and comorbidities. Multidisciplinary approaches are required to manage issues in diabetic foot ulcers (DFUs), including control of blood glucose, treatment of infection, pressure off‐loading, appropriate use of wound dressing, surgical debridement, and optimisation of vascular perfusion and oxygenation of the lower limbs.1, 2
Therefore, a variety of advanced treatments is needed. Various methods such as growth factor treatment (epidermal growth factor [EGF], platelet‐derived growth factor [PDGF], fibroblast growth factor [FGF]) and hyaluronic acid dressing are being studied and have shown about 30% accelerated wound‐healing rate.3, 4 The wound‐healing process is impaired in the diabetic foot as at least 30% of ulcers fail to heal within 20 weeks of treatment.5 It is the initial treatment that prevents secondary infection because of continuous ulcers and ultimately avoids amputation. Therefore, it has been a challenge to heal chronic DFUs.
Many studies investigating potential ulcer treatments are underway. Recently, cell therapies for diabetic ulcers, including cellular‐based treatment of tissue‐engineered products, have been developed, opening a new horizon for diabetic ulcer treatment. There are effective therapies utilising an underlying mechanism of stimulating cellular activity in the deteriorated diabetic foot. Currently, different types of skin substitutes are available for the treatment of diabetic foot ulceration.6 The most advanced products that are easy to produce with reasonable cost are epidermal substitutes obtained using in vitro expanded keratinocytes applied to a single‐layer carrier. Epithelial cells obtained from a healthy donor can be cultured to help self‐regeneration, and wound‐healing factors secreted from the epithelial cells and cell proliferation accelerate wound healing.
Among a number of alternative treatment options for ulceration, cultured allogeneic keratinocyte sheets have demonstrated efficacy and safety.7 Recently, phase III clinical trials using newborn foreskin products have been conducted.7 Therefore, we conducted a large‐scale, single‐institution observational study examining the effectiveness of allogeneic keratinocyte sheet (Kaloderm; Tego Science, Seoul, Korea) therapy for chronic DFUs. In addition to treatment efficacy, we analysed clinical and healing factors.
2. SUBJECTS, MATERIALS, AND METHODS
This was a prospective observational study in which patients with DFUs were targeted consecutively from a single centre. The Institutional Review Board approved the study protocol, and the study was conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all patients prior to enrolment. The skin substitutes were produced as a manufactured sheet at a cell factory. Those materials were obtained with authorisation from the Korean Food and Drug Administration.
2.1. Patients
Between April 2011 and June 2014, 102 patients with chronic DFUs that had not responded to conventional therapy were screened. The inclusion criteria included age >18 years, type 1 or 2 diabetes mellitus, foot ulcer >1.0 cm2 in size that persisted >6 weeks, Wagner grade of I or II, and transcutaneous oxygen pressure (TcPO2) ≥30 mm Hg or palpable pedal pulse. All patients agreed to participate in the study and signed consent forms. All enrolled patients had previously been subjected to compression dressing for 2 months, debridement, cleansing with saline, and advanced wound dressing without wound improvement. Exclusion criteria included signs of focal or systemic infection, cellulitis, osteomyelitis, systemic inflammatory disease such as rheumatoid arthritis or ankylosing spondylitis, and related spondyloarthropathy. Other exclusion criteria included pregnancy, ulcers with other aetiologies such as deep vein thrombosis and venous insufficiency, current corticosteroid or immunosuppressive agent treatment, present illness or systemic condition that could influence wound healing (e.g., Marfan syndrome, Charcot arthropathy, malignant tumours), and severe lack of nutrition (serum albumin <2.7 g/dL). We excluded 28 patients who did not meet the inclusion criteria (n = 21) or declined to participate (n = 7).
A total of 74 patients were included and observed prospectively. Of these 74 patients, 1 was lost to follow up, and 2 were removed from the study because of serious adverse event (SAE) intervention before completion of the study. Baseline information is shown in Table 1.
Table 1.
Patient baseline characteristicsa
| Demographic factors | ||
|---|---|---|
| Age (years) | 61.48 ± 15.97 | |
| Gender | Male | 49 (69%) |
| Female | 22 (31%) | |
| BMI (kg/m2) | 24.63 ± 6.74 | |
| Current smoker | 23 (32.4%) | |
| Medical status at screening | ||
| Duration of diabetes (years) | 8.56 ± 2.59 | |
| HbA1c (%) | 7.34 ± 2.11 | |
| Hb (g/dL) | 11.79 ± 2.14 | |
| Albumin (g/dL) | 3.71 ± 0.48 | |
| WBC (103/mm3) | 7.12 ± 1.76 | |
| Baseline DFU characteristics | ||
| Duration of ulcers (months) | Mean ± SD | 5.40 ± 8.52 |
| Median | 3.0 | |
| Range (min‐max) | 1.44‐21.6 | |
| Ulcer size (cm2) | Mean ± SD | 8.99 ± 13.99 |
| Median | 3.7 | |
| Range (min‐max) | 1.0‐78.9 | |
| Wagner grade | Grade I (%) | 25 (35.2%) |
| Grade II (%) | 46 (64.8%) | |
| Ulcer location | Dorsal aspect (forefoot: midfoot: hindfoot) | 38 (19: 5: 14) |
| Plantar aspect (forefoot: midfoot: hindfoot) | 33 (22: 4: 7) | |
Abbreviations: BMI, body mass index; DFU, diabetic foot ulcer.
Categorical variables are presented as number (%) of patients.
2.2. Study design
At the first visit, patient eligibility was determined by physical examination including foot radiography, general laboratory tests (routine chemistry screen, urinalysis, complete blood count) and vital signs (eg, blood pressure, body temperature), and a review of the past medical records. The 74 patients included in the study received weekly allogeneic keratinocyte therapy using a keratinocyte sheet (Kaloderm; Tego Science, Seoul, Korea) containing >2 × 107 cells derived from Korean infant foreskin and backed by Vaseline gauze. In the first week after use of the dressing material, daily follow up was performed to guarantee no hypersensitive response or increased exudate from the DFUs. Thereafter, weekly follow ups were considered. Each visit involved a general physical examination including vital signs, assessment of current medication status, DFU evaluation, and use of dressing material as indicated. All adverse events were recorded.
2.3. Treatment
Before applying skin substitutes, we assessed all DFUs for bacterial culture and performed extensive observations, including recording DFU size and gross photography. Wound evaluation was performed at each weekly visit with gross photography until complete ulcer healing or 12 weeks had elapsed. Ulcer size was measured by means of the Visitrak Digital Planimetry Wound Measurement System (Smith & Nephew, London, UK) by a trained wound care nurse who was not an investigator in the study. The Visitrak Digital Planimetry Wound Measurement System consists of a stylus that traced the margin of wound, a depth indicator, and a traced grid that allows marking of the wound.8 For wounds showing slow response to treatment, treatment was continued until complete healing. For wounds showing no response to treatment within 12 weeks, allogeneic keratinocyte treatment was discontinued, and other treatment modalities were initiated.
The following standard dressing protocol was applied1: the bed of the DFU was prepared by cleansing with normal saline and debridement of devitalised tissue.2 Extra debridement was conducted to evacuate necrotic tissue and observe fresh bleeding around the wound margin if necessary.3 The keratinocyte sheet was thawed at room temperature for 5 to 10 minutes.4 After cutting to wound size, the sheet was applied to completely and directly cover the entire ulcer, including the ends of wound.5 A secondary dressing material, polyurethane foam (Medifoam, Genewel, South Korea), was applied.
The dressing was changed at least weekly until ulcer healing or for a maximum of 12 weeks. Depending on the amount of exudation, dressing changes could be performed 2 to 3 times per week. Patient off‐loading was performed with foam dressing and use of an insole, a crutch, and a wheelchair. Any signs of adverse events (AEs) were observed using institutionalised conventions throughout the investigation period.
2.4. Evaluation
Wounds were examined weekly until complete wound closure or through 12 weeks of treatment. A 12‐week treatment period was chosen because a previous pilot study indicated 100% wound healing in the keratinocyte group.7 Complete healing was defined as complete reepithelialisation without discharge in the wound,3 which enabled the patient to shower.7
Clinical outcomes were evaluated with outcome parameters. The primary efficacy parameter was complete healing within the total treatment period after the initial dressing. Secondary efficacy parameters were ulcer healing velocity and time to achieve 50% wound size reduction.7, 8, 9 Safety outcomes were observed during patient visits via reported AEs, laboratory tests, and vital signs.
2.5. Statistical analysis
Data are reported as mean ± SD. A P‐value of <0.05 was considered to be statistically significant. The following variables were included in the analysis: age, gender, body mass index (BMI), duration of diabetes, HbA1c, Hb, WBC, albumin, duration of DFU prior to enrolment in the study, initial DFU size, Wagner grade, location of DFU, healing rate, percentage of wound area reduction, and time to complete ulcer healing. A Kaplan‐Meier survival analysis was adapted to evaluate the median time to 50% size reduction. An expert biomedical statistician analysed these data using SPSS statistical package (version 18.0; IBM corp, SPSS Inc, Chicago, Illinois).
3. RESULTS
3.1. Demographics
A total of 74 patients were enrolled. During the study period, one patient was lost to follow up, and two patients experienced SAEs. As a result, 71 patients were included in the final analysis. Patient demographics, medical status, baseline DFU, location of DFU, and characteristics are presented in Table 1. Patients presented with optimal glucose control as indicated by HbA1c level (g/dL). Other medical variables, including duration of diabetes, nutritional status indicated by serum albumin level, and smoking status, are also presented. The average patient was 61.48 ± 15.97 years of age. There were 23 (32.4%) patients who had a history of minor amputations. No patient experienced a major amputation.
DFU characteristics are described in Table 1. Concomitant conditions are presented in Table 2. Of the 71 patients, 15 (21.1%) were unable to achieve wound closure within the 12‐week period. Among them, 10 achieved complete healing within 1 to 9 weeks after the end of the study with keratinocyte sheet treatment. Two patients switched to hyaluronic acid dressing because there was no progression of healing, and the remaining three patients were lost to follow up.
Table 2.
Concomitant diseases
| Disease | Number (%) |
|---|---|
| Hypertension | 35 (49.3) |
| Renal disorder | 17 (23.9) |
| Cardiac disorder | 11 (15.5) |
| Metabolic disorder | 1 (1.4) |
| Cancer | 3 (4.2) |
| Ophthalmic disorder | 3 (4.2) |
3.2. Primary and secondary endpoints
The analysis of healing status progression and change is shown in Table 3. At baseline, the average wound size was 8.99 ± 13.99 cm2. The primary efficacy parameter was complete ulcer healing rate at week 12.3 The complete wound‐healing rate was 56 of 71 (78.9%). Forty‐six patients (64.8%) were healed within 12 weeks, which we considered the primary efficacy parameter, and 10 (14.1%) were healed after 12 weeks. The average time to complete wound healing was 7.8 weeks (range, 2‐16 weeks).
Table 3.
Diabetic foot ulcer (DFU) analysis: progression of healing status and change in size
| DFU size change (cm2) | |
|---|---|
| Initial assessment | |
| Mean ± SD | 8.99 ± 13.99 |
| Median (range) | 3.70 (1.0‐78.9) |
| Healing velocity (%/week) | |
| Mean ± SD | 22.88 ± 32.17 |
| Median (range) | 16.70 (−11.67‐245.00) |
| Time to 50% size reduction (days) | |
| Mean ± SD | 25.24 ± 22.68 |
| Median (range) | 14.00 (7‐84) |
We evaluated ulcer‐healing velocity and time to achieve 50% wound size reduction as secondary endpoints. The mean wound‐healing velocity was 3.2 ± 4.5% per day. Kaplan‐Meier survival analysis for median time to 50% ulcer size reduction (Figure 1) indicated that, among the patients who achieved complete wound healing, the median duration for 50% ulcer size reduction was 14 days (range, 7‐84 days).
Figure 1.
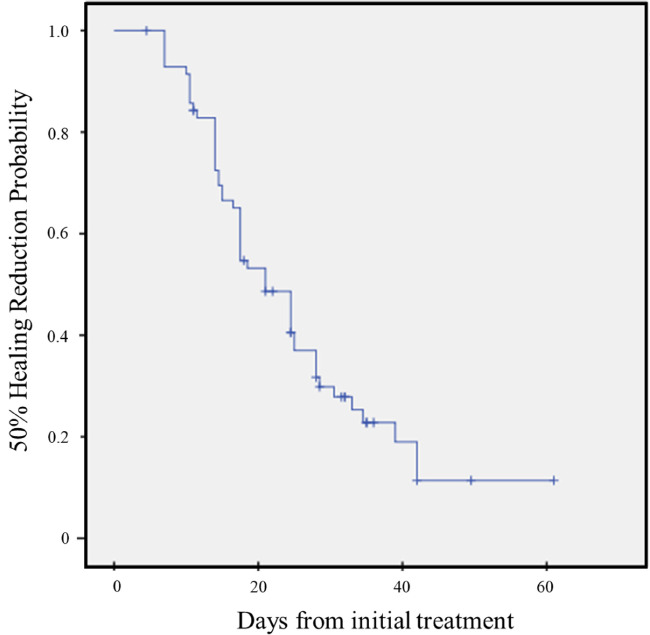
Kaplan‐Meier survival graph showing 50% healing reduction probability on time‐to‐wound closure
No side effects were noted. Two patients whose ulcers did not heal well with keratinocytes eventually underwent surgical debridement because of uncontrolled local infection not related to the study.
3.3. Safety
Two patients (2.8%) experienced AEs of minor local infection with serious discharge. One patient had undergone a surgical marginal resection at the 7th week of the study because of persistent exudation of the ulcer site, and the other patient was unresponsive to treatment because of local erythema and maceration around the ulcer. We also performed surgical debridement at the 6th week after enrolment in our study. However, these events were thought to be related to the patient's concomitant disease (end‐stage renal disease). Patients who developed SAEs were withdrawn from the study. No patient experienced changes from baseline in routine chemistry, haematological tests, urinalysis, or vital signs. In addition, no side effects related to the dressing material were reported.
4. DISCUSSION
In this observational study, we investigated whether newly developed dressing materials were effective in improving healing velocity with grades I or II DFUs that failed previous treatment modalities (Figure 2). To achieve successful treatment of DFUs, a combination of biological and tissue‐engineered dressing materials using keratinocytes have been introduced and examined in previous small pilot studies.7, 10 In this prospective, single‐centre, observational study with a relatively large sample size (74 patients), allogeneic keratinocyte products showed promising results in terms of complete wound‐healing rate, time to achieve 50% size reduction, and time to complete healing. There were no correlations between the consequence of healing velocity or wound healing with gender, age, location of ulcer, concomitant disease, or history of amputation.
Figure 2.
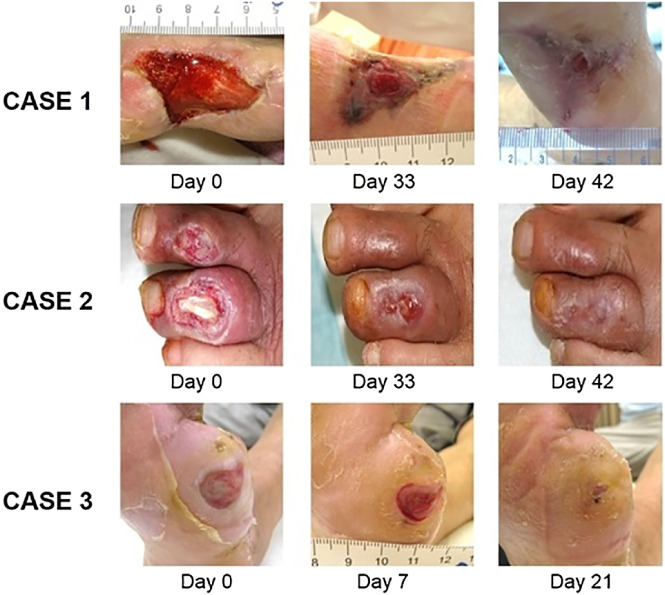
A, Case 1: A 55‐year‐old woman with a diabetic foot ulcer (DFU) that developed 8 weeks prior to the study on the plantar region of her right foot. Wagner classification of the DFU was grade I. Six weeks after allogeneic keratinocyte therapy, the wound was completely epithelialised. B, Case 2: A 45‐year‐old man with a Wagner grade II DFU on the dorsum of left third toe. Six weeks after allogeneic keratinocyte therapy, the wound was completely healed. C, Case 3: A 40‐year‐old woman with a Wagner grade I DFU that developed 6 weeks prior to the study on the plantar region of her right forefoot. Three weeks after allogeneic keratinocyte therapy, the wound was completely epithelialized
There are several reasons for the chronic nature and delayed healing of DFUs. First, within DFUs, keratinocyte proliferation is increased, but differentiation, cell migration, and activity are decreased. Second, extracellular matrix degradation occurs within DFUs. An extracellular matrix in which collagen, proteoglycan, glycosaminoglycan, glycoproteins, and growth factors interact with host cells is necessary for wound healing as these factors stimulate proliferation, differentiation, migration, and regulation mechanisms. Third, growth factors are down‐regulated in DFUs. During the process of ulcer healing, macrophages, neutrophils, fibroblasts, hepatocytes, and endothelial cells produce signals for cell activation. Almost all growth factors are down‐regulated in DFUs; for example, EGF, PDGF, transforming growth factor beta (TGF‐β), insulin‐like growth factor‐1 (IGF‐1), vascular endothelial growth factor (VEGF), and fibroblast growth factor (FGF) are decreased in the diabetic foot. Because of microcirculation dysfunction, essential angiogenesis for the healing DFU is disrupted. Finally, hyper‐regulation of inflammation is ongoing in patients with DFUs. Therefore, the implantation of healthy keratinocytes may be the solution for supplying the above‐mentioned factors to promote healthy wound healing in DFU.
Application of allogeneic keratinocytes has been used to treat chronic wounds that do not respond to conventional therapy. The first report of serial cultivation of allogeneic keratinocytes was in 1975 by Howard Green.11 Clinical uses of cultured allogeneic keratinocytes include treatment of burn injuries and chronic leg ulcers.9, 12 Using specific feeders and serum to inhibit stem cell differentiation, the cells maintain stemness and secrete various growth factors, cytokines, and extracellular matrix and also contain collagenase to inhibit excessive collagen formation and minimise scar formation. Currently, this treatment modality is commercially available in several countries. Allogeneic keratinocytes do not attach directly and do not cover the ulcer permanently and are soon replaced by healthy host keratinocytes. By releasing growth factors (transforming growth factor alpha [TGF‐α], PDGF, basic fibroblast growth factor [bFGF], VEGF, TGF‐β) and cytokines (interleukin [IL]‐1, IL‐6, IL‐8, IL‐10) and by synthesising the major components of the extracellular matrix (fibronectin, laminin) and basement membrane, they are known to promote the migration and proliferation of host keratinocytes.10
Recently, other types of cultured skin derivatives have been investigated. Edmonds et al13 reported a complete healing rate of 51.5% within 12 weeks after allogeneic keratinocyte and fibroblast therapy. Caravaggi et al14 reported a complete healing rate of 65.3% within 11 weeks after use of autologous fibroblast and keratinocyte grafts cultivated on a hyaluronic acid‐derived scaffold. Our study showed 78.9% complete healing in chronic DFUs that were unresponsive to conventional dressing. The current allogeneic keratinocyte dressing method, which is changed once weekly as opposed to the conventional once‐daily dressing method, may alleviate the burden for the patient and reduce the time and cost required for hospital visits. Although not investigated in this study, verbal inquiries from visiting patients indicated that patients were very satisfied with the tested dressing method. In addition, this method is capable of mass production using unrelated allogeneic cells, thus producing a uniform concentration of the product with stored keratinocytes in the Cell Bank.
This study has several limitations. First, the study was uncontrolled and non‐randomised. Second, we did investigate clinical evaluation for peripheral sensory neuropathy with 10 g Semmes‐Weinstein monofilament for some patients; however, the results were not clear. There are some limitations of evaluation with patients who experienced neuropathy combined with vascular insufficiency. Third, the investigators knew the enrolled patients as a result of their involvement in dressing the DFUs, introducing the possibility of observational case series bias. Finally, we did not consider the condition of the wound bed or environmental status around the ulcers, including wound depth and presence of exudate, which are crucial in the treatment of DFUs. However, the positive results presented here in a relatively large patient population compare favourably to previous results with conventional dressing materials. Future blinded, controlled, and randomised studies with a larger sample size and longer‐term follow up of ulcer recurrence and side effects in healed ulcers are needed. Moreover, comparative studies with different material combinations are required to determine the best way to treat DFU using keratinocytes.
In conclusion, our results indicate that cultured allogeneic keratinocyte sheets, which are advanced, safe, and easy to use, are an effective method for the treatment of DFUs.
ACKNOWLEDGEMENTS
This study was supported in part (dressing materials) by grants from Tego Science (Seoul, South Korea). None of the authors have any other conflicts of interest.
Hwang YG, Lee JW, Park KH, Han SH. Allogeneic keratinocyte for intractable chronic diabetic foot ulcers: A prospective observational study. Int Wound J. 2019;16:486–491. 10.1111/iwj.13061
REFERENCES
- 1. Jeffcoate WJ, Harding KG. Diabetic foot ulcers. Lancet. 2003;361(9368):1545‐1551. [DOI] [PubMed] [Google Scholar]
- 2. Apelqvist J, Bakker K, van Houtum WH, Schaper NC. Practical guidelines on the management and prevention of the diabetic foot: based upon the International Consensus on the Diabetic Foot (2007) prepared by the International Working Group on the Diabetic Foot. Diabetes Metab Res Rev. 2008;24(Suppl 1):S181‐S187. [DOI] [PubMed] [Google Scholar]
- 3. Lee M, Han SH, Choi WJ, Chung KH, Lee JW. Hyaluronic acid dressing (Healoderm) in the treatment of diabetic foot ulcer: a prospective, randomized, placebo‐controlled, single‐center study. Wound Repair Regen. 2016;24(3):581‐588. [DOI] [PubMed] [Google Scholar]
- 4. Noh K‐C, Liu XN, Zhuan Z, et al. Leukocyte‐poor platelet‐rich plasma‐derived growth factors enhance human fibroblast proliferation in vitro. Clin Orthop Surg. 2018;10(2):240‐247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Margolis DJ, Allen‐Taylor L, Hoffstad O, Berlin JA. Healing diabetic neuropathic foot ulcers: are we getting better? Diabet Med. 2005;22(2):172‐176. [DOI] [PubMed] [Google Scholar]
- 6. Santema T, Poyck PP, Ubbink DT. Systematic review and meta‐analysis of skin substitutes in the treatment of diabetic foot ulcers: highlights of a Cochrane systematic review. Wound Repair Regen. 2016;24:737‐744. [DOI] [PubMed] [Google Scholar]
- 7. You HJ, Han SK, Lee JW, Chang H. Treatment of diabetic foot ulcers using cultured allogeneic keratinocytes—a pilot study. Wound Repair Regen. 2012;20(4):491‐499. [DOI] [PubMed] [Google Scholar]
- 8. Gethin G, Cowman S. Wound measurement comparing the use of acetate tracings and VisitrakTM digital planimetry. J Clin Nurs. 2006;15(4):422‐427. [DOI] [PubMed] [Google Scholar]
- 9. Harvima IT, Virnes S, Kauppinen L, et al. Cultured allogeneic skin cells are effective in the treatment of chronic diabetic leg and foot ulcers. Acta Derm Venereol. 1999;79(3):217‐220. [DOI] [PubMed] [Google Scholar]
- 10. Vranckx JJ, Hoeller D, Velander PE, et al. Cell suspension cultures of allogenic keratinocytes are efficient carriers for ex vivo gene transfer and accelerate the healing of full‐thickness skin wounds by overexpression of human epidermal growth factor. Wound Repair Regen. 2007;15(5):657‐664. [DOI] [PubMed] [Google Scholar]
- 11. Green H, Rheinwald JG, Sun T‐T. Properties of an epithelial cell type in culture: the epidermal keratinocyte and its dependence on products of the fibroblast. Prog Clin Biol Res. 1977;17:493‐500. [PubMed] [Google Scholar]
- 12. Bolívar‐Flores YJ, Kuri‐Harcuch W. Frozen allogeneic human epidermal cultured sheets for the cure of complicated leg ulcers. Dermatol Surg. 1999;25(8):610‐617. [DOI] [PubMed] [Google Scholar]
- 13. Edmonds M. Apligraf in the treatment of neuropathic diabetic foot ulcers. Int J Low Extrem Wounds. 2009;8(1):11‐18. [DOI] [PubMed] [Google Scholar]
- 14. Caravaggi C, De Giglio R, Pritelli C, et al. HYAFF 11‐based autologous dermal and epidermal grafts in the treatment of noninfected diabetic plantar and dorsal foot ulcers a prospective, multicenter, controlled, randomized clinical trial. Diabetes Care. 2003;26(10):2853‐2859. [DOI] [PubMed] [Google Scholar]


